Abstract
Background
Whipworm (Trichuris trichiura) is a soil-transmitted helminth which infects over a billion people. It is a serious public health problem in many developing countries and can result in deficits in growth and cognitive development. In a follow-up study of a significant heritability for whipworm infection, we conducted the first genome scan for susceptibility to this important parasitic disease.
Methods
We assessed whipworm eggs per gram of feces in 1253 members of the Jirel population of eastern Nepal. All sampled individuals belonged to a single pedigree containing over 26,000 relative pairs that are informative for genetic analysis.
Results
Linkage analysis of genome scan data generated for the pedigree provided unambiguous evidence for two quantitative trait loci influencing susceptibility to whipworm infection, one located on chromosome 9 (LOD = 3.35, genome-wide p = 0.0138) and the other located on chromosome 18 (LOD = 3.29, genome-wide p = 0.0159). There was also suggestive evidence for two loci located on chromosomes 12 and 13 influencing whipworm infection.
Conclusion
The results of this first genome scan for susceptibility to whipworm infection may ultimately lead to the identification of novel targets for vaccine and drug development efforts.
Keywords: Genome scan, linkage analysis, soil-transmitted helminth, Jirel, tropical medicine, Jiri Helminth Project
INTRODUCTION
Whipworm (Trichuris trichiura) infection is estimated to affect over a billion people1. Heavy whipworm loads are associated with severe consequences such as rectal prolapse and dysentery that have an immediate and obvious health impact on the individual2,3. However, even infections that are classified as moderate or light by the World Health Organziation can have significant long-term health impacts3,4.
The relationship between whipworm infection and malnutrition has been documented in numerous human populations throughout the developing world3,5–9. While anthelminthic treatments may help achieve some recovery from the consequences of infection-associated malnutrition6,10,11, the deficits in childhood growth and development associated with whipworm infections have long-term implications8,12. Stunting is evident even in children with light worm loads13.
Trichuris infection has been associated with iron deficiency anemia, especially when other intestinal worm infections are present, in a number of studies3,14–16. Vitamin A deficiency has also been linked to whipworm infection17.
One of the most insidious effects of whipworm is on cognitive development2,3. A study of Filipino children aged between 7 and 18 years of age found that whipworm infection was associated with deficiencies in verbal fluency15. Similarly, a study conducted in Jamaica found significant effects of whipworm infection on cognitive functioning in children between the ages of 9 and 12 years18. The effects of deficits in cognitive development on learning ability may have lifetime consequences3,4.
Like other soil transmitted helminthic infections, whipworm is characteristically overdispersed in human populations, with a small proportion of the available hosts harboring the majority of the parasitic worms19–21. A genetic basis to this apparent predisposition of a small percentage of the population to whipworm infection has been supported by a number of studies22. Our previous work has demonstrated significant heritabilities for susceptibility to infection with Trichuris trichiura in two independent Asian populations23.
Susceptibility to whipworm infection was significantly heritable in a Han Chinese population from Jishan Island in the Jiangxi Province of the People’s Republic of China. Approximately 86% of the population was infected with T. trichiura. A quantitative genetic analysis of the data from the Jishan Island population revealed that 29% of the variation in egg counts was attributable to genetic factors23.
The heritability of susceptibility to whipworm infection was also assessed in the Jirel population of eastern Nepal, the focal population for the analyses reported in this paper. The analyses of data from over 1,200 individuals resulted in a significant heritability of whipworm eggs per gram of feces which indicated that between 28% and 36% of the variation was attributable to genetic factors23.
The earlier analyses of susceptibility to whipworm infection have indicated that genetic factors are involved in determining differential susceptibility to T. trichiura. However, the quantitative genetic approaches used cannot identify the specific genetic factors involved in determining the observed overdispersion of whipworm infections. The purpose of this paper is to present the results of the first genome-wide scan for quantitative trait loci influencing egg counts for Trichuris trichiura infection. The data for this analysis were generated by the Jiri Helminth Project, a long-term genetic study of soil-transmitted helminth infections in the Jirel population of eastern Nepal.
MATERIALS AND METHODS
Study Population
The focal population for this study was the Jirel ethnic group of eastern Nepal. This Tibeto-Burman language speaking group is a hybrid population derived from Sherpa and Sunwar ancestry. Members of the population reside in nine villages in the Jiri region of Dolakha district. The region is at an average elevation of 7500 feet and is approximately 190 kilometers east of the capital city of Kathmandu. Six of the Jirel villages were sampled for the study reported upon here.
Individuals were recruited into the study following a protocol that was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board and the Nepal Health Research Council. A total of 1253 individuals have data for whipworm infection, including 601 males and 652 females. Members of the sample were aged between 3 and 85 years with an average age of 25.4 years.
The Jirel population has been the subject of population genetic and genetic epidemiological research since 1985. As a result a vast amount of information is available on the genetic characteristics of the population and the kinship relationships among members of the population. Pedigree information was collected following the protocol described by Williams-Blangero and Blangero24. The detailed information available on relationships among individuals has allowed the assignment of all sampled individuals to a single highly complex extended pedigree which is extremely powerful for genetic analysis24. The pedigree used for the analyses described here contains over 26,000 pairs of relatives that are informative for genetic analysis23.
Sampling for quantitation of egg counts
Trichuris egg counts were determined as eggs per gram of feces from small fecal samples (approximately 5 grams). Of the 1253 participants, 94.7% provided fecal samples on two consecutive days. All samples were assessed within one hour of delivery to the field laboratory by a medical technologist. Egg counts were determined by the Kato-Katz thick smear technique using a kit developed in Nepal according to the methods outlined by the World Health Organization and implemented following the protocol defined by WHO (1994). Two egg counts were performed on each sample, and the results were averaged. There was no statistically significant difference in egg counts between days. For individuals for whom only one sample was available, the average of the two available egg counts was used as the measure. The trait used for analysis is quantitative egg count.
Analytic Methods
Genotyping Method
Genotyping was performed using the ABI Prism Linkage Mapping Set, version 2. All marker typing was done utilizing automated sequencers (ABI DNA Sequencer Models 377 and 3100). A total of 371 dinucleotide STR markers evenly distributed across the 22 autosomes were characterized for each individual in the sample resulting in a 10cM genome scan for linkage mapping.
Statistical Genetic Methods
Error checking
SimWalk225 was used to check for Mendelian consistency of the genotypic data. This program utilizes Markov chain Monte Carlo and simulated annealing algorithms to determine the probabilities of mistyping for each genotype.
Allele frequency estimation
Allele frequencies were estimated using maximum likelihood techniques as implemented in the computer program SOLAR26. Incorporating the pedigree structure in the estimation of allele frequencies is critical since gene frequency estimates can be significantly biased if the relationships among sample individuals are not taken into account27.
Linkage analysis
We use a variance components linkage method as implemented in the computer program SOLAR26 for all analyses of the genome scan data. We estimated all the elements of a location-specific IBD probability matrix jointly using a Markov chain Monte Carlo method incorporated into the program LOKI 28. Multipoint IBD matrices were then imported into SOLAR for the linkage analyses.
The standard likelihood framework for variance components was used for all parameter estimation and hypothesis testing. We tested the null hypothesis that the additive genetic variance due to the i-th QTL equals zero (no linkage) by comparing the likelihood of the restricted model with that of a model in which the variance due to the i-th QTL was estimated. The difference between the two log10 likelihoods provided the LOD score which is a measure of the support for the hypothesis of linkage over that of “no linkage” at a particular chromosomal location. P-values were determined using a test statistic that is twice the difference in loge likelihoods of the two models and which is asymptotically distributed as a ½:½ mixture of a χ21 variable with a point mass at zero29. Because of the large number of tests required to scan the genome using this linkage procedure, we calculated analytical genome-wide p-values30. Thus, all statistical results of linkage are provided with experiment-wide significance levels.
Because the data on whipworm tended to be leptokurtic, we employed robust LOD scores31,32 when evaluating the log-transformed Trichuris egg counts [ln(EPG + 1)] to avoid the potential problem of non-normality leading to excessive type I error33.
Within each model, we corrected for multiple covariates as potential confounders. For the current analyses, we simultaneously controlled for the effects of sex, age, age2, sex×age, sex×age2, and whether or not an individual had daily access to a latrine (as a marker of potential economic-related hygiene) on ln-transformed Trichuris egg count per gram of feces.
RESULTS
The prevalence of whipworm infection in the Jirel population was 14.4%. The heritability (or familiality) of whipworm egg count in this sample of the Jirel population is highly significant (h2 = 0.38 ± 0.06, p = 6.5×10−17). The genome-wide linkage analyses on the 1253 members of the Jirel population localized two significant QTLs influencing susceptibility to whipworm eggs per gram of feces. Figure 1 presents the string plot of the linkage results for the complete genome scan. Two regions provided genome-wide significance with LOD scores of 3.35 and 3.29 (chromosomes 9 and 18 respectively). Two additional suggestive signals with LOD scores of 2.28 and 2.04 respectively are found on chromosomes 12 and 13.
Figure 1.
String plot of the results of the genome scan for genes influencing susceptibility to infection with Trichuris trichiura.
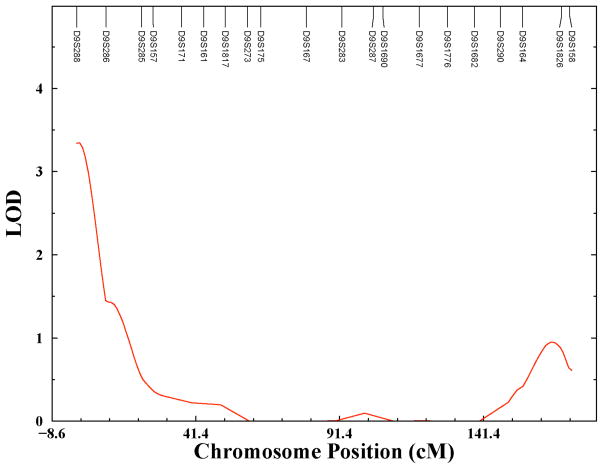
Figure 2 presents the linkage results for chromosome 9 in more detail. The linkage peak on chromosome 9 occurs at 2 cM. The LOD score for this QTL is 3.347 with a genome-wide p-vale of 0.0138 (nominal p-value = 4.3 x 10−5). The chromosomal region of the peak is 9p24. The 1-LOD support interval for the peak spans from the p-terminus to 7 cM, which corresponds approximately to a 14 Mb physical region on chromosome 9.
Figure 2.
Linkage plot for chromosome 9.
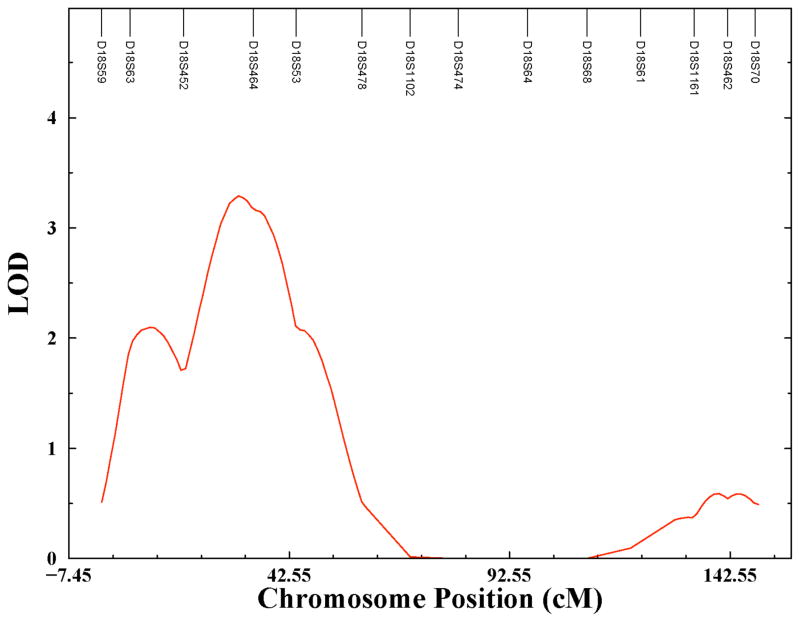
Figure 3 presents similar results for chromosome 18. The peak LOD score on chromosome 18 occurs at 31 cM. The approximate chromosomal location of the peak signal is 18p11. The LOD score for this QTL reaches 3.290 which is significant with a genome-wide p-value of 0.0159 (nominal p-value = 5 x 10−5). The one LOD support interval for this linkage signal is larger than the one on chromosome 9 and spans from 22 to 43 cM. This relatively large genetic region spans a relatively small physical region of approximately 6 Mb on chromosome 18.
Figure 3.
Linkage plot chromosome 18.
The linkage peak on chromosome 13 occurs at 37 cM with a LOD score of 2.276. This result is suggestive of a gene influencing susceptibility to whipworm infection (genome-wide p-value = 0.206, nominal p-value = 0.0006). Thus, in a given genome-scan, we would expect 0.21 occurrences of a LOD score at least this large by chance. Similarly, the other suggestive evidence of a QTL influencing whipworm is on chromosome 12 at 191 cM. This second linkage peak has a LOD score of 2.042 (genome-wide p-value = 0.373, nominal p-value = 0.0011).
Interestingly, there is no overlap between the QTLs influencing whipworm egg counts and the genes localized for infection with Ascaris lumbricoides (Williams-Blangero et al., in press).
DISCUSSION
This study reports the first genome-wide linkage scan for quantitative trait loci influencing susceptibility to infection with Trichuris trichiura. Our findings of at least two significant QTL regions is consistent with the previously reported significant heritability of susceptibility to whipworm infection in this population23. Localization of the quantitative trait loci involved in determination of a complex disease trait is the first step towards gene identification. Given the objective prioritization that such linkage analyses proffer, we now plan to dissect these genomic regions using a combined approach involving in-silico data mining and linkage disequlibrium mapping to nominate positional candidate genes for exhaustive resequencing and formal detection of the most likely functional variants34. Once the specific individual genes influencing whipworm infection have been identified, the information can be used to potentially identify novel drug and vaccine targets35,36. The analyses reported here have unambiguously localized two QTLs influencing whipworm infection, and also provided suggestive evidence for two additional QTLs that may be involved in determining differential susceptibility to whipworm infection.
Preliminary investigation into the potential positional candidate genes within the 1-LOD support interval of these two QTLs identified several interesting possibilities. Within the QTL region on chromosome 9, there are approximately 161 known or predicted genes, most of which have unknown functions. One potential candidate is the JAK2 gene which is found near the linkage peak (at approximately 4.9 Mb). Activation of the Janus-activated kinase 2 (JAK2)/STAT1alpha signaling pathway is suppressed in Leishmania-infected macrophages37 and JAKE2 appears to be involved in the nitric oxide killing response to parasitic infection38. Also in this immediate region on chromosome 9 can be found the PDCD1LG1 (also known as B7H1) genes that are known to be involved in the stimulation of T-cell response with a particularly strong effect on the production of interleukin-1039. IL-10 is well known to be critically involved has playing a role in Trichuris infection in mouse40. Additionally, in humans, IL-10 levels, among other cytokines, have been shown to be predictive factors in whipworm susceptibility41. The whipworm susceptibility QTL on chromosome 18 spans approximately 6 Mb and contains 86 known and predicted genes. One obvious candidate gene in this region is RALBP1 (or RIP1) which is involved in the direction of cell death following stimulation by pathogens42. Overall, as with most genomic regions, the 1-LOD support intervals contain a number of genes with some known function but little is known about most of the genes. Thus, it will be necessary to perform additional studies to probe these genes for functional signals in relation to whipworm susceptibility either by looking for associations with sequence variants or in combination with expression-based studies in relation to worm infection.
Whipworm infection has a huge public health impact, affecting over a billion people and resulting in the loss of millions of years of disability adjusted life years each year3, 43. Numerous studies have shown that individuals are readily reinfected after anthelminthic treatment, indicating that there is little acquired host immunity to whipworm19, 44, 45, 46.
As clearly noted by Bethony and colleagues2, whipworm infections are diseases of poverty and will continue to be major public health problems the developing world until substantial improvements in standard of living are achieved in these areas. The long-term efficacy of mass deworming programs will limited due to logisitical and compliance problems, and to emerging resistance in the parasites2,3. There is a critical need for improved understanding of the biological determinants of differential susceptibility to helminthic infections. The results of this study provide the first step towards the identification of the specific genes influencing differential susceptibility to whipworm infection.
Acknowledgments
The generous cooperation of the Jirel people is gratefully acknowledged. We thank the staff of the Jiri Helminth Project for their contributions to this research. We also thank Angie Olson, Liz Rainwater, Mary Jo Aivaliotis, Mari Hui, and Cheryl Raindl for their expert technical assistance. This study was supported by the National Institutes of Health under grants AI37091 and AI44406 awarded to SWB. The statistical methods applied in the study were developed under MH59490 awarded to JB. The work reported was conducted in part in facilities constructed with support from Research Facilities Improvement Program under grants C06 RR013556 and C06 RR017515 from the National Center for Research Resources, National Institutes of Health.
References
- Albonico M, Smith PG, Ercole E, Hall A, Chwaya HM, Alawi KS, Savioli L. Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Trans R Soc Trop Med Hyg. 1995;89:538–541. doi: 10.1016/0035-9203(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Allison DB, Neale MC, Zannolli R, Schork NJ, Amos CI, Blangero J. Testing the robustness of the likelihood-ratio test in a variance-component quantitative-trait loci-mapping procedure. Am J Hum Genet. 1999;65:531–544. doi: 10.1086/302487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy LA, Blangero J. Multipoint quantitative trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Blangero J, Goring HH, Kent JW, Jr, Williams JT, Peterson CP, Almasy L, Dyer TD. Quantitative trait nucleotide analysis using Bayesian model selection. Hum Biol. 2005;77:541–559. doi: 10.1353/hub.2006.0003. [DOI] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Quantitative trait locus mapping using human pedigrees. Hum Biol. 2000;72:35–62. [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Robust LOD scores for variance component-based linkage analysis. Genet Epidemiol. 2000;19(S1):S8–S14. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Variance component methods for detecting complex trait loci. Adv Genet. 2001;42:151–181. doi: 10.1016/s0065-2660(01)42021-9. [DOI] [PubMed] [Google Scholar]
- Boehnke M. Allele frequency estimation from data on relatives. Am J Hum Genet. 1991;48:22–25. [PMC free article] [PubMed] [Google Scholar]
- Bradley JE, Jackson JA. Immunity, immunoregulation, and the ecology of trichuriasis and ascariasis. Parasite Immunology. 2004;26:429–441. doi: 10.1111/j.0141-9838.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Cooper ES, Thompson DE, Didier JM, Anderson RM, Simmons I. Predisposition to Trichuris trichiura infections in humans. Epidemiol Infect. 1987;98:65–71. doi: 10.1017/s0950268800061719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casapia M, Joseph SA, Nunez C, Rahme E, Gyorkos TW. Parasite risk factors for stunting in grade 5 students in a community of extreme poverty in Peru. Int J Parasitol. 2006;36:741–747. doi: 10.1016/j.ijpara.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Chan MS. The global burden of intestinal nematode infections – Fifty years on. Parasitol Today. 1997;13:438–443. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- Cooper ES, Bundy DAP. Trichuriasis in St. Luci. In: McNeish AS, Walker-Smith JA, editors. Diarrhoea and Malnutrition in Children. Vol. 1986. London: Butterworths; 1986. pp. 91–96. [Google Scholar]
- Gilman RH, Davis C, Fitzgerald F. Heavy Trichuris infection and ameobic dysentery in Orang Asli children. A comparison of the two diseases. Trans R Soc Trop Med Hyg. 1976;70:313–316. doi: 10.1016/0035-9203(76)90085-7. [DOI] [PubMed] [Google Scholar]
- Cooper ED, Bundy DA, MacDonald TT, Golden MH. Growth suppression in the Trichuris dysentery syndrome. Eur J Clin Nutr. 1990;44:285–291. [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Ezeamama AE, Friedman JF, Olveda RM, Acosta LP, Kurtis JD, Mor V, McGarvey ST. Functional significance of low intensity polyparasite helminth infections in anemia. 2005;192:2160–2170. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- Feingold E, Brown PO, Siegmund D. Gaussian models for genetic linkage analysis using complete high-resolution maps of identity by descent. Am J Hum Genet. 1993;53:234–251. [PMC free article] [PubMed] [Google Scholar]
- Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- Forget G, Gregory DJ, Whitcombe LA, Olivier M. Role of host protein tyrosine phosphatase SHP-1 in Leishmania donovani-induced inhibition of nitric oxide production. Infect Immun. 2006;74:6272–6279. doi: 10.1128/IAI.00853-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget G, Gregory DJ, Olivier M. Proteasome-mediated degradation of STAT1alpha following infection of macrophages with Leishmania donovani. J Biol Chem. 2005;280:30542–30549. doi: 10.1074/jbc.M414126200. [DOI] [PubMed] [Google Scholar]
- Forrester JE, Scott ME, Bundy DAP, Golden MHN. Clustering of Ascaris lumbricoides and Trichuris trichiura infections within households. Trans R Soc Trop Med Hyg. 1988;82:282–288. doi: 10.1016/0035-9203(88)90448-8. [DOI] [PubMed] [Google Scholar]
- Gilgen D, Mascie-Taylor CG. The effect of anthelminthic treatment on helminth infection and anaemia. Parasitology. 2001;122:105–110. doi: 10.1017/s0031182000007113. [DOI] [PubMed] [Google Scholar]
- Guyatt H. The economics of worm control. In: Holland CV, Kennedy MW, editors. The Geohelminths: Ascaris, Trichuris, and hookworm. Boston: Kluwer; 2000. pp. 75–87. [Google Scholar]
- Hadju V, Satriono, Abadi K, Stephenson LS. Relationship between soil-transmitted helminthiases and growth in urban slum schoolchildren in Ujung Pandang, Indonesia. Int J Food Sci Nutr. 1997;48:85–93. doi: 10.3109/09637489709006966. [DOI] [PubMed] [Google Scholar]
- Harris T. Genetics, genomics, and drug discovery. Med Res Rev. 2000;20:203–211. doi: 10.1002/(sici)1098-1128(200005)20:3<203::aid-med4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Heath SC. Markov chain Monte Carlo segregation and linkage analysis of oligogenic models. Am J Hum Genet. 1997;61:748–760. doi: 10.1086/515506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RG, Sharp DS, Hughes MC, Akau’ola S, Heinsbroek P, Velayudhan R, Schulz D, Palmer K, Cavalli-Sforza T, Galea G. Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren. Int J Environ Health Res. 2004;14:163–177. doi: 10.1080/0960312042000218589. [DOI] [PubMed] [Google Scholar]
- Jackson JA, Turner JD, Rentoul L, Faulkner H, Behnke JM, Hoyle M, Grencis RK, Else KJ, Kamgno J, Boussinesq M, Bradley JE. T helper cell type 2 responsiveness predicts future susceptibility to gastrointestinal nematodes in humans. J Infect Dis. 2004;190:1804–18011. doi: 10.1086/425014. [DOI] [PubMed] [Google Scholar]
- Kightlinger LK, Seed JR, Kightlinger MB. The epidemiology of Ascaris lumbricoides, Trichuris trichiura, and hookworm in children in the Ranomafana rainforest, Madagascar. J Parasitol. 1995;81:159–169. [PubMed] [Google Scholar]
- Kongsbak K, Wahed MA, Friis H, Thilsted SH. Acute-phase protein levels, diarrhea, Trichuris trichiura and maternal education are predictors of serum retinol: A cross sectional study of children in a Dhaka slum, Bangladesh. Brit J Nutr. 2006;96:725–734. [PubMed] [Google Scholar]
- Larocque R, Casapia M, Gotuzzo E, Gyorkos TW. Relationship between intensity of soil-transmitted helminth infections and anemia during pregnancy. Am J Trop Med Hyg. 2005;73:683–789. [PubMed] [Google Scholar]
- Narain K, Medhi GK, Rajguru SK, Mahanta J. Cure and reinfection patterns of geohelminthic infections after treatment in communities inhabiting the tropical rainforest of Assam, India. Southeast Asian J Trop Med Pub Health. 2004;35:512–517. [PubMed] [Google Scholar]
- Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Robinson BA, Bundy DA. Moderate to light infections of Trichuris trichiura affect cognitive function in Jamaican school children. Parasitol. 1992;104:539–547. doi: 10.1017/s0031182000063800. [DOI] [PubMed] [Google Scholar]
- Ohlstein EH, Johnson AG, Elliott JD, Romanic AM. New strategies in drug discovery. Methods Mol Biol. 2006;316:1–11. doi: 10.1385/1-59259-964-8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quihui-Cota L, Valencia ME, Crompton DW, Phillip S, Hagan P, Diaz-Camacho SP, Triana Tejas A. Prevalence and intensity of intestinal parasitic infections in relations to nutritional status in Mexican schoolchildren. Trans R Soc Trop Med Hyg. 2004;98:653–659. doi: 10.1016/j.trstmh.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Quinnell RJ. Genetics of susceptibility to human helminth infection. Int J Parasitol. 2003;33:1219–1231. doi: 10.1016/s0020-7519(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr, Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- Self SG, Liang KY. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests using nonstandard conditions. J Am Stat Assoc. 1987;82:605–610. [Google Scholar]
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Simeon Dr, Grantham-McGregor SM, Callender JE, Wong MS. Treatment of Trichuris trichiura infections improves growth, spelling scores, and school attendance in some children. J Nutr. 1995;125:1875–1883. doi: 10.1093/jn/125.7.1875. [DOI] [PubMed] [Google Scholar]
- Sobel E, Lange K. Descent graphs in pedigree analysis: Applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet. 1996;58:1323–1337. [PMC free article] [PubMed] [Google Scholar]
- Stephenson LS, Holland CV, Cooper ES. The public health significance of Trichuris trichiura. Parasitology. 2000;121:S73–S95. doi: 10.1017/s0031182000006867. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121:S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- Tshikuka JC, Gray-Donald K, Scott M, Olela KN. Relationship of childhood protein-energy malnutrition and parasite infections in an urban African setting. Trop Med Int Health. 1997;2:374–382. doi: 10.1111/j.1365-3156.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, Blangero J. Collection of pedigree data for genetic analysis in isolate populations. Human Biology. 2006;78:89–101. doi: 10.1353/hub.2006.0023. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, McGarvey ST, Subedi J, Wiest PM, Upadhayay RP, Rai DR, Jha B, Olds GR, Guanling W, Blangero J. Genetic component to susceptibility to Trichuris trichiura Evidence from two Asian populations. Genet Epidemiol. 2002;11:254–264. doi: 10.1002/gepi.0187. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, VandeBerg JL, Subedi J, Aivaliotis MJ, Rai DR, Upadhayay RP, Jha B, Blangero J. Genes on chromosomes 1 and 13 have significant effects on Ascaris infection. Proc Natl Acad Sci, USA. 2002;99:5533–5538. doi: 10.1073/pnas.082115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WM, Dufour DL, Staten LK, Barac-Nieto M, Reina JC, Spurr GB. Gastrointestinal parasitic infection, anthropometrics, nutritional status, and physical work capacity in Colombian boys. Am J Hum Biol. 1999;11:763–771. doi: 10.1002/(SICI)1520-6300(199911/12)11:6<763::AID-AJHB6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:i–57. [PubMed] [Google Scholar]