Abstract
Background
Obesity is associated with increased mortality in the general population but, paradoxically, with decreased mortality in individuals with diabetes.
Methods
Among 88,373 French women participating in the E3N-EPIC study who were free of diabetes in 1990, we estimated the mortality hazard ratios (HR) and 95% confidence intervals (CI) for body-mass index (BMI) levels by diabetes status.
Results
During an average 16.7 years of follow-up, 3,750 deaths and 2,421 incident diabetes cases occurred. In overweight/obese versus normal weight women, the mortality HR (95%CI) was 1.42 (1.32,1.53) in women without diabetes, and 0.69 (0.40,1.18) in women with incident diabetes. Mortality increased with BMI among women without diabetes and decreased as BMI increased in women with diabetes.
Conclusions
We found a direct association between BMI and mortality among women without diabetes but not among those with incident diabetes in the same population. Selection bias may be a simple explanation for this “paradox”.
Obesity is associated with increased mortality in the general population but with decreased mortality in individuals with chronic disease (e.g. diabetes).2–4 This so-called obesity paradox has led some to suggest that patients with established chronic disease should avoid weight loss.5 However, the obesity “paradox” might just be a selection bias6 that arises from a misguided analysis.7–9
Interestingly, despite the increasing interest in this topic,10 the obesity “paradox” has not been empirically described in a prospective study of individuals without chronic disease at baseline. Here we (i)provide such description, (ii)propose a likely explanation for the “paradox” and, (iii)discuss its practical implications.
i)Empirical illustration of the “paradox”
Our analysis included 88,373 French women in the E3N Study11 followed through mailed questionnaires between 1990 (baseline) and 2007 who were free of diabetes and had a BMI ≥18.5 kg/m2 at baseline (see eAppendix). We defined normal weight in 1990 as BMI 18.5–24.9 kg/m2 and overweight/obesity as BMI ≥25 kg/m2. Self-reported cases of diabetes were confirmed using supplementary questionnaires and a drug reimbursement database (eAppendix Figure 1 and eAppendix Table 1). Deaths were identified through the health insurance plan, postal service, and next-of-kin. We estimated unadjusted incidence rates and fit Cox regression models adjusted for baseline covariates (marital status, education, menopause, hormone therapy use, physical activity, smoking, hypertension, cardiovascular disease, cancer) to estimate mortality hazard ratios (HR) for overweight/obesity versus normal weight, and for BMI categories 18.5–22.4, 25.0–27.4, 27.5–29.9, and ≥30 versus 22.5–24.9 kg/m2.
After an average 16.7 years of follow-up, 3,750 women died and 2,421 had incident diabetes. [see eAppendix Table 2 for age-adjusted characteristics by BMI group]. Overweight/obese women had higher mortality and diabetes incidence rates (38.3 and 56.0 per 10,000 person-years, respectively) than normal weight women (22.6 and 7.9 per 10,000 person-years, respectively). The adjusted HR (95% CI) for overweight/obesity versus normal weight was 6.10 (5.60, 6.64) for diabetes, and 1.33 (1.23, 1.43) for mortality (Table 1). Results did not materially change after excluding women with cancer/cardiovascular disease at baseline and smokers. Mortality increased with BMI (eAppendix Figure 2).
Table 1.
Hazard Ratios of Diabetes and All-cause mortality by overweight/obesity status at baseline, E3N study 1990–2007
| Cases | Person-years | Incidence rate per 10,000 person-years | Hazard Ratios (95%CI) | ||
|---|---|---|---|---|---|
| Baseline BMI | |||||
| Diabetes | <25 | 963 | 1,225,210 | 7.9 | 1.00 |
| ≥25 | 1,458 | 258,926 | 56.3 | 6.10 (5.60–6.64) | |
| Mortality | <25 | 2,740 | 1,214,797 | 22.3 | 1.00 |
| ≥25 | 1,010 | 263,618 | 38.3 | 1.33 (1.23–1.43) |
CI: Confidence interval; BMI: Body mass index.
Hazard ratios adjusted for marital status, highest level of educational level attainment, menopausal status and menopause hormone therapy use, physical activity, smoking status (never, past and current), treated hypertension, cardiovascular disease (stroke, myocardial infarction and angina) and cancer at baseline.
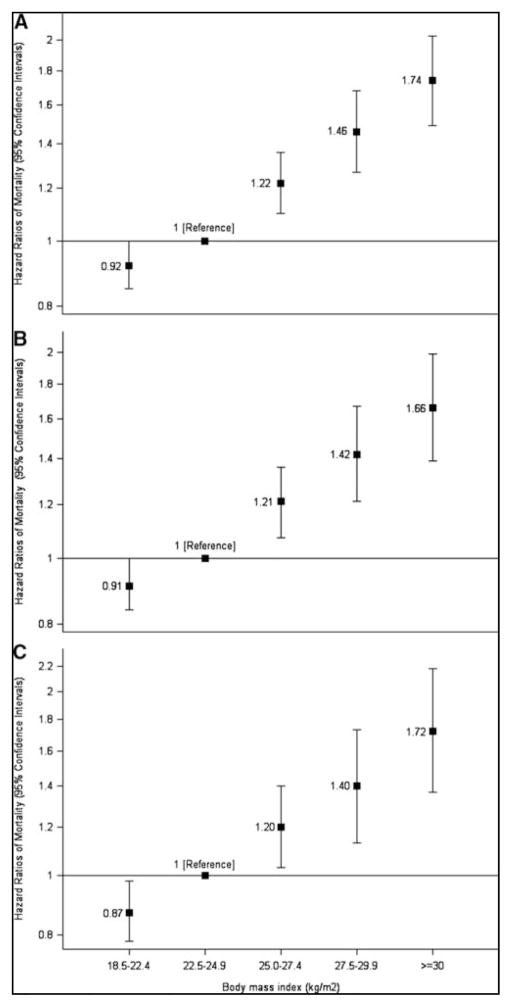
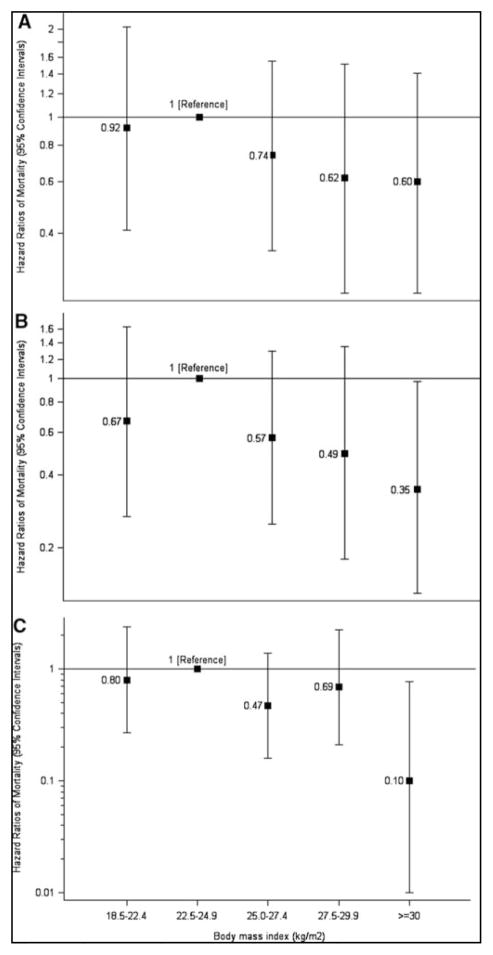
Among women without diabetes, mortality was higher in the overweight/obese than in normal-weight (38.8 vs. 22.5 per 10,000 person-years). The mortality HR was 1.42 (1.32, 1.53) and did not change after exclusion of smokers and women with cancer or cardiovascular disease (Table 2). Mortality increased with BMI (Figure 1). Conversely, among women with diabetes, mortality was lower in overweight/obese individuals than in normal-weight individuals (26.2 vs. 43.3 per 10,000 person-years). The mortality HR was 0.69 (0.40, 1.18) (Table 2). After excluding women with cancer/cardiovascular disease and smokers the HR was 0.41 (0.18, 0.92). Mortality decreased as BMI increased (Figure 2). These findings illustrate the “paradox” via a direct comparison between women with and without diabetes from the same population.
Table 2.
Hazard Ratios of All-Cause Mortality by Overweight/Obesity Status at Baseline, Stratified by Diabetes, E3N study 1990–2007
| No diabetes | Incident Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline BMI | Deaths | Person-years | Mortality rate per 10,000 person-years | Hazard Ratios (95%CI) | Deaths | Person-years | Mortality rate per 10,000 person-years | Hazard Ratios (95%CI) |
| All (n=83,373) | ||||||||
| <25 | 2,714 | 1,208,798 | 22.5 | 1.00 | 26 | 5,998 | 43.3 | 1.00 |
| ≥25 | 982 | 252,922 | 38.8 | 1.42 (1.32–1.53) | 28 | 10,695 | 26.2 | 0.69 (0.40–1.18) |
| Excluding women with chronic disease (n=83,288) | ||||||||
| <25 | 2,192 | 1,146,877 | 19.1 | 1.00 | 23 | 5,617 | 40.9 | 1.00 |
| ≥25 | 755 | 235,470 | 32.1 | 1.40 (1.28–1.52) | 19 | 9,850 | 19.3 | 0.54 (0.29–1.01) |
| Excluding women with chronic disease and smokers (n=45,195) | ||||||||
| <25 | 1,206 | 617,685 | 19.5 | 1.00 | 15 | 3083 | 48.7 | 1.00 |
| ≥25 | 445 | 132,064 | 33.7 | 1.43 (1.28–1.60) | 10 | 5381 | 18.6 | 0.41 (0.18–0.92) |
CI: Confidence interval; BMI: Body mass index in kg/m2 at baseline in 1990.
Hazard ratios adjusted for marital status, highest level of educational attainment, menopausal status and menopause hormone therapy use, physical activity, smoking status (never, past and current), treated hypertension, cardiovascular disease (stroke, myocardial infarction and angina) and cancer at baseline.
Figure 1. Hazard Ratios of All-Cause Mortality by Body Mass Index among Women without Diabetes (Panel A), excluding Women with Chronic Disease (Panel B), and additionally excluding Women Who Had Ever Smoked (Panel C).
Hazard ratios were adjusted for age, marital status, highest level of educational level attainment, menopausal status and menopause hormone therapy use, physical activity, smoking status (never, past and current), cardiovascular disease (stroke, myocardial infarction and angina), cancer and treated hypertension.
I bars denote 95 percent confidence intervals. P for trend: Panel A <0.0001; Panel B <0.0001; Panel C <0.0001.
Figure 2. Hazard Ratios of All-Cause Mortality by Body Mass Index among Women with Diabetes (Panel A), excluding Women with Chronic Disease (Panel B), and additionally excluding Women Who Had Ever Smoked (Panel C).
Hazard ratios were adjusted for age, marital status, highest level of educational level attainment, menopausal status and menopause hormone therapy use, physical activity, smoking status (never, past and current), cardiovascular disease (stroke, myocardial infarction and angina), cancer and treated hypertension.
I bars denote 95 percent confidence intervals. P for trend: Panel A 0.20; Panel B 0.08; Panel C 0.02.
In sensitivity analyses, we found similar estimates when we i)used the BMI just before diabetes diagnosis [eAppendix Table 3]; ii)used BMI as a time-varying exposure; iii)repeated the analyses starting follow-up in 1993 (when dietary information was first available) and adjusted for coffee, fruits and vegetables, and processed red meat intake [eAppendix Table 4]; iv)replaced BMI by waist circumference and started the follow-up in 1994[eAppendix Table 5]; and v)censored women after two missed questionnaires.
ii)Explanation of the “paradox”
Biological explanations for the “paradox” rely on potential benefits of obesity, 12,13,14 or differences2 (perhaps genetic15) between normal-weight and overweight/obese individuals with diabetes that put normal-weight individuals at a higher mortality risk. However, there is an explanation that does not require to posit any benefits of elevated BMI: selection bias due to conditioning on a variable affected by exposure.7–9
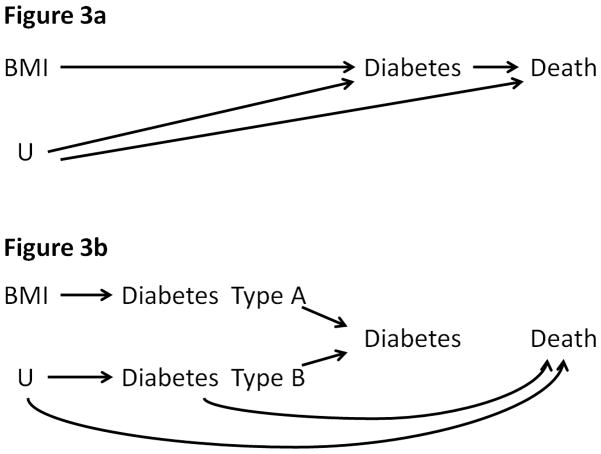
An analysis restricted to individuals with diabetes is conditioned on a variable (diabetes) that is affected by exposure (BMI) and unmeasured risk factors for mortality such as lifestyle and genes. According to the simplified causal diagram in Figure 3a, stratifying the analysis on diabetes (a collider) will generally induce an association between BMI and mortality through other common causes U, even if no association existed in the unstratified analysis. Similar causal diagrams have been proposed to explain the birth weight “paradox” 16 and some components of Simpson’s “paradox”.17
Figure 3. Simplified Causal Diagram to Represent the Association between BMI and Mortality when Conditioning on Diabetes.
U, other unmeasured risk factors. The graph is conditional on measured confounders and, for simplicity, assumes a null direct “effect” of BMI on mortality.
To see how the bias arises, first suppose that the only causes of diabetes are obesity and a genetic factor that, independently, increases the risk of mortality. In this oversimplified scenario, all individuals with diabetes are either obese or have the genetic factor (or both), and all normal-weight individuals with diabetes necessarily have the genetic factor that results in higher mortality. In other words, an inverse association between obesity and mortality---the “paradox”---is expected among individuals with diabetes. This inverse association is also expected in the more realistic setting with multiple causes of diabetes other than obesity and the genetic factor (e.g., lifestyle). In this case, normal-weight individuals with diabetes are more likely to have other risk factors for mortality.
The above explanation can also accommodate the possibility that diabetes may be a collection of similar diseases with different etiologies and with different effects on mortality. Suppose that Diabetes – Type A is a disorder caused by high BMI, that Diabetes – Type B is a condition caused by other causes U, and that there are no common causes of Types A and B. Also suppose that Diabetes –Type B, but not Type A, increases mortality. This scenario is depicted in Figure 3b, which is an elaboration of Figure 3a. Under this scenario an analysis restricted to patients with Diabetes –Type A would not introduce selection bias (Diabetes –Type A is not a collider). However, because the type of diabetes is unknown in practice, any analysis restricted to individuals with diabetes (without specifying the type) will be conditioned on a collider and may therefore introduce selection bias. The bias has a structure similar to that of the birth weight paradox16, some components of Simpson’s paradox17, Berkson’s fallacy18, adjustment for time-varying confounders affected by prior treatment6, and including prevalent users in drug safety studies19,20.
Our analysis has several strengths that help rule out alternative explanations---other than conditioning on a collider--- to the “paradox”. These strengths include measurement of BMI before the diagnosis of diabetes, a long-term follow-up, use of incident cases of confirmed diabetes, no differential access to health care among participants, and a detailed assessment of lifestyle factors and clinical diagnoses. However, compared with other cohorts,2 E3N participants are leaner, which limits our ability to study high levels of BMI.
iii)Practical implications of the “paradox”
If the “paradox” is indeed an example of selection bias, then the lower mortality risk in overweight diabetics should not be the basis for weight management recommendations to individuals with diabetes. Similarly, the lower mortality in low birth weight babies of smokers (the birth weight “paradox”16) should not be the basis to recommend maternal smoking, and the lower risk of heart disease in prevalent users of estrogen plus progestin hormone therapy should not be the basis to recommend preventive hormone therapy21. The argument that conditioning on diabetes is necessary to estimate the effect of BMI in diabetics and non-diabetics separately is invalid for the same reason that one cannot generally estimate effects of a randomized treatment within levels of a post-randomization variable.
The implications of the obesity paradox are harder to describe than those of similar paradoxes because “the causal effect of BMI” is an ill-defined concept.22,23 Even in the absence of confounding and other biases, it is unclear what causal effect, if any, is estimated when BMI is the exposure. For the same reason, conditioning on diabetes after baseline does not guarantee that the estimates can be interpreted as the direct effects of BMI on mortality, i.e., effects not mediated through diabetes. [If the effect of BMI were well defined, one would still need to condition on all common causes of diabetes and mortality (e.g., genetic and lifestyle factors),24,25 which is generally impossible, for an unbiased estimation of direct effects.]
Leaving aside causal inference considerations, suppose the goal is to estimate the association between baseline BMI and mortality in a population of patients without chronic disease at baseline. Then the “paradox” resolves itself by simply not conditioning on post-baseline disease. Studies restricted to patients with prevalent disease are conditioned on post-baseline disease by design and thus are not appropriate to estimate the association between BMI and mortality. One can attempt to reconstruct this association using external data and strong statistical assumptions,9 but a safer method is to refrain from conducting these analyses in studies restricted to patients with prevalent disease and to focus our research efforts on unrestricted studies. Of course, the same problem arises in studies with patients without chronic disease at baseline when the analysis is restricted to patients with disease.
In summary, the inverse association observed between BMI and mortality among patients with diabetes, and perhaps other chronic diseases, could be explained by selection bias due to stratification on a variable affected by the exposure. Being explicit about the causal question of interest and the causal assumptions helps clarify and interpret observations that may seem paradoxical at first sight.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the Mutuelle Générale de l’Education Nationale, French League against Cancer, Gustave Roussy Institute, French Institute of Health and Medical Research, and Inserm-INSP Associated International Lab. The validation of type 2 diabetes cases was supported by the European Union (Integrated Project LSHM-CT-2006-037197 in the Framework Program 6 of the European Community) InterAct project. This research was partly supported by NIH grant R01 HL080644. M. Lajous was supported by the National Council for Science and Technology (CONACYT, Mexico).
We are indebted to the participants in the Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale (E3N) for their continuing dedication and support.
References
- 1.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1. 46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308(6):581–90. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1):13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring) 2008;16(2):442–50. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, von Haehling S. The obesity paradox in heart failure: accepting reality and making rational decisions. Clin Pharmacol Ther. 2011;90(1):188–90. doi: 10.1038/clpt.2011.72. [DOI] [PubMed] [Google Scholar]
- 6.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira I, Stehouwer CD. Obesity paradox or inappropriate study designs? Time for life-course epidemiology. J Hypertens. 2012;30(12):2271–5. doi: 10.1097/HJH.0b013e32835b4fe0. [DOI] [PubMed] [Google Scholar]
- 8.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305(8):822–3. doi: 10.1001/jama.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banack HR, Kaufman JS. The “obesity paradox” explained. Epidemiology. 2013;24(3):461–2. doi: 10.1097/EDE.0b013e31828c776c. [DOI] [PubMed] [Google Scholar]
- 10.Lainscak M, von Haehling S, Doehner W, Anker SD. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2012;3(1):1–4. doi: 10.1007/s13539-012-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, Clavel-Chapelon F, Thiebaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, Gonzalez CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 12.Tseng CH. Obesity paradox: Differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2012 doi: 10.1016/j.atherosclerosis.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Kastorini CM, Panagiotakos DB. The obesity paradox: methodological considerations based on epidemiological and clinical evidence--new insights. Maturitas. 2012;72(3):220–4. doi: 10.1016/j.maturitas.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Wacholder S. Precursors in cancer epidemiology: aligning definition and function. Cancer Epidemiol Biomarkers Prev. 2013;22(4):521–7. doi: 10.1158/1055-9965.EPI-13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry JR, Voight BF, Yengo L, Amin N, Dupuis J, Ganser M, Grallert H, Navarro P, Li M, Qi L, Steinthorsdottir V, Scott RA, Almgren P, Arking DE, Aulchenko Y, Balkau B, Benediktsson R, Bergman RN, Boerwinkle E, Bonnycastle L, Burtt NP, Campbell H, Charpentier G, Collins FS, Gieger C, Green T, Hadjadj S, Hattersley AT, Herder C, Hofman A, Johnson AD, Kottgen A, Kraft P, Labrune Y, Langenberg C, Manning AK, Mohlke KL, Morris AP, Oostra B, Pankow J, Petersen AK, Pramstaller PP, Prokopenko I, Rathmann W, Rayner W, Roden M, Rudan I, Rybin D, Scott LJ, Sigurdsson G, Sladek R, Thorleifsson G, Thorsteinsdottir U, Tuomilehto J, Uitterlinden AG, Vivequin S, Weedon MN, Wright AF, Hu FB, Illig T, Kao L, Meigs JB, Wilson JF, Stefansson K, van Duijn C, Altschuler D, Morris AD, Boehnke M, McCarthy MI, Froguel P, Palmer CN, Wareham NJ, Groop L, Frayling TM, Cauchi S Magic Consortium D, Consortium G. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012;8(5):e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164(11):1115–20. doi: 10.1093/aje/kwj275. [DOI] [PubMed] [Google Scholar]
- 17.Hernan MA, Clayton D, Keiding N. The Simpson’s paradox unraveled. Int J Epidemiol. 2011;40(3):780–5. doi: 10.1093/ije/dyr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snoep JD, Morabia A, Hernandez-Diaz S, Hernan MA, Vandenbroucke JP. A structural approach to Berkson’s fallacy. International Journal of Epidemiology. 2013 doi: 10.1093/ije/dyu026. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernan MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, Manson JE, Robins JM. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–79. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250–62. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernan MA, Taubman SL. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int J Obes (Lond) 2008;32 (Suppl 3):S8–14. doi: 10.1038/ijo.2008.82. [DOI] [PubMed] [Google Scholar]
- 23.Hernan MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22(3):368–77. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–5. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 25.Robins JM. A new approach to causal inference in mortality studies with sustained exposure periods - Application to control of the healthy worker survivor effect. Mathematical Modelling. 1986;7:1393–1512. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.