Abstract
Introduction
It is unknown how evoked myotonia varies with stimulus frequency or train length, or how it compares to voluntary myotonia in myotonic dystrophy type 1 (DM1).
Methods
First dorsal interosseous (FDI) tetanic contractions evoked by trains of 10–20 ulnar nerve stimuli at 10–50 Hz were recorded in 10 DM1 patients and 10 normals. For comparison, maximum voluntary handgrip contractions were also recorded. An automated computer program placed cursors along the declining (relaxation) phase of the force recordings at 90 and 5% of peak force (PF) and calculated relaxation times (RTs) between these points.
Results
For all stimulus frequencies and train lengths, evoked RTs were much shorter, and evoked PFs were much greater in normals than in DM1. In normals, evoked RT was independent of stimulus frequency and train length, while in DM1, RT was longer for train lengths of 20 stimuli (mean:9 seconds in DM1; 0.20 in normals) than for 10 stimuli (mean:3 seconds in DM1, 0.19 in normals), but it did not change with stimulus frequency. In both groups, PF increased greatly as stimulus frequency rose from 10 to 50 Hz but only slightly as train length rose from 10 to 20 stimuli. Voluntary handgrip RT (mean:1.9 seconds) was less than evoked FDI RT (mean:9 seconds).
Conclusions
In DM1, evoked RT can be “dialed up” by increasing stimulus train length. Evoked myotonia testing utilizing a stimulus paradigm of at least 20 stimuli at 30–50 Hz may be useful in anti-myotonic drug trials, particularly when grip RT is normal or equivocal.
Keywords: myotonia, myotonic dystrophy, handgrip, relaxation time
INTRODUCTION
In a previous study of evoked myotonia of the first dorsal interosseous muscle in DM1 patients,1 it was shown that myotonia can be quantified reliably using an automated analysis of muscle relaxation time (RT). Using this technique, it was found that evoked RT is prolonged in most DM1 patients. It is most prolonged in the terminal rather than the initial phase of relaxation, and is there is a positive correlation with CTG repeat length. It was also shown that RT of tetanic contraction is disproportionately prolonged compared to that of single twitch contractions, but only one tetanic stimulus paradigm was employed: 10 stimuli at 10 Hz. The purpose of this study was to explore the dependence of RT on stimulus frequency and train length to determine the optimal stimulus parameters for evoked myotonia, and to compare the severity of evoked RT with contralateral voluntary grip RT. These assessments have practical implications for clinical trials that evaluate ant-myotonic drugs. In addition, to further elucidate the pathophysiology of myotonia in DM1, we were interested to know if RT varies predictably with stimulus frequency and train length in a manner that is consistent with the potassium hypothesis2 that has been proposed to explain the development of myotonia in the presence of chloride channel dysfunction and was recently demonstrated in DM1.3,4
MATERIALS and METHODS
Study Participants
After approval by our medical center institutional review board, 10 DM1 patients and 10 age- and gender-matched controls were evaluated prospectively. The DM1 patients were part of a larger cohort of patients in a single arm NIH-funded trial to assess the safety of recombinant human insulin-like growth factor 1 complexed with IGF binding protein 3 (rhIGF1/IGFBP3) IPLEXtm as a treatment for myotonia. Patients who participated in the study were clinically diagnosed with DM1.5 They also possessed an abnormal expansion of the CTG repeat in the myotonic dystrophy gene (DMPK) as demonstrated by genetic analysis of leukocyte DNA.6–8 Inclusion Criteria were: age between 18 and 80 years; [CTG]n repeat size in DM gene >100 repeats; delayed relaxation of grip of three seconds or more after maximum voluntary contraction; percussion myotonia of forearm extensor and thenar muscles. Exclusion criteria were coexistence of another neuromuscular disease; drug or alcohol abuse within three months of enrollment; treatment with growth hormone, insulin-like growth factor, or testosterone in the previous 6 months; any orthopedic, rheumatologic, cardiac, or pulmonary disorders that would preclude proper positioning on the myometry testing table or restrict the patient’s ability to tolerate evoked or maximum voluntary muscle contractions; and inability to give informed consent as a result of a serious neurocognitive disorder or a major psychiatric illness. The normal volunteers were healthy staff members of the University of Rochester Medical Center.
Evoked Myotonia Measurements in the left first dorsal interosseous
Except for the stimulus paradigm, the method employed to measure evoked myotonia in this study was the same as that described previously.1 In brief, the left arm of each subject was placed in an adjustable support with the left forearm secured to the armrest with Velcro wrap, the left elbow flexed at 90 degrees, forearm pronated, hand supported at the wrist, second digit secured in a ring clamp attached to a force transducer (Daytronic, model 3570), and the thumb abducted and secured to an adjustable rod with paper tape. The ring force transducer was oriented horizontally and medial to the forearm thus selectively measuring, by compression of the load cell, the contraction force of index finger abduction. A bipolar surface bar electrode placed over the left ulnar nerve just proximal to the wrist was used to stimulate the nerve supramaximally. Each of the standardized trials consisted of one single stimulus trial, followed by 5 tetanic stimulus trials, each separated by a ten-minute rest period. The tetanic trials were in the following order: 10 stimuli at 10 Hz, 10 stimuli at 50 Hz, 10 stimuli at 30 Hz, 20 stimuli at 50 Hz, 20 stimuli at 30 Hz. The output of the force transducer was received and digitized by a Model 3570 Daytronic DC Strain Gauge Conditioner and in real-time transmitted to a stimulator-triggered Nicolet 420 Digital Oscilloscope that recorded and stored each trial. Surface temperature of the dorsum of the hand was monitored throughout the testing.
In 7 of the 10 patients, myotonia evoked by 20 stimulus trains at 30 and 50 Hz was repeated 6–12 weeks after the initial study; the remaining 3 patients were evaluated only once.
Voluntary grip myotonia measurements in the contralateral right finger flexors
The contralateral right arm was used for measuring grip RTs after a maximal voluntary isometric contraction as described previously.9,10 In brief, study participants were seated with standardized forearm and hand placement using labeled pegboards, with their fingers grasping the handle of an ergometer.9 Squeezing the ergometer produced an analog force signal that was digitized by an analog-to-digital converter, graphically displayed in real-time on a monitor, and stored for later off-line analysis. Each trial consisted of six maximal voluntary squeezes on the ergometer. Each squeeze lasted 3 seconds, with a 10-second rest period between each squeeze. Three sets of measurements (trials) were performed, but only the initial squeeze of the initial trial was used for this analysis. The standardized voluntary muscle contraction protocol was the same for all 10 patients. The normal control subjects did not undergo handgrip testing.
Off-line analysis of isometric force recordings
For both the evoked first dorsal interosseous and voluntary grip muscle contractions, an automated computer program first determined peak force (PF), and then placed cursors on the declining, relaxation phase of the force recording at various levels of PF: 100 %, 90%, 50%, 25%, 10%, and 5%.1,9,10 RT was then calculated between these designated points. The RTs measured in the current study were the times required for decline in force from 90 to 5% of PF. Finally, in order to normalize evoked RT for the actual drop in PF in grams rather than percent, the ratio of RT (second) / PF (g) was calculated for both normal subjects and patients. As in our previous studies, we reported force in g or Kg units rather than converting the force to Newtons.
Statistical analysis
Evoked myotonia
After confirming that the data was normally distributed, the Student’s t test was used to compare the PF, RT and RT/PF values for the normal versus the patient groups. The paired student’s t test (for normally distributed data) or paired Wilcoxan signed rank test (for non-normally distributed data) were used to compare PF, 90-5% RT, and RT/PF in one stimulus paradigm versus another for patients and controls in order to determine whether: 1) these measurements increased with increase in stimulus frequency (e.g., RT values from 10 Hz - 10 stimuli versus that for 30 and 50 Hz - 10 stimuli), or 2) with stimulus train length (e.g., RT values from 50 Hz - 10 stimuli versus 50 Hz - 20 stimuli, and 30 Hz - 10 stimuli versus 30 Hz – 20 stimuli).
In the 7 patients who underwent repeat evoked myotonia testing, test re-test reproducibility of the 90-5% RT was determined as before1: (max RT – min RT)/max RT). In addition, the correlation between the first and second RT measurement was determined using the non-parametric Spearman correlation coefficient.
Grip myotonia
After confirming that the data were normally distributed, the Student’s t test was used to compare the PF and RT values for the patient group with the RT and PF obtained from our previous normal control group.10 In the patient group, grip RT was compared to first dorsal interosseous evoked RT at the various stimulus frequencies and train lengths.
All p-values represented 2-tailed comparisons and were adjusted for asymmetries in variance.
RESULTS
The normal control group was well matched to the patient group with respect to age [mean (range):38 (24–58) for normals, 39 (27–54) for patients], gender (5 women and 5 men for each group), and surface hand temperature [mean (range): 29.8 (26.5–32.6) for normals and 28.8 (26.5–31.6) for patients]. The CTG repeat size for the patient group ranged from 159–866 (mean 435). Evoked PF was reduced, and RT and RT/PF was prolonged in patients compared to normals for all stimulus paradigms (Table). Similarly, grip PF was reduced, and grip RT (90-5 %) was prolonged in our current patient group versus our historical control subjects.10
Table 1.
Physiologic Data
| Normal Controls | DM1 Patients | ||
| 90-5% Relaxation Time (RT, sec) | Mean (Range) | Mean (Range) | p-value (NIs vs DM1) |
| Single Stimulus (Twitch) | 0.18 (0.11 – 0.21) | 0.23 (0.11 – 0.35) | 0.03 |
| 10 stimuli, 10 Hz | 0.18 (0.14 – 0.25) | 1.96 (0.20 – 5.73) | 0.001 |
| 10 stimuli, 30 Hz | 0.19 (0.13 – 0.29) | 3.83 (0.18 – 11.7) | 0.01 |
| 10 stimuli, 50 Hz | 0.19 (0.13 – 0.25) | 2.60 (0.19 – 9.10) | 0.001 |
| 20 stimuli, 30 Hz | 0.21 (0.12 – 0.28) | 8.62 (0.31 – 19.30) | 0.003 |
| 20 stimuli, 50 Hz | 0.20 (0.12 – 0.28) | 9.33 (0.23 – 23.40) | 0.0007 |
| Grip | 1.92 (0.18 – 6.25) | ||
| Peak Force (PF, g) | Mean (Range) | Mean (Range) | p-value (NIs vs DM1) |
| Single stimulus (Twitch) | 568: 265–1,580 | 147: 31–310 | 0.007 |
| 10 stimuli, 10 Hz | 1,250: 821–2,100 | 454: 111–916 | <0.0001 |
| 10 stimuli, 30 Hz | 2,390: 1,620–4,320 | 971:566–1,740 | 0.0002 |
| 10 stimuli, 50 Hz | 2,450:1,520–4,250 | 959: 279–1,170 | <0.0001 |
| 20 stimuli, 30 Hz | 2,190:1,580–3,560 | 1,120: 532–1,960 | 0.0005 |
| 20 stimuli, 30 Hz | 2,190:1,580–3,560 | 1,120: 532–1,960 | 0.0005 |
| 20 stimuli, 50 Hz | 2,550:1,680–4,660 | 1,190: 569–1,930 | 0.0005 |
| Grip | 10,880 (4,490–23,400) | ||
| RT / PF (sec/g) | Mean | Mean | p-value (NIs vs DM1) |
| Single Stimulus (Twitch) | 0.0004 | 0.0021 | 0.001 |
| 10 stimuli, 10 Hz | 0.0002 | 0.0052 | 0.0002 |
| 10 stimuli, 30 Hz | 0.0001 | 0.004 | 0.01 |
| 10 stimuli, 50 Hz | 0.0001 | 0.003 | 0.002 |
| 20 stimuli, 30 Hz | 0.0001 | 0.009 | 0.008 |
| 20 stimuli, 50 Hz | 0.0001 | 0.007 | 0.003 |
| Grip | 0.18 |
Effect of increasing stimulus train length on evoked RT, PF, and RT/PF in controls and patients (Figures 1, 2; Table)
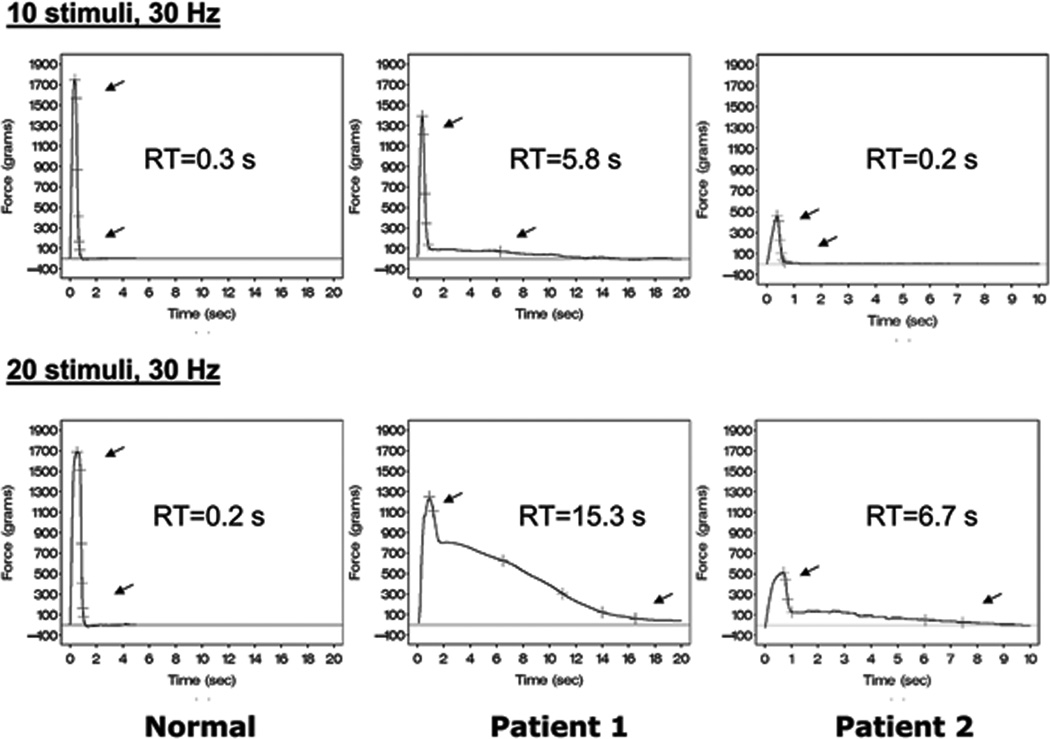
Figure 1.
Examples of evoked first dorsal interosseous muscle contractions with 2 stimulus paradigms: 10 stimuli 30 Hz (top row), and 20 stimuli 30 Hz (bottom row) for a control subject (left panels) and 2 DM1 patients: #1 (middle panels), and #2 (right panels). Time (seconds) on the x-axis and Force (grams) on the y-axis Cursors are automatically placed along the relaxation phase of the contraction curves. Top arrows indicate the point that corresponds to 90% peak force (PF), while the bottom arrows indicate 5% PF. The 90-5% relaxation time (RT) between these arrows is shown in the center of each panel.
For the control subject, RT is about the same for both stimulus paradigms: 0.2 – 0.3 seconds. For patient #1, the RT is prolonged with a stimulus train of 10, but it dramatically rises when the train length is increased to 20 stimuli. For patient #2, RT is within the normal range for a train length of 10 stimuli, but it is quite prolonged when the train length is increased from 10 to 20 stimuli.
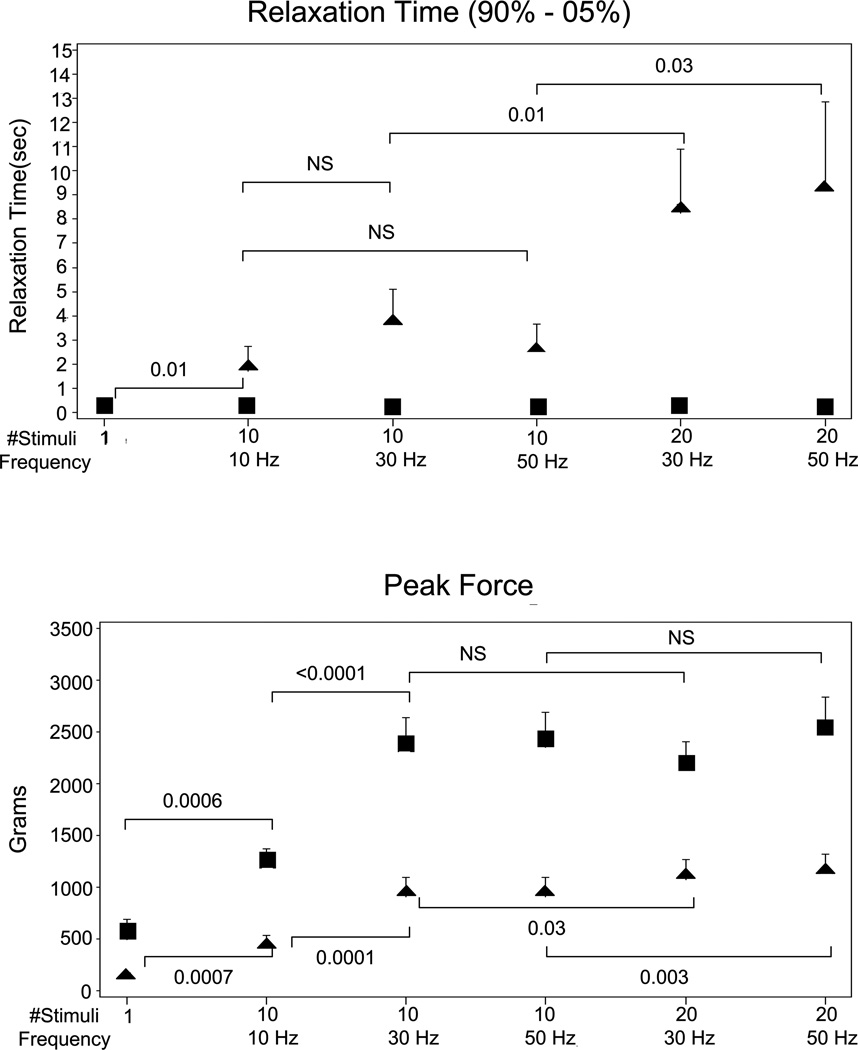
Figure 2.
Group evoked contraction data in the 10 normals (■) and 10 DM1 patients (▲) are shown. Evoked mean relaxation time (RT, top), and peak force (PF, bottom) are plotted with 1 SE error bars varying stimulus train length: 1, 10 and 20, and stimulus frequency 10, 30 and 50 Hz. P-values for two-tailed paired t-test comparisons are shown.
For normals, RT is independent of stimulus frequency and train length even though peak force dramatically rises with increase in stimulus frequency from 10 to 30 Hz and with train length from 1 to 10. By contrast, in DM1 patients, RT increases when train length increases from 1 to 10, and again from 10 to 20, but it does not change significantly in the 10 stimulus condition when frequency increases from 10 to 30 to 50 Hz, or in the 20 stimulus condition when frequency increases from 30 to 50 Hz. (Note that the error bars in normals for RT are very small and buried within the width of the symbol.)
RT did not increase in the control group as train length increased from 1 to 10 stimuli or from 10–20 stimuli. By contrast, RT increased dramatically as train length increased in the patient group by about 800% as train length increased from 1 to 10 stimuli and a further 100–350% as train length increased from 10 to 20 stimuli.
In control subjects and patients, PF increased significantly 200–300 % as train length increased from 1 to 10 stimuli, but only slightly as train length increased from 10 to 20 stimuli (there was a significant increase in patients, but not in controls).
In normal subjects, RT/PF declined significantly as train length increased from 1 to 10 (p<0.001), but it did not change significantly as train length increased from 10 to 20. The patient group showed the opposite finding. There was an insignificant increase from 1 to 10, but there was a significant increase as train length rose from 10 to 20 (p<0.05 for 30 Hz stimuli and p<0.01 for 50 Hz stimuli).
Effect of increasing stimulus frequency on evoked RT, PF, and RT/PF in controls and patients (Figure 2, Table)
In both the control and patient groups, RT did not increase significantly as frequency increased from 10–50 Hz. By contrast, PF increased dramatically in both patients and controls as frequency increased from 10 to 30 Hz, and it plateaued as frequency increased from 30 to 50 Hz. RT/PF was much greater in patients compared with controls as expected. It declined significantly as stimulus frequency increased from 10 to 30 Hz in control subjects, but in patients it did not change significantly as stimulus frequency was increased from 10–50 Hz
Test retest reproducibility of myotonia evoked by trains of 20 stimuli
For the 20 stimulus 30 Hz condition, the mean (max RT – minRT) / max RT was 0.21 (range: 0.10 – 0.56), and the Spearman correlation coefficient was 0.79 (p<0.037). For the 20 stimulus 50 Hz trials, the mean (max RT – minRT)/max RT was 0.21 (range: 0.04 – 0.35), and the Spearman correlation coefficient was 0.86 (p<0.015).
Comparison of voluntary grip and evoked RT in patients
Mean voluntary 90-5% Grip RT in the right hand was about 2 seconds in duration. This was not significantly different from the contralateral first dorsal interosseous myotonia evoked by 10 stimuli at 10, 30 or 50Hz. In contrast, evoked first dorsal interosseous RTs utilizing 20 stimulus train lengths at 30 and 50 Hz were 400–500% longer (p<0.01 and p<0.05, respectively) than contralateral grip myotonia. Historical controls from our laboratory had mean voluntary grip RTs of 0.37 sec, which was significantly greater than normal evoked first dorsal interosseous RTs (about 0.2 sec for stimulus trains of 20). This indicates that the longer evoked versus voluntary grip RTs in our patient group cannot be explained by systematic variation in RTs between different muscles or methodologies, since if this were the case, DM1 grip RTs would be expected to be longer than DM1 evoked RTs as is the case in normals.
DISCUSSION
The major observation in our study was that evoked myotonia can be “dialed up or down” by varying stimulus train lengths. Specifically, while evoked RT in normal subjects remained relatively flat with increase in stimulus frequency and train length, in DM1 patients, RT increased when train length was increased from 1 to10 and then 10 to 20, but it did not increase significantly as stimulus frequency rose from 10–50Hz. (This latter observation is consistent with a preliminary study of evoked first dorsal interosseous myotonia in 7 DM1 patients who underwent 10, 20, 30, 40, and 50 Hz stimulation utilizing a fixed stimulus train length of 10. RT was found to be independent of stimulus frequency (Logigian, unpublished data)). By contrast, PF increased when train length was increased from 1 to 10, but it changed very little from 10 to 20 and with stimulus frequency from 10 to 30 Hz (but not 30 to 50) in both groups. To eliminate the remote possibility that prolongation of RT is dependent on the slight increase in PF as stimulus train length increased, we also calculated RT/PF to control for this effect. As train length increased from 10 to 20, RT/PF remained flat in normals, but it increased in patients. This excludes the slight increase in PF as a cause for train length-related increase in RT (particularly for train lengths in the 10 to 20 range). Finally, the test retest reproducibility of the 90-5% RT for the 20 stimulus 30 Hz and 20 stimulus 50 Hz paradigms is comparable to what we have reported earlier for the 10 Hz 10 stimulus condition,1 with a statistically significant correlation between measurements taken 6–12 weeks apart.
Recent investigations using a transgenic mouse model of DM1 have shown that myotonia results from reduced chloride conductance in muscle fibers.3,11–13 In this model, and in human DM1, it appears that a toxic, triplet repeat-containing RNA produced from the DM gene accumulates in the nucleus where it binds and inactivates Muscleblind 1 protein14 and ultimately results in abnormal splicing of the C1C-1 chloride channel pre-mRNA. This effect on RNA splicing in turn leads to loss of functional chloride channels in the muscle membrane.3,4 This observation fits with a longstanding, classical explanation of myotonia, in which loss of hyperpolarizing chloride channel conductance, combined with the normal accumulation of extracellular potassium in the T-tubule, results in progressive muscle membrane depolarization and ultimately the spontaneous development of repetitive muscle fiber discharges (e.g., electrical myotonia).2,15,16
We propose that, as stimulus train length increases from 1 to 2, 2 to 3, ….and 10 to 20, the T-tubule membrane becomes progressively depolarized due to a gradual rise in T-tubule potassium in proportion to the number of stimuli. In DM1 patients, this in turn results in post-stimulus train spontaneous repetitive muscle fiber discharges in chloride channel-deficient muscle fibers, the duration and intensity of which is in proportion to the number of stimuli in the train. This hypothesis is supported by aforementioned studies of myotonic goat and chloride deficient intercostal muscle fibers, in which: 1) the muscle membrane “after-depolarization” increases linearly with the number of muscle fiber action potentials in a train of potentials (about 1 mV per action potential) induced by a constant current applied to the muscle fibers,2 and 2) the “after-depolarization” is eliminated by treatment with glycerol, which is known to disrupt the T-tubule system.2
We do not know why evoked RTs appeared to be independent of stimulus frequency. It is possible that the longer half-life of potassium in the T tubule relative to all tested inter-stimulus intervals may explain this in part. In normal frog muscle, the half-life of potassium in the T-tubule is in the range of 400 milliseconds.17 Similarly, the decline of the muscle fiber after-depolarization in the myotonic chloride channel-deficient goat described above has a half-life of about 500 milliseconds.2 Assuming a similar half-life for potassium in DM1 patients, all stimulus trains with frequencies between 10 – 50 Hz in this study are adequate to result in a progressive rise in T-tubule potassium, since the respective inter-stimulus intervals are all 100 msec or less. However, this explanation alone is inadequate, since the buildup of T tubule potassium would occur faster with higher stimulus frequencies than with lower.
We found that the first dorsal interosseous RT evoked by trains of 20 stimuli at 30–50 Hz was, on average, about 3 times that of contralateral maximum voluntary grip RT. The explanation for this observation is likely due to differences in the measurement technique, differences in the myotonic properties of extrinsic finger flexors (grip) versus intrinsic hand muscles (first dorsal interosseous), or both. With respect to differences in technique, maximum finger flexor motor unit firing rates would be expected to be in the 30–50 Hz range,18 which are comparable to the ulnar stimulation frequencies, but motor unit train lengths are significantly longer (90 – 150 motor unit spikes) than that of the evoked testing (since the duration of the hand grip was standardized at 3 seconds). But compared to quantification of voluntary grip myotonia, evoked first dorsal interosseous myotonia testing requires less patient cooperation and avoids the problem of unintended voluntary activation of antagonist muscles (e.g., finger extensors) to help open a myotonically-closed hand. To the extent that patients cannot relax finger flexors without simultaneously contracting finger extensors, voluntary grip RTs will be spuriously shortened, and they could account for shortened grip versus evoked RTs, at least in part. With respect to differences in myotonic properties of the two muscle groups, it is possible that the more distal hand muscles in the evoked studies are more myotonic than are the more proximal finger flexor muscles utilized in the voluntary grip studies. In two previous needle EMG studies in patients with DM1,19,20 electrical myotonia was found to be more severe in distal hand muscles (e.g., first dorsal interosseous), than in proximal forearm and upper arm muscles. Regardless of the reason for the differences in evoked versus grip RT, the data in this study suggests that evoked myotonia may be useful in anti-myotonic drug trials or for following disease progression, particularly when grip RT is minimally prolonged or normal.
There are important limitations in this study. As noted before,1 percutaneous repetitive ulnar nerve stimulation is painful for some patients and requires customized equipment and software. Secondly, it may be that 90-5% RT, while fairly simple to calculate, is not the optimal measurement of myotonia. For example, measurement of relative square area (kilogram-second) of the relaxation phase might provide a more sensitive detection of myotonia. Finally, because the lowest stimulus frequency used in this study was 10 Hz, with an inter-stimulus interval of 100 milliseconds, and well below the proposed T-tubule half-life of potassium (400–500 milliseconds), we did not determine if the “cut-off” stimulus frequency required to amplify RT is in the range predicted by the tubular potassium hypothesis (e.g. about 2 Hz). Still, despite these limitations, our study supports this hypothesis and also provides practical information on performance of evoked myotonia testing in clinical trials of anti-myotonic drugs.
Acknowledgement
This study was supported in part by the Paul D. Wellstone Muscular Dystrophy Cooperative Research Center (NIH 5U54 NSO48843-05), and by a General Clinical Research Center grant (5 MO1 RR00044) from the National Center for Research Resources, NIH. The authors also thank Mr. Marty Swanton for help with preparation of the figures.
ABBREVIATIONS
- DM1
Myotonic dystrophy type I
- FDI
First dorsal interosseous
- PF
Peak force
- RT
Relaxation time
REFERENCES
- 1.Logigian EL, Moxley RT, IV, Blood CL, Barbieri C, Martens WB, Wiegner AW, et al. Leucocyte CTG Repeat Length Correlates with Severity of Myotonia in Myotonic Dystrophy Type I. Neurology. 2004;62:1081–1089. doi: 10.1212/01.wnl.0000118206.49652.a3. [DOI] [PubMed] [Google Scholar]
- 2.Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibers. J Physiol. 1974;240:505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 Chloride Channel and hyperexcitability of skeletal muscle in myotonic dystrophy. Molecular Cell. 2002;10:1–20. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- 4.Charlet BN, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in Type 1 myotonic dystrophy due to misregulated alternative splicing. Molecular Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 5.Griggs RC, Wood DS. Criteria for establishing the validity of genetic recombination in myotonic dystrophy type 1 (DM1) Neurology. 1989;39:420–421. doi: 10.1212/wnl.39.3.420. [DOI] [PubMed] [Google Scholar]
- 6.Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3’ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 7.Fu YH, Pizzuti A, Fenwick RG, Jr, King J, Rajnarayan S, Dunne PW, et al. An unstable triplet repeat in the gene related to myotonic dystrophy. Science. 1992;255:1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 8.Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3’ untranslated region of the gene. Science. 1992;255:1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- 9.Logigian EL, Blood CL, Dilek N, Martens WB, Moxley RT, IV, Wiegner AW, et al. A Quantitative analysis of the warm-up phenomenon in Myotonic dystrophy Type 1 (DM1) Muscle Nerve. 2005;32:35–42. doi: 10.1002/mus.20339. [DOI] [PubMed] [Google Scholar]
- 10.Moxley RT, III, Logigian EL, Martens WB, Annis CL, Pandya S, Moxley RT, IV, et al. Computerized Hand Grip Myometry Reliably Measures Myotonia and Muscle Strength in Myotonic Dystrophy (DM1) Muscle Nerve. 2007;36:320–328. doi: 10.1002/mus.20822. [DOI] [PubMed] [Google Scholar]
- 11.Mankodi A, Logigian E, Callahan L, McClain C, White R, Henderson D, et al. Myotonic Dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289:1769–1772. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- 12.Lueck JD, Mankodi A, Swanson MS, Thornton CA, Dirksen RT. Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy. J Gen Physiol. 2007;129:79–94. doi: 10.1085/jgp.200609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler TM, Lueck JD, Swanson MS, Dirksen RT, Thornton CA. Correction of ClC-1 splicing eliminates chloride channelopathy and myotonia in mouse models of myotonic dystrophy. J Clin Invest. 2007;117:3952–3957. doi: 10.1172/JCI33355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin X, Miller JW, Mankodi A, Kanadia RN, Yuan Y, Moxley RT, et al. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genetics. 2006;15:2087–2097. doi: 10.1093/hmg/ddl132. [DOI] [PubMed] [Google Scholar]
- 15.Barchi RL. Myotonia: an evaluation of the chloride hypothesis. Arch Neurol. 1975;32:175–180. doi: 10.1001/archneur.1975.00490450055007. [DOI] [PubMed] [Google Scholar]
- 16.Barchi RL. The pathophysiology of excitation in skeletal muscle. In: Karpati G, Hilton Jones D, Griggs RC, editors. Disorders of Voluntary Muscle. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 17.Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972;225:33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellemare F, Woods JJ, Johansson R, Biglan-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol. 1983;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- 19.Streib EW, Sun SF. Distribution of electrical myotonia in myotonic muscular dystrophy. Ann Neurol. 1983;14:80–82. doi: 10.1002/ana.410140113. [DOI] [PubMed] [Google Scholar]
- 20.Logigian EL, Ciafaloni E, Quinn LC, Dilek N, Pandya S, Moxley RT, III, et al. Severity, Type, and Distribution of myotonic discharges are different in Type 1 and Type 2 Myotonic Dystrophy. Muscle Nerve. 2007;35:479–485. doi: 10.1002/mus.20722. [DOI] [PubMed] [Google Scholar]