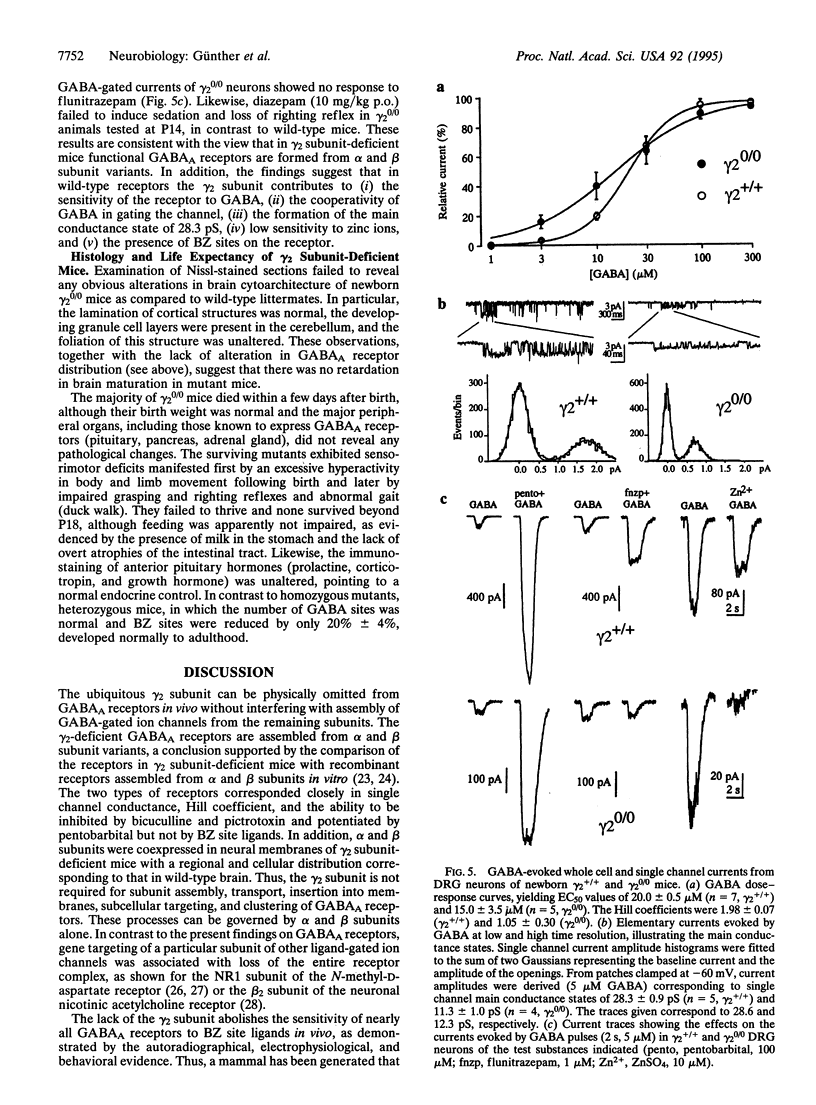
Abstract
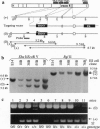
Vigilance, anxiety, epileptic activity, and muscle tone can be modulated by drugs acting at the benzodiazepine (BZ) site of gamma-aminobutyric acid type A (GABAA) receptors. In vivo, BZ sites are potential targets for endogenous ligands regulating the corresponding central nervous system states. To assess the physiological relevance of BZ sites, mice were generated containing GABAA receptors devoid of BZ sites. Following targeted disruption of the gamma 2 subunit gene, 94% of the BZ sites were absent in brain of neonatal mice, while the number of GABA sites was only slightly reduced. Except for the gamma 2 subunit, the level of expression and the regional and cellular distribution of the major GABAA receptor subunits were unaltered. The single channel main conductance level and the Hill coefficient were reduced to values consistent with recombinant GABAA receptors composed of alpha and beta subunits. The GABA response was potentiated by pentobarbital but not by flunitrazepam. Diazepam was inactive behaviorally. Thus, the gamma 2 subunit is dispensable for the assembly of functional GABAA receptors but is required for normal channel conductance and the formation of BZ sites in vivo. BZ sites are not essential for embryonic development, as suggested by the normal body weight and histology of newborn mice. Postnatally, however, the reduced GABAA receptor function is associated with retarded growth, sensorimotor dysfunction, and drastically reduced life-span. The lack of postnatal GABAA receptor regulation by endogenous ligands of BZ sites might contribute to this phenotype.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bloom F. E. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967 Dec;6(4):716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Angelotti T. P., Macdonald R. L. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993 Apr;13(4):1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A. S., Pannell L., Jaouni T., Gammal S. H., Fales H. M., Jones E. A., Skolnick P. Brain concentrations of benzodiazepines are elevated in an animal model of hepatic encephalopathy. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5263–5267. doi: 10.1073/pnas.87.14.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D., Fritschy J. M., Trzeciak A., Bannwarth W., Mohler H. Distribution, prevalence, and drug binding profile of gamma-aminobutyric acid type A receptor subtypes differing in the beta-subunit variant. J Biol Chem. 1994 Oct 28;269(43):27100–27107. [PubMed] [Google Scholar]
- Bowie D., Feltz P., Schlichter R. Subpopulations of neonatal rat sensory neurons express functional neurotransmitter receptors which elevate intracellular calcium. Neuroscience. 1994 Jan;58(1):141–149. doi: 10.1016/0306-4522(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H. P., DeArmond S. J., Prusiner S. B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992 Apr 16;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci. 1991;49(5):325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- Del Rio J. A., Soriano E., Ferrer I. Development of GABA-immunoreactivity in the neocortex of the mouse. J Comp Neurol. 1992 Dec 22;326(4):501–526. doi: 10.1002/cne.903260403. [DOI] [PubMed] [Google Scholar]
- Forrest D., Yuzaki M., Soares H. D., Ng L., Luk D. C., Sheng M., Stewart C. L., Morgan J. I., Connor J. A., Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994 Aug;13(2):325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Fritschy J. M., Benke D., Mertens S., Oertel W. H., Bachi T., Möhler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy J. M., Paysan J., Enna A., Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994 Sep;14(9):5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A., Wisden W., Lüddens H., Puia G., Vicini S., Seeburg P. H. The third gamma subunit of the gamma-aminobutyric acid type A receptor family. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1433–1437. doi: 10.1073/pnas.89.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Knoflach F., Backus K. H., Giller T., Malherbe P., Pflimlin P., Möhler H., Trube G. Pharmacological and Electrophysiological Properties of Recombinant GABAA Receptors Comprising the alpha3, beta1 and gamma2 Subunits. Eur J Neurosci. 1992 Oct;4(1):1–9. doi: 10.1111/j.1460-9568.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Knoflach F., Rhyner T., Villa M., Kellenberger S., Drescher U., Malherbe P., Sigel E., Möhler H. The gamma 3-subunit of the GABAA-receptor confers sensitivity to benzodiazepine receptor ligands. FEBS Lett. 1991 Nov 18;293(1-2):191–194. doi: 10.1016/0014-5793(91)81184-a. [DOI] [PubMed] [Google Scholar]
- Lauder J. M., Han V. K., Henderson P., Verdoorn T., Towle A. C. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986 Oct;19(2):465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- Li Y., Erzurumlu R. S., Chen C., Jhaveri S., Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994 Feb 11;76(3):427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Malherbe P., Sigel E., Baur R., Persohn E., Richards J. G., Mohler H. Functional characteristics and sites of gene expression of the alpha 1, beta 1, gamma 2-isoform of the rat GABAA receptor. J Neurosci. 1990 Jul;10(7):2330–2337. doi: 10.1523/JNEUROSCI.10-07-02330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Mertens S., Benke D., Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by alpha 5- and delta-subunit-specific immunopurification. J Biol Chem. 1993 Mar 15;268(8):5965–5973. [PubMed] [Google Scholar]
- Mohler H., Knoflach F., Paysan J., Motejlek K., Benke D., Lüscher B., Fritschy J. M. Heterogeneity of GABAA-receptors: cell-specific expression, pharmacology, and regulation. Neurochem Res. 1995 May;20(5):631–636. doi: 10.1007/BF01694546. [DOI] [PubMed] [Google Scholar]
- Olasmaa M., Rothstein J. D., Guidotti A., Weber R. J., Paul S. M., Spector S., Zeneroli M. L., Baraldi M., Costa E. Endogenous benzodiazepine receptor ligands in human and animal hepatic encephalopathy. J Neurochem. 1990 Dec;55(6):2015–2023. doi: 10.1111/j.1471-4159.1990.tb05790.x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., McCabe R. T., Wamsley J. K. GABAA receptor subtypes: autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J Chem Neuroanat. 1990 Jan-Feb;3(1):59–76. [PubMed] [Google Scholar]
- Picciotto M. R., Zoli M., Léna C., Bessis A., Lallemand Y., Le Novère N., Vincent P., Pich E. M., Brûlet P., Changeux J. P. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995 Mar 2;374(6517):65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Garland W., Puia G., Guidotti A., Weber R. J., Costa E. Purification and characterization of naturally occurring benzodiazepine receptor ligands in rat and human brain. J Neurochem. 1992 Jun;58(6):2102–2115. doi: 10.1111/j.1471-4159.1992.tb10952.x. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Guidotti A., Tinuper P., Cortelli P., Avoni P., Plazzi G., Lugaresi E., Schoch P., Montagna P. Endogenous benzodiazepine receptor ligands in idiopathic recurring stupor. Lancet. 1992 Oct 24;340(8826):1002–1004. doi: 10.1016/0140-6736(92)93011-b. [DOI] [PubMed] [Google Scholar]
- Schlumpf M., Richards J. G., Lichtensteiger W., Möhler H. An autoradiographic study of the prenatal development of benzodiazepine-binding sites in rat brain. J Neurosci. 1983 Jul;3(7):1478–1487. doi: 10.1523/JNEUROSCI.03-07-01478.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G., Xie X., Krishek B. J. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994 Feb;42(3):393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. GABAA receptor channels: from subunits to functional entities. Curr Opin Neurobiol. 1992 Jun;2(3):263–269. doi: 10.1016/0959-4388(92)90113-y. [DOI] [PubMed] [Google Scholar]