Abstract
Activity-regulated cytoskeleton-associated protein (Arc) is an immediate early gene that is expressed almost exclusively in glutamatergic neurons. Arc protein is enriched in the postsynaptic density (PSD) and colocalizes with the N-methyl-D-aspartate receptor (NMDAR) complex. Arc transcription is positively modulated by NMDAR activity and is important for dendritic spine plasticity. Genetic ablation of serine racemase (SR−/−), the enzyme that converts L-serine to D-serine, a coagonist at the NMDAR, reduces dendritic spine density in the hippocampus. Here we demonstrate that SR deficient (SR−/−) mice also have reduced Arc protein expression in the hippocampus that can be reversed with chronic D-serine administration in adulthood. Furthermore, D-serine treatment partially rescues the hippocampal spine deficit in SR−/− mice. These results demonstrate the importance of D-serine in regulating the hippocampal expression of Arc in vivo. In addition, our findings underscore the potential utility of using the glycine modulatory site agonist D-serine to treat disorders that exhibit Arc and dendritic spine dysregulation as a consequence of NMDAR hypofunction, such as schizophrenia.
Keywords: serine racemase, activity-regulated cytoskeleton-associated protein, dendritic spines, dentate gyrus, schizophrenia
1. Introduction
Activity-regulated cytoskeleton-associated protein (Arc; Arg3.1) is a non-transcription factor immediate early gene that is robustly induced by many learning paradigms. Its expression is confined almost exclusively to excitatory neurons of the hippocampus and neocortex, with little or no expression in glia (Vazdarjanova et al., 2006). Arc is also involved in regulating dendritic spines via actin remodeling, as mice lacking Arc have reduced dendritic spine density (Peebles et al., 2010). Arc mRNA, which is induced by calcium influx through voltage-gated calcium channels and N-methyl-D-aspartate receptors (NMDARs), is trafficked to dendrites and synthesized at synaptic sites (Korb and Finkbeiner, 2011).
In addition to glutamate, NDMARs require the binding of either glycine or D-serine to the glycine modulatory site (GMS) for activation. The enzyme serine racemase (SR) converts L-serine to D-serine. SR deficient transgenic (SR−/−) mice display NMDAR hypofunction that is coupled with deficits in hippocampal long-term potentiation (LTP), dendritic spine plasticity, and memory (Balu et al., 2012; Balu et al., 2013; DeVito et al., 2011). There is substantial evidence that hypofunction of the NMDAR, as well as genetic alterations of proteins enriched in the postsynaptic density (PSD), including Arc (Fromer et al., 2014; Kirov et al., 2012; Purcell et al., 2014), contribute to the pathophysiology of schizophrenia. In this manuscript, we investigated whether D-serine could reverse the Arc protein and synaptic deficits in SR−/− mice.
2. Experimental procedures
2.1. Animals
SR−/− mice were generated as previously described (Basu et al., 2009). SR+/− sires and dams were bred to produce wild-type (WT) and SR−/− offspring. Adult male mice (3–5 months old) were used for all the experiments. Animals were housed in groups of 2–4 in polycarbonate cages and maintained on a 12:12 h light/dark cycle in a temperature (22°C) and humidity controlled vivarium. Animals were given access to food and water ad libitum. All animal procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee.
2.2. D-serine treatment
Mice received once daily, subcutaneous (s.c.) injections of vehicle or D-serine for 20 days at a volume of 5ml/kg. WT mice received vehicle (0.9% sodium chloride) and SR−/− mice received either vehicle or D-serine (Sigma-Aldrich, St. Louis MO). D-serine was given at an initial dose of 300 mg/kg on day 1, followed by 150mg/kg for the remaining 20 days. Mice were sacrificed on day 21 without receiving an injection. One cohort (WT: n=7, SR−/−: n=5, SR−/− + D-serine: n=6) was used for Golgi staining and another cohort (WT: n=12, SR−/−: n=6, SR−/− + D-serine: n=6) was used for Western blotting.
2.3. Golgi staining and quantification of dendritic spine density
Golgi staining was performed using the FD Rapid GolgiStain Kit (FD NeuroTechnologies, Ellicot City, MD) as previously described (Balu et al., 2013). Neurons were located between approximately −1.46 mm to −2.20 mm posterior to bregma (Paxinos & Franklin, 2001) and within the middle third of the section. Spines were counted on two unobscured apical dendritic branches in the middle molecular layer (minimum 2nd order) per neuron, with the average spine density used as the value for that neuron. Spines were counted on 5–6 neurons per animal. Only neurons in the outer two-thirds of the GCL of the DG were chosen for spine density analysis. Neurons in the outer layers of the GCL are not derived from the actively dividing cells of the subgranular zone associated with adult neurogenesis. The average dendritic length analyzed for spine density did not significantly differ between groups (WT=17.5 ± 0.9 μm, SR−/−=18.54 ± 0.5 μm, SR−/− treated with D-serine=16.2 ± 0.6 μm, p>0.05). Dendrites were visualized at 100x (oil-immersion) on a Zeiss Axioskop40 microscope and the number of spines was quantified using Neurolucida (MBF Bioscience, Williston, VT). The experimenter was blind to genotype during tracing.
2.4. Western blotting
Hippocampi were removed and flash frozen on dry ice and stored at −80C until lysis. Protein concentrations were measured using the Bradford protein assay (Bio-Rad). Immunoblotting was performed as previously described (Balu et al., 2012). Mouse anti-Arc (Santa Cruz sc-17839 (C7); 1:1000) and rabbit anti-β-actin (1:8000; Abcam) were used for primary antibody incubations. Chemiluminescent values of the protein of interest were divided by its corresponding β-actin chemiluminescent values. The ratio of each WT sample was divided by the average of all the WT sample values in each gel and multiplied by 100. The average of the normalized WT values from each gel was 100% ± SEM. The mutant values were normalized to WT values (% WT) collected in parallel from the same gel. The normalized values were then averaged and used for statistical analysis.
2.5 Statistical analysis
One-way ANOVA was used to analyze dendritic spine length and density, as well as Western blot results. Significant F values were subject to Bonferroni’s Multiple Comparison post hoc analysis. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Chronic D-serine reverses Arc deficit in SR−/− mice
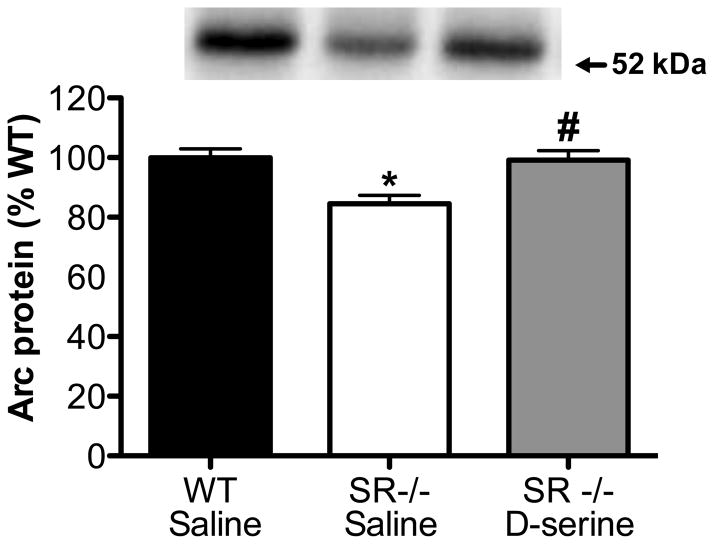
Arc protein levels in the hippocampus, as measured by Western blot, were reduced in SR−/− mice (Fig. 1). Three weeks of D-serine treatment during adulthood completely normalized Arc expression to WT levels.
Fig. 1.
Chronic D-serine administration restores Arc protein levels in the hippocampus of SR−/− mice. Protein levels of Arc were measured in the hippocampus of WT (n=12; black bars), SR−/− (n=6; white bars), and SR−/− mice treated with D-serine (n=6; gray bars). Asterisk (*) indicates significant difference from the WT vehicle group and # indicates significant difference from the SR−/− group (p < 0.01). All values represent the means ± SEM.
3.2. Chronic D-serine partially rescues the dendritic spine deficit in SR−/− mice
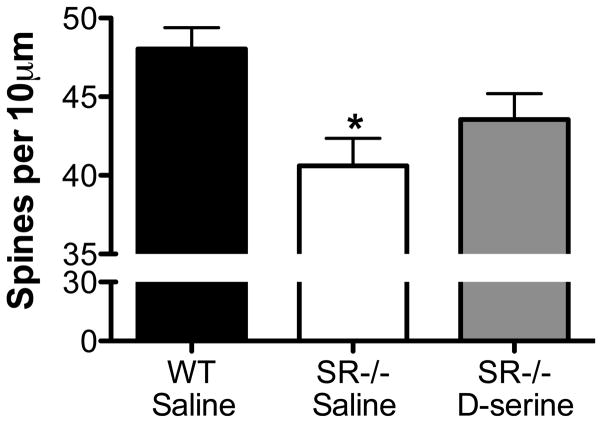
In agreement with what we previously demonstrated (Balu et al., 2013), SR−/− mice had significantly fewer spines on the apical dendrites of hippocampal dentate granule neurons (Fig. 2). Although D-serine treatment increased the number of spines in SR−/− mice, it did not fully restore their density to WT levels (Fig. 2).
Fig. 2.
Chronic D-serine administration partially rescues the dendritic spine deficit in the dentate gyrus of SR−/− mice. Spine density on dentate granule neurons (5–6 neurons/animal) was compared between WT (n=7; black bars), SR−/− (n = 5; white bars), and SR−/− mice administered D-serine (n=6; gray bars). Spine density is expressed as the number of spines per 10μm of dendrite. Asterisk (*) indicates significant difference from the WT vehicle group (p < 0.01). All values represent the means ± SEM.
4. Discussion
SR−/− mice have reduced levels of Arc protein in the hippocampus that are associated with fewer dendritic spines on hippocampal dentate granule neurons. We found that chronic treatment with D-serine during adulthood was able to fully restore Arc expression. In addition, D-serine administration partially rescued the spine deficit in SR−/− mice.
In the hippocampus, Arc is expressed primarily in glutamatergic neurons at low levels under basal conditions. Arc expression is robustly induced following synaptic activity in response to a wide range of learning and behavioral paradigms. Arc transcription and/or translation is positively regulated by BDNF/TrkB signaling (Ying et al., 2002) and NMDAR (Steward and Worley, 2001) activity. SR−/− mice exhibit NMDAR hypofunction, as well as reduced BDNF protein and TrkB signaling in the hippocampus (Balu et al., 2013). Our current findings suggest that D-serine mediated NMDAR activity and/or TrkB signaling regulate hippocampal Arc protein levels under basal conditions. Future studies will be needed to determine the precise contribution of these pathways to Arc translational regulation under basal conditions. It will also be important to determine if Arc induction following neuronal activity or learning is blunted in SR−/− mice.
SR−/− mice have reduced dendritic spine density on granule cells of the dentate gyrus. Arc is one of many factors that contribute to dendritic spine plasticity. In vitro and in vivo evidence demonstrates that Arc is a positive modulator of spine density in the hippocampus (Peebles et al., 2010). Thus, our finding of reduced hippocampal Arc protein levels in SR−/− mice is consistent with fewer dendritic spines in the hippocampus. D-serine treatment fully rescued the deficit in hippocampal Arc protein in SR−/− mice, similar to its restorative effects on BDNF and Akt signaling (Balu et al., 2013). Future studies will investigate the mechanisms underlying D-serine-induced changes in Arc protein. However, D-serine only partially reversed the spine deficit in SR−/− mice, suggesting that factors outside of the examined signaling pathways contribute to the reduced number of spines in these mutants.
There are increasingly more large-scale studies showing various types of mutations in genes encoding postsynaptic signaling proteins, including those in NMDAR and Arc complexes, being associated with schizophrenia (Fromer et al., 2014; Kirov et al., 2012; Purcell et al., 2014). Our data here demonstrate the convergence of D-serine mediated NMDAR transmission with the neuronal-activity sensitive, postsynaptic protein Arc. The ability of D-serine to reverse the deficits in Arc and dendritic spines highlights the glycine modulatory site as a potential drug target for disorders in which the perturbed NMDAR and Arc signaling contribute to its etiology.
Acknowledgments
We thank Jiamin Feng for animal colony maintenance and genotyping. A Phyllis & Jerome Lyle Rappaport Mental Health Research Scholars Award and 1K99MH099252-01A1 (DTB), as well as grants R01MH05190 and P50MH0G0450 (JTC) supported this work. JTC has served as a consultant for EnVivo, and Abbvie in the last 2 years. A patent owned by Massachusetts General Hospital for the use of D-serine as a treatment for serious mental illness could yield royalties for Dr. Coyle.
Footnotes
Conflict of interest
Dr. Balu has no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balu DT, Basu AC, Corradi JP, Cacace AM, Coyle JT. The NMDA receptor co-agonists, d-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex. Neurobiology of disease. 2012;45:671–682. doi: 10.1016/j.nbd.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A. 2013;110:E2400–2409. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Balu DT, Kanter BR, Lykken C, Basu AC, Coyle JT, Eichenbaum H. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 2011;10:210–222. doi: 10.1111/j.1601-183X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014 doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Grozeva D, Fjodorova M, Wollerton R, Rees E, Nikolov I, Lagemaat LN, Bayes A, Fernandez E, Olason PI, Bottcher Y, Komiyama NH, Collins MO, Choudhary J, Stefansson K, Stefansson H, Grant SG, Purcell S, Sklar P, O’Donovan MC, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011;34:591–598. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotactic Coordinates. 2 Academic Press; San Diego: 2001. [Google Scholar]
- Peebles CL, Yoo J, Thwin MT, Palop JJ, Noebels JL, Finkbeiner S. Arc regulates spine morphology and maintains network stability in vivo. Proc Natl Acad Sci U S A. 2010;107:18173–18178. doi: 10.1073/pnas.1006546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Kahler A, Duncan L, Stahl E, Genovese G, Fernandez E, Collins MO, Komiyama NH, Choudhary JS, Magnusson PK, Banks E, Shakir K, Garimella K, Fennell T, DePristo M, Grant SG, Haggarty SJ, Gabriel S, Scolnick EM, Lander ES, Hultman CM, Sullivan PF, McCarroll SA, Sklar P. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]