Abstract
The molecular events that precede the development of osteosarcoma, the most common primary malignancy of bone, are unclear, and concurrent molecular and genetic alterations associated with its pathogenesis have yet to be identified. Recent studies suggest that activation of β-catenin signaling may play an important role in human tumorigenesis. To investigate the potential role of β-catenin deregulation in human osteosarcoma, we analyzed a panel of 47 osteosarcoma samples for β-catenin accumulation using immunohistochemistry. Potential activating mutations were investigated by sequencing exon 3 of the β-catenin gene in genomic DNA isolated from tumor samples. Our findings revealed cytoplasmic and/or nuclear accumulation of β-catenin in 33 of 47 samples (70.2%); however, mutation analysis failed to detect any genetic alterations within exon 3, suggesting that other regulatory mechanisms may play an important role in activating β-catenin signaling in osteosarcoma. In our survival analysis, β-catenin deregulation conferred a hazard ratio of 1.05, indicating that β-catenin accumulation does not appear to be of prognostic value for osteosarcoma patients. When analyzed against other clinicopathologic parameters, β-catenin accumulation correlated only with younger age at presentation (26.4 vs. 39.8 years). Nevertheless, our results demonstrate that the deregulation of β-catenin signaling is a common occurrence in osteosarcoma that is implicated in the pathogenesis of osteosarcoma.
Keywords: Wnt signal, β-catenin, osteosarcoma, tumorigenesis, bone tumor
Osteosarcoma is the most common primary malignant tumor of bone, encompassing a class of osteoid-producing neoplasms that range in clinical behavior and responsiveness to therapeutic regimens.1 Best known of these lesions, the classic high-grade osteosarcoma, primarily afflicts individuals in the second decade of life and is distinguished by its locally aggressive character and early metastatic potential. Despite advances in chemotherapy, 5-year survival has remained around 60%.2
The molecular events that precede the development of osteosarcoma are poorly understood. Given its relatively early age at presentation and predilection for early metastasis, osteosarcoma is likely the result of underlying early somatic and/or germline mutations. Alterations in tumor-suppressor genes such as retinoblastoma (Rb1) and p53 have been identified in osteosarcoma and hypothesized to predispose individuals to this malignancy at an especially early age.3–6 Although several putative chromosomal regions have been suggested to harbor potential tumor-suppressor genes, the molecular nature and identity of these genes have not been elucidated.
β-Catenin is a cellular protein with multiple functions. As an important component of the adherens junction complex, it helps to anchor E-cadherin to the intracellular actin cytoskeleton through interactions with β-catenin.7,8 As an important Wnt signal transducer, β-catenin plays an important role in many developmental processes. In normal cells, β-catenin protein is maintained at a very low level and thereby restricted to the cellular membrane. Wnt ligands initiate their signaling pathway by binding to the frizzled receptors, leading to phosphorylation of the disheveled protein.9 Through its association with Axin and the adenomatous polyposis coli (APC) tumor-suppressor, phosphorylated disheveled protein then prevents glycogen synthase kinase 3β (GSK3β) from phosphorylating β-catenin.10,11 Unphosphorylated β-catenin is stabilized by escaping recognition by β-TrCP, a component of an E3 ubiquitin ligase.7,8 Stabilized β-catenin accumulates in the cytoplasm and eventually translocates to the nucleus, where it engages transcription factors LEF and Tcf-4 to activate expression of downstream target genes, such as c-Myc12 and cyclin D1.13
The involvement of β-catenin in tumorigenesis was first established in colorectal cancer, where it was found to form a complex with the APC tumor-suppressor.14,15 The importance of β-catenin in regulating cell proliferation has been further highlighted by the discovery of oncogenic mutations of the β-catenin gene in colon cancers containing the wild-type APC gene.16–18 Mutant β-catenin protein becomes more stable because it is capable of bypassing APC-targeted degradation. Oncogenic forms of β-catenin induce tumor formation in transgenic animals, whereas mutations in the β-catenin gene have been frequently uncovered in tumors induced by either carcinogens or activated oncogenes.19,20 Furthermore, cytoplasmic and/or nuclear accumulation of the β-catenin protein has been extensively documented in the vast majority of human tumors, though β-catenin mutations have been uncovered at a low frequency in several forms of human tumor.21,22 Thus, these collective genetic data suggest that deregulation of β-catenin signaling may be involved in the development of a broad range of human malignancies. To increase our understanding of the molecular mechanisms underlying the development of osteosarcoma, we investigated the potential involvement of β-catenin signaling in human osteosarcoma. Immunohistochemical analysis of 47 osteosarcoma samples revealed significant cytoplasmic and/or nuclear accumulation of the β-catenin protein in 33 cases (70.2%). Interestingly, mutations of β-catenin exon 3 were not detected in any of the analyzed samples, indicating that while elevation of cytoplasmic and nuclear β-catenin protein is prevalent, mutations in the β-catenin gene are rare in osteosarcoma. Nevertheless, our results strongly imply that deregulation of β-catenin signaling is associated with the pathogenesis of osteosarcoma.
MATERIAL AND METHODS
Osteosarcoma tumor samples
The use and selection of human tumor specimens followed guidelines approved by the Institutional Review Board of the University of Chicago. Forty patients diagnosed with osteosarcoma and treated at the University of Chicago Hospitals were selected, and 4 µm sections of each sample were prepared from archival paraffin blocks. Samples from different stages of treatment were available from 6 patients, for a total of 47 samples. These samples represented a cross-section of histologic subtypes of osteosarcoma and included both primary and metastatic lesions. They were selected to evaluate the extent of β-catenin expression in the various manifestations of osteosarcoma.
Immunohistochemical staining with β-catenin
Paraffin-embedded sections were deparaffinized using xylene at room temperature and then rehydrated in a graduated fashion. For antigen retrieval, deparaffinized samples were immersed in a 0.1 M citrate buffer (pH 6.0) and microwaved for 10 min. After fixation, slides were incubated with a mouse anti-β-catenin antibody (against the C terminus of the protein; Transduction Laboratories, Lexington, KY) at a dilution of 1:200 for 1 hr at room temperature. Super Sensitive Multilink and Super Sensitive Label (both from BioGenex, San Ramon, CA) were then applied to each slide for 30 min. To visualize the β-catenin protein, a diaminobenzidine substrate (Pierce, Rockford, IL) was added for approximately 5 min, followed by counterstaining with light green (Fisher, Pittsburgh, PA) and mounting. A negative control exposed to mouse IgG antibody accompanied each specimen, and at least 1 positive control (i.e., a known positive section from human prostate cancer) was run with each series of specimens to ensure the validity of positive and negative findings. Stained samples were evaluated independently by 3 investigators. Samples were considered positively stained for β-catenin if significant staining was detected in the nucleus and/or cytoplasm. Conversely, staining restricted to the cytoplasmic membrane or no staining at all was considered negative.
Isolation of genomic DNA and DNA sequencing
Based on the immunostaining results of the β-catenin protein, a total of 23 cases were selected for genomic DNA isolation and subsequent sequencing analysis. Two 4 µm sections of each sample were prestained with hematoxylin and eosin (HE). Upon inspection of the original HE section, the specimens selected for sequence analysis consisted entirely of homogenous sheets of pleomorphic spindle cells, without any identifiable background stromal cells. Microdissection, therefore, was not used. The tumor region was carefully collected by scraping with a scalpel blade and then deparaffinized by placing in 100 µl of 0.5% Tween-20 and heating at 90°C for 10 min. Samples were allowed to cool down to 50°C, and proteinase K (Life Technologies, Gaithersburg, MD) was added to each sample at a final concentration of 200 mg/ml. Digestion was carried out at 50°C overnight. The following day, 100 µl of 5% Chelex-100 (Bio-Rad, Hercules, CA) were added to each sample, followed by heating at 99°C for 10 min. Samples were then centrifuged and the supernatants collected. Standard phenol:chloroform:isoamyl alcohol extraction was performed for each sample, followed by ethanol precipitation. PCR amplification of exon 3 of β-catenin was performed using the following primer pair: 5′-TATTTCAATGGGTCATATCACAG-3′ and 5′-CTGTTCCCACTCATACAGGAC-3′. A reaction volume of 25 µl was used, and touchdown PCR amplifications were performed on a PCR Express thermocycler (Hybaid, Franklin, MA) as follows: 94°C for 2 min, 1 cycle; 92°C for 20 sec, 67–56°C (10°C decrease/cycle) for 30 sec and 70°C for 30 sec, 12 cycles; 92°C for 20 sec, 55°C for 30 sec and 70°C for 30 sec, 30 cycles; 70°C for 5 min, 1 cycle. Amplification products were purified from 0.5% agarose gels. The sequencing primer was 5′-CTGATTTGATGGAGTTGGACATG-3′. Sequence reactions were performed using the ThermoSequenase kit (USB, Cleveland, OH) and resolved on 6% DNA sequencing gels. DNA sequence was radiographically visualized.
Statistical analysis
Fisher’s exact test was performed on comparisons of noncontinuous variables (gender, stage, tumor location, grade and histologic subtype); Student’s t-test was performed to compare the means for age. Survival analysis and Kaplan-Meier curves were generated on a subset of 30 prebiopsy specimens from patients for whom outcome data were available. All patients were treated at the University of Chicago with preoperative chemotherapy and wide resection. Mean follow-up was 60.03 months (range 1–166 months). Statistical analyses were conducted using Stata5 Statistics/Data Analysis software (Stata Corporation, College Station, TX).
RESULTS
Immunohistochemical evaluation of β-catenin in 47 samples of human osteosarcoma
Forty-seven osteosarcoma samples, representing a cross-section of histologic subtypes of osteosarcoma as well as both primary and metastatic lesions, were derived from 40 patients. Immunohistochemical staining was carried out using a well-characterized β-catenin antibody. Staining results were graded independently by 3 investigators according to the location and intensity of the signal. Samples where significant signal was detected in the nucleus and/or cytoplasm but no staining was observed in the negative control (i.e., mouse IgG added) were designated positive. Negative staining was defined as no detectable signal or staining of the cytoplasmic membrane only. A representative case from each category is illustrated in Figure 1. HE staining was also used as a histologic reference to ensure that tumor regions were properly analyzed.
Figure 1.
Representative immunohistochemical analyses of β-catenin in human osteosarcoma. Three cases of osteosarcoma were selected and 4 µm sections prepared. Immunohistochemical staining was performed using an anti-β-catenin antibody (see Material and Methods). Immunostaining results were graded according to the location and intensity of the signal. Slides were examined at ×40 magnification under a light microscope. Negative specimens had no nuclear/cytoplasmic staining, though many had isolated membranous staining (top row). Positive samples showed significant staining within the nucleus (middle row) and/or cytoplasm (bottom row). Control staining was performed in the same conditions except that a mouse IgG was used. After immunostaining, each section was counterstained with light green to reveal cellular structures. HE staining was also performed to reveal the histology of the selected samples. See text for details. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
As summarized in Table I, 33 cases of 47 osteosarcoma samples (or 70.2%) exhibited significant cytoplasmic and/or nuclear accumulation of the β-catenin protein. The immunohistochemical staining results were then tabulated and compared to the following clinical variables: age, gender, Enneking stage, disease stage, histologic subtype, histologic grade and tumor location. Statistical analyses were performed to assess whether β-catenin accumulation was associated with specific histologic subtypes of osteosarcoma, disease stage (e.g., primary lesions, local recurrence or distal metastases) or age at presentation of disease. There was no clear association between β-catenin accumulation and any clinicopathologic variable (p > 0.20, Fisher’s exact test), with the exception of patient age at presentation. Specifically, patients with elevated β-catenin in tumors were, on average, younger (mean age 26.38 vs. 39.78, p = 0.02).
TABLE I.
SUMMARY OF CLINICOPATHOLOGIC DATA AND Β-CATENIN IMMUNOSTAINING RESULTS ON SELECTED OSTEOSARCOMA SPECIMENS
| Total | β-Catenin+ | β-Catenin− | |
|---|---|---|---|
| Total | 47 | 33 (70.2%) | 14 (29.8%) |
| Gender | |||
| Male | 28 | 19 | 9 |
| Female | 19 | 14 | 5 |
| Mean age (years) | 31.37 | 26.38 | 39.78 |
| Histology of primary tumor | |||
| Osteoblastic | 21 | 17 | 4 |
| Chondroblastic | 9 | 6 | 3 |
| Fibroblastic | 3 | 1 | 2 |
| Telangiectatic | 2 | 1 | 1 |
| Paget’s sarcoma | 2 | 1 | 1 |
| Small cell | 1 | 1 | 0 |
| Soft tissue | 1 | 1 | 0 |
| Grade of primary tumor | |||
| High | 37 | 31 | 6 |
| Low | 2 | 1 | 1 |
| Location of primary tumor | |||
| Pelvis | 8 | 7 | 1 |
| Lower extremity | 23 | 19 | 4 |
| Upper extremity | 6 | 4 | 2 |
| Skull | 1 | 1 | 0 |
| Soft tissue | 1 | 1 | 0 |
| Disease stage | |||
| Prechemotherapy primary tumor | 17 | 13 | 4 |
| Postchemotherapy primary tumor | 19 | 13 | 6 |
| Local recurrence | 3 | 3 | 0 |
| Metastasis (lung) | 7 | 5 | 2 |
| Metastasis (chest wall) | 1 | 0 | 1 |
In a few selected cases, we analyzed tumor samples from the same patient at different stages of treatment. Interestingly, 2 cases consisted of prechemotherapy biopsies compared to postchemotherapy resections. One case demonstrated detectable β-catenin accumulation before chemotherapy as opposed to afterward, and the other demonstrated the reverse. Overall, no distinct pattern existed among the comparisons within the same patient.
Mutational analysis of exon 3 of the β-catenin gene
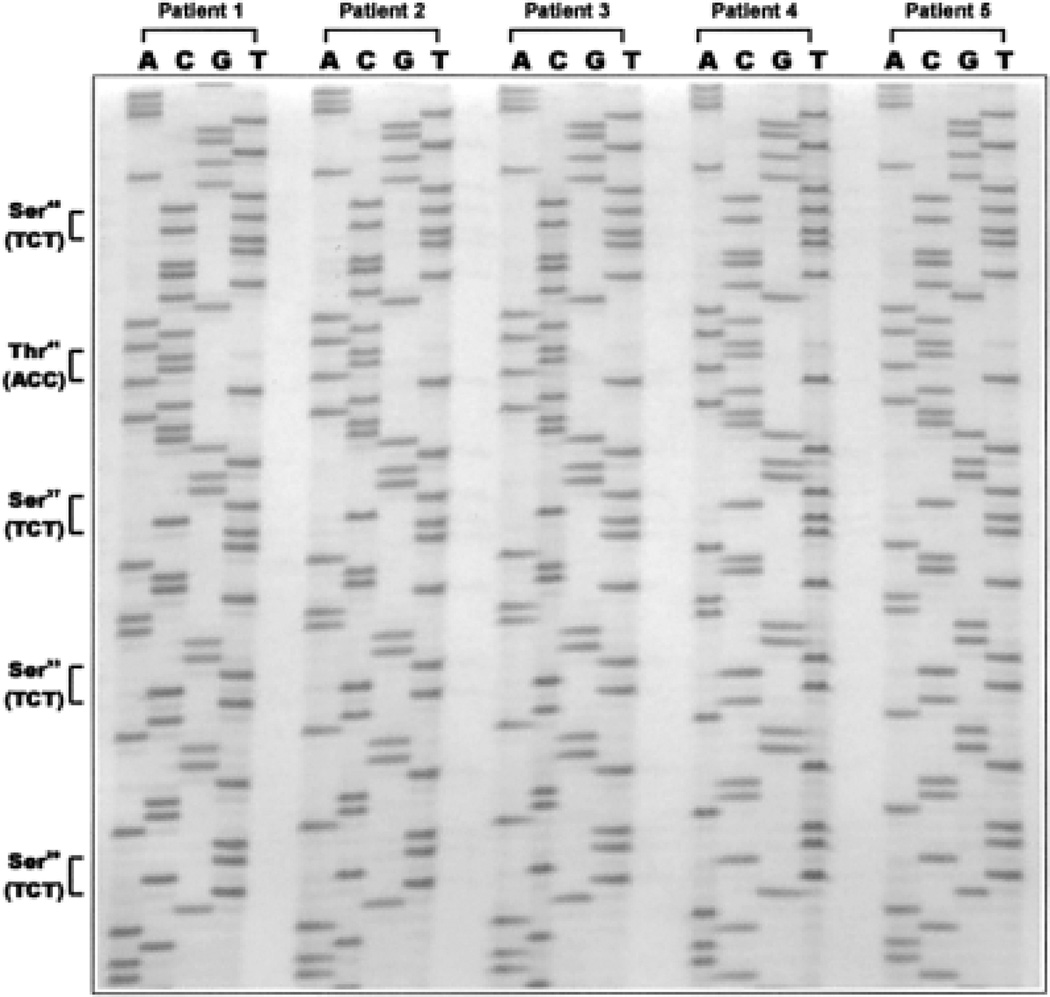
To investigate the cause of β-catenin deregulation, we performed a mutational analysis in 23 of the 33 samples that exhibited marked accumulation of β-catenin in the cytoplasm and/or nucleus. Exon 3 of β-catenin was selected for analysis because it harbors the majority of β-catenin mutations identified in other human malignancies. Genomic DNA was isolated from paraffin-embedded sections and amplified using oligonucleotides that flanked the region of exon 3 containing each of the GSK3β phosphorylation sites of β-catenin where mutations have been documented in other malignancies. In all analyzed samples, we failed to detect any apparent genetic alterations within this region. Figure 2 illustrates the wild-type genomic DNA sequences of the hot spots in 5 cases with markedly elevated β-catenin staining. These results suggest that the β-catenin protein may be frequently elevated, but mutations of the β-catenin gene are rare in osteosarcoma.
Figure 2.
Mutational analyses of the exon 3 region of the β-catenin gene in human osteosarcoma. Genomic DNA was purified from the tumor regions of paraffin-embedded sections of the selected samples. Exon 3 of the β-catenin gene was amplified by PCR and sequenced using the USB ThermoSequenase kit (see Material and Methods). Representative DNA sequences from 5 samples are shown. Tumor samples derived from patients 1, 3 and 4 exhibited significant cytoplasmic accumulation of β-catenin protein; the tumor sample from patient 2 was notable for marked nuclear accumulation of this protein, whereas the tumor sample from patient 3 exhibited strong nuclear and cytoplasmic staining of the β-catenin protein. Except for the specimen from patient 4 (from pulmonary metastatic tumors), all other samples were primary tumors. The frequently mutated GSK3β phosphorylation sites within exon 3 reported in other types of human cancer are indicated.
Statistical correlation analysis of β-catenin accumulation with clinical outcome in 30 patients
We performed a survival analysis on a subset of 30 patients for whom follow-up data were available and found a hazard ratio of 1.05 (SD = 0.563). Furthermore, the Kaplan-Meier curves that were generated were almost identical between the 2 cohorts (data not shown). Although the sample size was relatively small, these results suggest that the prognostic value of β-catenin accumulation may be limited in osteosarcoma.
DISCUSSION
The molecular events leading up to the development of osteosarcoma remain an area of continuing investigation. Mutations in the Rb1 and p53 tumor-suppressor genes, e.g., have been widely documented in osteosarcoma,5,6,23–26 as with most sarcomas in general. While Rb1 loss of heterozygosity may be a poor prognostic sign,27 p53 has not emerged as a meaningful prognostic marker,27–30 and neither has been effectively used as a therapeutic target in osteosarcoma. The INK4 products p14ARF and p16 were underexpressed in over 50% of cases of osteosarcoma.31 These data support a central role for both p53 and Rb1 in the development of osteosarcoma; however, mutational analysis of INK4 revealed no sequence alteration, suggesting that the observed downregulation of p14ARF and p16 was not central to the tumorigenesis process. Furthermore, cytogenetic analyses of osteosarcoma reveal a broad variety of chromosomal abnormalities, including allelic loss of 3q, 6p, 10q, 13q, 15q, 17p and 18q, each of which occurs in 50–75% of cases.32 While Rb1 and p53 largely account for the allelic loss reported on 13q and17p, respectively, further attempts to identify tumor-suppressor genes that map to other regions of chromosomal loss have failed to identify additional genes critical to the development of osteosarcoma. In this respect, osteosarcoma has largely eluded our attempts to generate molecular markers for clinical surveillance.
The contribution of β-catenin deregulation in the pathogenesis of sarcomas has only recently been explored. In a broad survey of several types of sarcoma, including a small group of osteosarcomas, Iwao et al.33 reported that 4 of 6 tumors exhibited significant elevation of β-catenin. Their results are comparable to our data, suggesting that β-catenin deregulation occurs in the majority of osteosarcomas. Furthermore, our findings are consistent with similar studies in other human tumors, in which β-catenin accumulation was a frequent event but β-catenin mutations were infrequent.34–38 It is conceivable that β-catenin accumulation can also be caused by loss-of-function mutations of the negative regulators of β-catenin signaling (e.g., APC, Axin and GSK3β). However, thus far, mutations of the APC tumor-suppressor gene have not been reported in noncolon cancer, and genetic alterations of Axin have been reported only in a small fraction of hepatocellular carcinomas, colon adenocarcinomas and sporadic medulloblastomas.39–41 Thus, the lack of mutations in β-catenin and its negative regulators strongly suggests that deregulation of β-catenin signaling may be caused by other mechanisms.
Our data, therefore, indicate that β-catenin deregulation is common in osteosarcoma, indeed more common than loss of heterozygosity for either Rb1 or p53. However, our data do not support the use of β-catenin accumulation as a clinical prognostic marker. Although the sample used for survival analysis was relatively small, clinical outcome was nearly identical between patients with tumors exhibiting β-catenin accumulation and those with β-catenin-negative tumors. This similarity was evident in both the estimated hazard ratio as well as the Kaplan-Meier curves. This lack of prognostic value should not diminish the importance of β-catenin deregulation in human osteosarcoma. For instance, although activation of β-catenin signaling is the primary cause for most human colorectal cancers, its activation has not been shown to correlate with the clinical prognosis of colon cancer patients.42 Alternatively, other tumors have demonstrated a correlation between elevated cytoplasmic/nuclear β-catenin and poor prognosis or disease progression.43–46 When we compared β-catenin accumulation to a variety of clinicopathologic data, no identifiable pattern emerged, with the important exception of age at presentation.
In summary, our results demonstrate that deregulation of β-catenin signaling is a common occurrence in osteosarcoma and appears to correlate with younger age at initial presentation. The lack of association with other clinicopathologic parameters may reflect the possible early involvement of β-catenin signaling in osteosarcoma. Taken together, our findings suggest that deregulation of β-catenin signaling may represent an important new mechanism underlying the development of osteosarcoma.
ACKNOWLEDGEMENTS
We thank Dr. D. Huo for assistance with the statistic analysis.
Grant sponsor: American Cancer Society Illinois Division; Grant sponsor: Brinson Foundation; Grant sponsor: Cancer Research Foundation; Grant sponsor: Concern Foundation.
REFERENCES
- 1.Mirra JM, Picci P, Gold RH. Bone tumors: clinical, radiologic, and pathologic correlations. Philadelphia: Lea & Febiger; 1989. [Google Scholar]
- 2.Glasser DB, Lane JM, Huvos AG, Marcove RC, Rosen G. Survival, prognosis, and therapeutic response in osteogenic sarcoma. The Memorial Hospital experience. Cancer. 1992;69:698–708. doi: 10.1002/1097-0142(19920201)69:3<698::aid-cncr2820690317>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Danckwerth F, Wuisman P, Ritter J, Blasius S, Jurgens H, Ozaki T, Winkelmann W. Osteosarkom bei zwei Geschwistern. Eine kasuistische Darstellung. Klin Padiatr. 1995;207:298–301. doi: 10.1055/s-2008-1046555. [DOI] [PubMed] [Google Scholar]
- 4.Chauveinc L, Mosseri V, Quintana E, Desjardins L, Schlienger P, Doz F, Dutrillaux B. Osteosarcoma following retinoblastoma: age at onset and latency period. Ophthalmic Genet. 2001;22:77–88. doi: 10.1076/opge.22.2.77.2228. [DOI] [PubMed] [Google Scholar]
- 5.Miller CW, Aslo A, Won A, Tan M, Lampkin B, Koeffler HP. Alterations of the p53, Rb and MDM2 genes in osteosarcoma. J Cancer Res Clin Oncol. 1996;122:559–565. doi: 10.1007/BF01213553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreck RR. Tumor suppressor gene (Rb and p53) mutations in osteosarcoma. Pediatr Hematol Oncol. 1992;9:ix–x. doi: 10.3109/08880019209018322. [DOI] [PubMed] [Google Scholar]
- 7.Cowin P, Burke B. Cytoskeleton–membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- 8.Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. doi: 10.1023/a:1006200102166. [DOI] [PubMed] [Google Scholar]
- 9.Noordermeer J, Klingensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez I, Itoh K, Sokol SY. Role of glycogen synthase kinase 3 β as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Saint-Jeannet JP, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 12.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 13.Tetsu O, McCormick F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 14.Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 15.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 16.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 17.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 18.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 19.Tsujiuchi T, Tsutsumi M, Sasaki Y, Murata N, Konishi Y. Mutations of adenomatous polyposis coli and β-catenin genes during progression of lung tumors induced by N-nitrosobis(2-hydroxypropyl)amine in rats. Cancer Res. 2000;60:6611–6616. [PubMed] [Google Scholar]
- 20.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker N, Clevers H. Catenins, Wnt signaling and cancer. Bioessays. 2000;22:961–965. doi: 10.1002/1521-1878(200011)22:11<961::AID-BIES1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 23.Castresana JS, Rubio MP, Gomez L, Kreicbergs A, Zetterberg A, Barrios C. Detection of TP53 gene mutations in human sarcomas. Eur J Cancer. 1995;5:735–738. doi: 10.1016/0959-8049(95)00121-x. [DOI] [PubMed] [Google Scholar]
- 24.Scholz RB, Kabisch H, Weber B, Roser K, Delling G, Winkler K. Studies of the RB1 gene and the p53 gene in human osteosarcomas. Pediatr Hematol Oncol. 1992;9:125–137. doi: 10.3109/08880019209018328. [DOI] [PubMed] [Google Scholar]
- 25.Toguchida J, Yamaguchi T, Ritchie B, Beauchamp RL, Dayton SH, Herrera GE, Yamamuro T, Kotoura Y, Sasaki MS, Little JB. Mutation spectrum of the p53 gene in bone and soft tissue sarcomas. Cancer Res. 1992;52:6194–6199. [PubMed] [Google Scholar]
- 26.Wadayama B, Toguchida J, Yamaguchi T, Sasaki MS, Yamamuro T. p53 expression and its relationship to DNA alterations in bone and soft tissue sarcomas. Br J Cancer. 1993;68:1134–1139. doi: 10.1038/bjc.1993.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feugeas O, Guriec N, Babin-Boilletot A, Marcellin L, Simon P, Babin S, Thyss A, Hofman P, Terrier P, Kalifa C, Brunat-Mentigny M, Patricot LM, et al. Loss of heterozygosity of the RB gene is a poor prognostic factor in patients with osteosarcoma. J Clin Oncol. 1996;14:467–472. doi: 10.1200/JCO.1996.14.2.467. [DOI] [PubMed] [Google Scholar]
- 28.Gokgoz N, Wunder JS, Mousses S, Eskandarian S, Bell RS, Andrulis IL. Comparison of p53 mutations in patients with localized osteosarcoma and metastatic osteosarcoma. Cancer. 2001;92:2181–2189. doi: 10.1002/1097-0142(20011015)92:8<2181::aid-cncr1561>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Kakar S, Mihalov M, Chachlani NA, Ghosh L, Johnstone H. Correlation of c-fos, p53, and PCNA expression with treatment outcome in osteosarcoma. J Surg Oncol. 2000;73:125–126. doi: 10.1002/(sici)1096-9098(200002)73:2<125::aid-jso14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama R, Schneider-Stock R, Radig K, Wex T, Roessner A. Clinicopathologic implications of MDM2, p53 and K-ras gene alterations in osteosarcomas: MDM2 amplification and p53 mutations found in progressive tumors. Pathol Res Pract. 1998;194:615–621. doi: 10.1016/s0344-0338(98)80096-4. [DOI] [PubMed] [Google Scholar]
- 31.Benassi MS, Molendini L, Gamberi G, Magagnoli G, Ragazzini P, Gobbi GA, Sangiorgi L, Pazzaglia L, Asp J, Brantsing C, Picci P. Involvement of INK4A gene products in the pathogenesis and development of human osteosarcoma. Cancer. 2001;92:3062–3067. doi: 10.1002/1097-0142(20011215)92:12<3062::aid-cncr10161>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Toguchida J, Yamamuro T, Kotoura Y, Takada N, Kawaguchi N, Kaneko Y, Nakamura Y, Sasaki MS, Ishizaki K. Allelotype analysis in osteosarcomas: frequent allele loss on 3q, 13q, 17p, and 18q. Cancer Res. 1992;52:2419–2423. [PubMed] [Google Scholar]
- 33.Iwao K, Miyoshi Y, Nawa G, Yoshikawa H, Ochi T, Nakamura Y. Frequent β-catenin abnormalities in bone and soft-tissue tumors. Jpn J Cancer Res. 1999;90:205–209. doi: 10.1111/j.1349-7006.1999.tb00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimachi K, Taguchi K, Aishima S, Tanaka S, Shimada M, Kajiyama K, Tsuneyoshi M. Altered expression of β-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900–905. doi: 10.1038/modpathol.3880409. [DOI] [PubMed] [Google Scholar]
- 35.Schlosshauer PW, Pirog EC, Levine RL, Ellenson LH. Mutational analysis of the CTNNB1 and APC genes in uterine endometrioid carcinoma. Mod Pathol. 2000;13:1066–1071. doi: 10.1038/modpathol.3880196. [DOI] [PubMed] [Google Scholar]
- 36.Polakis P. The oncogenic activation of β-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara A, Yokoyama Y, Wan X, Takahashi Y, Mori Y, Takami T, Shimokawa K, Tamaya T. Cytoplasmic/nuclear expression without mutation of exon 3 of the β-catenin gene is frequent in the development of the neoplasm of the uterine cervix. Gynecol Oncol. 2001;82:450–455. doi: 10.1006/gyno.2001.6298. [DOI] [PubMed] [Google Scholar]
- 38.Omholt K, Platz A, Ringborg U, Hansson J. Cytoplasmic and nuclear accumulation of β-catenin is rarely caused by CTNNB1 exon 3 mutations in cutaneous malignant melanoma. Int J Cancer. 2001;92:839–842. doi: 10.1002/ijc.1270. [DOI] [PubMed] [Google Scholar]
- 39.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 40.Webster MT, Rozycka M, Sara E, Davis E, Smalley M, Young N, Dale TC, Wooster R. Sequence variants of the axin gene in breast, colon, and other cancers: an analysis of mutations that interfere with GSK3 binding. Genes Chromosomes Cancer. 2000;28:443–453. [PubMed] [Google Scholar]
- 41.Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sorensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T. Deletions of AXIN1 a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res. 2001;61:7039–7043. [PubMed] [Google Scholar]
- 42.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Salas N, Palacios J, de Castro J, Moreno G, Gonzalez-Baron M, Gamallo C. β-catenin expression pattern in small cell lung cancer: correlation with clinical and evolutive features. Histol Histopathol. 2001;16:353–358. doi: 10.14670/HH-16.353. [DOI] [PubMed] [Google Scholar]
- 44.Wong CM, Fan ST, Ng IO. β-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 45.Hasegawa T, Yokoyama R, Matsuno Y, Shimoda T, Hirohashi S. Prognostic significance of histologic grade and nuclear expression of β-catenin in synovial sarcoma. Hum Pathol. 2001;32:257–263. doi: 10.1053/hupa.2001.22764. [DOI] [PubMed] [Google Scholar]
- 46.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]