Abstract
The concept of inflammatory back pain (IBP) evolved in the 1970s, coincident with the discovery of the HLA-B27 association with ankylosing spondylitis (AS), leading to the development of criteria to determine the presence of IBP. The concept of IBP and it relationship with AS and axial spondyloarthritis (AxSpA) has further evolved, and an instrument developed (the Spondylitis Association of America Back Pain Tool), which was further modified and field tested for use in the 2009-2010 National Health and Nutrition Examination Survey (NHANES). This has shown the frequency of chronic back pain to have risen to 19.4%, with nearly one-third having IBP. The prevalence of AxSpA has been defined at 1.0-1.4% and AS at 0.52-0.55%. The national prevalence of HLA-B27 in the U.S. is 6.1%, and intriguing data from NHANES 2009 suggest a decreasing frequency with increasing age. From this arise new questions and a work agenda ahead.
Keywords: Epidemiology, Spondyloarthritis, HLA-B27, Back Pain, Ankylosing Spondylitis
Introduction
Population studies have shown that chronic back pain is among the most common problems that cause patients to seek medical care. Earlier data from the second National Health and Nutrition Examination Survey (NHANES II), conducted between 1976 and 1980, demonstrated that the cumulative lifetime prevalence of low back pain (LBP) lasting at least 2 weeks was 13.8%1. LBP is second only to the common cold in frequency among adult ailments, and represents the fifth most common reason for an office visit. Recent data from NHANES 2009-2010 report the frequency of chronic back pain (defined as being present on most days for at least three months) to have risen in frequency to 19.3% of the population between the ages of 20 and 65 years, inclusively2.
The high frequency of chronic LBP has engendered a host of diagnostic tests and treatments, many widely used and expensive, not necessarily evidence based, and at times associated with significant morbidity in their own right. One recent review reported a 629% increase in Medicare expenditures for epidural steroid injections in the U.S.; a 423% increase in expenditures for opioids for back pain; a 307% increase in the number of lumbar MRIs among Medicare beneficiaries; and a 220% increase in spinal fusion surgery rates3. The limited studies available suggest that these increases have not been accompanied by population-level improvements in patient outcomes or disability rates3.
The awareness of inflammatory back pain (IBP) as a discrete entity in the U.S. goes back to the 1970s4, coinciding with the discovery of the association of HLA-B27 with AS5,6, for which instruments to gauge it have been refined and validated for use in the clinic as a case-ascertainment tool. Now that such are available the true frequency of IBP, as well as conditions associated with it, including axial spondyloarthritis (AxSpA) and ankylosing spondylitis (AS), in the U.S. can be estimated. With the introduction of effective but costly new medications in the treatment of AxSpA and AS, it is clear that more comprehensive ascertainment of these conditions is necessary to plan a health-care agenda and to facilitate diagnosis and maximize cost-effectiveness of the treatment of these diseases7.
This review will focus on this in the context of how a case-ascertainment tool was converted into a population-based screening instrument of chronic back pain, IBP, AxSpA and AS in the NHANES story. It will further focus on the consequences of carrying out HLA-B27 testing in a population-based sample in NHANES 2009, compare this with other studies in this regard, and address unanswered questions left by these new data.
Developing Instruments to Study Inflammatory Back Pain
In developing the initial instrument characterizing IBP, the Stanford group administered a questionnaire relating to the presence and nature of back pain to all 10,150 employees of an industrial complex8. The questionnaire was returned by 2,892 subjects (65% men). Of these, 1,880 (65% of responders or 19% of total) reported a history of back pain. One hundred twenty-four described their back pain as insidious in onset, persisting for at least three months, developing at less than 40 years of age, being associated with morning stiffness, and showing improvement with exercise. Three hundred sixty-seven subjects scored four of these five features. Pelvic radiographs of 342 persons were available for blind evaluation. Sixteen patients (12 men) were shown to have definite ankylosing spondylitis (Grade III or IV sacroiliitis or HLA-B27-associated grade II sacroiliitis). Only one of these persons was known to have spondylitis. The majority of these symptomatic patients had been seen by both medical and nonmedical practitioners.
Then, questionnaires containing 17 questions relating to back pain were given to three groups of subjects: 42 known HLA-B27 positive subjects with AS by New York criteria, 21 patients from an orthopedic clinic who were B27 negative, with normal X-rays of the sacroiliac joints, and 75 controls, including healthy volunteers or patients attending non-rheumatology clinics4. They found back pain was pretty common in all groups (60% of controls), and the following five characteristics distinguished AS from the rest: age less than 40 years, insidious onset, duration at least three months, morning stiffness, and improvement with exercise.
In two studies from this era, AS was found in 20% of healthy HLA-B27positive blood donors (at Stanford9 and from Chicago10), and, with a reported 6% population frequency of HLA-B27, led to the conclusion that AS occurs in 1% of the population9,10. A subsequent population-based study, however, suggested this to be an overestimation11.
Since back pain was ubiquitous (20-50% of the population) and genetic testing was expensive, cumbersome (the microcytotoxicity assays for B27 typing in that era required fresh living cells) and reagents were in short supply, the aim was to use a practical alternative to identify patients with AS. Moreover, there were no other laboratory diagnostic tests, and it was recognized then that X-rays were normal in early stage illness and pelvis X-rays frequently misread. Thus Calin, et al. proposed to use the clinical history as a practical screening alternative and emphasize the difference between back pain of an inflammatory nature (AS) from back pain of a non-specific (mechanical) type. In 1977, their conclusions were: 1) reliance on four or more features provided a diagnostic screening test for AS with 95% sensitivity and 85% specificity vs. control groups; 2) if affirmative responses for all five features were required, sensitivity declined (only 60% of AS patients fulfilled all criteria) but specificity increased. Calin's group felt they created a simple, cheap, reproducible screening technique compared to HLA-B27 typing, which was felt alone to be 95% sensitive but only 20% specific. Their overall plan, never achieved, eventually was to validate this test in the general population, where AS is less common. However, their results did concentrate the group for subsequent investigation by a factor of between six and 30, increasing the efficiency of case-finding.
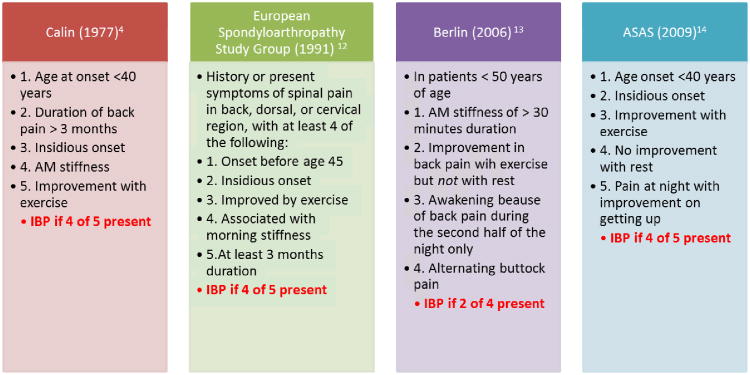
Since this time, the concept of inflammatory back pain has been further explored and defined by other groups using largely similar criteria11-13 (Table 1).
Table 1. Features of Axial Pain in NHANES 2009-2010.
| N | % | |
|---|---|---|
| Chronic low back pain prevalence* | 980/5103 | 19.4 |
| Total Axial Pain Sample | 980 | 100.0 |
| Current Pain | 873 | 89.1 |
| No Current Pain | 107 | 10.9 |
| Age-at-Onset of Pain | ||
| < 20 Years | 164 | 16.8 |
| 20-29 Years | 247 | 25.3 |
| 30-44 Years | 306 | 31.4 |
| 45+ Years | 258 | 26.5 |
| Duration of Pain | ||
| < 1 Year | 50 | 5.2 |
| 1-2 Years | 154 | 16.0 |
| 3-10 Years | 343 | 35.7 |
| > 10 Years | 415 | 43.1 |
| Temporal Pattern of Pain | ||
| Constant, never goes away | 660 | 67.5 |
| Episodic, no relief > 1mo | 178 | 18.2 |
| Episodic, pain relief > 1 mo | 123 | 12.6 |
| One pain episode only | 17 | 1.7 |
Chronic back pain is defined as at least one episode of neck, upper-, mid- or lower back or buttock pain lasting at least three months
Now, 35 years later, the question is whether this approach is still valid or if we need to do more work? With biologic agents, advanced imaging techniques, and recent successes in defining genetic susceptibility, it is even more important to identify disease earlier. Besides, the initial instrument was never utilized to define how many patients have AS in the population. Even if AS is identified early, important questions remain: Can early intervention change the course of the disease (to the extent to which the natural history of the disease is even known)? Do new drugs make a difference in real outcome effects (bone proliferation, comorbidities) or is there a great deal of health-care resources being allocated for nothing that alters the natural history of the disease in return?7 So, there are plenty of reasons to repeat these studies with a larger sample size and utilizing a more sophisticated analytic approach.
To improve case-identification and expand our efforts toward a true population-based survey, the Spondylitis Association of America (SAA) in 2008 initiated the SAA back pain study, ultimately aimed at developed an online screening tool to facilitate the diagnosis of AS15. A three-phase approach was undertaken, beginning with literature review, expert panel, and cognitive testing with AS patients followed by initial feasibility testing, and then a case control study for validation, item reduction, and creation of a scoring algorithm. One hundred forty-five AS cases and >300 chronic back pain controls made up the final set15. The final model provided a 12-question pool with a sensitivity of 70% and a specificity of 99%. The strongest discriminators were male gender, pain/stiffness in the neck and/or hip, pain/stiffness decreasing with daily physical activity, and a history of iritis15. This tool performed similarly to other case-ascertainment instruments such as mammography, PAP smears, and various nuclear cardiology tests for coronary artery disease.
This study employed additional aspects beyond traditional symptom-based questionnaires, including items such as gender, location of pain/stiffness, and responsiveness to NSAIDs12-14. Instead of binary questions, gradations of input were examined. The concept of “exercise” related to pain/stiffness, however, is complex–the differences between intensive physical exercise and activities of daily living should be distinguished. The concept of awakening from sleep is not just in the morning, given people's different sleep patterns. Much more cognitive testing needed to be done for a population-based instrument. The role of diagrams for location of symptoms and signs would be important and provide much more granularity.
NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States (http://www.cdc.gov/nchs/nhanes.htm). The survey is unique in that it combines interviews, physical examinations, and laboratory assessments. Begun in the 1960s, NHANES is a major program of the National Center for Health Statistics (NCHS). NCHS is part of the Centers for Disease Control and Prevention (CDC) and has the responsibility for producing vital and health statistics for the nation. The NHANES interview includes demographic, socioeconomic, dietary, and health-related questions. The examination component consists of medical, dental, and physiological measurements, as well as laboratory tests administered by highly trained medical personnel. Currently, available U.S. population-based data for AS, SpA and IBP from the nationally representative NHANES include both NHANES I (1971-1975)16 and NHANES II (1976-1980) surveys1. The pelvic radiographs obtained in NHANES I provided U.S. prevalence estimates for radiographic sacroiliitis, an important component of the AS case definition. AS and SpA prevalence's cannot readily be calculated from NHANES I survey data; however, IBP prevalence (Rudwaleit, et al. criteria 7b)13 can be estimated from NHANES II. The NHANES II estimate for IBP is 0.8% of the adult population ages 25 to 49 years2. The prevalence of IBP in the subset of persons with a history of a back pain episode lasting two or more weeks was 6.7%1.
The goals of the NHANES 2009-10 musculoskeletal component were threefold: 1) to provide a comprehensive U.S. national IBP estimate; 2) to provide a provisional U.S. national AxSpA estimate; and 3) to conduct the first U.S. national study of HLA-B27 prevalence, population distribution and associations.
In estimating the current U.S. IBP prevalence, four published case definitions4,12-14 are available. An IBP data collection instrument, specifically designed for NHANES 2009-10 and derived from the SAA Back Pain Screening Tool, was developed and field tested in 550 individuals at 15 sites around the U.S. The participants were 5,103 U.S. adults ages 20-69 with complete data. IPB prevalence was determined by questions from Calin, et al criteria4, European Spondyloarthropathy Study Group (ESSG) criteria12, and two of the Berlin criteria13. The ASAS Criteria for the Classification of Axial Spondyloarthritis14 had not been finalized by the time the NHANES instrument was field tested and were not included in the study.
NHANES 2009-2010 showed the age adjusted U.S. prevalence of IBP by Calin, et al criteria was 5.0% (95% CI 4.2% to 5.8%), 5.6% by ESSG criteria12, and 5.8% or 6.0% by various Berlin criteria13. (Table 2). There was no difference among age groups or between men and women. There were differences among ethnicities: IBP prevalence was significantly lower among non-Hispanic black persons compared to non-Hispanic white persons. Non-Hispanic white persons had higher frequency of IBP compared to Mexican-Americans.
Table 2. NHANES 2009-2010 Prevalence of IBP.
| Overall U. S. Prevalence | Calin et al (1977) % | ESSG(1991) % | Berlin (2006) % | Berlin (2006) % |
|---|---|---|---|---|
| Age group | 5.0 | 5.6 | 5.8 | 6.0 |
| 20-35 years | 5.0 | 5.0 | 4.8 | 6.2 |
| 36-49 years | 5.9 | 6.8 | 6.8 | 5.9 |
| 40-69 years | 4.1 | 5.0 | n.a | n.a |
| Sex | ||||
| Male | 5.2 | 5.6 | 5.6 | 5.4 |
| Female | 4.9 | 5.6 | 6.1 | 6.7 |
| Racial/ethnic groups | ||||
| Mexican-Americans | 4.1 | 4.4 | 4.9 | 4.2 |
| Caucasians-not Hispanic | 5.9 | 6.5 | 6.6 | 7.2 |
| Afro-Americans | 3.3 | 4.1 | 5.5 | 5.1 |
What was learned about inflammatory back pain from these efforts? First of all–the term IBP is used without knowing what it really is or what the gold standard is–is this circular reasoning? The NHANES 2009-2010 numbers are higher than prior estimates, probably reflecting use of a pain diagram and questions designed to capture IBP. Another big question is: What constitutes the gap between the 5-6% with IBP and the prevalence of AS estimated to be 1%? Is IBP a different disease or condition? Calin's original definitions were created to separate AS from mechanical back pain; but not all AS patients have IBP, especially at different stages of the disease. This raises concerns as to whether IBP should even be used as diagnostic criteria for AS.
NHANES and the Prevalence of Axial Spondyloarthritis
Different criteria have been proposed for SpA, including those of Amor17 and ESSG12 over 20 years ago, and more recently by the ASAS group14. The Amor and ESSG criteria have been widely used in population studies; however, the requirement for MRI scanning and/or HLA-B27 typing has made the ASAS criteria unfeasible for population studies. Because of this (and that the ASAS criteria were published after the development and fielding of the NHANES study instrument), NHANES used Amor and ESSG classification criteria. The NHANES data collection fully supported the ESSG inflammatory spinal pain case definition and the Amor back pain/stiffness case definition, but some items could not be fielded by NHANES (such as getting pelvic radiographs to determine radiographic sacroiliitis). The overall age-adjusted prevalence of definite and probable SpA by the Amor criteria was 0.9% (95% CI 0.7%-1.1%) and by ESSG criteria was 1.4%18 (Table 3). There were no sex differences. These estimates were in the range of 0.35 to 1.30%published in 2008 by the U.S. National Arthritis Data Workgroup, which basically summed up published prevalence estimates for the various SpA subgroups19. One limitation is that the validity of the NHANES prevalence estimates rests entirely on the prior validation of the two published sets. Therefore, the current estimates probably represent a lower boundary for the true prevalence of AxSpA in the U.S.
Table 3. Estimated Prevalence of Spondyloarthritis by ESSG Criteria in U.S. Adults Ages 20-69 years: NHANES 2009-10.
| Case Type | N | N | % | SE | L 95% CI | U 95% CI |
|---|---|---|---|---|---|---|
| Overall AS | 5103 | 28 | 0.55 | (reporting as having a dx of AS) | ||
| Overall AxSpA | 5103 | 70 | 1.4 | 0.2 | 1.0 | 1.9 |
| 20-49 Years | 3188 | 49 | 1.5 | 0.2 | 1.1 | 2.0 |
| 50-69 Years | 1915 | 21 | 1.3 | 0.4 | 0.7 | 2.5 |
| Gender | ||||||
| Males | 2472 | 24 | 1.1 | 0.3 | 0.6 | 2.0 |
| Females | 2631 | 46 | 1.7 | 0.3 | 1.2 | 2.5 |
| Race/Ethnicity Groups | ||||||
| Mexican-Americans | 1024 | 15 | 1.5* | 0.5 | 0.7 | 3.0 |
| Non-Hispanic Whites | 2244 | 38 | 1.5 | 0.3 | 1.0 | 2.3 |
| Non-Hispanic Blacks | 963 | 9 | 0.9 | 0.3 | 0.4 | 1.8 |
NHANES and the Prevalence of AS
In NHANES I (1971-1975), 6,913 participants between the ages of 25 and 74 years were evaluated, in whom pelvic radiographs were obtained in all but 2,010 (women of childbearing age—i.e., under the age of 50 years—in whom obtaining pelvic radiographs was felt to be unethical). The prevalence of severe or moderate radiographic sacroiliitis (roughly corresponding to grade IV or III, respectively) in men was 4.0/1,000 for ages 25–34 years, 3.0/1,000 for ages 35–44 years, 27/1,000 for ages 55–64 years, 6.0/1,000 for ages 65–74 years, and 7.3/1,000 for ages 25–74 years (Table 2). Among women, the prevalence was 3.0/1,000 for ages 55–64 years, 4.0/1,000 for ages 65–74 years, and 3.0/1,000 for ages 50–74 years.
Of note, 54% of those who had moderate to severe radiographic sacroiliitis reported never having been treated for joint problems, and only 7.6% were currently experiencing “significant pain in their lower backs on most days for at least one month.” How many had AS by modified New York criteria was not ascertained, as questions regarding IBP or measurements of spinal mobility were not done. These may be underestimates for radiographic sacroiliitis, as the knee and hip osteoarthritis readings by the same observers were found to be under-read16,19; moreover, the overall grade for disease was averaged between bilateral sites, which may have diluted the scores if there was only unilateral disease. Of note, 28 of the 5,013 participants in the NHANES 2009-2010 admitted to carrying a diagnosis of AS. These data are in striking contrast to an old, clinic-based, pre-criteria study showing a much lower U.S. frequency of AS19.
The Importance of HLA-B27 in AxSpA and AS
The critical role that HLA-B27 plays in the pathogenesis of ankylosing spondylitis is little disputed. Epidemiologic studies have shown that the frequency of AS and SpA in a given population parallels the frequency of HLA-B2722-36 (Tables 3-4). In early axial SpA, one recent study has shown that HLA-B27 is associated with earlier onset of IBP, less delay in diagnosis, axial inflammation (spine and sacroiliac joints), radiographic damage of the sacroiliiac joint, decreased disease activity, and lower frequency of psoriasis, but not with physical function and MRI structural lesions of the sacroiliac joints37. In another early axial SpA cohort, HLA-B27 positivity was only associated with age at symptom onset38.
Table 4. Prevalence of AS and HLA-B27 in Different Population Groups.
| Group | Year | Frequency of AS (%) | HLA-B27 Population Frequency (%) | Reference |
|---|---|---|---|---|
| Hungary | 1977 | 0.24 | 13 | 22 |
| Haida (Canada) | 1984 | 20 | 50 | 23 |
| Netherlands | 1984 | 0.24 | 8 | 11 |
| Norway | 1985 | 1.2-1.4 | 14 | 24 |
| Sami (Norway) | 1992 | 1.2 | 24 | 25 |
| Taiwan (three groups) | 1994 | 0.19-0.54 | 2.1-9.2 | 26 |
| Chukotka (Siberia) | 1994 | 1.1 | 34 | 27 |
| Eskimo (Alaska) | 1994 | 0.4 | 37-50 | 28 |
| Berlin (Germany) | 1998 | 0.55 | 9 | 29 |
| Japan | 2001 | 0.0065 | 0.5 | 30 |
| Greece | 2005 | 0.24 | 6 | 31 |
| Italy | 2007 | 0.37 | 5 | 32 |
| Turkey | 2008 | 0.49 | 6.8-8.0 | 33 |
| China | 2008 | 0.1-0.5 | 3.6-5.7 | 34 |
| Iceland | 2010 | 0.13 | 15 | 35 |
| Mexico | 2011 | 0.02 | 4.6 | 36 |
| United States | 2012 | 0.54 | 6.1 | 18 |
NHANES 2009 has shown that the age-adjusted U.S. prevalence of HLA-B27 was 6.1%39 (Table 5). HLA-B27 occurred in 7.5% of non-Hispanic whites and 3.5% of all other U.S. races and ethnicities combined. In Mexican-Americans, the prevalence was 4.6%. In blacks, the numbers were too low to make any definitive prevalence statements, although the observed frequency of 1.1% was strikingly similar to older estimates40. For adults 50-69 years, the prevalence was 3.6%, suggesting a decrease in HLA-B27 with age. However, there was no linear trend when this was analyzed by decade. Multiple logistic regression analysis of the independent effects of gender, race/ethnicity, and age group showed significantly lower HLA-B27 prevalence estimates for older as opposed to younger U.S. adults (3.6% for those 50-69 years of age vs. 7.3% for those 20-49 years, respectively [OR 0.4, 95% CI 0.3-0.8]). The lower prevalence of B27 in this group is suggestive, but does not prove, that B27 is a risk factor for early mortality. Numerous studies have shown that patients with AS have a reduced life span, especially those with persistently active disease41. Moreover, in NHANES I the prevalence of moderate-to-severe sacroiliitis fell in men, from 2.7% in men 55-64 years of age to 0.6% in men in the 65-74 age group16. One interpretation of these data is that these individuals have reduced survival.
Table 5. The Prevalence of HLA-B27 for U.S. Adults Ages 20-69 Years, by Selected Characteristics, NHANES 2009*.
| Selected Characteristic | n | N | % | SE | 95% CI |
|---|---|---|---|---|---|
| Overall U.S. Prevalence | 124 | 2320 | 6.1 | 0.8 | (4.6-8.2) |
| Gender | |||||
| Males | 53 | 1123 | 5.8 | 1.0 | (3.9-8.4) |
| Females | 71 | 1197 | 6.5 | 1 | (4.7-8.9) |
| Race/Ethnic Groups | |||||
| Non-Hispanic Whites | 79 | 1021 | 7.5 | 1.2 | (5.3-10.4) |
| Mexican-Americans | 27 | 622 | 4.6 | 0.6 | (3.4-6.1) |
| Non-Hispanic Blacks | 4 | 345 | 1.1* | 0.5* | (0.4-3.1)* |
| Age Groups | |||||
| 20-29 Years | 39 | 498 | 8.0 | 2.0 | (4.6-13.4) |
| 30-39 years | 26 | 471 | 5.6 | 1.3 | (3.4-9.2) |
| 40-49 Years | 34 | 508 | 8.1 | 1.2 | (5.8-11.2) |
| 50-59 Years | 11 | 404 | 2.9** | 0.9** | (1.4-5.8)** |
| 60-69 Years | 14 | 439 | 4.6** | 1.9** | (1.9-10.7)** |
This is an estimate, as the small numbers lack statistical power to derive a definitive frequency
Multiple logistic regression analysis of the independent effects of gender, race/ethnicity and age group showed significantly lower HLA-B27 prevalence estimates for older as opposed to younger U.S. adults (3.6% for those 50-69 years of age vs. 7.3% for those 20-49 years, respectively (OR 0.4, 95% CI 0.3-0.8).
On top of this are recent data from The Australo-Anglo-American Spondylitis Consortium (TASC) and Wellcome Trust Case Control Consortium II showing that the combination of HLA-B27 and a specific (and common) endoplasmic reticulum-associated aminopeptidase (ERAP1) polymorphism results in aberrant presentation of intracellularly derived peptides42. HLA-B27 positive individuals are at a disadvantage in dealing with infections with certain bacteria that can survive intracellularly, such as those associated with reactive arthritis, including Salmonellae, Yersiniae, Shigellae, Campylobacteriae and Chlamydiae43,44. A recent study also suggests that HLA-B27 positive individuals may be less effective against malaria47. This and prior data showing impaired intracellular killing44 and enhanced intracellular bacterial replication45 in HLA-B27 positive cell lines reinforce the hypothesis that people with HLA-B27 (and perhaps the appropriate ERAP1 polymorphism) have a selective immunodeficiency against certain intracellular microbes. This may result in chronic and often subclinical infection that gives rise to a chronic inflammatory state, which is ultimately proatherogenic. Still, this question is by no means resolved, and requires further study.
Conclusion
The original concept of IBP from 35 years ago (1977) has stood the test of time. Clearly, this concept works well in the clinic: It has contributed to the development of an excellent case-ascertainment tool that justifies additional genetic and imaging testing. Does IBP work as a tool for population-based research? What is the relationship between IBP and SpA? The gap between prevalence of IBP and SpA suggests it may be even more heterogeneous than we originally thought. HLA-B27 testing, when merged with the NHANES data, should narrow the gap in our understanding of these issues. But the new data from NHANES, including data showing age-related differences in the frequency of HLA-B27, raises numerous new questions that will require further confirmation and definition.
Figure 1. Inflammatory Back Pain By Different Criteria.
Acknowledgments
This work was supported by 2P01AR052915-06A1 (J. Reveille PI) and by the Spondyloarthritis Research and Treatment Network and the Spondylitis Association of America
Footnotes
Conflict of Interest Statement: Neither author has any conflict of interest nor source of funding relevant to the material contained in this manuscript
Presented at the annual research and education meeting of SPondyloArthritis Research and Treatment Network (SPARTAN), Portland, Oregon, July 27-28, 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila, Pa 1976) 1987;12(3):264–68. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Weisman MH, Witter JP, Reveille JD. The prevalence of inflammatory back pain: population-based estimates from the US National Health and Nutrition Examination Survey, 2009-10. Ann Rheum Dis. 2012 Jul 11; doi: 10.1136/annrheumdis-2012-201403. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62–68. doi: 10.3122/jabfm.2009.01.080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calin A, Porta J, Fries JF, Schurman DJ. Clinical history as a screening test for ankylosing spondylitis. J Am Med Assoc. 1977;237(24):2613–14. [PubMed] [Google Scholar]
- 5.Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288(14):704–06. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 6.Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing spondylitis and HL-A 27. Lancet. 1973;301(7809):904–07. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 7.Reveille JD, Ximenes A, Ward MM. Economic considerations of the treatment of ankylosing spondylitis. Am J Med Sci. 2012;343(5):371–74. doi: 10.1097/MAJ.0b013e3182514093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calin A, Kaye B, Sternberg M, et al. The prevalence and nature of back pain in an industrial complex: a questionnaire and radiographic and HLA analysis. Spine (Phila, Pa 1976) 1980;5(20):201–05. doi: 10.1097/00007632-198003000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Calin A, Fries JF. Striking prevalence of ankylosing spondylitis in “healthy” w27 positive males and females. N Engl J Med. 1975;293(17):835–39. doi: 10.1056/NEJM197510232931701. [DOI] [PubMed] [Google Scholar]
- 10.Cohen LM, Mittal KK, Schmid FR, et al. Increased risk for spondylitis stigmata in apparently healthy HL-AW27 men. Ann Intern Med. 1976;84(1):1–7. doi: 10.7326/0003-4819-84-1-1. [DOI] [PubMed] [Google Scholar]
- 11.van der Linden S, Valkenburg H, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals: a family and population study. Br J Rheumatol. 1983;22(4 Suppl 2):18–19. doi: 10.1093/rheumatology/xxii.suppl_2.18. [DOI] [PubMed] [Google Scholar]
- 12.Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34(10):1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 13.Rudwaleit M, Metter A, Listing J, et al. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum. 2006;54(2):569–78. doi: 10.1002/art.21619. [DOI] [PubMed] [Google Scholar]
- 14.Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 15.Weisman MH, Chen L, Clegg DO, et al. Development and validation of a case ascertainment tool for ankylosing spondylitis. Arthritis Care Res (Hoboken) 2010;62(1):19–27. doi: 10.1002/acr.20009. [DOI] [PubMed] [Google Scholar]
- 16.Maurer K. Basic data on arthritis: knee, hip, and sacroiliac joints in adults ages 25–74 years, United States, 1971–1975. Vital Health Stat. 1979;11(213):1–31. [PubMed] [Google Scholar]
- 17.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57:85–89. [PubMed] [Google Scholar]
- 18.Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondyloarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken) 2012;64(6):905–10. doi: 10.1002/acr.21621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part 1. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 20.Dillon CF, Hirsch R. The United States National Health and Nutrition Examination Survey and the epidemiology of ankylosing spondylitis. Am J Med Sci. 2011;341(4):281–83. doi: 10.1097/MAJ.0b013e31820f8c83. [DOI] [PubMed] [Google Scholar]
- 21.Carter ET, McKenna CH, Brian DD, et al. Epidemiology of ankylosing spondylitis in Rochester, Minnesota, 1935-1973. Arthritis Rheum. 1979;22(4):365–70. doi: 10.1002/art.1780220408. [DOI] [PubMed] [Google Scholar]
- 22.Gomor B, Gyodi E, Bakos L. Distribution of HLA B27 and ankylosing spondylitis in the Hungarian population. J Rheumatol Suppl. 1977;3:33–5. [PubMed] [Google Scholar]
- 23.Gofton JP, Chalmers A, Price GE, Reeve CE. HL-A 27 and ankylosing spondylitis in B.C. Indians. J Rheumatol. 1984;11(5):572–73. [PubMed] [Google Scholar]
- 24.Gran JT, Husby G, Hordvik M. Prevalence of ankylosing spondylitis in males and females in a young middle-aged population of Tromsø, northern Norway. Ann Rheum Dis. 1985;44(6):359–67. doi: 10.1136/ard.44.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnsen K, Gran JT, Dale K, et al. The prevalence of ankylosing spondylitis among Norwegian Samis (Lapps) J Rheumatol. 1992;19(10):1591–94. [PubMed] [Google Scholar]
- 26.Chou CT, Pei L, Chang DM, et al. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural differences. J Rheumatol. 1994;21(2):302–06. [PubMed] [Google Scholar]
- 27.Alexeeva L, Krylov M, Vturin V, et al. Prevalence of spondyloarthropathies and HLA-B27 in the native population of Chukotka, Russia. J Rheumatol. 1994;21(12):2298–300. [PubMed] [Google Scholar]
- 28.Kaipiainen-Seppanen O, Aho K, Heliovaara M. Incidence and prevalence of ankylosing spondylitis in Finland. J Rheumatol. 1997;24(3):496–99. [PubMed] [Google Scholar]
- 29.Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67. doi: 10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 30.Hukuda S, Minami M, Saito T, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol. 2001;28(3):554–59. [PubMed] [Google Scholar]
- 31.Trontzas P, Andrianakos A, Miyakis S, et al. Seronegative spondyloarthropathies in Greece: a population-based study of prevalence, clinical pattern, and management. The ESORDIG study Clin Rheumatol. 2005;24(6):583–89. doi: 10.1007/s10067-005-1106-9. [DOI] [PubMed] [Google Scholar]
- 32.De Angelis R, Salaffi F, Grassi W. Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand J Rheumatol. 2007;36(1):14–21. doi: 10.1080/03009740600904243. [DOI] [PubMed] [Google Scholar]
- 33.Onen F, Akar S, Birlik M, et al. Prevalence of ankylosing spondylitis and related spondyloarthritides in an urban area of Izmir, Turkey. J Rheumatol. 2008;35(2):305–09. [PubMed] [Google Scholar]
- 34.Zeng QY, Chen R, Darmawan J, et al. Rheumatic diseases in China. Arthritis Res Ther. 2008;10(1):R17. doi: 10.1186/ar2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geirsson AJ, Eyjolfsdottir H, Bjornsdottir G, et al. Prevalence and clinical characteristics of ankylosing spondylitis in Iceland - a nationwide study. Clin Exp Rheumatol. 2010;28(3):333–40. [PubMed] [Google Scholar]
- 36.Alvarez-Nemegyei J, Peláez-Ballestas I, Sanin LH, et al. Prevalence of musculoskeletal pain and rheumatic diseases in the southeastern region of Mexico. J Rheumatol Suppl. 2011;86:21–5. doi: 10.3899/jrheum.100954. [DOI] [PubMed] [Google Scholar]
- 37.Chung HY, Machado P, van der Heijde D, et al. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis. 2011;70(11):1930–36. doi: 10.1136/ard.2011.152975. [DOI] [PubMed] [Google Scholar]
- 38.Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60(3):717–27. doi: 10.1002/art.24483. [DOI] [PubMed] [Google Scholar]
- 39.Reveille JD, Hirsch R, Dillon CF, et al. The prevalence of HLA-B27 in the United States: data from the U.S. National Health and Nutrition Examination Survey, 2009. Arthritis Rheum. 2012;64(5):1407–11. doi: 10.1002/art.33503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan MA, Kushner I, Braun WE. Letter: low incidence of HLA-B27 in American blacks with spondyloarthropathies. Lancet. 1976;1(7957):483. doi: 10.1016/s0140-6736(76)91504-x. [DOI] [PubMed] [Google Scholar]
- 41.Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011;70(11):1921–25. doi: 10.1136/ard.2011.151191. [DOI] [PubMed] [Google Scholar]
- 42.Australo-Anglo-American Spondyloarthritis Consortium (TASC) and the Wellcome Trust Case Control Consortium 2 (WTCCC2) Genome-wide association study in ankylosing spondylitis identifies further non-MHC associations, and demonstrates that the ERAP1 association is restricted to HLA-B27 positive cases implicating peptide presentation as the likely mechanism underlying the association of HLA-B27 with the disease. Nat Genet. 2011;43:761–67. [Google Scholar]
- 43.Mathieu A, Cauli A, Fiorillo MT, Sorrentino R. HLA-B27 and ankylosing spondylitis geographic distribution versus malaria endemic: casual or causal liaison? Ann Rheum Dis. 2008;67(1):138–40. doi: 10.1136/ard.2007.072488. [DOI] [PubMed] [Google Scholar]
- 44.Laitio P, Virtala M, Salmi M, et al. HLA-B27 modulates intracellular survival of Salmonella enteritidis in human monocytic cells. Eur J Immunol. 1997;27(6):1331–38. doi: 10.1002/eji.1830270606. [DOI] [PubMed] [Google Scholar]
- 45.Penttinen MA, Heiskanen KM, Mohapatra R, et al. Enhanced intracellular replication of Salmonella Enteritidis in HLA-B27-expressing human monocytic cells: dependency on glutamic acid 45 in the B pocket of HLA-B27. Arthritis Rheum. 2004;50(7):2255–63. doi: 10.1002/art.20336. [DOI] [PubMed] [Google Scholar]