Abstract
The gastrointestinal (GI) tract is in constant “negotiation” with the microbial flora present in the lumen. Resident hematopoietic cells (i.e. lymphocytes, mast cells and eosinophils) are part of this ongoing and silent homeostatic battle. Eosinophilic GI diseases (EGID) are characterized by an increased number of eosinophil infiltrates with no identified cause. In this review, we describe the past and present knowledge regarding the chemotactic factors involved in GI eosinophilia.
Keywords: Eosinophil, gastrointestinal, chemokine, cytokine, pathogenesis
A. CHEMOKINES INVOLVED IN THE HEALTHY GI TRACT
Eosinophils in the healthy GI tract
In normal conditions, most tissues have low levels of eosinophils. However some organs are rich in eosinophils, such as the gastrointestinal tract, spleen, lymph nodes, and thymus, mammary glands and uterus. Interestingly, while present in these multiple tissues, only GI eosinophils are associated with a marked degranulation [1]. At baseline, in healthy patients or normal mice, eosinophils are present in the lamina propria throughout the GI tract from the stomach to the colon [1,2]. However, eosinophils are not found in peyers patches, or intra-epithelial locations [3-6]. Since eosinophils can infiltrate these sites in EGID, the knowledge of the distribution of eosinophils and the chemokines responsible for their presence in the GI tract at baseline is thus essential to identify a possible cause of EGID [1-3]. DeBrosse et al, have shown an increasing level of eosinophils from the esophagus to the colon with barely any eosinophils detected in the esophagus [2]. In the lamina propria, a maximum of 8 eos/hpf were noted in the antrum, 11 in the fundus, up to 26 in the duodenum. Finally, up to 50 eos/hpf were seen in the colon with a high variability inside the different colon segments and between individuals [2].
Chemokine receptor expression
The intestinal tract expresses numerous chemokines at baseline (such as eotaxin-1 and 2, RANTES). In addition to the expression of chemokines in the GI tract, eosinophils expressed numerous chemokine receptors (i.e. CCR3, CCR1 constitutively, and CXCR2, CXCR3, CXCR6 when activated with IL-5). Blood eosinophils are thus under a continual chemotactic gradient originating from the GI tract. The fine balance between the chemokine released, the density of receptor, and the adhesion molecules expression determine the entry of eosinophils in the gut tissue. Eosinophils highly express CCR3; the role for CCR3 and CCR3 ligands in GI eosinophilia have been studied in animal model using gene deficient mice (See figure 1 A).
Figure 1. Chemotactic factors involved in eosinophil infiltration in the GI track.
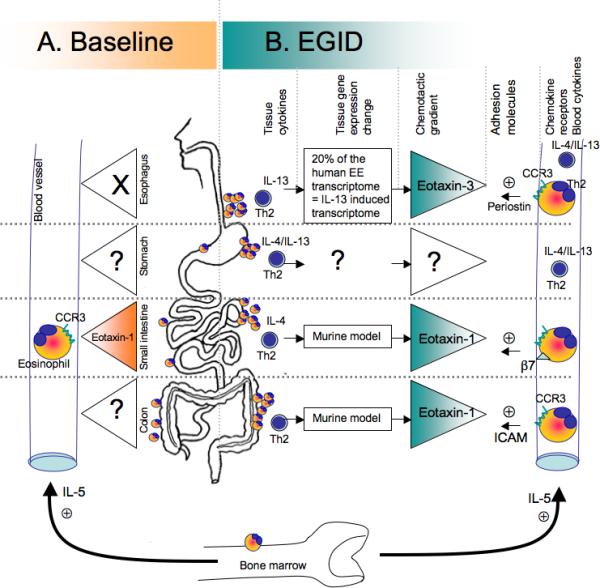
A. Hematopoietic cells are present at baseline in all segments of the GI track. The distribution of eosinophils differs from one segment to another. Basal IL-5 level allows maturation and survival of eosinophils. In the esophagus, no eosinophils are seen, however, the eosinophil levels in the tissue increase from the stomach to the colon. Eotaxin-1 expression in the GI tract and CCR3 expression on the eosinophils are involved in the eosinophilia in the jejunum. B. Eosinophil infiltration in EGID has been associated to Th2 diseases and increased Th2 cytokine production (IL-4, IL-13), has been shown in the blood in EE, EGE, and EG patients. CCR3 expressed on eosinophils have been shown critical for intestinal and esophageal eosinophilic diseases. The eotaxins 1 and 3 are responsible in part for EGID in the intestines and the esophagus, respectively. Others molecules, such the adhesion molecules B7 integrin and ICAM have been shown involved in EGE and EC models but no data document their role EE and EG.
Chemokine expression and involvement in the GI eosinophils at baseline (human and mice)
Murine models have convincingly demonstrated that eosinophilic infiltration is not dependent upon the colonic flora or the endotoxin load of the gut as assessed by the high eosinophil level observed in prenatal mice [7]. Indeed, germ-free mice have normal levels of gastrointestinal eosinophils. Eotaxin 1 and eotaxin 2 are constitutively expressed in a variety of tissues, but are expressed at high levels in the GI tract [7-10]. Interestingly, eotaxin-3 mRNA is not detected at baseline in the GI tract using northern blot [11]. The use of animals deficient in the eotaxin genes has enlightened the field regarding the baseline chemotactic signals responsible for baseline eosinophilia in the gut. For example, it has been shown that the intestine of eotaxin-1 deficient mice is almost completely devoid of eosinophils suggesting that the baseline intestinal eosinophilia is the consequence of the constitutive expression of this chemokine [7,12]. Similar results were observed in CCR3 deficient mice, (deficient for the receptor of eotaxin-1) which have a decreased eosinophil level in the jejunum at baseline (1.3 vs 9 eosinophils / hpf). The residual presence of eosinophils in the GI tract of the CCR3 deficient mice (1.3/hpf) and eotaxin-1 deficient mice (0.3/villus) suggest a modest involvement of other chemotactic factors for eosinophils in the jejunum [12-14].
B. CHEMOTACTIC FACTORS IN EOSINOPHILIC GI DISEASES
In EGID, the eosinophils number is elevated in one or more segment of the esophago-gastroinstestinal tract.
1) Known stimulus in EGID diseases responsible for the induction of chemotactic signal
A Th2 disease
EGID inflammation are believed to be driven by CD4+ Th2 cells [15,16]. These cells are the key component in the production of IL-4, IL-13, IL-5, IL-10 and the control of allergic inflammation responses. IL-4 and 13 are responsible for Th2 cell production and IgE by B cells and IL- 5 controls eosinophil production, activation and survival. Interestingly, resident eosinophils have different cytokine expression patterns under inflammatory or non-inflammatory conditions [15], and esophageal eosinophils from EE patients express relatively high levels of Th2 cytokines [15,16]. Th2 cytokines have been shown to regulate the expression of a large variety of chemokines in vitro.
Chemotactic factors in EE
Murine models have demonstrated that IL-5 maintains the systemic eosinophil levels needed for esophageal eosinophilia accumulation [7,17]. Eosinophil accumulation has been shown to be CCL11/eotaxin-1 and CCR3 dependent using the respective KO mice [18,19]. Remarkably, eosinophils are still infiltrating the esophagus of CD2-IL-5tg/CCL11KO mice, suggesting that other factors may be involved [7]. In human EE, eotaxin-3 expression strongly correlates with eosinophils numbers [19]. Other factors such as chemokines, extracellular matrix component (periostin) or adhesion molecules may facilitate the entry of eosinophils in esophageal tissue [19-21]. Lymphocytes, mast cells, dendritic cells are also increased in EE and microarray analysis have revealed the presence of multiple other chemokines overexpressed in EE patients such as CXCL1, IL-8 and CXCL6 [19] although eotaxin-3 is most strongly upregulated gene of the EE transcriptome. Additionally, IL-13 has recently been shown to induce 20% of the EE transcriptome, and more particularly to induce eotaxin-3 expression in primary esophageal epithelial cells [16].
Chemotactic factors in eosinophilic gastritis and gastroenteritis
The paucity of studies on the molecular pathogenesis of EG and EGE is striking. In clinical studies, increased secretion of IL-4 and IL-5 by peripheral blood T cells has been reported in patients with eosinophilic gastroenteritis. [22]. Similarly, in the duodenum, lamina propria mononuclear cells are more primed to secrete Th2 cytokines in EGID compared to control patients when stimulated with milk proteins [23]. The allergic component of EGE and EG is also emphasized by the increased atopy rate in EGID [24] and by the increased presence of tissue mast cells in human and murine models of allergic diarrhea suggesting a critical role for mast cells in the development of EGID symptoms [25]. In mice, the overexpression of eotaxin-1 by epithelial cells is sufficient to induce intestinal eosinophilia in eotaxin-deficient mice suggesting a possible role for eotaxin-1 in small bowel eosinophilia [17].
Interestingly, RANTES expression strongly correlates with eosinophilia levels in food allergy model in mice (OVA) [26] and, like eotaxin-1, RANTES mRNA is highly expressed in the jejunum of mice [26]. RANTES is also expressed at baseline in the human GI tract and may contribute to hematopoietic cell recruitment at baseline and in EGID [23]. It is also increased in the colon of atopic dermatitis patients [27] and in a rat colitis model [28]. No studies on RANTES deficient mice have determined the ultimate role of this cytokine in eosinophil recruitment at baseline and in EGID.
Chemotactic factors in eosinophilic colitis
Eosinophilic colitis is divided into allergic and non-allergic colitis, but is usually not an IgE-associated disease. While the exact causes of the disease are still unknown, T cell function has been suggested in human studies [23]. T cell has also been shown, in mice, to transfer the disease in a model of ovalbumin-induced colonic inflammation. The development of the intestinal inflammation model was strongly associated with the transcription factor STAT6, involved in the signaling of IL-13 and IL-4 [29]. These cytokines, IL-13 and IL-4, have been implicated in this model and may drive TH2 induced chemokine expression (such as eotaxins). Using an experimental gastrointestinal allergy model in deficient animals, an essential role for eotaxin-1 in regulating eosinophil-associated gastrointestinal pathology, has been demonstrated [30].
Additionally, a critical role for eotaxin-1 in the development of eosinophilia in DSS-induced colitis, suggests a possible role for this cytokine in eosinophilic associated colonic diseases [31].
2) Murine models of EGID: knowledge gained and limitation
It is compelling to note the paucity of murine models that characterize or reproduce the human EGID diseases. EGID is a relatively common disease and very few formal models exist. As a consequence, molecular mechanisms involved in human EGID are still, to this day, not well understood.
In addition, the imperfect overlap between the murine and human chemokines and chemokine receptor genetic map, may reveal some discrepancies between murine models and human diseases. Eotaxin-3, not expressed in mice [32], and highly involved in human EE, is one example of such a limitation.
3)Cellular localization of the chemokines involved in eosinophils chemoattraction in EGID
Due to the various structures present in the GI tract, the cell types involved in the production of these chemokines are quite different. From the top to the bottom, the esophageal epithelial cell has been shown to overexpress eotaxin-3. In the stomach, no study has formally defined the chemokine or the source of chemokines responsible for the eosinophilic accumulation. In the small intestine, eotaxin-1 is expressed by inflammatory cells (mononuclear cell at the neck of the crypt in mice). Finally, in the human colon, eotaxin-1 is expressed by inflammatory cells [33] and epithelial cells. RANTES mRNA was found expressed by intraepithelial lymphocytes and subepithelial lamina propria [34].
4) Paradox of eotaxin-1, 2 and 3
Still, a conundrum exists; eotaxin-1 -2 and -3 can be regulated by the same Th2 stimuli in vitro and all interact with the same receptor CCR3. Studies suggest that a tissue and cell specificity of the expression of these chemokines, in addition to a different kinetic expression and affinity for CCR3, influences the course of asthma pathogenesis. The individual role of these chemokines in the different GI segments requires further study. Although the global paucity of information regarding the involvement of these chemokines in murine models and in human EGID, studies indicates that the baseline eotaxin-1 highly expressed in the GI tract might promote most of the tissue dwelling eosinophils. In lower eosinophilic GI disease, eotaxin-1 might be the main chemotactic signal while eotaxin-3 is the key player in upper EGID (esophagus) (see figure 1B).
5) Other molecules (adhesion molecules, integrins) involved in cell recruitment in EGID
While chemokine and chemokine receptors are essential for the recruitment of eosinophils, a critical role in eosinophil GI infiltration has been attributed to integrins.
Colonic eosinophils express the integrins alphaL, alphaM, and ß2, counter receptors for the adhesion molecule-1 (ICAM-1). Using ICAM-1-deficient mice and anti-ICAM-1 neutralizing antibodies, Forbes et al showed that hapten-induced colonic eosinophilic inflammation is critically dependent upon ICAM-1 suggesting that β2-integrin/ICAM-1 are key components to eosinophil recruitment into the colon during GI inflammation associated with colonic injury [35]. The intestinal eosinophilia induced by the eotaxin-1 intestine transgene has been shown to be dependent upon the β7-integrin [36]. β7-integrin has been shown to be important for eosinophil recruitment to the intestinal tract under inflammatory conditions in an intestinal allergy model where β7 gene-deficient mice displayed impaired eosinophilia compared with wild type mice without affecting blood eosinophilia [37].
Similarly, scattered studies have shown that leukotriene receptor antagonists are able to clinically improve EGID clinical symptoms [38], although their effect on the eosinophil level is controversial [38-42].
Finally, in eosinophilic esophagitis, it has recently been shown that the extracellular matrix protein periostin, an IL-13 induced gene that is highly overexpressed in EE patients compared to control biopsy samples, correlates with eosinophil numbers in the biopsies. Interestingly, in experimental EE, periostin-deficient mice have decreased eosinophil recruitment to the esophagus. A direct role of periostin on eosinophil adhesion was shown. This study suggests that periostin may facilitate eosinophil infiltration in the esophagus of EE patients [21].
C. CONCLUSION: blockage of chemokine or chemokine receptor (use of inhibitors and antibodies) and potential results
Although studies indicate that Th2 cytokines, (IL-5, IL-13, IL-4), and chemokines (eotaxins) are involved in pathogenesis; allergen elimination and corticosteroids is/are still the gold standard therapy for EGID. Anti-IL-5 therapy has been used in EE and hypereosinophilic syndrome-associated EGID [43-46]. We envision that molecular diagnostics, similar to the approach currently being taken to classify cancer [19], where therapeutic approaches and outcomes can be predicted, will be applied to EGID and become useful for diagnosis and prediction of therapeutic responsiveness and prognosis. It is important to note that the mechanisms by which any therapeutic intervention improves EGID pathological symptoms have not been established, highlighting the value of translational research aiming to develop optimal EGID therapy. We anticipate that mechanism-based therapeutic intervention (e.g. anti-eotaxin, CCR3 antagonists, anti-IL-5, and anti-IL-13) will prove to be successful therapies for EGID.
Synopsis.
Eosinophils are permanent residents of the gastrointestinal tact. A high eosinophil accumulation in the GI tract, without a known cause (e.g. infection, IBD), is observed in eosinophilic GI diseases (EGID). Known chemokines and chemotactic factors associated with this eosinophilic infiltration are presented in this review.
Acknowledgments
This work was supported by in part by the American Heart Association 0625296B (C.B.), the Thrasher Research Fund NR-0014 (C.B.), the PHS Grant P30 DK0789392 (C.B.) the NIH AI079874-01 (C.B.) AI070235, AI45898, and DK076893 (M.E.R.), the Food Allergy and Anaphylaxis Network (M.E.R.), Campaign Urging Research for Eosinophil Disorders (CURED), the Buckeye Foundation (M.E.R.) and the Food Allergy Project (M.E.R).
Reference
- 1.Kato M, Kephart GM, Talley NJ, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252:418. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.DeBrosse CW, Case JW, Putnam PE, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210. doi: 10.2350/11-05-0130.1. [DOI] [PubMed] [Google Scholar]
- 3.Mishra A, Hogan SP, Brandt EB, et al. Peyer's patch eosinophils: identification, characterization, and regulation by mucosal allergen exposure, interleukin-5, and eotaxin. Blood. 2000;96:1538. [PubMed] [Google Scholar]
- 4.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113:11. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg ME, Mishra A, Brandt EB, et al. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139. doi: 10.1034/j.1600-065x.2001.790114.x. [DOI] [PubMed] [Google Scholar]
- 6.Rothenberg ME, Mishra A, Brandt EB, et al. Gastrointestinal eosinophils in health and disease. Adv Immunol. 2001;78:291. doi: 10.1016/s0065-2776(01)78007-8. [DOI] [PubMed] [Google Scholar]
- 7.Mishra A, Hogan SP, Lee JJ, et al. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg ME, Luster AD, Lilly CM, et al. Constitutive and allergen-induced expression of eotaxin mRNA in the guinea pig lung. J Exp Med. 1995;181:1211. doi: 10.1084/jem.181.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, et al. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol. 1996;157:5613. [PubMed] [Google Scholar]
- 10.Zimmermann N, Hogan SP, Mishra A, et al. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000;165:5839. doi: 10.4049/jimmunol.165.10.5839. [DOI] [PubMed] [Google Scholar]
- 11.Kitaura M, Suzuki N, Imai T, et al. Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem. 1999;274:27975. doi: 10.1074/jbc.274.39.27975. [DOI] [PubMed] [Google Scholar]
- 12.Matthews AN, Friend DS, Zimmermann N, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998;95:6273. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurish MF, Humbles A, Tao H, et al. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J Immunol. 2002;168:5730. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- 14.Humbles AA, Lu B, Friend DS, et al. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci U S A. 2002;99:1479. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straumann A, Kristl J, Conus S, et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11:720. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Mishra A, Hogan SP, Brandt EB, et al. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 18.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard C, Wang N, Rothenberg ME. Eosinophilic esophagitis: pathogenesis, genetics, and therapy. J Allergy Clin Immunol. 2006;118:1054. doi: 10.1016/j.jaci.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard C, Mingler MK, MacBride M, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunology. 2008;1:289. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe JS, James SP, Mullins GE, et al. Evidence for an abnormal profile of interleukin-4 (IL-4), IL-5, and gamma-interferon (gamma-IFN) in peripheral blood T cells from patients with allergic eosinophilic gastroenteritis. J Clin Immunol. 1994;14:299. doi: 10.1007/BF01540983. [DOI] [PubMed] [Google Scholar]
- 23.Beyer K, Castro R, Birnbaum A, et al. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002;109:707. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 24.Katz AJ, Twarog FJ, Zeiger RS, et al. Milk-sensitive and eosinophilic gastroenteropathy: similar clinical features with contrasting mechanisms and clinical course. J Allergy Clin Immunol. 1984;74:72. doi: 10.1016/0091-6749(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 25.Brandt EB, Strait RT, Hershko D, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JB, Matsumoto T, Shin YO, et al. The role of RANTES in a murine model of food allergy. Immunol Invest. 2004;33:27. doi: 10.1081/imm-120027682. [DOI] [PubMed] [Google Scholar]
- 27.Yamada H, Izutani R, Chihara J, et al. RANTES mRNA expression in skin and colon of patients with atopic dermatitis. Int Arch Allergy Immunol. 1996;111(Suppl 1):19. doi: 10.1159/000237408. [DOI] [PubMed] [Google Scholar]
- 28.Ajuebor MN, Hogaboam CM, Kunkel SL, et al. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- 29.Kweon MN, Yamamoto M, Kajiki M, et al. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000;106:199. doi: 10.1172/JCI8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan SP, Mishra A, Brandt EB, et al. A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc Natl Acad Sci U S A. 2000;97:6681. doi: 10.1073/pnas.97.12.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes E, Murase T, Yang M, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004;172:5664. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 32.Pope SM, Fulkerson PC, Blanchard C, et al. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 33.Wagsater D, Lofgren S, Hugander A, et al. Analysis of single nucleotide polymorphism in the promoter and protein expression of the chemokine eotaxin-1 in colorectal cancer patients. World J Surg Oncol. 2007;5:84. doi: 10.1186/1477-7819-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzucchelli L, Hauser C, Zgraggen K, et al. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Forbes E, Hulett M, Ahrens R, et al. ICAM-1-dependent pathways regulate colonic eosinophilic inflammation. J Leukoc Biol. 2006;80:330. doi: 10.1189/jlb.1105643. [DOI] [PubMed] [Google Scholar]
- 36.Mishra A, Hogan SP, Brandt EB, et al. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem. 2002;277:4406. doi: 10.1074/jbc.M110424200. [DOI] [PubMed] [Google Scholar]
- 37.Brandt EB, Zimmermann N, Muntel EE, et al. The alpha4bbeta7-integrin is dynamically expressed on murine eosinophils and involved in eosinophil trafficking to the intestine. Clin Exp Allergy. 2006;36:543. doi: 10.1111/j.1365-2222.2006.02456.x. [DOI] [PubMed] [Google Scholar]
- 38.Quack I, Sellin L, Buchner NJ, et al. Eosinophilic gastroenteritis in a young girl--long term remission under Montelukast. BMC Gastroenterol. 2005;5:24. doi: 10.1186/1471-230X-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attwood SE, Lewis CJ, Bronder CS, et al. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut. 2003;52:181. doi: 10.1136/gut.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daikh BE, Ryan CK, Schwartz RH. Montelukast reduces peripheral blood eosinophilia but not tissue eosinophilia or symptoms in a patient with eosinophilic gastroenteritis and esophageal stricture. Ann Allergy Asthma Immunol. 2003;90:23. doi: 10.1016/S1081-1206(10)63609-5. [DOI] [PubMed] [Google Scholar]
- 41.Gupta SK, Peters-Golden M, Fitzgerald JF, et al. Cysteinyl leukotriene levels in esophageal mucosal biopsies of children with eosinophilic inflammation: are they all the same? Am J Gastroenterol. 2006;101:1125. doi: 10.1111/j.1572-0241.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 42.Urek MC, Kujundzic M, Banic M, et al. Leukotriene receptor antagonists as potential steroid sparing agents in a patient with serosal eosinophilic gastroenteritis. Gut. 2006;55:1363. doi: 10.1136/gut.2006.099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrett JK, Jameson SC, Thomson B, et al. Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113:115. doi: 10.1016/j.jaci.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 44.Sutton SA, Assa'ad AH, Rothenberg ME. Anti-IL-5 and hypereosinophilic syndromes. Clin Immunol. 2005;115:51. doi: 10.1016/j.clim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Simon D, Braathen LR, Simon HU. [Anti-interlekuin-5 therapy for eosinophilic diseases.]. Hautarzt. 2007;58:122. doi: 10.1007/s00105-006-1273-x. [DOI] [PubMed] [Google Scholar]


