Abstract
Epidemiological and animal studies suggest that environmental toxins including paraquat (PQ) increase the risk of developing Parkinson's disease (PD) by damaging nigrostriatal dopaminergic neurons. We previously showed that overexpression of a group of microRNAs (miRs) affects the antioxidant promoting factor, Nrf2 and related glutathione-redox homeostasis in SH-SY5Y dopaminergic neurons. Although, dysregulation of redox balance by PQ is well documented, the role for miRs and their impact have not been elucidated. In the current study we investigated whether PQ impairs Nrf2 and its related cytoprotective machinery by misexpression of specific fine tune miRs in SH-SY5Y neurons. Real time PCR analysis revealed that PQ significantly (p<0.05) increased the expression of brain enriched miR153 with an associated decrease in Nrf2 and its function as revealed by decrease in 4× ARE activity and expression of GCLC and NQO1. Also, PQ and H2O2-induced decrease in Nrf2 3′ UTR activity was restored on miR153 site mutation suggesting a 3′ UTR interacting role. Overexpression of either anti-miR153 or Nrf2 cDNA devoid of 3′ UTR prevented PQ and H2O2-induced loss in Nrf2 activity confirming that PQ could cause miR153 to bind to and target Nrf2 3′ UTR thereby weakening the cellular antioxidant defense. Adenovirus mediated overexpression of cytoplasmic catalase (Ad cCAT) confirmed that PQ induced miR153 is hydrogen peroxide (H2O2) dependent. In addition, Ad cCAT significantly (p<0.05) negated the PQ induced dysregulation of Nrf2 and function along with minimizing ROS, caspase 3/7 activation and neuronal death. Altogether, these results suggest a critical role for oxidant mediated miR153-Nrf2/ARE pathway interaction in paraquat neurotoxicity. This novel finding facilitates the understanding of molecular mechanisms and to develop appropriate management alternatives to counteract PQ-induced neuronal pathogenesis.
Keywords: Paraquat, miR153, H2O2, Nrf2, neurotoxicity
1. Introduction
Herbicides and pesticides are becoming increasingly potent environmental threats and their usage has increased by 527 million pounds and 404 million pounds respectively from 1996 to 2011 (Benbrook, 2012). Importantly, exposures to these environmental toxins are not only restricted to occupational setting but can stretch out to general residential background (CDC, 2009). Among agricultural chemicals, paraquat (PQ) is a well known herbicide that chiefly affects dopamine systems causing severe pathophysiological alterations and irreversible injuries to dopaminergic neurons (Betarbet et al., 2000; Peng et al., 2004; Ramachandiran et al., 2007). Of note, nigral dopaminergic neuronal loss is documented to be the reason for dysfunction of dopamine transport and associated functional impairments in subjects with Parkinson's disease (PD), Alzheimer's disease (AD) and dementia with Lewy bodies (O'Brien et al., 2004). Though, the striatonigral system is preferentially affected in PD, extensive neurotoxicity and degeneration is also seen in striatum, hippocampus, and neocortex in cases with cognitive impairment associated with PD (Harding et al., 2002; McKeith et al., 2004). PQ has also been reported to elicit neurobehavioral syndrome (Brooks et al., 1999). PQ has been shown to cross the blood brain barrier (BBB) and is suggested to enter brain through a neutral amino acid carrier due to its structural homology to amino acids (McCormack and Di Monte, 2003; Shimizu et al., 2001; Widdowson et al., 1996). Long-term studies demonstrated that oral ingestion of PQ resulted in brain accumulation of PQ with a much longer half-life (Prasad et al., 2007). ROS generation and the resultant oxidative stress are suggested as one of the primary mechanisms for PQ-induced selective neuronal degeneration. Typically, following cellular exposure of PQ, a cascade of oxidative events are elicited leading to NADPH utilization generating reactive oxygen species (ROS) mainly harmful H2O2 and hydroxyl radical (HO.) resulting in lipid peroxidation (Burk et al., 1980).
In general, ROS generated in response to any endogenous or exogenous stimuli is counterbalanced by redox-active cellular factors including enzymatic as well as non-enzymatic antioxidants. Nuclear factor erythroid-2 related factor 2 (Nrf2), a redox-sensitive transcription factor is well documented to tune the expression of array of antioxidant response element (ARE) containing cytoprotective genes and control the physiological and pathophysiological perturbations of oxidants (Yates and Kensler, 2007). Under basal state, Nrf2 is retained in cytoplasm and is maintained at a low level by proteasomal degradation via Keap-1-Cul3/Rbx1/E3-dependent mechanism (Kobayashi et al., 2004). During oxidative stress, the cysteine residue in Keap1 is oxidized and the subsequent conformational change untethers Nrf2 from its latch. Following this event, Nrf2 gains nuclear entry and binds to ARE sequence containing target genes, transactivating them (Nguyen et al., 2000). However, this adaptive response of Nrf2 is only likely in mild to moderate oxidative stress which is evident from dampened Nrf2 activity and low expression of antioxidant genes in several prolonged/chronic oxidative stress conditions (Gounder et al., 2012; Ramsey et al., 2007). Dysregulation of Nrf2 expression and/or disrupted Nrf2 signaling is suggested to play a critical role in several neurodegenerative conditions including AD and PD (Ramsey et al., 2007). Genetic evidence indicates a link between a complete haplotype (SNPs) of Nrf2 and Keap1 genes in relation to the risk of PD (von Otter et al., 2010). In addition, in severe neurodegenerative conditions, the nuclear trafficking of Nrf2 has been shown to be impaired (Ramsey et al., 2007).
Relevant to the current study, it is important to note that deficiency of Nrf2 and/or its activity could aggravate the Parkinsonism like phenotype and the sustained ROS generated by PQ causes similar patterns of selective vulnerability of dopaminergic neurons as that seen in PD (Betarbet et al., 2000; Brooks et al., 1999; Henchcliffe and Beal, 2008). Keap1 based control of Nrf2 is well studied in various experimental scenarios while the post-transcriptional regulation of Nrf2 which occurs chiefly via microRNA's (miRs) is poorly studied in a given context especially in neurons. Recently, we reported that enforced expression of miRs: 144, 153, 27a and 142-5p elicited a post-transcriptional repression of Nrf2 and its activity in dopaminergic SH-SY5Y neurons (Narasimhan et al., 2012). Thus, the goal of this study was to determine whether PQ exposure would dampen Nrf2 based cytoprotection by a miR-related mechanism in human dopaminergic neuronal SH-SY5Y cells.
2. Materials and Methods
2.1. Materials
SH-SY5Y neuroblastoma cells (CRL-2266) were obtained from ATCC (Manassas, VA). Eagle's minimum essential medium (MEM), antibiotic/antimycotic solution, Trizol were from Invitrogen (Carlsbad, CA). Nutrient mixture F-12 Ham was obtained from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum was from Atlanta biologicals (Lawrenceville, GA) and plasmocin from Invivogen (San Diego, CA). Scramble control anti-miR, anti-miR153 and siPort™ Amine NeoFX were purchased from Ambion (Austin, TX). FuGENE HD was from Roche Diagnostics (Indianapolis, IN). QuantiTect reverse transcription kit for first-strand synthesis and endo free plasmid maxi kit was purchased from QIAGEN (Valencia, CA). Antibodies for Nrf2, GCLC, tubulin, GAPDH, lamin b1 and HRP-conjugated goat secondary antibody were from SantaCruz Biotechnologies Inc. (Santa Cruz, CA). HRP conjugated rabbit secondary antibody was bought from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Caspase 3/7 glo assay and Dual Luciferase assay kit was obtained from Promega (Madison, WI). H2O2 and Supersignal West Pico Chemiluminescence detection system was purchased from Thermo Fisher Scientific Inc., (Rockford, IL). 4× ARE Luc plasmid from Dr. Roland C. Wolf (Dundee, UK) and Nrf2 3′ UTR WT and Nrf2 devoid of its endogenous full-length 3′ UTR region (Nrf2(-3′ UTR) constructs were kind gift from Dr. Jen-Tsan Chi (Durham, USA). Adenovirus encoding cytoplasmic catalase (Ad cCAT) was a kind gift from Dr. Arthur Cederbaum (New York, USA). The recombinant adenovirus vector containing CMV promoter β-galactosidase (Ad-CMV-β-gal) was obtained from Vector Biolabs (Philadelphia, PA). Paraquat and all other reagents were from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture
SH-SY5Y cells were grown in minimum essential medium (MEM)/F-12 HAM nutrient medium with 10% FBS, antibiotic/antimycotic and plasmocin at 37°C in a humidified incubator containing 5% CO2. All experiments were performed at cell confluency of 70% to 80% between passages 26–33.
2.3. Dose justification of stressors used
SH-SY5Y cells were previously demonstrated to generate more ROS at a PQ concentration ranging from 50 μM-400 μM in the absence of marked cell death (Alvarez-Erviti et al., 2013; Gabbianelli et al., 1999; Maracchioni et al., 2007). Based on these reports, a sub-toxic concentration of 50 μM PQ was used in the present study. During aerobic cerebral metabolism, H2O2 is continuously generated reaching up to 100 μM under certain prooxidative conditions (Hyslop et al., 1995). Importantly, this is the concentration that showed impaired Nrf2 activity (Rojo et al, 2008).
2.4. Transfection and Luciferase assay
Transfection experiments were carried out as previously described (Narasimhan et al., 2011; Narasimhan et al., 2012). The concentrations of different constructs and anti-miRs used per well were as follows: (i) 200 ng each of 4× ARE reporter, and 3′ WT UTR and mutant reporter (ii) 100 nM of scramble control anti-miR or anti-miR153 (iii) 1 μg of either pcDNA3 or pcDNA3-Nrf2 without 3′ UTR and (iv) 3 ng of renilla reporter used to control for transfection efficiency. Cells were transfected using either siPort amine or Fugene HD. Briefly, miRs (or) vector constructs along with renilla vector and respective transfection reagents were appropriately diluted in OPTI-MEM I medium separately and incubated for 5 min. The plasmid solution is mixed with transfection solution and incubated at room temperature for 20 min allowing the formation of transfection complex. Transfection was performed in serum free, antibiotic free media and 1.5 h post-transfection media containing serum, antibiotics was added and the plates were returned to incubator. After 24 h, cells were treated with indicated concentrations of PQ and H2O2 for additional 24 h. At the end of the experiments, gene reporter activity was determined using dual-luciferase reporter assay system according to the manufacturer's instructions (Promega, Madison, WI). The luciferase activity was measured using Glomax 20/20 luminometer (Promega, Madison, WI) and the luciferase activity was normalized to renilla activity or protein concentration obtained from the corresponsing samples.
2.5. Adenovirus infection
Cells seeded in six-well plates at 75% confluency were infected with either Ad CMV-β-gal or Ad cCAT. Virus infection was performed in 1 ml of serum free media at a multiplicity of infection (MOI) of 15. After infection, the cells were incubated in the culture hood at room temperature for 1 h following which 1 ml of media containing 20% serum was added to obtain a final concentration of 10 % FBS. The infected plates were then returned to a 37°C humidified 5% CO2 incubator. Cells were harvested after 24 and 48 h for initial Western analysis of catalase expression. All experiments involving PQ and H2O2 were performed at 24 h post-infection (Narasimhan et al., 2011).
2.6. Immunoblotting
After treatment, cells were washed with cold PBS and lysed in ice-cold RIPA lysis buffer containing protease inhibitor cocktail. The lysates were sonicated and cleared of debris by centrifugation. Equal amounts of protein lysates from various treated groups were separated on 8% polyacrylamide gels using SDS-PAGE, and transferred to a polyvinylidene difluoride membrane, and blocked with 5% nonfat dry milk at room temperature. After washing once with TBS containing 0.1% Tween 20, the membrane were hybridized with primary antibodies against rat Nrf2, GCLC, GAPDH, tubulin and lamin b1. The extensively washed blots were then incubated with horseradish peroxidase-conjugated anti-rabbit or mouse IgG or goat IgG for 1 h. The antigen-antibody complex was detected using an ECL chemiluminescence kit. The immunoreactive bands were quantified by scanning densitometry using ImageJ software and were normalized to the signal intensity of either GAPDH or tubulin.
2.7. Subcellular fractionation
Cells grown in 100 mm petri dish were treated with H2O2or PQ and at the end of experiment, cytosolic and nuclear protein fractions were extracted from cell lysates using NE-PER Nuclear and Cytoplasmic fractionation kit (Thermo Scientific Inc., Rockford, IL), following the manufacturer's instructions. Equal amounts of protein were electrophoresed and immuoblotted for Nrf2 as described above. The quality of the isolation of cytosolic and nuclear fractions was confirmed by immunoblotting analysis using antibody probes specific for GAPDH and lamin b1 respectively.
2.8. RNA isolation and Semi-quantitative real time PCR analysis
Total cellular RNA was extracted using Trizol reagent. 1.5 μg of RNA was incubated with gDNA wipe out buffer (Qiagen, Valencia, CA) at 42°C for 2 min to remove any genomic DNA contamination. Following gDNA elimination, the RNA is reverse transcribed to cDNA using Quantitect reverse transcription kit according to instructions (Qiagen).
For real time PCR analysis of Nrf2, NQO1, GCLC, GAPDH mRNA expression, 1/10th of cDNAs prepared as above was used. Taqman gene expression assays consisting of predesigned primer, probe sets specific for the genes tested and the TaqMan universal mastermix were from Applied Biosystems (Bedford, MA). Real time PCR amplification was performed in 386-well optical plates in a final volume of 20 μL containing 10 μL of TaqMan universal mastermix, 20 pmol of respective primers and 1/10th of reverse transcribed RNA. Real time PCR was conducted on a Biorad CFX384 Real time system (Hercules, CA). The thermal cycling conditions used consisted of 50° C/2 min; 95° C/10 min followed by 40 PCR cycles at 95° C/15 sec and 60° C/1 min. The mRNA expression levels of genes of interest were calculated on the basis of cycle threshold (Ct) value using GAPDH as normalization control. Relative amplification of each transcript was further calculated as fold difference from untreated samples using ΔΔ Ct method (Livak and Schmittgen, 2001). miRNA detection by real time analysis involved reverse transcription of cDNA using a small RNA specific stem-loop RT primer (hsa-miR153: RT-000476; U6 snRNA: RT-001973). Once specific cDNA was generated, individual miRNA was detected using Taqman small RNA assay real time PCR analysis (hsa-miR153: TM-000476; U6 snRNA: TM-001973). Results were normalized to small nuclear RNA U6 that served as control and the data was expressed as Log 2 fold change in respective miR153/U6 snRNA levels.
2.9. ROS detection by DCF fluorescent assay
Cells were treated with indicated concentrations of the stressors and at the end of the experiments, gently washed once with PBS and then incubated at 37°C for 30 min in dark conditions with 25 μM of cell-permeant, fluorogenic probe, 2′, 7′-dichlorofluorescein diacetate (DCF-DA) in PBS. Cells were then gently washed with PBS and scraped with PBS containing 2 mM EDTA in a 96-well clear bottom-white walled plate. Reactive oxygen species oxidize this non-fluorescent DCF-DA to brightly green fluorescent compound 2′, 7′-dichlorofluorescein whose fluorescence intensity was measured on GLOMAX Multi Detection System with Optical Kit Blue (Excitation 490 nm and Emission 510–570).
2.10. Caspase 3/7 glo assay
The combined activity of caspases-3/7 was estimated using Caspase-glo 3/7 assay. Briefly, atfter treatment, cells were washed with PBS and 300 μL of caspase-glo 3/7 reagent was added to each well and cells were scraped, collected in a microfuge tube in dark. The cell lysate was incubated in dark for 30 min and luminescence was read in a Glomax luminometer (Promega). RLU was recorded and results were expressed as fold change in caspase 3/7 activity from control.
2.11. MTT assay
Cell viability was assessed using MTT assay. After incubation of indicated concentration of the stressors for appropriate time, cells were gently washed with phenol red free RPMI-1640 media. MTT was then added at a final concentration of 0.5 mg/ml and incubated at 37° C for 2 h. The purple colored insoluble formazon crystals generated by viable cells were dissolved using an equal volume of solubilizing solution (isopropanol/HCl/Triton X-100). The optical density was recorded at 570 nm in Model 680 microplate reader (BioRad, Hercules, CA). The resultant data was expressed as the percentage of viable cells relative to that of untreated controls.
2.12. Statistical analysis
Data obtained from the experiments were analyzed using Graph Pad Prism software (San Diego, CA). Results are presented as means ± s.e.m. Statistical significance for multiple-group comparisons was assessed using one-way ANOVA followed by a post hoc (Newman-Keuls) test. Two-tailed, paired Student's t-test was used to determine the significance of differences between treated samples and controls. A probability value less than 0.05 was regarded to be statistically significant.
3. Results
3.1. Paraquat upregulates brain enriched, miR153 expression and affect Nrf2 and its transactivation function
Prior research from our laboratory has demonstrated that forced expression of group of miRs viz. miR144, miR153, miR27a and miR142-5p impairs basal Nrf2 and its dependent redox control in SH-SY5Y neurons (Narasimhan et al., 2012). Endogeneous level of miR144 and miR142-5p in this cell is inherently low and was undetectable (Narasimhan et al., 2012). Of note, miR153 is reported to be a brain-specific miRNA and is prominently expressed in neurons (Doxakis, 2010; Sempere et al., 2004). Importantly, a very recent study demonstrated that miR153 overexpression altered neuronal morphogenesis and neuronal development (Wei et al., 2013). On the basis of this premise and PQ being strongly implicated as dopaminergic toxin, we focused herein to test whether PQ influence the expression of brain-enriched, miR153 and target Nrf2 dependent antioxidant function in SH-SY5Y cells. Real time PCR analysis for miR153 was performed on the lysates obtained from untreated, PQ treated cells. miR153 expression, normalized to U6 levels, revealed that PQ significantly (p<0.05) induced miR153 expression by ∼ 60% (Fig. 1A). We next examined whether the increase in miR153 might have resulted in suppression of its endogenous target, Nrf2. As expected, real time PCR analysis showed that the miR153 expression was associated with significant (p<0.05) downregulation of Nrf2 message normalized to GAPDH levels (Fig. 1B). Nuclear levels of Nrf2 are critical that determines the functionality of Nrf2 and therefore, we next assessed as to whether PQ-induced miR153/Nrf2 message alterations reflected in decline in Nrf2 protein levels in the nuclear compartment. Immunoblotting of Nrf2 using the antibody (sc-722) resulted in multiple bands. Prior studies suggested that Nrf2 anomalously migrates at ∼100 kDA in SDS-PAGE as against its actual predicted molecular weight 68 kDA due to its inherent high acidic charges (Moi et al., 1994). Other studies report that the ∼100 kDA form of Nrf2 is ubiquitinated (Li et al., 2005) or is an actin-Nrf2 dimer (Kang et al., 2002). Thus, in our Western analysis, we quantified both ∼100 kDA and 68 kDA Nrf2 specific bands in the nuclear fractions and were normalized to the levels of lamin-b1. It was observed that ∼100 kDA and 68 kDA Nrf2-specific bands were significantly decreased in PQ exposed cells (Fig. 1C). Such a regulation of both ∼100 kDA and 68 kDA for Nrf2 in a given context was reported by us and others (Narasimhan et al., 2012; Rushworth et al., 2011). Next we addressed if the downregulation of Nrf2 is accompanied by its impairment in transactivation function by using two measures: (i) 4× ARE reporter assay in which the luciferase construct has 4× Nrf2 recognition sites (ii) semi-quantitative real time PCR analysis of Nrf2 target genes, GCLC and NQO1. As measured by luciferase assay, the 4× ARE transcriptional activity was significantly decreased approximately ∼1.6 fold in PQ treated cells when compared to untreated controls (Fig. 1D). In parallel with luciferase transcription assay, the expression of ARE-bearing genes such as NQO1 and GCLC (transcriptional target of Nrf2) was also significantly (p<0.05) downregulated in PQ exposed cells (Fig. 1E). The reduction in Nrf2 and its target gene expression triggered by PQ at the tested dosage were significant but the decrease was only about ∼20% which does not correlate to the increase in miR153 (60%) (Fig. 1A vs Fig. 1B; Fig. 1D). Such a fine regulation is typical with the miR:mRNA interaction wherein miRs can greatly impact biological outcome with subtle changes on target genes (Mukherji et al., 2011; Chatterjee et al., 2013; van Jaarsveld et al., 2013). This could be a result of distribution of miR153 among its gene targets as a single miR is known to target several genes (Doxakis, 2010; Broadie and Patton, 2013). Together, these results indicated that PQ induced miR153 is associated with dysregulation of Nrf2 levels and its transactivation function.
Fig. 1. Paraquat induced upregulation of brain enriched miR-153 expression is associated with downregulation of Nrf2 and its transactivation function.

SH-SY5Y cells were treated with indicated concentrations of paraquat for 24 h. (A) At the end of the experiment, cDNA was reverse transcribed using a small RNA specific stem-loop RT primer (for miR153 and U6). Semi-quantitative real time PCR analysis was performed using Taqman small RNA assay and results were normalized to small nuclear RNA U6 that served as control. The analyzed data was expressed as Log 2 fold change in miR153/U6 snRNA levels from 4 independent samples. (B) TaqMan based semi-quantitative real time RT-PCR analysis of Nrf2 mRNA level in SH-SY5Y cells treated with indicated concentration of PQ. GAPDH mRNA levels were used to normalize those of Nrf2 mRNA (n=4). (C) Nuclear extracts were immunoblotted for Nrf2, lamin-b1 and GAPDH. The panel below the representative image depicts the quantification of ∼100 kDA (represented by *) and 68 kDA (represented by #) specific bands corrected to lamin b1 expression using Image J software (n=3). Low and high exposure was provided to note the changes in ∼100 kDA and 68 kDA respectively. (D) Cells were transfected with 200 ng of luciferase construct containing 4× ARE sequences along with 3 ng of pRL-renilla construct. 24 h post-transfection, cells were treated with indicated concentration of PQ for additional 24 h. Nrf2 transactivation was determined in terms of measuring the reporter activity. Percentage change in luciferase activity normalized to renilla was plotted (n=6). (E) Endogenous NQO1 and GCLC mRNA expression from cells treated with and without PQ were estimated by Taqman based semi-quantitative real-time RT-PCR analysis. NQO1 and GCLC mRNA levels were normalized to GAPDH (n=4). In panel (A-D), values are expressed as means + s.e.m and two-tailed paired Student's t-test was used to establish the statistical significance. * - p<0.05 compared with untreated control.
3.2. H2O2/PQ-induced miR153 resulted in direct targeting of Nrf2 in SH-SY5Y neurons
Typically most forms of cellular oxidative stress lead to copious generation of endogenous H2O2, the most stable and highly reactive ROS candidate. As PQ generates ROS (Burk et al., 1980) and H2O2 being a pivotal messenger in a given stress-response signaling events, we next tested if H2O2 can induce a similar response as that of PQ with reference to miR153/Nrf2 changes. SH-SY5Y cells were treated with H2O2 for 24 h and real time PCR analysis demonstrated that H2O2 treatment elicited a significant (p<0.05) level of miR153 expression (Fig. 2A). Generally, miRs bind to a ∼7 to 8 nucleotide match sequences located in the 3′ untranslated region (3′ UTR) of their target mRNA and control their expression (Fabian et al., 2010). In order to experimentally determine whether H2O2 induced miR153 directly targets Nrf2 by binding to its 3′ UTR sequence, we employed reporter assays. Two different reporter constructs were used in this assay: (i) an artificial WT Nrf2 3′ UTR construct that harbors intact miR153 site and (ii) miR153 mutated Nrf2 3′ UTR luciferase constructs in which the miR153 binding site is three-point mutated (Narasimhan et al., 2012). Both the constructs were individually introduced into the cells following which exposed to H2O2. In the wild type transfected conditions, H2O2 decreased the luciferase output significantly (p<0.05) by ∼35% as H2O2-induced miR153 can bind to its intact cognate site. In contrast, the suppressive effect induced by H2O2 treatment was lost in mutant miR153 reporter transfected cells (lane 2 vs lane 4; Fig. 2B), suggesting that miR153 directly target these sequences and could suppress gene expression. Next, we verified whether H2O2 induced targeting of Nrf2 3′ UTR was because of miR153 amplification using anti-miR153 strategy. Cells were transfected with WT Nrf2 3′ UTR construct along with antisense-miR153 inhibitor and the effect of H2O2 was tested. A non-targeting inhibitor miR (scramble) was used as negative control. Scramble antisense-miR treated with H2O2 exhibited relatively low basal luciferase activity (lane 1; Fig. 2C). While, Nrf2 UTR activity was found to be increased in anti-miR153 transfected cells treated with H2O2 (lane 2 vs lane 1; Fig. 2C). Further, the results for PQ treatment following similar experimental strategies using WT and mutant Nrf2 3′ UTR (Fig. 2D) and anti-miR153 + WT Nrf2 3′ UTR (Fig. 2E) were akin to H2O2 treatment. This indicates that repression of Nrf2 3′UTR activity by PQ/H2O2 could occur due to specific increase in activity of miR153. Overall, these results suggest the following: (i) in order for PQ/H2O2-induced miR153 to target Nrf2, the respective miR binding site in Nrf2 3′ UTR must be intact. In other words, PQ/H2O2 induced miR153 can bind to and directly target Nrf2 3′ UTR and (ii) functional silencing of miR153 using specific anti-miR could relieve PQ/H2O2-induced repression of Nrf2 3′ UTR activity.
Fig. 2. PQ/H2O2 induced miR153 targets Nrf2 in SH-SY5Y cells.

(A) SH-SY5Y cells were treated with H2O2 for 24 h and semi-quantitative real time RT-PCR analysis for miR153 and U6 was performed. Data is expressed as relative intensity of miR153 normalized to U6 over untreated control (n=4). (B) SH-SY5Y cells were co-transfected with 200 ng of reporter gene (either wild type or miR 153 site mutated Nrf2 3′ UTR) constructs along with 3 ng of pRL-renilla constructs. 24 h following transfection, cells were exposed to 100 μM H2O2. At the end of the treatment, firefly luciferase activity normalized to renilla activity was determined. The results are represented as percentage change over respective controls (n=6). (C) Luciferase reporters containing wild-type human Nrf2 3′ UTR were co-transfected with scramble control anti-miR or anti-miR-153 into SH-SY5Y cells. 24 h after transfection, cells were treated with 100 μM H2O2 and following which luciferase activity was measured. After normalization for protein levels, the results were plotted (n=6). (D) Cells were transfected and processed as in Panel B but for treatment with PQ (n=4). (E) Cells were transfected and processed as in Panel C but for treatment with PQ (n=4). In (A-E), values are expressed as means + s.e.m. * indicates significant differences at p<0.05 by either one way ANOVA/Newman Keul's post-test or two-tailed paired Student's t-test; ns-not significant.
3.3. miR153/Nrf2/ARE pathway alterations in response to H2O2 are coupled with cell death
Having shown that H2O2-induced miR153 directly targeted Nrf2 3′ UTR, we next investigated whether this is associated with impairment in Nrf2 levels and its function. To test this we examined the expression of Nrf2 message using real time PCR and Fig. 3A shows that Nrf2 mRNA normalized to GAPDH expression was significantly downregulated approximately ∼2 fold in H2O2-treated samples as compared with controls. Since cytosolic and nuclear levels of Nrf2 are critical that determines the functionality of Nrf2, we next assessed as to whether H2O2-induced miR153/Nrf2 message alterations reflected in decline in Nrf2 protein levels in this sub-cellular compartments. Immunoblotting of Nrf2 using the antibody (sc-722) demonstrated significant decrease in Nrf2 in both the compartments of H2O2 exposed cells (Fig. 3B & 3C). Next, we assessed if the decreased nuclear Nrf2 in response to H2O2 was associated with impaired 4× ARE transcriptional regulation. Fig. 3D illustrates that H2O2 effected a significant (p<0.05) downregulation of Nrf2-dependent transcription which is evident from ∼30% decrease in 4× ARE luciferase activity over basal. Reporter gene overexpression based transcriptional activity measures does not represent the true endogenous setting and thus we verified Nrf2's transcriptional activity by measuring endogenous expression of GCLC, an Nrf2 target. In parallel with 4× ARE luciferase estimates, GCLC protein content was found to be significantly decreased in H2O2 treated cells (∼40% decrease; p<0.05; Fig. 3E). Since imbalance of cellular antioxidant pathway in response to H2O2 has been shown to affect cell survival (Zhang et al., 2007), we next assessed cell death using MTT assay. In accordance with several findings, our results demonstrate that the cell viability was significantly reduced by ∼30% (Fig. 3F). Altogether these results along with Fig. 2 demonstrate that H2O2 impairs Nrf2/ARE defense network through the induction of miR153 and all of these events are associated with cell death of these dopaminergic neurons.
Fig. 3. Nrf2 is a functional downstream target of miR153 during H2O2 induced cell death.

(A) Semi-quantitative RT-PCR analysis of Nrf2 expression in SH-SY5Y cells 24 h post-treatment of 100 μM H2O2. Endogenous Nrf2 mRNA levels were normalized to GAPDH (n=4).(B) Cytosolic and (C) nuclear extracts from either untreated or H2O2 exposed cells were immunoblotted for Nrf2. Tubulin and lamin b1 served as controls for purity of cytosolic and nuclear fraction respectively. * and # indicate 100 kDA and 68 kDA Nrf2 bands respectively. In panel C, low exposure and high exposure was provided to appreciate the reduction in ∼100 kDA and 68 kDA respectively. Using ImageJ based densitometric analysis, the intensity of 100 kDA and 68 kDA Nrf2 levels were normalized to either tubulin or lamin b1 and plotted. A representative blot is given in B & C from 3 independent experiments. (D) 4× ARE luciferase reporter construct transfected cells were treated with or without H2O2 for 24 h followed by luciferase measurements. Luciferase activity normalized to protein was plotted and values are the averages of n=6. (E) Immunoblotting analysis of GCLC expression levels in SH-SY5Y cells treated with 100 μM H2O2 and a representative image is given (n=3). The respective densitometric scanning ratio of GCLC/tubulin is depicted in the lower panel. (F) SH-SY5Y cells were treated with indicated concentration of H2O2 and MTT assay was performed as described in materials and methods. The data are represented as percentage of cell viability relative to untreated cells. Each bar represents the mean ± s.e.m from 6 independent determinations. In panels (A-F), Student's t-test determined the significance of treatment. * - represents P<0.05 vs untreated controls (mean ± s.e.m).
3.4. miR153 mediated blunting of Nrf2-directed ARE activity influences cell death
To further investigate whether PQ/H2O2 induced miR153 mediated suppression of 4× ARE transactivation activity occurs through Nrf2 downregulation, we performed co-transfection experiments. In this assay, cells were transfected with plasmids encoding Nrf2 devoid of its endogenous full-length 3′ UTR region (Nrf2(-3′ UTR); not containing miR sites) facilitating a miR153 non-targetable Nrf2 mRNA. pcDNA3 empty vector (without Nrf2 cDNA sequence) was used as control. These constructs were individually introduced into cells along with 4× ARE reporter construct. 24 h post-transfection, cells were exposed to either PQ or H2O2. We observed significant (p<0.05) suppression in 4× ARE transcriptional activity in PQ or H2O2-treated control vector transfected cells (lane 2 vs lane 1; Fig. 4A, Fig. 4B respectively) indicating a PQ/H2O2 augmented miR153 and its accessibility for Nrf2 3′ UTR that in turn, can lower Nrf2 and its activity. In contrast, a significant (p<0.05) inverse correlation between PQ/H2O2-induced reduction of luciferase activity of 4× ARE construct with the overexpression of Nrf2(-3′ UTR) was observed (lane 4 vs lane 2; Fig. 4A & Fig. 4B). In this case, though PQ/H2O2 induced miR153 can bind to and target endogenous Nrf2 3′ UTR, since the cells were primed with Nrf2 lacking 3′ UTR, PQ/H2O2 elicited reduction in 4× ARE activity were rescued. This result supports the notion that altered expression of Nrf2 by miR153 is indeed responsible for PQ/H2O2 evoked repression of ARE activity. Having shown this, we next investigated whether blocking miR153 prevents the loss of PQ/H2O2 induced 4× ARE activity. To study this, either scramble anti-miR or anti-miR153 inhibitor along with 4× ARE reporter construct was introduced into SH-SY5Y cells. 24 h post-transfection, PQ or H2O2 treatment was performed for additional 24 h and the lysates were assessed for luciferase activity. A ∼2 fold decrease (p<0.05) in 4× ARE transcriptional activity was noted in scramble transfected cells (lane 2 vs lane 1; Fig. 4C, Fig. 4D respectively). On the other hand, overexpression of anti-miR specific against miR153 drastically antagonized the PQ/H2O2-induced reduction in 4× ARE transcriptional activity (p<0.05) (compare fold difference lane 1, 2 vs lane 3, 4; Fig. 4C & Fig. 4D). We next verified the endogenous expression of GCLC (one of the ARE promoted antioxidants), since luciferase assay uses exogenous expression of ARE containing elements thus not representing the actual cellular ARE driven gene expression. Similar anti-miR strategy with and without H2O2 treatment (as in Fig. 4D) was carried out followed by immunoblotting for GCLC. Analogous to luciferase assays, H2O2 induced diminution of cellular GCLC levels observed in scramble miR transfected cells (lane 1 vs lane 2; Fig. 4E) was abolished in anti-miR153 overexpressed cells (lane 2 vs 4; Fig. 4E). Thus, data from Fig. 4A-4E denotes that PQ/H2O2 prompted suppression of ARE-driven gene expression could be restored by inhibiting miR153 in SH-SY5Y dopaminergic neurons. Since H2O2 induces death of dopaminergic neurons and maintenance of redox balance affords cellular protection (Zhang et al., 2007), we next sought to determine whether Nrf2(-3′ UTR) that lacks miR153 binding site may confer protection against H2O2. Empty vector transfected cells displayed ∼30% (p<0.05) cell death in response to H2O2, while, Nrf2(-3′ UTR) overexpression normalized H2O2 induced cell death (Fig. 4F). Overall these results illustrate that (i) PQ/H2O2 induced miR153 hinders ARE activity via impairing Nrf2 and this can be reversed by inhibiting miR153 (ii) H2O2 induced miR153-mediated Nrf2 repression could play a key role in oxidant stress related dopaminergic neuronal death.
Fig. 4. Role for intact Nrf2 3′ UTR/miR153 interaction in H2O2 induced cell death.

(A) 4× ARE luciferase reporter and pRL renilla constructs were co-transfected with either Nrf2 devoid of its endogenous full-length 3′ UTR region (Nrf2(-3′ UTR); lacking miR sites) or empty vector. 24 h post-transfection, cells were treated with or without PQ for 24 h. Dual luciferase measurements were performed and the data are expressed as fold change relative to respective control. Luciferase activity normalized to renilla activity was plotted and values are the averages of n=4. (B) Cells were transfected and processed for luciferase activity as in Panel A but treated with H2O2 for 24 h after transfection (n=6). (C) Co-transfection assays involving 4× ARE reporter constructs along with anti-miR153 was performed as indicated in materials and methods. 24 h post-transfection, cells were treated with PQ for additional 24 h. Extracts prepared at the end of the experiment was processed for dual luciferase assay. Relative luciferase units were normalized to renilla activity and the percentage change over respective controls were plotted (n=4). (D) Cells were transfected and processed for luciferase activity as in Panel C but underwent 24 h H2O2 treatment post-transfection (n=6). (E) Scramble control anti-miR or anti-miR153 transfected cells were treated with or without H2O2 and immunoblotting for GCLC and GAPDH was performed. Relative fold change in expression of GCLC normalized to GAPDH was indicated. (F) Transfection and treatment without 4× ARE and pRL renilla reporter constructs were performed as in Fig. 4A. Cell viability was measured using MTT assay and % live cells were plotted (n=6). In panels (A, B, C, D, F), values are expressed as mean ± s.e.m and statistical significance was established using one way ANOVA/Newman Keul's post-test. * - represents P<0.05 vs untreated controls and ns indicates not significant.
3.5. Paraquat- induced deregulation of miR153/Nrf2/ARE network is ROS (H2O2)-dependent
Since we have shown that PQ and exogenous H2O2 treatment induce miR153 and affect Nrf2/ARE pathway (Fig. 1-4), we next evaluated the role for intracellular H2O2 in PQ elicited damages to miR153/Nrf2/ARE pathway. Typically, intracellular level of H2O2 is delicately balanced by action of several antioxidant proteins including catalase (Rhee et al., 2000). Thus to test this we performed overexpression experiments with adenovirus encoding cytoplasmic catalase (Ad cCAT). The recombinant adenovirus vector containing CMV promoter β-galactosidase (Ad-CMV-β-gal) was used as control. The efficiency of catalase virus was first verified by immunoblotting in SH-SY5Y cells. Fig. 5A demonstrates that increase in catalase expression was noted as early as 24 h post-infection of Ad cCAT when compared with Ad-CMV-β-gal infection. This increase in catalase level was found to be sustained till 48 h (Fig. 5A). Given that PQ triggers H2O2 production and catalase serving as the major scavengers of H2O2, it follows that overexpression of catalase could abolish PQ-induced amplification of miR153. To evaluate this, we infected the cells with either Ad-CMV-β-gal or Ad cCAT 24 h prior to PQ treatment and analyzed the expression of miR153. As expected, the real time PCR analysis demonstrated significant suppression of PQ-induced miR153 by ectopic expression of catalase (p<0.05) (lane 4 vs lane 2; Fig. 5B). These cells exhibit low GP× activity (Filomeni et al., 2012) and thus expected to have higher intrinsic H2O2 levels regulating the basal miR153 expression. So, catalase expression would hypothetically decrease the basal miR153. In view of this anticipation, we observed a moderate but not significant decrease in basal miR153 levels on Ad cCAT overexpression (lane 3 vs lane 1; Fig. 5B) indicating a partial role for H2O2 in basal regulation of miR153. We next validated whether or not the catalase mediated inhibition of PQ-induced miR153 results in recovery of its target gene, Nrf2. In association with blocking miR153 levels, catalase overexpression significantly limited the PQ induced decline in Nrf2 mRNA level (p<0.05) (lane 4 vs lane 2; Fig. 5C). Though the miR153 induction response to PQ is higher in adenovirus based experiments when compared to uninfected experimental set up (∼ 3 fold in Fig. 5B vs ∼1.9 fold Fig. 1A), the magnitude of Nrf2 repression was almost identical. Notably, replication-defective recombinant adenovirus containing no foreign gene (Ad CMV/Ad-CMV-β-gal) has been demonstrated to induce oxidative stress that are marked by the activation of AP-1 binding, c-jun along with decrease in GSH/GSSG ratio, alterations in mitochondrial and host-cell environment (Zhang et al., 2001; Alesci et al., 2002; Hoenig et al., 1974; Margolis et al., 1974). Thus, we speculate that adenovirus transduction could prime (oxidant) basal ROS machinery as well as its dependent transcription factors that could enhance miR153 response to PQ (Fig. 5B). Further, the reason for identical strength of Nrf2 repression among the uninfected and adenovirus experimental set up despite higher miR153 response to PQ may be ascribed to co-induction of a “sponge” phenomenon in mammalian and non-mammalian organisms (Ebert and Sharp, 2010) (yet other reasons detailed in the discussion could also play a role). Subsequently, the involvement of H2O2 in PQ-mediated impairment of Nrf2 function was confirmed by assessing the expression of GCLC, an Nrf2 target. It is clear from immunoblotting analysis that PQ induced significant downregulation of GCLC expression in Ad gal transfected control cells (p<0.05) (lane 3 vs 1; Fig. 5D). On the contrary, infection of cells with Ad cCAT restored the PQ-induced loss of GCLC levels (compare lane 3 vs lane 4; Fig. 5D). These data suggests that stressors like PQ that stimulates H2O2 generation can upregulate miR153 thereby impairing Nrf2 dependent cellular antioxidant capacity. This can be attenuated by catalase overexpression.
Fig. 5. Paraquat-induced H2O2 induces miR153 and impairs Nrf2/ARE pathway.

(A) Cells were infected with 15 MOI of adenovirus encoding cytoplasmic catalase (Ad cCAT) or Ad-CMV-β-gal. 24 h and 48 h later, cell extract was prepared and 25 μg protein was immunoblotted for catalase and GAPDH. As described in Fig. 5A, cells were infected with the viruses for 24 h and PQ was treated for additional 24 h. At the end of treatment, cells were processed for semi-quantitative real time PCR analysis for miR153 and U6 (B); Nrf2 and GAPDH (C). miR153 and Nrf2 were normalized to U6 and GAPDH levels respectively. (D) Infection and treatment was carried out as in Fig. 5B, and proteins were analyzed by immunoblot with anti-GCLC, anti-catalase and anti-tubulin. Panel below the representative image depicts the quantification of GCLC expression corrected to tubulin using Image J software. In panels (B, C & D), data are expressed as mean ± s.e.m and one way ANOVA/Newman Keul's post-test was used for statistical analysis. * - represents P<0.05.
3.6. Catalase inhibits PQ-evoked miR153 mediated redox-related cellular alterations
Above data indicate that alterations in Nrf2/ARE pathway is an outcome of increased miR153 during PQ exposure (Fig. 5). Since we and others have reported that dysregulation of the Nrf2/ARE network enhances intracellular ROS (Aw Yeang et al., 2012; Harvey et al., 2009; Narasimhan et al., 2011; Narasimhan et al., 2012), we next sought to evaluate whether the Nrf2/ARE changes induced by PQ elevate intracellular ROS levels. In agreement, the DCF based fluorescence assay revealed that PQ significantly (p<0.05) induced endogenous ROS levels by ∼25% (lane 2 vs lane 1; Fig. 6A). However, in cells overexpressing catalase, PQ failed to invoke ROS production (lane 2 vs lane 4; Fig. 6A) suggesting that the cellular redox equilibrium could be shielded by neutralizing H2O2 and its associated disturbances in miR153/Nrf2/ARE cascades. To strengthen our hypothesis that ROS induced cell death precedes activation of effector caspases namely caspase 3 and caspase 7, we measured the activity of caspase 3/7 by glo assay. Fig. 6B shows that PQ-induced caspase 3/7 activity (lane 2 vs lane 1) was significantly (p<0.05) minimized by catalase overexpression (lane 2 vs lane 4; Fig. 6B). To ascertain that these events follow cell death, we utilized the MTT assay. It is evident that Ad cCAT infected SH-SY5Y neurons underwent minimal apoptosis following PQ treatment (lane 2 vs lane 4; Fig. 6C) as compared to Ad-CMV-β-gal infected cells (lane 2 vs lane 1; Fig. 6C). Taken together, these results suggest that if PQ-triggered H2O2 is inactivated, the subsequent H2O2-dependent miR153 mediated impairment in Nrf2/ARE signaling cascade could be corrected thus conferring neuroprotection.
Fig. 6. Effect of catalase in preventing paraquat-induced ROS, caspase3/7 and cell death.
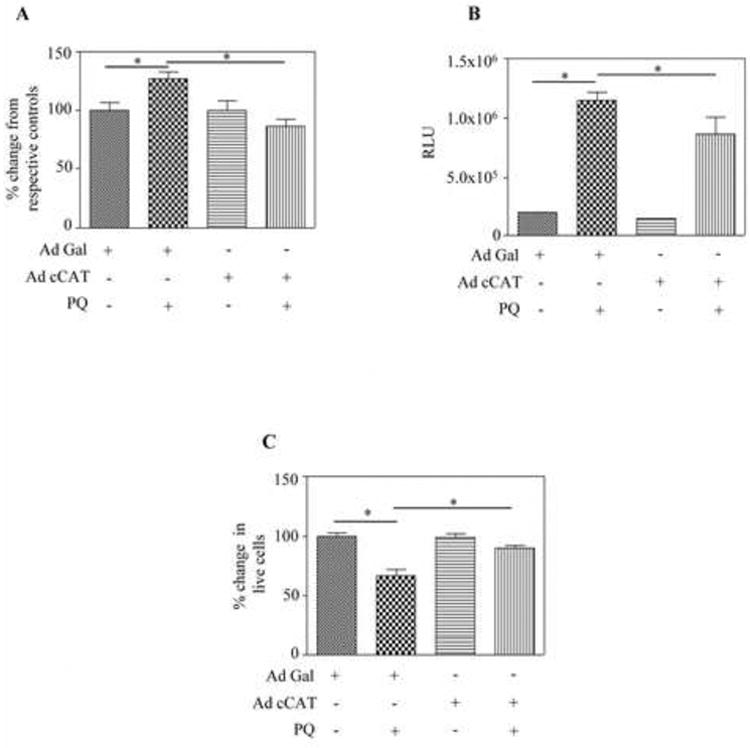
Infection of catalase virus and paraquat treatment was performed as described in Fig. 5B. At the end of the treatment, cells were processed for intracellular ROS generation by DCF based fluorescence assay (A), caspase 3/7 activity by glo assay (B) and cell viability by MTT assay (C). Data are expressed as mean ± s.e.m (n=6) and one way ANOVA/Newman Keul's post-test was performed to establish statistical significance. * - represents P<0.05.
4. Discussion
Despite extensive progress in the field of Nrf2-redox biology, mechanisms underlying how persistent and/or prolonged oxidative stress dampens Nrf2 cytoprotective responses enabling cells to undergo death are not fully elucidated. In this study, we report for the first time that miR153 suppresses Nrf2/ARE cascade and its associated cytoprotection during PQ-induced toxicity in dopaminergic neurons. The major findings of this study are: (i) miR153 is significantly upregulated in response to a physiologically relevant level of PQ (ii) This miR153 increase elicits inactivation of Nrf2 and its antioxidant functions (iii) Silencing of miR153 by anti-miR153 rescued PQ and H2O2-induced Nrf2 3′ UTR targeting and Nrf2's transcriptional activity (iv) PQ-elicited miR153 is H2O2 dependent (v) Catalase-mediated reduction of cellular H2O2 prevented PQ-related induction of miR153 thereby safeguarding Nrf2/ARE cytoprotective machinery and reducing oxidative cellular damage. Thus, minimizing miR153 levels could be a critical factor in governing cellular integrity during PQ-induced neurotoxicity. The overall findings of the current study are summarized in a schematic (Fig. 7).
Fig. 7. Schematic showing the role of miR153 in paraquat elicited neurotoxicity.

H2O2 generated on paraquat exposure, can evoke the expression of miR153. Increased miR153 could then target Nrf2 mRNA by binding to its 3′ UTR resulting in decreased levels of Nrf2 protein. Further, this could impair transcription of Nrf2 dependent antioxidant genes leading to cascade of adverse events such as increased ROS and activation of effector caspases 3/7 affecting the survival of dopaminergic neurons.
A plethora of studies have documented that Nrf2 protects multiple organs against diverse toxic insults, functioning as a multi-organ protector (Lee et al., 2005). We and many others have shown that Nrf2 is activated during oxidative stress in a variety of settings (Harvey et al., 2009; Kang et al., 2002; Li et al., 2005; Narasimhan et al., 2011). However, it is evident that such Nrf2 activation is a typical stress response that can occur only during mild to moderate oxidative stress. This was recently supported in a classical non-neuronal cell culture based study wherein short-term exposure of astrocyte-rich cultures to a ROS-generating cytokine, TNFα was shown to upregulate Nrf2. However, this increase reversed following 72 h treatment of TNFα (Correa et al., 2012). In addition, the Nrf2 system can be dysfunctional in severe and/or chronic oxidative neurodegenerative pathologies such as PD, AD and Lewy body variant of AD (LBVAD) (Ramsey et al., 2007). So far, mechanisms like nuclear trafficking involving GSK-3β signaling changes or Keap1 are suggested to inactivate Nrf2 during persisting oxidant milieu or post-induction repression of antioxidant response (Rojo et al., 2008; Sun et al., 2007). Notably, each cellular system is unique and several pathways could intersect in controlling a pivotal molecule like Nrf2 in a given context. Equally important is that miR mediated post-transcriptional control of a gene product occurs relatively rapid when compared to transcriptional or translational based regulatory mechanisms.
In line with such views, we identified miR153 as a key regulator that is induced during paraquat exposure and it disrupts Nrf2-redox control in dopaminergic cells. Though we observed a significant reduction in the levels of Nrf2 in response to PQ, the decrease was only about ∼20% which was not comparable to the percentage increase in miR153 (Fig. 1A; Fig. 1B). Recently it has been demonstrated that the absolute expression level of miRNA need not necessarily predict its inhibitory potential as the availability of repertoire of mRNA targets level could titrate away the miRNAs and thus its association with RISC complex (De et al., 2013; Flores et al., 2014) hence, saturating the level of RISC loading of a cognate miRNA in a given stress context (herein, PQ). Further, PQ could likely co-induce the expression of miR153 and its specific competing endogenous “miRNA sponges” (ceRNAs) [such as endogenously transcribed pseudogenes, long noncoding RNAs, and recently discovered circular RNAs that could sequester a fraction of a particular miRNA]. This could likely fix a high threshold above which miR153 must rise to effectively repress the expression of a critical cytoprotective gene like Nrf2. In general, stress-induced sponges could act as a quality control/buffer mechanism and ensure that the critical genes fundamental to cell survival are not readily targeted (Bak and Mikkelsen, 2014; Poliseno et al., 2010; Cesana et al., 2011) instead undergo a programmed damage averting a sudden collapse by knocking off Nrf2 hugely. In otherwords, this is to maintain a gene expression thresholding, one of the key features of cell-fate decisions thus providing an recovering opportunity in case of waning off the toxin, PQ, This is also evident from our earlier report, wherein ectopic expression of miR153 resulted in log (2) 8.5 fold in levels of miR153 with only ∼25% of Nrf2 targeting in SH-SY5Y cells (Narasimhan et al., 2012). In such situations where changes in miRNA levels may not be exactly reflected in the target gene regulation, it is recommended that a miRNA-gene regulation be supported by evaluating protein levels and/or any functional output of the gene. To this end, we have supported our gene expression data with protein level (Nrf2) (Fig. 1C), 4× ARE functional assay- (Nrf2 dependent) (Fig. 1D) and gene expression of GCLC, NQO1 (targets of Nrf2) (Fig. 1E), all of which points out an inverse correlation between miR153 to Nrf2 and its associated functions in response to PQ. In addition, this novel finding of miR153 based Nrf2 regulation could be yet another mechanism through which PQ continues to maintain oxidative stress and severe mitochondrial dysfunction (Alvarez-Erviti et al., 2013; Fukushima et al., 1995; Gabbianelli et al., 1999; Maracchioni et al., 2007). Although the focus of the present study addresses miR153-Nrf2 antioxidant machinery, it is likely that other redox and non-redox aspects of cellular changes are impacted by induction of miR153.
In the last few years, miR based mechanisms have been demonstrated in several neurodegenerative diseases e.g. AD, PD, spinocerebellar ataxia type I etc. (Hebert et al., 2008; Kim et al., 2007; Lee et al., 2008). Very recently, enforced expression of miR153 was shown to reduce the expression of Nrf2, bassoon, SNAP25 and SNCA in SH-SY5Y neuronal cells (Mandemakers et al., 2013; Narasimhan et al., 2012). In the current study, we have determined a previously unknown mechanism of PQ-mediated neurotoxicity with respect to increased miR153 and altered Nrf2 system suggesting miR153 targeting could be beneficial. However, a recent report showed that miR153 is essential for proper neuronal patterning and associated functions during early zebrafish development (Wei et al., 2013). This raises an important issue as to the need to identify specific functions of this miR within the context of species and specific cell types. A vital function would be as a regulator during development of neuronal system, ROS based mechanisms are required for appropriate lineage specification of neural progenitors, cellular growth, differentiation, neuronal plasticity (Klann and Thiels, 1999; Le Belle et al., 2011; Prozorovski et al., 2008; Suzukawa et al., 2000; Tsatmali et al., 2006). Further, ROS-induced apoptosis may be a means to selectively remove superfluous neurons during brain development thereby sculpting the size and ultimately, functionality of the nervous system (Dekkers et al., 2013; Oppenheim, 1991). If the above hold true, regulating miR153 as an intervention will need to take into account development dependent balances where a specific manipulation is favorable at one stage and detrimental at the other e.g. "antagonistic pleiotropy" (Williams, 1957). Thus, it is possible that miR153-Nrf2 fine tuning may fall under this interesting category, as recently found for E74A gene-miR34 (Liu et al., 2012) and hinting at a need for more focused contextual based approach to modulate endogenous miR153 levels. Our results using mutation and anti-miR strategies provide additional insights as to how miR153 can directly target Nrf2 and its related antioxidant system, and emphasize the need for controlling miR153 for dopaminergic neuronal survival during imminent oxidative stress.
Mechanistically, ROS can alter the structure of DNA, protein. H2O2, a relatively stable ROS, can induce greater structural perturbation of cellular RNA than DNA (Hofer et al., 2006). However, the means by which ROS regulates non-coding RNAs like miRs in the nervous system is still under investigation. That this occurs for the mature miR153 determined in our studies, was confirmed by H2O2 and gain-of-function experiments with catalase overexpression in dopaminergic neurons. In the PQ setting, H2O2 was indispensable for miR153 upregulation and associated Nrf2 impairment that is evident from catalase experiments. The mechanism by which PQ-induced ROS/H2O2 mediates miR153 expression is unknown at this point. However, we speculate that a ROS responsive transcription factor p53 could either induce direct upregulation as in the case of miR34a (Raver-Shapira et al., 2007) or increase Drosha-mediated post-transcriptional maturation (Suzuki et al., 2009). Studies are underway in our laboratory to investigate as to how ROS/H2O2 elicits endogenous miR153.
Previously several studies have sought to determine the effect of PQ on neuronal function and death. Suggested mechanisms for this neurotoxin-induced cell death included energy failure, mitochondrial dysfunction, redox imbalance, autophagy etc., (Alvarez-Erviti et al., 2013; Gabbianelli et al., 1999; Maracchioni et al., 2007; Mollace et al., 2003). Within the context of PQ toxicity, we are not aware of any reports describing how miRNA based fine tuning events can modulate a key Nrf2 dependent nodal regulatory point in cytoprotective signaling in a dopaminergic neuron model. In this connection, the current study is the first to reflect a very important mode of miR153 based Nrf2 dampening response occurring in protracted oxidative stress. Of note is that the current model of PQ addresses only the neurotoxicity aspect (not the late degeneration stage). As ROS is continuously being generated and the cells are in progressive stage of cell death (∼35 % cell death; Fig. 6C), de novo biogenesis of miR153 is still a possibility that enables the cells to maintain miR153 at a fairly high level. Notably, elevated and prolonged oxidative stress occurs after a single intraperitoneal exposure to PQ, with brain levels ranging between 0.1-0.2 ng PQ/mg tissue (Prasad et al., 2007). Therefore, disrupting miR153 could be a reasonable means to restore Nrf2/ARE signaling-related cytoprotection. Since ROS generation is a trigger for miR153 increase and at the same time a mild/moderate oxidative stress induces Nrf2, we propose an interesting co-regulation of a miR153 and its target during early PQ exposure. In such a case could this be used as a sensor for the upcoming degenerative events? Studies are emerging detailing detection of cellular miRs in plasma, whole blood and leukocytes from PD, AD patients and suggested a non-invasive strategy to diagnose early PD and AD (Cardo et al., 2013; Khoo et al., 2012; Leidinger et al., 2013; Soreq et al., 2013). Figure 7 illustrates the overall schema of miR153 working in sync to regulate the Nrf2/ARE network in PQ neurotoxicity.
Conclusions
Our study suggests that PQ-evoked miR153 targets the Nrf2-antioxidant network resulting in aberrant cellular survival events. Further, changes in miR153 levels may likely predict the progression of PQ-induced redox related neuropathology.
Implications
Dopaminergic neuron systems regulate critical functions including motor control and cognition. Damage to these is linked to a plethora of psychiatric and neurological disorders. Considering the neurotoxic effects of PQ and the emerging roles of miRs in neuro-pathogenic mechanisms, studies of this type will provide a better understanding of the mechanistic intricacies of dopaminergic neuron protective systems and could potentially lead to means of enhancing their neuroprotective responses.
Supplementary Material
Highlights.
Paraquat and H2O2 induces miR153 in SH-SY5Y dopaminergic neuronal model
Paraquat and H2O2 decreased Nrf2 gene expression and associated transactivation function is miR153 dependent
Catalase-mediated reduction of cellular H2O2 prevented PQ-related induction of miR153 and reinstated Nrf2/ARE cytoprotection
Acknowledgments
This work was supported by RO1 AA010114 (National Institutes of Health Research Project Grant Program) to George I. Henderson. We thank Dr. Jen-Tsan Chi (Durham, USA) for Nrf2 3′ UTR WT; Dr. Roland C. Wolf (Dundee, UK) for 4× ARE Luc plasmid; Dr. Arthur Cederbaum (New York, USA) for adenovirus encoding cytoplasmic catalase (Ad cCAT).
Footnotes
Preliminary results from these studies were presented at the Lone Star Chapter of the Society of Toxicology (LSSOT) annual meeting, October 17-18, Lubbock, Texas, USA.
Conflict of interest statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, Cooper JM. Influence of microRNA deregulation on chaperone-mediated autophagy and alpha-synuclein pathology in Parkinson's disease. Cell death and disease. 2013;4:e545. doi: 10.1038/cddis.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw Yeang HX, Hamdam JM, Al-Huseini LM, Sethu S, Djouhri L, Walsh J, Kitteringham N, Park BK, Goldring CE, Sathish JG. Loss of transcription factor nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) leads to dysregulation of immune functions, redox homeostasis, and intracellular signaling in dendritic cells. The Journal of biological chemistry. 2012;287(13):10556–10564. doi: 10.1074/jbc.M111.322420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak RO, Mikkelsen JG. miRNA sponges: soaking up miRNAs for regulation of gene expression. Wiley Interdisciplinary Reviews: RNA. 2014;5(3):313–333. doi: 10.1002/wrna.1213. [DOI] [PubMed] [Google Scholar]
- Benbrook CM. Impacts of genetically engineered crops on pesticide use in the U.S. -- the first sixteen years. Environmental Sciences Europe. 2012;24:24. [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature neuroscience. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain research. 1999;823(1-2):1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Burk RF, Lawrence RA, Lane JM. Liver necrosis and lipid peroxidation in the rat as the result of paraquat and diquat administration. Effect of selenium deficiency. The Journal of clinical investigation. 1980;65(5):1024–1031. doi: 10.1172/JCI109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardo LF, Coto E, de Mena L, Ribacoba R, Moris G, Menendez M, Alvarez V. Profile of microRNAs in the plasma of Parkinson's disease patients and healthy controls. Journal of neurology. 2013;260(5):1420–1422. doi: 10.1007/s00415-013-6900-8. [DOI] [PubMed] [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. 2009 [PubMed] [Google Scholar]
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Wang WL, Conklin T, Chittur S, Tenniswood M. Histone deacetylase inhibitors modulate miRNA and mRNA expression, block metaphase, and induce apoptosis in inflammatory breast cancer cells. Cancer Biology and Therapy. 2013;14(7):658–671. doi: 10.4161/cbt.25088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Mallard C, Nilsson M, Sandberg M. Dual TNFalpha-induced effects on NRF2 mediated antioxidant defence in astrocyte-rich cultures: role of protein kinase activation. Neurochemical research. 2012;37(12):2842–2855. doi: 10.1007/s11064-012-0878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De N, Young L, Lau PW, Meisner NC, Morrissey DV, MacRae IJ. Highly complementary target RNAs promote release of guide RNAs from human Argonaute2. Molecular Cell. 2013;50:344–355. doi: 10.1016/j.molcel.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers MP, Nikoletopoulou V, Barde YA. Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. The Journal of cell biology. 2013;203(3):385–393. doi: 10.1083/jcb.201306136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. The Journal of biological chemistry. 2010;285(17):12726–12734. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annual review of biochemistry. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Piccirillo S, Rotilio G, Ciriolo MR. p38(MAPK) and ERK1/2 dictate cell death/survival response to different pro-oxidant stimuli via p53 and Nrf2 in neuroblastoma cells SH-SY5Y. Biochemical Pharmacology. 2012;83(10):1349–1357. doi: 10.1016/j.bcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Flores O, Kennedy EM, Skalsky RL, Cullen BR. Differential RISC association of endogenous human microRNAs predicts their inhibitory potential. Nucleic Acids Research. 2014;42(7):4629–4639. doi: 10.1093/nar/gkt1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Tawara T, Isobe A, Hojo N, Shiwaku K, Yamane Y. Radical formation site of cerebral complex I and Parkinson's disease. Journal of neuroscience research. 1995;42(3):385–390. doi: 10.1002/jnr.490420313. [DOI] [PubMed] [Google Scholar]
- Gabbianelli R, Ferri A, Rotilio G, Carri MT. Aberrant copper chemistry as a major mediator of oxidative stress in a human cellular model of amyotrophic lateral sclerosis. Journal of neurochemistry. 1999;73(3):1175–1180. doi: 10.1046/j.1471-4159.1999.0731175.x. [DOI] [PubMed] [Google Scholar]
- Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KS, Odelberg SJ, Firpo MA, Paine R, 3rd, Hoidal JR, Abel ED, Rajasekaran NS. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PloS one. 2012;7(9):e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Lakay B, Halliday GM. Selective hippocampal neuron loss in dementia with Lewy bodies. Annals of neurology. 2002;51(1):125–128. doi: 10.1002/ana.10071. [DOI] [PubMed] [Google Scholar]
- Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free radical biology & medicine. 2009;46(4):443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(17):6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nature clinical practice. Neurology. 2008;4(11):600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- Hoenig EM, Margolis G, Kilham L. Experimental adenovirus infection of the mouse adrenal gland. II. Electron microscopic observations. American Journal of Pathology. 1974;75(2):375–394. [PMC free article] [PubMed] [Google Scholar]
- Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biological chemistry. 2006;387(1):103–111. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- Hyslop PA, Hinshaw DB, Scraufstatter IU, Cochrane CG, Kunz S, Vosbeck K. Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free radical biology & medicine. 1995;19(1):31–37. doi: 10.1016/0891-5849(95)00005-i. [DOI] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Molecular pharmacology. 2002;62(5):1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- Khoo SK, Petillo D, Kang UJ, Resau JH, Berryhill B, Linder J, Forsgren L, Neuman LA, Tan AC. Plasma-based circulating MicroRNA biomarkers for Parkinson's disease. Journal of Parkinson's disease. 2012;2(4):321–331. doi: 10.3233/JPD-012144. [DOI] [PubMed] [Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Progress in neuro-psychopharmacology & biological psychiatry. 1999;23(3):359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and cellular biology. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell stem cell. 2011;8(1):59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19(9):1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Lee Y, Samaco RC, Gatchel JR, Thaller C, Orr HT, Zoghbi HY. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nature neuroscience. 2008;11(10):1137–1139. doi: 10.1038/nn.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidinger P, Backes C, Deutscher S, Schmitt K, Mueller SC, Frese K, Haas J, Ruprecht K, Paul F, Stahler C, Lang CJ, Meder B, Bartfai T, Meese E, Keller A. A blood based 12-miRNA signature of Alzheimer disease patients. Genome biology. 2013;14(7):R78. doi: 10.1186/gb-2013-14-7-r78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicological sciences : an official journal of the Society of Toxicology. 2005;83(2):313–328. doi: 10.1093/toxsci/kfi027. [DOI] [PubMed] [Google Scholar]
- Liu N, Landreh M, Cao K, Abe M, Hendriks GJ, Kennerdell JR, Zhu Y, Wang LS, Bonini NM. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature. 2012;482(7386):519–523. doi: 10.1038/nature10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mandemakers W, Abuhatzira L, Xu H, Caromile LA, Hebert SS, Snellinx A, Morais VA, Matta S, Cai T, Notkins AL, De Strooper B. Co-regulation of intragenic microRNA miR-153 and its host gene Ia-2 beta: identification of miR-153 target genes with functions related to IA-2beta in pancreas and brain. Diabetologia. 2013;56(7):1547–1556. doi: 10.1007/s00125-013-2901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maracchioni A, Totaro A, Angelini DF, Di Penta A, Bernardi G, Carri MT, Achsel T. Mitochondrial damage modulates alternative splicing in neuronal cells: implications for neurodegeneration. Journal of neurochemistry. 2007;100(1):142–153. doi: 10.1111/j.1471-4159.2006.04204.x. [DOI] [PubMed] [Google Scholar]
- Margolis G, Kilham L, Hoenig EM. Experimental adenovirus infection of the mouse adrenal gland. I. Light microscopic observations. American Journal of Pathology. 1974;75(2):363–374. [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. Journal of neurochemistry. 2003;85(1):82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, Carlos Machado J, O'Brien J, Playfer J, Reid W. Dementia with Lewy bodies. Lancet neurology. 2004;3(1):19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollace V, Iannone M, Muscoli C, Palma E, Granato T, Rispoli V, Nistico R, Rotiroti D, Salvemini D. The role of oxidative stress in paraquat-induced neurotoxicity in rats: protection by non peptidyl superoxide dismutase mimetic. Neuroscience letters. 2003;335(3):163–166. doi: 10.1016/s0304-3940(02)01168-0. [DOI] [PubMed] [Google Scholar]
- Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nature Genetics. 2011;43(9):854–859. doi: 10.1038/ng.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan M, Mahimainathan L, Rathinam ML, Riar AK, Henderson GI. Overexpression of Nrf2 protects cerebral cortical neurons from ethanol-induced apoptotic death. Molecular pharmacology. 2011;80(6):988–999. doi: 10.1124/mol.111.073262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PloS one. 2012;7(12):e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Huang HC, Pickett CB. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. The Journal of biological chemistry. 2000;275(20):15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- O'Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D, Aarsland D, McKeith IG. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Archives of neurology. 2004;61(6):919–925. doi: 10.1001/archneur.61.6.919. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annual review of neuroscience. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Peng J, Mao XO, Stevenson FF, Hsu M, Andersen JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. The Journal of biological chemistry. 2004;279(31):32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K, Winnik B, Thiruchelvam MJ, Buckley B, Mirochnitchenko O, Richfield EK. Prolonged toxicokinetics and toxicodynamics of paraquat in mouse brain. Environmental health perspectives. 2007;115(10):1448–1453. doi: 10.1289/ehp.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, Zipp F, Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nature cell biology. 2008;10(4):385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Ramachandiran S, Hansen JM, Jones DP, Richardson JR, Miller GW. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicological sciences : an official journal of the Society of Toxicology. 2007;95(1):163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL. Expression of Nrf2 in neurodegenerative diseases. Journal of neuropathology and experimental neurology. 2007;66(1):75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Molecular cell. 2007;26(5):731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Science's STKE : signal transduction knowledge environment. 2000;2000(53):pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. Journal of neurochemistry. 2008;105(1):192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Shah S, MacEwan DJ. TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol. 2011;187(2):702–707. doi: 10.4049/jimmunol.1004117. [DOI] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome biology. 2004;5(3):R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, Suno M, Ogawa K, Hayase N, Kimura K, Shiono H. Carrier-mediated processes in blood--brain barrier penetration and neural uptake of paraquat. Brain research. 2001;906(1-2):135–142. doi: 10.1016/s0006-8993(01)02577-x. [DOI] [PubMed] [Google Scholar]
- Soreq L, Salomonis N, Bronstein M, Greenberg DS, Israel Z, Bergman H, Soreq H. Small RNA sequencing-microarray analyses in Parkinson leukocytes reveal deep brain stimulation-induced splicing changes that classify brain region transcriptomes. Frontiers in molecular neuroscience. 2013;6:10. doi: 10.3389/fnmol.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Zhang S, Chan JY, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Molecular and Cellular Biology. 2007;27(18):6334–6349. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. The Journal of biological chemistry. 2000;275(18):13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460(7254):529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- Tsatmali M, Walcott EC, Makarenkova H, Crossin KL. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Molecular and cellular neurosciences. 2006;33(4):345–357. doi: 10.1016/j.mcn.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Jaarsveld MT, Helleman J, Boersma AW, van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH, Berns EM, Verweij J, Pothof J, Wiemer EA. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32(36):4284–4293. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- von Otter M, Landgren S, Nilsson S, Celojevic D, Bergstrom P, Hakansson A, Nissbrandt H, Drozdzik M, Bialecka M, Kurzawski M, Blennow K, Nilsson M, Hammarsten O, Zetterberg H. Association of Nrf2-encoding NFE2L2 haplotypes with Parkinson's disease. BMC medical genetics. 2010;11:36. doi: 10.1186/1471-2350-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Thatcher EJ, Olena AF, Cha DJ, Perdigoto AL, Marshall AF, Carter BD, Broadie K, Patton JG. miR-153 regulates SNAP-25, synaptic transmission, and neuronal development. PloS one. 2013;8(2):e57080. doi: 10.1371/journal.pone.0057080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson PS, Farnworth MJ, Simpson MG, Lock EA. Influence of age on the passage of paraquat through the blood-brain barrier in rats: a distribution and pathological examination. Human & experimental toxicology. 1996;15(3):231–236. doi: 10.1177/096032719601500308. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11(4):398–411. [Google Scholar]
- Yates MS, Kensler TW. Chemopreventive promise of targeting the Nrf2 pathway. Drug news & perspectives. 2007;20(2):109–117. doi: 10.1358/dnp.2007.20.2.108343. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Drake VJ, Xu L, Hu J, Domann FE, Oberley LW, Kregel KC. Redox regulation of adenovirus-induced AP-1 activation by overexpression of manganese-containing superoxide dismutase. Journal of Virology. 2002;76(1):355–363. doi: 10.1128/JVI.76.1.355-363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. European journal of pharmacology. 2007;564(1-3):18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


