Abstract
Regulatory T cells are a subset of T cells with inhibitory function that are critical for protection against autoimmunity and immunopathology. A failure to maintain adequate regulatory T cell numbers in the periphery results in autoimmune manifestations, highlighting the importance of the continuous maintenance of peripheral regulatory T cells. The cellular and molecular requirements for regulatory T cell homeostasis and expansion are not fully understood but involve a complex interplay among dendritic cells, conventional T cells, and regulatory T cells. In addition, soluble factors such as the cytokine granulocyte macrophage colony-stimulating factor may play a role in enhancing these interactions. In this review, we discuss our National Blood Foundation-funded studies relating to the role of granulocyte macrophage colony-stimulating factor and dendritic cells in controlling regulatory T cell homeostasis and expansion.
Adequate numbers of regulatory T cells are necessary to prevent autoimmunity
T cells are key effectors of an immune response and are vital to protection from environmental threats. Occasionally, T cells become inappropriately activated, which may lead to a state of chronic inflammation and immunopathology. Thus, several mechanisms are in place to avoid aberrant activation of T cells. One important mechanism that governs appropriate T cell reactivity lies in thymic education, which involves positive selection and negative selection of T cells. This process generates T cells that weakly react to self major histocompatibility complex (MHC)/peptide complexes (positive selection) but deletes T cells that recognize self MHC/peptide complexes too strongly (negative selection)1,2. However, a small fraction of T cells may avoid negative selection, which could potentially cause autoimmunity. When this occurs, peripheral mechanisms act to avoid the activation of T cells and development of autoimmunity.
One peripheral mechanism involves the suppression of T cell activation by regulatory T cells (Treg)s, a subset of T cells with inhibitory function. The importance of Tregs is portrayed by disorders that result in a deficiency in their numbers or function, which leads to autoimmune disease3–5. For example, Scurfy mice and immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome patients lack a functional form of the transcription factor forkhead box P3 (Foxp3)6–8, which is essential for Treg development and function3,4,9. As a result, conventional T cell (Tconv) activation is left uncontrolled and a fatal autoimmune syndrome ensues4,9,11. In addition, an inadequate number of Tregs can result from a defect in maintaining their survival and/or proliferation in the periphery3. Mice deficient in several proteins, including CD28, TGF-β, and interleukin (IL)-2, display an inability to maintain their peripheral Treg pool in the steady state and also display autoimmune manifestations3,13. Thus, understanding the mechanisms that generate Tregs in the thymus and regulate peripheral Treg numbers is of critical importance.
The study of mast cell-T cell interactions reveals the role of IL-3 family member cytokines IL-3, IL-5, and granulocyte macrophage colony-stimulating factor (GMCSF) as potential enhancers of Treg proliferation in vitro
Our interest in Treg biology sprung from investigations involving mast cell/T cell interactions. Much of the studies in our laboratory prior to funding by the National Blood Foundation had focused on the function of mast cells in immune responses. Mast cells are well known for their pivotal role in the allergic hypersensitivity reaction, where most of their activation is mediated through FcεRI, a high affinity receptor that binds to monomeric IgE. Activation of mast cells through this receptor leads to the release of inflammatory mediators such as histamine, which are responsible for the symptoms seen in an allergic response. Although most infamous for their role in allergic disease, mast cells are also involved in anti-bacterial14,15 and anti-parasitic16–19 host defense, as well as in the development of T cell-mediated autoimmune/hypersensitivity disorders20–27 suggesting that mast cells may promote T cell activation. The effect of mast cells on T cell responses may also be inhibitory under certain circumstances, as mast cells are vital for T cell-mediated skin allograft tolerance28. Although a direct correlation between the activation of T cells and the presence of mast cells has been established in some of these models16,28,29, the mechanisms that allow mast cells to influence T cell responses still remain obscure.
As activation of T cells primarily occurs through the interaction of the antigenspecific T cell receptor (TCR) on T cells with cognate antigen/MHC on antigen presenting cells (APC), we hypothesized that mast cells might affect T cell responses directly by acting as an APC. To this end, we investigated the fate of antigens that bound to FcεRI and tested whether these antigen-treated mast cells could activate T cells in an antigen-specific manner30. Through these studies, we found that mast cells could be involved either indirectly or directly in antigen presentation. Mast cells did not constitutively express MHC class II and therefore could not present antigen to CD4+ T cells in the resting state. However, we found that mast cells incorporated antigens efficiently through FcεRI and transferred these incorporated antigens to dendritic cells (DC)s for presentation to T cells. Thus, we proposed that mast cells could act as an antigen depot by concentrating antigens through antigen-specific IgE molecules.
In addition to antigen transfer, our subsequent studies revealed that mast cells could inducibly express MHC class II molecules when stimulated by TLR agonists in vitro or in vivo31. MHC class II-positive mast cells were found in lymph nodes of LPS-injected mice and were capable of re-activating antigen-experienced T cells. Because the interaction of mast cells with Tregs was documented in vivo32 and was shown to be important for induction of allograft tolerance28, we also tested whether antigen-loaded mast cells expanded Tregs. Indeed, when antigen-specific Tregs were co-cultured with antigen-pulsed LPS/IFNγ-activated mast cells, they preferentially divided over antigen-specific Tconvs. Thus, mast cells appeared to promote the antigen-specific activation and proliferation of Tregs.
We next tested whether alloreactive Tregs expanded in a similar manner when co-cultured with MHC-mismatched mast cells. As shown in Figure 1, proliferation (CFSE dilution) of Tregs was observed when allogeneic splenocytes were co-cultured with mast cells. Surprisingly, however, Tregs diluted carboxyfluorescein succimidyl ester (CFSE) even when the splenocytes were cultured without mast cells, suggesting that the expansion of Tregs in this setting was mast cell-independent. This observation was intriguing given that no exogenous antigens or TCR stimuli were added to the splenocyte cultures. However, one factor that was exogenously added to keep the mast cells alive was the cytokine IL-3, suggesting that IL-3 might potentiate Treg proliferation. Indeed, compared to untreated splenocyte cultures, Tregs diluted CFSE more robustly when IL-3 was given (Fig. 2). IL-3 belongs to the short chain helical cytokine family and shares the signaling component of its receptor (common βreceptor; βc) with GMCSF and IL-533. To extend our findings to other cytokines within the IL-3 family, we examined the proliferation of Tregs in splenocyte cultures treated with or without IL-3, IL-5, and GMCSF. Although all three cytokines were capable of enhancing Treg proliferation, GMCSF exhibited the strongest effect (Fig. 2). In comparison to Tregs, minimal proliferation of FoxP3− Tconvs was observed when the cytokines was added to the splenocyte cultures.
Figure 1. Co-culture of Balb/c splenocytes with C57BL/6-derived mast cells promotes Treg proliferation.
Single cell suspensions from spleens of Balb/c mice were CFSE-labeled and incubated with or without C57BL/6 bone marrow-derived mast cells with IL-3 for 4 days. T cell proliferation was measured by analyzing CFSE-diluted cells by flow cytometry. The plots are gated on Tregs (CD4+FoxP3+; top plots) or Tconvs (CD4+FoxP3−; bottom plots). The number in the each plot represents the % of divided (CFSE-diluted) cells.
Figure 2. IL-3, IL-5, and GMCSF selectively induce Treg proliferation in vitro.
Single cell suspensions from spleens of Balb/c mice were CFSE-labeled and incubated without (left) or with IL-3 (10ng/ml; 2nd left), IL-5 (10ng/ml; 2nd right), or GMCSF (10ng/ml; right) for 4 days. T cell proliferation was measured by analyzing CFSE-diluted cells by flow cytometry. The plots are gated on Tregs (CD4+FoxP3+; top plots) or Tconvs (CD4+FoxP3−; bottom plots). The number in the each plot represents the % of divided (CFSE-diluted) cells.
In addition to proliferation, we examined the inhibitory activity of Tregs in the GMCSF-treated splenocyte cultures. Thus, Tregs from GMCSF-treated splenocyte cultures and freshly isolated splenic Tregs were co-cultured with Tconvs at multiple Treg:Tconv ratios and stimulated with anti-CD3 antibody. GMCSF-treated Tregs suppressed the proliferation of Tconvs at lower Treg:Tconv ratios compared to freshly isolated Tregs34. Together, these data suggested that IL-3 family cytokines, in particular GMCSF, markedly and preferentially augment the proliferation and function of Tregs.
The enhancement of Treg proliferation by GMCSF requires DCs
Because of these findings, we became interested in investigating how GMCSF promoted Treg proliferation in the splenocyte cultures, since these studies could potentially identify novel molecules and pathways that could modulate Treg numbers and function. Such information is beneficial for in vivo manipulation of Tregs as well as in the development of novel strategies for expanding Treg ex vivo as cell-based immunotherapy. Ultimately, we hoped that these findings could yield new treatment strategies for individuals with T cell-mediated autoimmune disorders or those suffering from graft-versus-host disease (GVHD), and help patients attenuate rejection of allogeneic transplants. We were successful at obtaining funding from the National Blood Foundation to embark on these studies.
In starting our investigation, we first sought to examine whether GMCSF could induce the proliferation of purified T cells. In contrast to GMCSF-treated splenocyte cultures, Tregs did not proliferate when GMCSF was added to fluorescence-activated cell sorting (FACS)-sorted T cells. Tregs regained their ability to proliferate when T cell-depleted splenocytes were added back to the FACS-sorted T cells, suggesting that GMCSF-enhanced Treg proliferation required a non-T cell spleen cell type34. Since DCs are potent stimulators of antigen-specific T cells and have also been shown to expand and differentiate in the presence of GMCSF or IL-335, we reasoned that DCs may be a likely intermediary in this process. Indeed, adding back purified DCs but not B cells or monocytes to FACS-sorted T cells was sufficient to induce GMCSF-mediated Treg expansion. T cells from βc-deficient mice proliferated equally to wildtype (WT) T cells when co-cultured with WT DCs suggesting that GMCSF receptor expression by T cells was not required for Treg proliferation34. Collectively, these data suggested that DCs were needed for GMCSF-induced proliferation of Tregs.
The marked proliferation observed in the T cell/DC co-cultures suggested that Treg proliferation might be indexed to DC numbers in vivo. To test this notion, DCs were expanded in vivo by inoculation of a tumor cell line (B16 melanoma) engineered to express FMS-like tyrosine kinase 3 ligand (FLT3L). Compared to mice injected with WT B16 melanoma, B16.FLT3L-inoculated mice exhibited a >10-fold increase in splenic DC numbers. Concomitant with this increase, the fraction of Tregs among all CD4+ T cells doubled from ~10% to ~20%34. The increase in Treg numbers was likely due to proliferation in B16.FLT3L-inoculated mice, since Bromodeoxyuridine (BrdU) incorporation by Tregs was also significantly elevated (Fig. 3). The increase in Treg proliferation and numbers was secondary to DCs, because DC depletion of B16.FLT3Linoculated mice significantly decreased Treg proliferation in these mice (Fig. 3). These findings are consistent with data by others using similar approaches36,37. These data suggested that Treg proliferation correlates positively with DC numbers in vivo.
Figure 3. Expansion of DCs in vivo causes increased Treg proliferation.
CD11c-DTR mice (transgenic mice with diphtheria toxin receptor expression driven by the CD11c promoter) were injected subcutaneously with B16 or B16-FLT3L tumor cells and nine days later administered BrdU for three days. DC depletion was performed by daily diphtheria toxin administration started 2 days before BrdU treatment. BrdU incorporation of splenic Tregs from these mice was measured by flow cytometry. The graphs show BrdU incorporation by Tregs (CD4+FoxP3+ cells).
IL-2 production by Tconvs through MHC II-dependent interactions with DC is required for GMCSF-induced Treg proliferation
The continuing presence of adequate numbers of Tregs is required for the suppression of autoimmunity. In contrast to Tconvs that proliferate in response to TCR stimulation, Tregs do not proliferate with TCR stimulation alone38. This is because Tregs cannot make their own IL-2, although they are critically dependent on IL-2 for their proliferation. Tregs constitutively express CD25, the high affinity αsubunit of the IL-2 receptor, and thus can respond to low levels of IL-2. IL-2-deficient mice or mice lacking components of the IL-2 receptor display heightened Tconv activity and succumb to autoimmune disease39–41, which is caused by a decrease of Tregs in these mice42,43. The source of IL-2 that maintains Tregs in the steady state has not been fully identified. IL-2-deficient bone marrow chimera complementation studies suggest that T cells are the source of IL-2, since the addition of WT but not TCR-deficient bone marrow rescued Treg numbers in mixed bone marrow chimeras44. Among T cells, CD4+ T cells expressing intermediate levels of CD25 express the highest amount of IL-2 mRNA45, suggesting that these T cells might be the prime source of IL-2 for Treg homeostasis.
Using our GMCSF-induced Treg proliferation in vitro system, we attempted to tease out the requirement of Tconvs in GMCSF-induced Treg proliferation. When co-cultured with syngeneic DCs, CD4+ Tconvs produced detectable amounts of IL-234. This IL-2 was necessary for driving DC-induced Treg proliferation in these cultures. IL-2 production by Tconvs occurred in the absence of any exogenous antigens but in an MHC class II-dependent manner, suggesting that Treg proliferation was dependent on the activation of Tconvs by self-derived antigens presented by DCs. Indeed, Tconvs lacking a key TCR signaling adaptor molecule SH2 domain-containing leukocyte protein of 76 kD (SLP-76) showed diminished capacity to induce Treg proliferation when co-cultured with DCs (T. Zou, unpublished observations). Together, these data suggest that TCR stimulation of Tconvs with self-reactivity is necessary for the elaboration of IL-2 required for Treg maintenance.
DCs but not MHC class II is required for IL-2-induced Treg proliferation
Since Tconv-derived IL-2 production was required to drive GMCSF-induced Treg proliferation, we tested whether the provision of IL-2 would bypass the requirement of Tconvs. Although the addition of IL-2 allowed Tregs to proliferate in the absence of Tconvs, Tregs still required contact with DCs for IL-2-induced proliferation34. We speculated that Tregs required contact with DCs in order to receive TCR stimulation by MHC class II-expressing DCs. Tregs are generated in the thymus through interaction with APCs bearing cognate antigens46,47 and thus a significant proportion of Tregs are presumed to have specificity for self-derived peptides. Because of this, one may imagine that Tregs are continuously being stimulated through their TCR in the periphery. Freshly isolated Tregs from mice display increased phosphorylation of TCRζ chains compared to Tconvs, suggesting increased encounter of the TCR with cognate ligands in vivo48.Moreover, TCR stimulation of Tregs was thought to be necessary for Treg proliferation in vivo, since earlier studies testing the role of MHC class II in Treg homeostasis demonstrated that Tregs failed to proliferate and expand upon adoptive transfer of T cells into MHC class II-deficient mice38,49.
Surprisingly, however, we found that Tregs still expanded, albeit to a lesser extent, when co-cultured with IL-2 and MHC class II-deficient DCs34 or when the Tregs were inducibly deleted of SLP-76 (T. Zou, unpublished observations). Thus, the failure of Treg expansion in MHC class II-deficient mice could be due to the lack of IL-2 production by Tconvs rather than a requirement of TCR stimulation by Tregs. This finding was not merely an in vitro phenomenon as our preliminary data suggest that Tregs do not require MHC class II expression by host cells but still require DCs for IL-2-induced Treg proliferation in vivo (T. Zou and A. Satake, unpublished observations).
To determine which molecules on DCs might provide the MHC class II-independent signals to Tregs, the surface phenotype of DCs cultured with GMCSF was examined. In addition to MHC class II, GM-CSF treatment upregulated the expression of co-stimulatory molecules such as CD80 and CD86, suggesting that co-stimulatory molecules might play a role in GMCSF-induced Treg proliferation. The blockade of CD80 and CD86 by CTLA-4-Ig or the addition of anti-OX40L antibody but not anti-CD40L antibody partially attenuated the proliferation of FACS-sorted Tregs co-cultured with MHC class II-deficient DCs in the presence of IL-2 (Fig. 4). Moreover, the combination of both anti-OX40L and CTLA-4-Ig almost completely inhibited IL-2-induced Treg proliferation (Fig. 4). These data suggest that DC-derived B7 and OX40L signals synergize with IL-2, which contributes to MHC class II-independent Treg proliferation.
Figure 4. Blockade of CD80, CD86, and OX40L inhibits IL-2-induced Treg proliferation in vitro.
FACS-sorted CFSE-labeled C57BL/6 Tregs (CD4+CD25+) were co-cultured with purified MHC class II-deficient DCs (CD11c+) and IL-2 with or without CTLA4Ig, αOX40L, αCD40L, or all 3 antibodies combined each at 20 µg/ml for 4d ays. CFSE dilution was analyzed in CD4+FoxP3+ Tregs by flow cytometry. Plots are gated on live CD4+FoxP3+ cells and the number in the each plot represents the % of divided (CFSE-diluted) cells.
A Treg-intrinsic and -extrinsic role of the TCR in Treg homeostasis
Our experiments investigating how GMCSF expands Tregs in splenocyte cultures highlighted the central role of DCs in coordinating the events necessary for Treg proliferation in two critical ways. First, DCs were required for stimulation of Tconvs that provide IL-2 to Tregs. Second, in conjunction with this IL-2, DCs supplied the co-stimulatory signals necessary for IL-2-induced Treg proliferation. In addition to providing co-stimulatory signals, DCs also engaged the TCR of the Tconvs and Tregs through MHC class II. The elaboration of IL-2 by Tconvs was entirely dependent on MHC class II expression by DCs, since IL-2 was not detected when co-culturing Tconvs with MHC class II-deficient DCs. Moreover, Tconv/DC co-cultures separated from Treg/DC co-cultures by a transwell induced Treg proliferation only when the Tconvs were co-cultured with MHC class II-expressing DCs. In contrast to Tconvs, expression of MHC class II by DCs was not an absolute requirement for IL-2-induced Treg proliferation. However, the presence of MHC class II or the addition of a TCR stimulus significantly enhanced Treg proliferation suggesting that optimal Treg proliferation requires TCR engagement. Thus, Tregs may be maintained by both TCR-dependent and – independent pathways (Fig. 5). We propose that while TCR stimulation allows the preferential expansion of Tregs with relevant antigen specificities, the MHC class II/TCR-independent mode of Treg proliferation might be important to ensure survival of Tregs with TCR specificity against sequestered or inaccessible antigens, so that a diverse TCR repertoire can be continuously represented in the Treg pool. Our future studies include the investigation of how these two potential modes of Treg proliferation contribute to Treg homeostasis and to determine how the signals from the TCR, DCs, and IL-2 synergize to yield Treg proliferation.
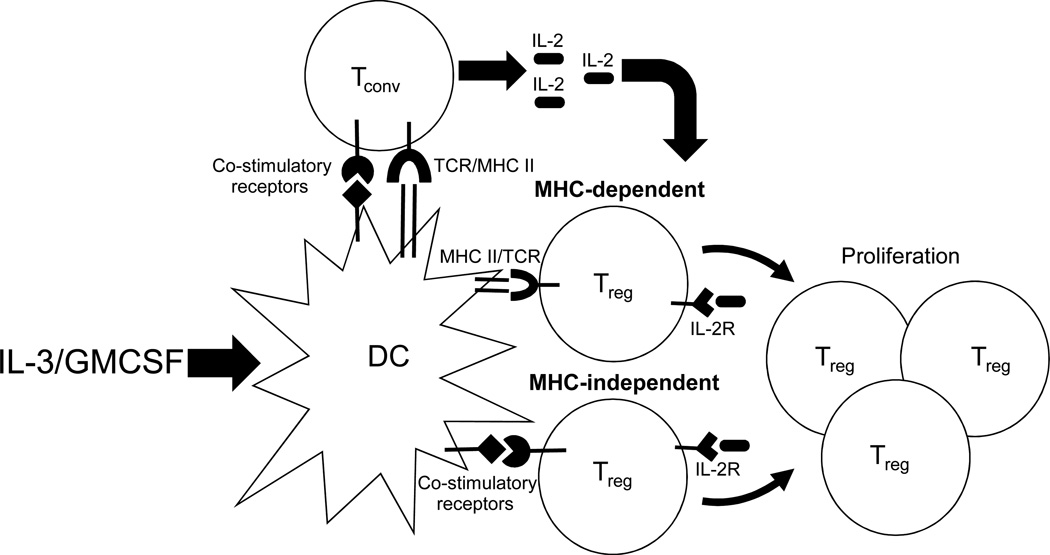
Figure 5. Interactions among DCs, Tconvs, and Tregs are necessary for Treg proliferation.
Tconvs are induced to produce IL-2 upon interaction with syngeneic DCs in an MHC class II/TCR and co-stimulatory molecule-dependent manner. This IL-2 in turn binds to the IL-2 receptor (IL-2R) on Tregs and induces their proliferation. IL-2-induced Treg proliferation requires contact with DCs but can occur in an MHC class II– dependent and –independent manner. Co-stimulatory molecules play an important role in the MHC-independent mode of Treg proliferation. The cytokines IL-3 and GMCSF facilitate this process by upregulating MHC class II and co-stimulatory molecule expression by DCs.
In addition to studying the Treg-intrinsic role of the TCR in Treg proliferation and maintenance, we are currently investigating the TCR signals that are required for Tconvs to produce IL-2 when interacting with syngeneic DCs (Treg-extrinsic role of TCR). Our data suggest that TCR stimulation of Tconvs expressing a diverse TCR repertoire with self-reactivity is necessary for the elaboration of IL-2 required for Treg maintenance. This implies that positive selection of Tconvs may serve a purpose to select Tconvs with moderate self-reactivity to ensure that an adequate number of Tconvs make IL-2 to support Treg homeostasis. Thus, a failure to maintain Tregs by inadequately positively selected Tconvs could be a mechanism that predisposes to autoimmunity. Further studies relating to the nature of the TCR signals and the interactions of Tconvs with DCs will be important to understand how Tregs are maintained in the steady state.
The role of IL-3 family cytokines in Treg homeostasis in vivo
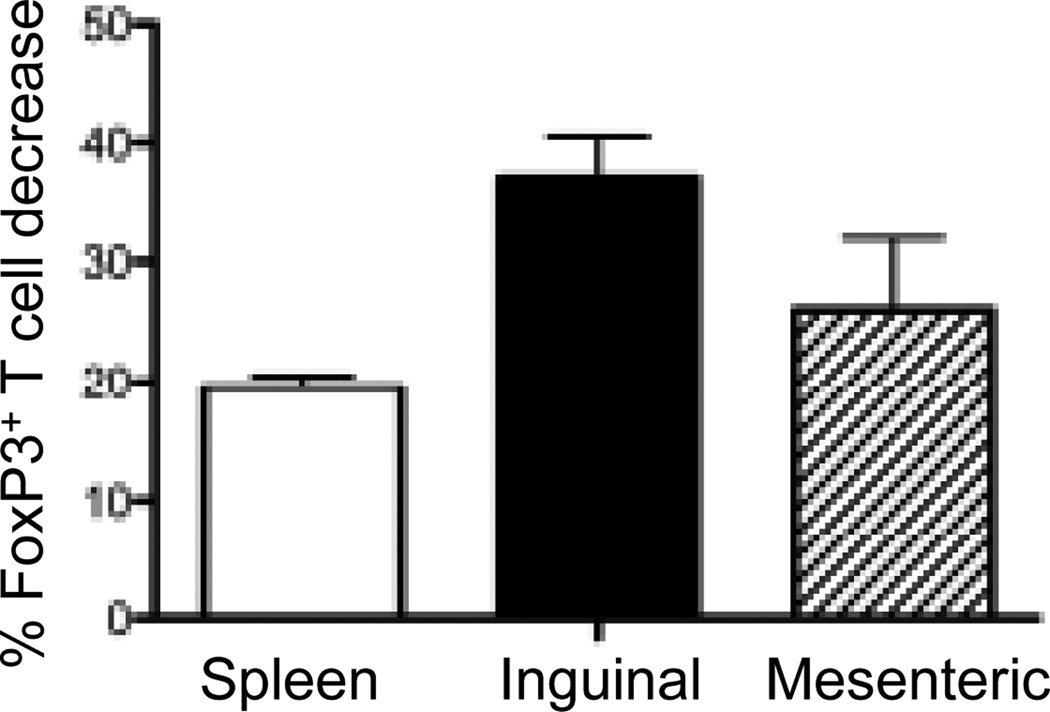
Although IL-3 and GMCSF have been shown to promote the development of a wide variety of myeloid cells including mast cells, DCs, and macrophages in vitro, the phenotypes of mice singly lacking IL-3 or GMCSF are relatively mild with little effect on steady-state hematopoiesis50–53. It was postulated that the mild phenotype was perhaps due to redundancies in IL-3 and GMCSF function, which would allow these cytokines to compensate for each other in their absence. However, this was not the case as mice lacking both IL-3 and GMCSF still have relatively normal hematopoiesis54. Yet, an unexpected finding was that these mice develop spontaneous insulitis and a systemic lupus erythematous (SLE)-like disorder55, suggesting that IL-3 and GMCSF contribute to protection against autoimmunity by maintaining Treg numbers. To test this notion, we examined mice lacking the receptors for IL-3/IL-5/GMCSF (IL-3β/βc−/−) mice. The secondary lymphoid organs (spleen, inguinal lymph nodes, and mesenteric lymph nodes) were removed from IL-3β/βc−/− mice and the proportion of Tregs was compared to wildtype mice. The proportion of CD4+FoxP3+ Treg in IL-3β/βc−/− was decreased by 20–40% compared to wildtype mice (Fig. 6), suggesting that receptors for IL-3/IL-5/GMCSF may play a role in maintaining numbers of Treg in vivo.
Figure 6. IL-3β−/−βc−/− mice contain fewer Treg compared to wildtype mice.
The spleen, inguinal lymph nodes, and mesenteric lymph nodes of C57BL/6 and IL-3β−/−βc−/− mice were harvested and analyzed for CD4+FoxP3+ T cells. Using wildtype mice as baseline, the % decrease in FoxP3+ T cells among total CD4+ T cells from IL-3β−/−βc−/− mice was determined. Results are expressed as mean ± SD of N = 4 mice/group.
In addition to Treg homeostasis, GMCSF could potentially be used therapeutically to increase Treg numbers. The administration of GMCSF has been shown to inhibit the development of autoimmune thyroiditis56,57, myasthenia gravis58, and type I diabetes in non-obese diabetic (NOD) mice59. The protective effect of GMCSF has been associated with an increase in Treg numbers in recipient mice, although the precise mechanism by which this occurs is not fully understood. Based on our findings, it is likely that GMCSF increases Treg numbers and protects against autoimmunity through the modulation of DCs. As mentioned earlier, an increase in DC numbers by FLT3L causes an expansion of Tregs. A recent study showed that this increase in Tregs by FLT3L protects against lethal graft-versus-host disease36. When comparing the efficacy of FLT3L and GMCSF in enhancing Treg proliferation and expansion, our preliminary data suggests that GMCSF is more potent in enhancing Treg proliferation in vivo (A. Satake, unpublished observations). We are currently investigating how GMCSF could be utilized in vivo and ex vivo for increasing Treg numbers in the prevention of a variety of T cell-mediated diseases.
Concluding remarks
Maintenance of an adequate number of Tregs is vital for limiting over-activation and inappropriate activation of T cells. As proliferation of Tregs contributes to the Treg homeostasis, understanding of the cellular and molecular requirements of this process is critical. Using funds from the National Blood Foundation, we performed experiments to identify the complex interplay among DCs, Tconvs, and Tregs that was necessary for Treg proliferation. Further studies identifying the precise mechanisms underlying these processes are needed to devise novel strategies for the manipulation of Treg numbers in patients with autoimmune diseases, transplant rejection, and graft-versus-host disease. Moreover, such studies will yield insight into new approaches for more efficient expansion of Tregs ex vivo for use in adoptive immunotherapies.
Acknowledgements
We thank members of the Kambayashi and Koretzky laboratory for helpful discussions and Mariko Okumura and Mercy Gohil for excellent technical support. We also thank support from the National Blood Foundation and the University of Pennsylvania for funding of our studies.
Source of support: National Blood Foundation, University of Pennsylvania Internal Funds
Footnotes
Conflicts of interest: None
References
- 1.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, Bluthmann H, Staerz UD, et al. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 3.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells Curr Opin Immunol. 2007:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25) Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases J Immunol. 1995:1151–1164. [PubMed] [Google Scholar]
- 6.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 7.Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3 Science. 2003:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 10.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 11.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells Nat Immunol. 2003:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3 Nat Immunol. 2007:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 13.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis Immunol Lett. 2007:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 15.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 16.Maurer M, Lopez Kostka S, Siebenhaar F, et al. Skin mast cells control T celldependent host defense in Leishmania major infections. Faseb J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 17.Li E, Zhou P, Petrin Z, Singer SM. Mast cell-dependent control of Giardia lamblia infections in mice. Infect Immun. 2004;72:6642–6649. doi: 10.1128/IAI.72.11.6642-6649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe T, Nawa Y. Reconstitution of mucosal mast cells in W/WV mice by adoptive transfer of bone marrow-derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti-infection. Parasite Immunol. 1987;9:31–38. doi: 10.1111/j.1365-3024.1987.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya M, Oku Y, Itayama H, Ohbayashi M. Prolonged expulsion of adult Trichinella spiralis and eosinophil infiltration in mast cell-deficient W/Wv mice. J Helminthol. 1985;59:233–239. doi: 10.1017/s0022149x00008002. [DOI] [PubMed] [Google Scholar]
- 20.Biedermann T, Kneilling M, Mailhammer R, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askenase PW, Van Loveren H, Kraeuter-Kops S, et al. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol. 1983;131:2687–2694. [PubMed] [Google Scholar]
- 22.Kobayashi T, Miura T, Haba T, et al. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J Immunol. 2000;164:3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 23.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DM, Friend DS, Gurish MF, et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 25.Nigrovic PA, Lee DM. Mast cells in inflammatory arthritis. Arthritis Res Ther. 2005;7:1–11. doi: 10.1186/ar1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbie-Ryan M, Brown M. The role of mast cells in allergy and autoimmunity. Curr Opin Immunol. 2002;14:728–733. doi: 10.1016/s0952-7915(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 28.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 29.Gregory GD, Robbie-Ryan M, Secor VH, et al. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis. Eur J Immunol. 2005;35:3478–3486. doi: 10.1002/eji.200535271. [DOI] [PubMed] [Google Scholar]
- 30.Kambayashi T, Baranski JD, Baker RG, et al. Indirect involvement of allergen-captured mast cells in antigen presentation. Blood. 2008;111:1489–1496. doi: 10.1182/blood-2007-07-102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kambayashi T, Allenspach EJ, Chang JT, et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gri G, Piconese S, Frossi B, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653–665. doi: 10.1016/S0091. quiz 66. [DOI] [PubMed] [Google Scholar]
- 34.Zou T, Caton AJ, Koretzky GA, Kambayashi T. Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. J Immunol. 185:2790–2799. doi: 10.4049/jimmunol.0903740. [DOI] [PubMed] [Google Scholar]
- 35.Lutz MB. IL-3 in dendritic cell development and function: a comparison with GM-CSF and IL-4. Immunobiology. 2004;209:79–87. doi: 10.1016/j.imbio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Swee LK, Bosco N, Malissen B, et al. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009;113:6277–6287. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 37.Darrasse-Jeze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gavin MA, Clarke SR, Negrou E, et al. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 39.Schorle H, Holtschke T, Hunig T, et al. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 40.Willerford DM, Chen J, Ferry JA, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 42.Malek TR, Yu A, Vincek V, et al. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 43.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 44.Almeida AR, Zaragoza B, Freitas AA. Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. J Immunol. 2006;177:192–200. doi: 10.4049/jimmunol.177.1.192. [DOI] [PubMed] [Google Scholar]
- 45.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 47.Walker LS, Chodos A, Eggena M, et al. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson J, Stefanova I, Stephens GL, Shevach EM. CD4+CD25+ regulatory T cells are activated in vivo by recognition of self. Int Immunol. 2007;19:557–566. doi: 10.1093/intimm/dxm021. [DOI] [PubMed] [Google Scholar]
- 49.Bhandoola A, Tai X, Eckhaus M, et al. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17:425–436. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 50.Mach N, Lantz CS, Galli SJ, et al. Involvement of interleukin-3 in delayed-type hypersensitivity. Blood. 1998;91:778–783. [PubMed] [Google Scholar]
- 51.Lantz CS, Boesiger J, Song CH, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 52.Stanley E, Lieschke GJ, Grail D, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dranoff G, Crawford AD, Sadelain M, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 54.Gillessen S, Mach N, Small C, et al. Overlapping roles for granulocyte-macrophage colony-stimulating factor and interleukin-3 in eosinophil homeostasis and contact hypersensitivity. Blood. 2001;97:922–928. doi: 10.1182/blood.v97.4.922. [DOI] [PubMed] [Google Scholar]
- 55.Enzler T, Gillessen S, Dougan M, et al. Functional deficiencies of granulocyte-macrophage colony stimulating factor and interleukin-3 contribute to insulitis and destruction of beta cells. Blood. 2007;110:954–961. doi: 10.1182/blood-2006-08-043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasu C, Dogan RN, Holterman MJ, Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol. 2003;170:5511–5522. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 57.Gangi E, Vasu C, Cheatem D, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 58.Sheng JR, Li LC, Ganesh BB, et al. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaudreau S, Guindi C, Menard M, et al. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]