Abstract
Free fatty acids (FFAs) serve not only as nutrients that provide energy but also as extracellular signaling molecules that manipulate intracellular physiological events through FFA receptors (FFARs) such as FFAR4. FFAR4 is also known as G-protein coupled receptor 120 (GPR120). The main role of GPR120 is to elicit FFA regulation on metabolism homeostasis. GPR120 agonism correlates with prevention of the occurrence and development of metabolic disorders such as obesity and diabetes. GPR120 activation directly or indirectly inhibits inflammation, modulates hormone secretion from the gastrointestinal tract and pancreas, and regulates lipid and/or glucose metabolism in adipose, liver, and muscle tissues, which may help prevent obesity and diabetes. This review summarizes recent advances in physiological roles of GPR120 in preventing insulin resistance and protecting pancreatic islet function, and examines how resident GPR120 in the pancreas may be involved in modulating pancreatic islet function.
Keywords: fatty acid, G protein-coupled receptors, inflammation, pancreas, obesity
Introduction
Metabolic diseases, such as obesity and type 2 diabetes mellitus (T2DM), are ever increasing in prevalence.1,2 Hence, researchers are working to develop pharmaceutical treatments that can alleviate these conditions. In this regard, G-protein coupled receptor 120 (GPR120), which plays important roles in regulating energy metabolism and eliciting anti-inflammatory effects, is an attractive target for diabetes treatments, in particular.
Binding of GPR120 by ω-3 fatty acids (ω-3 FAs) results in various physiological activities that have a stabilizing effect on metabolic homeostasis and may help prevent diabetes.3,4 Patients with T2DM exhibit insufficient pancreatic insulin secretion and insulin resistance in adipose, liver, and muscle; insulin resistance correlates with abnormal energy metabolism, such as a sequela of hepatic steatosis.5 GPR120 activation relieves insulin resistance by enabling adipogenesis in adipose tissue and thereby maintaining a lipid metabolism balance between adipose and liver tissues.3,4,6 Additionally, GPR120 stimulation can also support maintenance of insulin sensitivity through inhibition of inflammation, since GPR120 plays an important role in mediating the anti-inflammatory effects of ω-3 FAs on macrophages.3,4,7–10 GPR120 activation may also promote pancreatic β-cell survival and proliferation and stimulate pancreatic insulin secretion indirectly through induction of glucagon-like peptide-1 (GLP-1) and cholecystokinin (CCK) secretion from the intestine.11–14 Generally speaking, GPR120-mediated modulation on insulin sensitivity, pancreatic insulin secretion, and β-cell mass suggests that GPR120 could be targeted as an anti-diabetic treatment. However, it is not known yet whether pancreatic GPR120 has a role in direct modulatory effects on pancreatic and islet function.
In this review, we first describe the structure, tissue distribution, and agonists of GPR120. We then summarize advances in research on the roles of GPR120 in modulating physiological activities in the gastrointestinal tract and tongue, and in influencing the cellular activities of adipocytes and macrophages. Next, we discuss how GPR120 activation may maintain insulin sensitivity and indirectly promote pancreatic islet function. Finally, in light of evidence suggesting that pancreatic GPR120 stimulation might regulate islet function directly,15,16 we examine the potential mechanism by which local pancreatic GPR120 may modulate islet function.
Structure and distribution of GPR120 Structure
Human GPR120 has two splice variants, a short isoform (NM_181745) and a long isoform (BC101175) with 16 additional amino acids in the third intracellular loop.17 Both isoforms are phosphorylated similarly in the presence of ω-3 FAs, and both recruit β-arrestin-2 readily and undergo internalization,17,18 while the longer intracellular loop of the long isoform prevents G-protein-dependent intracellular calcium mobilization.18 Rat, mouse, and cynomolgus monkey have a GPR120 genetic sequence comprised of a single 1,086 nucleotide sequence encoding a protein that is 361 amino acids long.17,19 Not surprisingly, the cynomolgus monkey’s complementary DNA (cDNA) for GPR120 has higher homology with human GPR120 long isoform than does the mouse or rat genes.19
Similar to other G protein-coupled receptors (GPCRs), GPR120 contains an extracellular N-terminal domain, seven transmembrane domains, and an intracellular C-terminal domain.13,18,20,21 Both N-terminal and transmembrane domains may mediate interaction between GPCRs and their ligands. It has been identified that Arg99 in the transmembrane domain II of GPR120 is an essential residue for binding GPR120 to its ligands.20,22,23 The intracellular C-terminal region of GPR120 is important for its interaction with G-protein or scaffold protein, such as β-arrestin-2. It has been demonstrated that there are several putative β-arrestin-2-binding motifs in the C-terminal domain of GPR120.24 It is noteworthy that the C-terminal fusion of tag or protein (such as hemagglutinin, enhanced yellow fluorescent protein, and Gα16) does not impair the biological function of GPR120.11,20,25
Until now, the spatial structure of GPR120 has not been identified.23 To develop selective GPR120 agonists, researchers have thus constructed GPR120 homology model by utilizing the crystal structure of other GPCRs, such as the β2-adrenoceptor and bovine rhodopsin.20,22,23
Distribution
Expression of GPR120 differs between species (Table 1). In the rat, the highest expression of GPR120 was found in the colon, whereas in mouse and human, the highest GPR120 expression is found in the lung.11,13 Beyond the lung, GPR120 is expressed mainly in tissues and cells related to energy metabolism, such as the gastrointestinal tract, tongue, and adipose tissue. Various macrophages also express high levels of GPR120.
Table 1.
Tissue distribution of GPR120 in human, mouse, rat, and cynomolgus monkey
| Tissue(s) | Species
|
Cell/cell line | Coexpressed protein | References | |||
|---|---|---|---|---|---|---|---|
| H | M | R | CM | ||||
| Adipose | + | + | N | N | 3T3-L1 | N | 10,29,40 |
| Adrenal gland | + | + | N | N | N | N | 11 |
| Brain | + | + | − | + | N | N | 11,19 |
| Cecum | N | + | + | N | N | N | 11,13 |
| Colon | + | + | + | + | Colonic cell | GLP-1 | 3,11,13,19,28,29,33 |
| Duodenum and jejunum | − | + | + | N | L cell, K cell | Ghrelina | 3,11,13,26,30 |
| Heart | − | + | − | N | N | N | 11,13 |
| Ileum | + | + | + | N | N | N | 11,13,40 |
| Kidney | + | + | N | + | N | N | 11,19 |
| Liver | − | + | − | + | Kupffer cell | N | 3,9,11,13,19 |
| Lung | + | + | + | + | N | Mouse CC10 | 11,13, 19, 29 |
| Pancreas | + | ± | − | N | MIN6b, INS-1, BRIN-BD11 | N | 11,13,15,16,39,40 |
| Pituitary | N | + | N | N | N | N | 40 |
| Rectum | + | + | + | N | N | N | 11,13 |
| Musclec | N | − | + | N | N | N | 3,41 |
| Spleen | + | + | + | N | N | N | 11,13 |
| Stomach | N | + | − | + | SG-1, MGN3-1, GOC | Ghrelin | 11,13,19,30,31,32 |
| Thalamus | N | N | + | N | N | N | 13 |
| Thymus | + | + | + | N | N | N | 11,13 |
| Tongue | + | + | + | N | TBC | α-gustducin, CD36, GLP-1, PLCβ2, TRPM5 | 33–38 |
Notes: The tissue distribution is classed as follows: N = not reported; + = presence; − = absence.
The colocalization of GPR120 with ghrelin is in the duodenum
the expression of GPR120 in the MIN6 cell line is controversial
skeletal muscle.
Abbreviations: CC10, Clara cells expressed the Clara cell 10-kDa protein; GOC, gastric oxyntic cell; TBC, taste bud cell; TRPM5, transient receptor potential channel type M5; CD36, cluster of differentiation 36; GLP-1, glucagon-like peptide-1; PLC, phospholipase-C; H, human; M, mouse; R, rat; CM, cynomolgus monkey; GPR120, G-protein coupled receptor 120; 3T3-L1, mouse embryonic fibroblast - adipose like cell line; L cell, intestinal; enteroendocrine cell; K cell, intestinal enteroendocrine cell; Kupffer cell, hepatic fixed macrophage; MIN6, mouse pancreatic β cell line; INS-1, rat pancreatic β cell line; BRIN-BD11, rat insulin-secreting cell line; SG-1, stomach ghrelinoma cell line; MGN3-1, mouse gastric ghrelinoma cell line.
GPR120 expression has been confirmed in mouse intestinal endocrine cell lines (STC-1 and GLUTag), mouse intestinal glucose-dependent insulinotropic polypeptide (GIP)-secreting K-cells and human colorectal carcinoma tissue.11,26–28 GPR120 colocalizes with GLP-1 in mouse large intestine epithelial cells and human colonic intraepithelial neuroendocrine cells.11,29 GPR120 expression is also detected in mouse gastric ghrelinoma cell lines (MGN3-1 and SG-1), and it colocalizes with ghrelin in stomach ghrelin-secreting cells.30–32 It is also expressed in taste bud cells (TBCs) of circumvallate, fungiform, and foliate papillae in rat, mouse, and human.33–38 GPR120 has been reported to be co-expressed with phospholipase-Cβ2 (PLCβ2), α-gustducin, transient receptor potential channel type M5 (TRPM5), and GLP-1 in mouse TBCs of circumvallate papillae, indicating that it is mainly in type II TBCs.34–36 In addition, GPR120 also colocalizes with cluster of differentiation 36 (CD36) in mouse and human TBCs.36,37 Interestingly, Ozdener et al showed that human GPR120 and CD36 have non-overlapping roles in TBC signaling during oro-gustatory perception of dietary lipids.37
GPR120 messenger RNA (mRNA) has been detected in mouse islets, the murine pancreatic β-cell lines (MIN6, INS-1 and BRIN-BD11), and human pancreas and islets.15,16,39,40 However, others have reported no, or negligible, expression of GPR120 in MIN6 cells and rat pancreas.11,13 Thus, GPR120 expression in murine pancreatic islets and β-cells needs to be further confirmed. Negligible GPR120 expression has been detected in mouse soleus and extensor digitorum longus (EDL) skeletal muscle,3 but GPR120 mRNA levels in cardiac muscle and EDL skeletal muscle of Sprague Dawley rats can be increased by feeding with a high fat diet (HFD).41 GPR120 is also expressed in various mouse white adipose tissues, including subcutaneous, perineal, mesenteric and epididymal adipose, and GPR120 mRNA expression in mouse adipose tissues can also be upregulated with HFD feeding.40 In addition, it has been shown that the differentiated human adipocytes have greater GPR120 mRNA levels than preadipocytes.40 These findings suggest that expression level of GPR120 is associated with lipid metabolism and adipogenesis.
GPR120 expression is also identified in macrophages, such as mouse bone marrow-derived macrophages and intraperitoneal macrophages.3 In liver, GPR120 expression is limited to resident macrophages (Kupffer cells [KCs]) and its expression can be increased significantly in macrophages of adipose and liver by HFD-feeding in mice.3,9 Table 1 summarizes the tissue distribution of GPR120 in various species.
GPR120 signaling
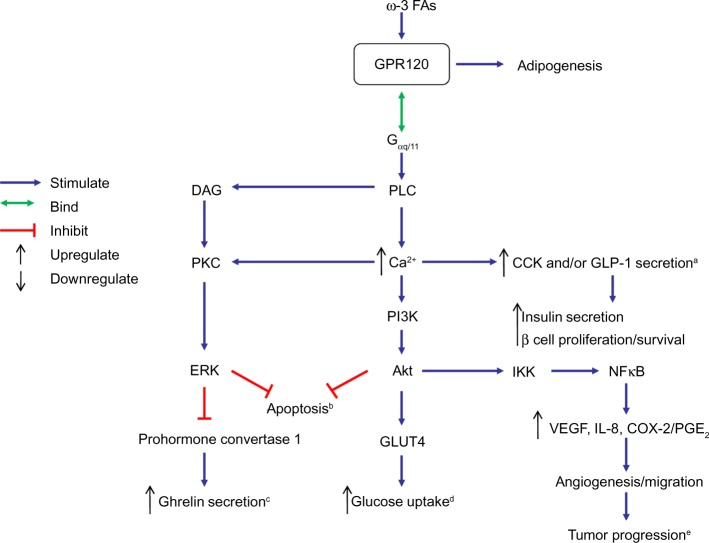
In gastrointestinal cells, TBCs, and adipocytes, agonist-stimulated GPR120 interacts with Gαq/11, a heterotrimeric G-protein,14,37 leading to PLC activation and subsequent elevation in intracellular Ca2+ concentration ([Ca2+]i). Not only can the Ca2+ mobilization promote GLP-1 and/or CCK secretion from gastrointestinal cells,11,13,14 it can also activate phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), which further induces glucose transporter 4 (GLUT4) translocation to cell membranes in adipocytes,3 provokes tumor progression of colorectal carcinoma (CRC) cells,27 inhibits gastric ghrelin secretion,31,32 and elicits an anti-apoptotic effect in STC-1.42 The mechanism(s) by which GPR120 stimulation regulates these physiological and pathological events is summarized in Figure 1.
Figure 1.
Schematic overview of the potential mechanism by which GPR120-Gαq/11 signaling may affect various physiological and pathological processes.
Notes: Binding of ω-3 FA-activated GPR120 to Gαq/11 stimulates PLC, leading to an increase in intracellular Ca2+ concentration ([Ca2+]i), which may influence diverse physiological processes, such as CCK, GLP-1, and ghrelin secretion, glucose uptake, tumor progression, and apoptosis. aCCK and/or GLP-1 secretion in taste bud cells, intestinal tract and STC-1 cells. bApoptosis of STC-1 cells. cGhrelin secretion of stomach. dGlucose uptake of adipocytes. eTumor progression of colorectal carcinoma.
Abbreviations: ω-3 FAs, ω-3 fatty acids; Akt, protein kinase B; CCK, cholecystokinin; COX-2, cyclooxygenase 2; DAG, diacylglycerol; ERK, extracellular-signal-regulated kinase; FA, fatty acid; Gαq/11, heterotrimeric G-protein; GLP-1, glucagon-like peptide 1; GLUT4, glucose transporter 4; GPR120, G-protein coupled receptor 120; IKK, IκB kinase; IL-8, interleukin 8; NFκB, nuclear factor kappa B; PGE2, prostaglandin E2; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PLC, phospholipase-C; PKC, protein kinase C; VEGF, vascular endothelial growth factor; STC-1, mouse intestinal enteroendocrine cell line; Ca2+, intracellular calcium level.
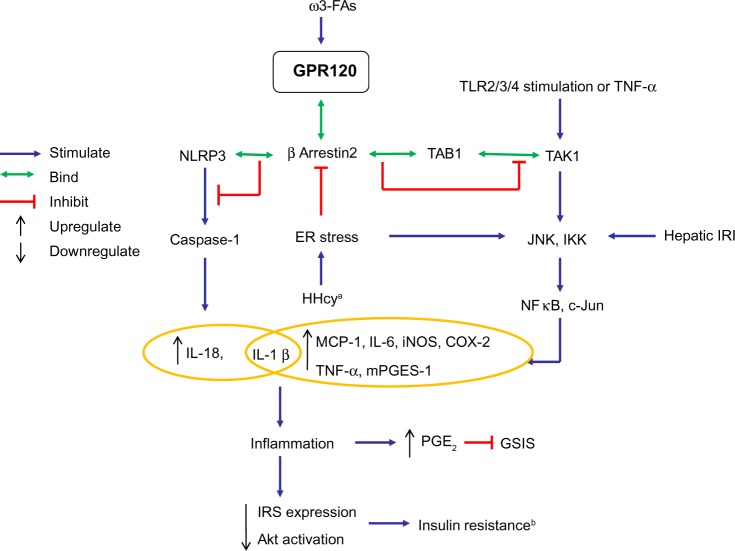
When stimulated by extracellular ω-3 FA ligands, GPR120 on the cell surface can be phosphorylated by kinases, leading to the increase in affinity of GPR120 intracellular domain to scaffold protein β-arrestin-2. Once GPR120 is bound to β-arrestin-2, the G-protein coupling to GPR120 is prevented, and then GPR120-β-arrestin-2 complex is internalized to the cytoplasm where downstream signaling can proceed.3,18 Thus far, all anti-inflammatory effects of GPR120 ligands on macrophages appear to be mediated by GPR120–β-arrestin-2 signaling.3,7,8 The activation of this signaling can inhibit production of pro-inflammatory factors – such as monocyte chemotactic protein-1 (MCP-1), cyclooxygenase-2 (COX-2), interleukin (IL)-18, and IL-1β – by binding TAB1 (transforming growth factor-β activated kinase 1 [TAK1]-binding protein 1) or an inflammasome known as nucleotide-binding oligomerization leucine-rich repeat and pyrin domain-containing protein (NLRP)3/1b.3,7 Figure 2 summarizes the mechanism by which GPR120 activation elicits anti-inflammatory effects in various macrophages and adipocytes.
Figure 2.
Schematic overview of the GPR120-β-arrestin-2 signaling pathway mediating anti-inflammatory effects.
Notes: ω-3 FA-activated GPR120 binds β-arrestin-2, and the resultant complex can interact with TAB1 or NLRP3, leading to inhibition of downstream inflammatory signals, which produce insulin resistance. aHHcy in adipose tissue. bInsulin resistance in liver and adipose tissue.
Abbreviations: ω-3 FAs, ω-3 fatty acids; Akt, protein kinase B; COX-2, cyclooxygenase 2; ER, endoplasmic reticulum; GPR120, G-protein coupled receptor 120; GSIS, glucose-stimulated insulin secretion; HHcy, hyperhomocysteinemia; IKK, IκB kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; IRI, ischemia-reperfusion injury; IRS, insulin receptor substrate; JNK, c-Jun N-terminal kinase; NLRP3, nucleotide-binding oligomerization leucine-rich repeat and pyrin domains-containing protein 3; MCP-1, monocyte chemotactic protein-1; mPGES-1, microsomal prostaglandin E synthase-1; PGE2, prostaglandin E2; TAB1, TAK1-binding protein 1; TAK1, transforming growth factor-β activated kinase 1; TLR2/3/4, toll-like receptor 2/3/4; TNF-α, tumor necrosis factor α.
GPR120 agonists
Natural agonists
Unsaturated free fatty acids (FFAs) with a chain length of 16–22 carbons and saturated FFAs of 14–18 carbons have been identified as ligands of GPR120, and the carboxylate anion of these FFAs is indispensable for their activity at GPR120.11 Upon binding, these FFAs induce internalization of GPR120 into the cytoplasm (as demonstrated by a fluorescent protein marker), provoke an elevation of [Ca2+]i in HEK293 cells and enhance survival of serum-starved STC-1 cells.11,42
ω-3, ω-6 and ω-9 FAs belong to unsaturated FFAs, some members of these FAs are GPR120 ligands. ω-3 FAs appear to be more potent than ω-6 and ω-9 FAs in stimulating GPR120. The ω-3 FAs docosahexaenoic acid (DHA) and α-linolenic acid (ALA) are the most potent and most common GPR120 agonists,11 but their selectivity is limited in that they interact with GPR40 as well.22 On the other hand, unlike ω-3 FAs, ω-6 and ω-9 FAs have failed to exert anti-inflammatory effects through GPR120 signaling in human macrophage THP-1 cells,7 and ω-6 FAs also have failed to attenuate COX-2 expression in RAW 264.7 cells.8
In terms of pharmaceutical application, it would be desirable to find ligands with selective binding of GPR120 only. Grifolin derivatives (ie, grifolic acid and grifolic acid methyl ether) activate GPR120 selectively, but they are less efficacious than ALA and can inhibit the ALA-induced [Ca2+]I response and extracellular-signal-regulated kinase (ERK) phosphorylation. Thus, they are classified as partial GPR120 agonists with high selectivity.43 Similar to the situation with other natural GPR120 ligands,11 carboxyl groups in grifolin derivatives are necessary for GPR120 stimulation.43
Synthetic agonists
To develop selective agonists of GPR120 with high potency, homology model of GPR120 was constructed by utilizing the crystal structure of other GPCRs;20,22,23 in this regard, the hydrogen bonds between the carboxylic acid residue of ligands and the guanidine of Arg99 in GPR120 have been found to be essential for the activity of ligands at GPR120.
The first synthetic ligand of GPR120, GW9508, was identified by Briscoe et al.44 However, GW9508 is not selective for GPR120, which restricts its application. NCG21, a peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist derivative, shows high (albeit incomplete) selectivity for GPR120 over GPR40.22 To investigate the roles of GPR120 in physiological processes, GW9508 and NCG21 can be used with cell lines and tissues that express GPR120 but not GPR40, such as RAW 264.7 cells.3 Knockdown or knockout of GPR120 can be performed to further verify the involvement of this receptor in biological responses induced by its agonists.11 GSK137647A, which can stimulate human, mouse, and rat GPR120, has been used to study the role of GPR120 in regulating GLP-1 secretion from mouse TBCs, although the selectivity of this agonist is not clear.36
Based on structure-activity relationship experiments, Shimpukade et al20 identified TUG-891 [3-(4-((4-fluoro-49-methyl-[1,19-biphenyl]-2-yl)methoxy)phenyl) propanoic acid] as a GPR120 agonist. This compound has high potency on both human and mouse GPR120 and is a more selective and potent agonist for human GPR120 than ALA, GW9508, or NCG21.25 However, the selectivity of TUG-891 for mouse GPR120 is still limited, and the potency and selectivity of TUG-891 for rat GPR120 is unknown. Hence, the potential utility of TUG-891 in animal studies may be restricted.
Differences between natural and synthetic agonists of GPR120
In most cases, natural ligands of FFAs, but not synthetic agonists, are chosen to perform ex vivo and in vivo studies to investigate the role of GPR120 in physiological processes. FFAs can activate GPR120 to exhibit their biological activities in cells, tissues, and animals.3,4 It is unclear whether the potency of natural and synthetic ligands in GPR120 stimulation is different in tissues and/or animals. Nevertheless, it has been reported that the potency of FFAs and synthetic agonists to activate GPR40 is not identical; in MIN6 cells and mouse islets, FFAs can activate GPR40 to enhance glucose-stimulated insulin secretion, while GW9805 only exhibits its stimulatory effect on glucose-stimulated insulin secretion in MIN6 cells and not in mouse islets.44
When using FFAs to study roles of GPR120, it is necessary to manifest the biological responses that are specific for GPR120. As nutrients, FFAs exert their bioactivities through several potential mechanisms. Besides GPCRs, metabolites of FFAs and other FFA-binding proteins, such as long-chain acyl-CoA and PPAR-γ, can also manipulate physiological processes.45–47 In contrast, most of the synthetic agonists of GPR120 merely activate GPR120 and GPR40. In view of this, it would be better to select synthetic ligands in order to avoid the interference that could be caused by other factors.
Compared with synthetic ligands, FFAs have other disadvantages. Due to their poor solubility in water, FFAs are bound to the carrier (ie, fatty acid-free bovine serum albumin [BSA]).45,48,49 On the other hand, BSA blunts the potency of FFAs to activate GPCRs, as it can influence interaction between FFAs and GPCRs.11,50,51
Role of GPR120 in regulating cell and tissue functions
Intestinal cells and tissues
Incretin secretion
GLP-1 and CCK are intestinal incretins; that is, proteins that stimulate pancreatic insulin secretion.52–54 GPR120 activation can induce GLP-1 and CCK secretion, which may further regulate pancreatic islet function and β-cell proliferation and survival.
The GPR120 agonists ALA or TUG-891 induce GLP-1 secretion in murine enteroendocrine cell lines (STC-1 and GLUTag) via stimulating GPR120.11,13,14,25 ALA administration has also been shown to increase plasma GLP-1 levels in mice and rats, and FFA supplementation or incubation increases mouse plasma CCK levels as well as CCK secretion of STC-1 cells.11–14 This stimulatory effect of FFAs on GLP-1 and CCK secretion is dependent on a GPR120-mediated rise in [Ca2+]i.14 FFA-stimulated GPR120 interacts with Gαq/11, leading to activation of PLCβ and generation of inositol trisphosphate, which in turn causes intracellular calcium release from the endoplasmic reticulum (Figure 1). This calcium release activates TRPM5, which elicits membrane depolarization via cation influx. The resultant depolarization induces opening of voltage-gated calcium channels, allowing calcium influx, which then promotes secretion of GLP-1 and CCK.14
Thus far, there has been no direct evidence confirming whether GPR40, which can also be stimulated by FFAs,11 is involved in FFA-induced CCK and GLP-1 secretion in STC-1 cells. However, GPR40 agonists (ie, AM-1638 and AM-6226) can stimulate GLP-1 secretion from primary rat intestinal cells.55 It may be that FFAs have a preference for GPR120 over GPR40 on STC-1 cells in the modulation of GLP-1 and CCK secretion.
Long-term ALA supplementation has been shown to promote pancreatic insulin secretion and β-cell proliferation in rats.13 This finding is highly encouraging, since it suggests that GPR120 may be harnessed for anti-diabetic activities via not only modulation of insulin secretion but also via an effect on β-cell mass. It has been suggested that ALA effects on insulin secretion and β-cell proliferation may involve an indirect effect through GPR120 stimulation of GLP-1 and CCK secretion.13 However, it is still controversial whether GPR120 is expressed in pancreatic islets/β-cells of mouse and rat.16 If GPR120 is expressed in rodent islets, it would raise the possibility of these effects being realized through a direct mechanism.
High levels of GPR120 mRNA were detected in primary murine K-cells, and incubation of K-cells with linoleic acid (LA) can increase GIP secretion significantly, suggesting that GPR120 may be involved in the induction of GIP secretion.26 GIP is an intestinal K-cell-secreting incretin hormone with anti-apoptotic effects on pancreatic β-cells.26 Thus, it is possible that intestinal GPR120 may mediate an anti-diabetic (anti-apoptotic) effect on β-cells by promoting GIP secretion.
Anti-apoptosis
GPR120 was implicated in LA-induced anti-apoptotic effects in STC-1 cells: LA can enhance cell survival and inhibit caspase-3 activity and DNA fragmentation in nutrient-deprived STC-1 cells. Interestingly, in STC-1 cells, transient transfection with GPR120 cDNA enhances the inhibitory effect of LA on caspase-3 inhibition, but knockdown of GPR120 attenuates LA effect.42 The potential mechanism is that LA binding to GPR120 leads to activation of two independent pathways, ERK and PI3K-Akt (also known as protein kinase B) signaling, which in turn may mediate anti-apoptotic effects (Figure 1).42
The ERK pathway is associated with growth and differentiation,56,57 whereas Akt signaling is involved in cell survival and proliferation.58 Unsaturated FFAs have been shown to promote proliferation, enhance cell survival, and inhibit apoptosis via activation of ERK and/or PI3K-Akt in two GPR120-expressing human breast cancer cell lines, namely MDA-MB-231 cells and MCF-7 cells.42,59,60 Pancreatic β-cells also undergo apoptosis during the development of diabetes,61,62 and activation of ERK and Akt can protect against β-cell apoptosis and increase insulin secretion.63,64 Therefore, it is possible that activation of pancreatic GPR120 may mediate the anti-apoptotic effects of FFAs in diabetic-islet β-cells via induction of ERK and Akt phosphorylation.
Angiogenesis
In human CRC cells and tissues, GPR120 acts as a tumor-promoting factor that augments angiogenesis and migratory capacity.27 It has been shown that, in human CRC cells and tissues, GPR120 expression is highly inducible and correlates strongly with CRC differentiation and clinical progression.27 Moreover, in human CRC cell lines (HCT116, SW480) and in an HCT116 xenograft mouse model, GPR120 activation upregulates expression and/or secretion of several pro-angiogenic factors, namely vascular endothelial growth factor, IL-8, prostaglandin E2 (PGE2) and COX-2. In addition, GPR120 stimulation can also enhance CRC motility and promote epithelial-mesenchymal transition of CRCs. The role of GPR120 in the pro-angiogenic influence has been attributed to GPR120 binding of Gαq/11, leading to a rise in [Ca2+]i and subsequent activation of the PI3K/Akt pathway, which favors activation of IκB kinase (IKK)/nuclear factor (NF)-κB signaling, and subsequent induction of pro-angiogenic factors (Figure 1).27
It is noteworthy that, although GPR120 stimulation in human CRCs enhance their migratory ability and increase their expression of COX-2, activation of GPR120 in intraperitoneal macrophages reduce macrophage motility and COX-2 expression.3 These contrasting effects may be due to differential downstream signaling responses being triggered in the different cell lines. In macrophages (RAW 264.7 cell), GPR120 binds β-arrestin-2, leading to inhibition of IKK/NF-κB and c-Jun N-terminal kinase (JNK)/c-Jun pathways. On the other hand, in CRC cells, stimulated GPR120 binds Gαq/11, which consequently activates the IKK/NF-κB pathway. Hence, GPR120 linkage to intracellular signaling mechanism may be cell-type specific.
Stomach
Ghrelin secretion
It has been shown that GPR120, as a receptor of FFAs, is involved in inhibition of secretion of the gastric hormone ghrelin.31,32 FFAs have been shown to inhibit ghrelin secretion both in vitro (transgenic cells in which ghrelin carries a fluorescent marker) and in vivo (serum ghrelin levels of mice with pylorus ligation).31 In addition, Gong et al32 demonstrated that GPR120 stimulation by GW9508 or ALA reduces intracellular acyl-ghrelin content, inhibits acyl-ghrelin secretion in stomach ghrelinoma cell line (SG-1), and decreases fasting-induced plasma acyl-ghrelin levels of mice. These inhibitory effects have been attributed to GPR120 binding of Gαq/11, leading to phosphorylation of ERK via PLC, which may reduce expression of prohormone convertase 1 (the enzyme that converts proghrelin into ghrelin, which upon octanoylation by ghrelin O-acyltransferase [GOAT] becomes acyl-ghrelin) (Figure 1). However, in another ghrelin-producing cell line (MGN3-1), GPR120 was shown to be uninvolved in the inhibitory effect of ALA on ghrelin secretion.30 Thus, it seems that GPR120 involvement in modulating ghrelin secretion is influenced by cell type, the ligand used, or duration of treatment.
The hormone ghrelin is secreted primarily by stomach cells, and to a far lesser extent by pancreatic cells.65 It stimulates hunger, appetite, and weight gain.66–68 Similar to GLP-1, CCK, and insulin, ghrelin can modulate glucose homeostasis levels, and ghrelin levels can be altered dramatically by food intake. Hence, GPR120 activation could regulate metabolic homeostasis through modulation of stomach ghrelin secretion.68 Moreover, it has been reported that blockade of pancreatic ghrelin secretion can increase insulin secretion.69 Given the inhibitory effects of GPR120 activation on stomach ghrelin secretion,31,32 it is possible that GPR120 stimulation may promote insulin secretion by repressing pancreatic ghrelin secretion.
Taste buds
Taste preference and perception
In human subjects, individuals’ overall preference for fatty foods correlates with percentage body fat. That is, relative to lean individuals, obese individuals show, in general, a greater preference for fatty foods.70,71 GPR120 is involved in regulating spontaneous preference for fat content in one’s diet. Compared with wild-type controls, GPR120 knockout mice exhibit a lower oro-gustatory preference for FFAs and reduced taste nerve responses to several FFAs.35 Researchers have speculated that decreased GPR120 expression may account for diminished responsiveness to an FFA-rich diet in ghrelin knockout mice and GOAT knockout mice.72
Taste perception plays a crucial role in modulating food preference and energy homeostasis. GLP-1 signaling can enhance sweet and lipid detection sensitivity.36,73 It has been recently reported that lipids can induce GLP-1 secretion in mouse circumvallate papillae and, consequently, regulate taste perception threshold for sweets and lipids via activation of GPR120.36 FFAs can also induce GLP-1 secretion to modulate fat preference through stimulation of GPR120 in human TBCs37 through a mechanism that is similar to that which occurs in STC-1 cells (Figure 1).14 Differential perception sensitivity for lipids or sweets may affect interest in fat- or carbohydrate-rich food, which may contribute to fat intake and, consequently, affect risk for obesity.74,75 According to this model, GPR120-mediated modulation of taste perception in TBCs may alter an individual’s susceptibility to obesity. GPR120 activation may increase intake of high-lipid/high-sugar foods by upregulating GLP-1-induced of fat and sweet taste sensitivity in TBCs. However, given the important role of other long-chain fatty acid receptors (ie, GPR40 and CD36) in fat taste preference, it seems that GPR120 activation may not be the primary event in fat taste preference signaling.76,77 Thus, we cannot predict that upregulation or activation of GPR120 in TBCs would produce increased fat/carbohydrate intake.
Adipose tissues
Adipogenesis
GPR120 plays an important role in modulating adipose tissue development and adipocyte differentiation. Liu et al78 and Oh et al3 have shown that differentiation of 3T3-L1 cells is associated with increased levels of GPR120 mRNA. Gotoh et al40 has demonstrated that GPR120 expression in human differentiated adipocytes is much higher than that in human preadipocytes. Ichimura et al4 has shown that adipogenesis is suppressed in mouse embryonic fibroblast-derived adipocytes from GPR120 knockout mice.
During differentiation of 3T3-L1 cells, mRNA levels of GPR120 are influenced by regulation of PPAR-γ2, a crucial transcription factor in adipose tissue differentiation.40 In addition, GPR120 knockdown was found to decrease mRNA levels of PPAR-γ2.40 Moreover, Ichimura et al4 has demonstrated in adipocytes derived from mouse embryonic fibroblasts of GPR120 knockout mice, adipocyte differentiation marker genes (fatty acid binding protein 4, PPAR-γ, and sterol regulatory element binding protein 1) are reduced. It seems GPR120 may interact with these adipogenesis-related proteins, especially PPAR-γ2, during adipocyte differentiation, although, if so, the specific mechanism has not been clarified. It is noteworthy that, besides its role in differentiation, PPAR-γ is also an important modulator of pancreatic β-cell function, glycometabolism, and lipid metabolism.79 Given the interactions between GPR120 and PPAR-γ in adipose, it would be of interest to investigate whether GPR120 can regulate islet function and energy metabolism via interaction with PPAR-γ in other tissues, such as pancreas and liver.
Glucose uptake
GPR120 is involved in glucose metabolism in adipocytes and adipose tissue via regulation of GLUT4.3,78 Oh et al3 has shown in 3T3-L1 adipocytes and primary adipose tissue the stimulated GPR120 binds Gαq/11, leading to activation of the PI3K/Akt pathway, which in turn causes GLUT4 translocation to the cell membrane and, finally, triggers glucose uptake (Figure 1). Interestingly, GPR120 knockdown can reduce expression of GLUT4 and insulin receptor substrate 1 (IRS1),78 suggesting that GPR120 dysfunction may impair glucose uptake in adipose tissue.
Gαq/11 and IRS1 play crucial, although independent, roles in the activation of the PI3K/Akt/GLUT4 pathway.3 IRS1 transmits extracellular insulin signals to the cell interior, producing intracellular responses that alter glucose metabolism. Based on the observation that DHA did not stimulate IRS1 phosphorylation, Oh et al3 speculated that the stimulatory effect of DHA-induced GPR120 activation on GLUT4 translocation in 3T3-L1 adipocytes must be occurring downstream of IRS1. However, Liu et al78 has shown that IRS1 expression can be decreased by down-regulation of GPR120 expression in differentiated 3T3-L1 adipocytes. Hence, although normal GPR120 stimulation may not activate IRS1 to induce GLUT4 translocation, dysfunctional GPR120 can result in a downregulation of IRS1 expression and, thus, repression of insulin signaling, preventing normal induction of GLUT4-regulated glucose uptake.
Macrophages
Anti-inflammation
GPR120 activation has been shown to produce anti-inflammatory effects in macrophages (RAW 264.7 cells) by repressing activation of IKKβ/NF-κB and JNK/c-Jun signaling, which is induced by toll-like receptor 4 (TLR4) activation or TNF (tumor necrosis factor)-α. This repression can inhibit downstream pro-inflammatory cascades, including induction of MCP-1, TNF-α, IL-6, COX-2, PGE2, and microsomal PGE synthase-1 (mPGES-1).3,8 The mechanism for this anti-inflammatory effect involves DHA-activated GPR120 binding to scaffolding protein β-arrestin-2; GPR120–β-arrestin-2 complex is then internalized into the cytoplasm and binds TAB1, which in turn blocks TAB1/TAK1 association, induction of downstream pro-inflammatory signaling, and generation of inflammatory products (Figure 2).3
It has been demonstrated, in primary rat adipocytes, that GPR120 activation can reverse hyperhomocysteinemia (HHcy)-induced insulin resistance by inhibiting inflammation signaling.10 HHcy is a condition characterized by abnormally high levels of homocysteine, which correlates with insulin resistance and can elevate risk of death in T2DM.80–82 HHcy-induced stress on the endoplasmic reticulum activates JNK phosphorylation, triggers transcription of pro-inflammation markers (eg, MCP-1 and TNF-α) in adipose tissue and plasma, and inhibits Akt activation (shown in primary rat adipocytes); all of which may further exacerbate insulin resistance (Figure 2).10 GPR120 likely mediates its anti-inflammatory effects on JNK activation through the β-arrestin-2 signaling pathway mentioned above.3 In addition, since homocysteine can repress β-arrestin-2 levels,83 it would be of interest to determine whether GPR120 stimulation might also be involved in anti-inflammatory effects via restoration of β-arrestin-2 expression.
It has been reported that GPR120 expression in liver is limited to resident macrophage KCs, and indeed, GPR120 plays a key role in mediating anti-inflammatory effect in KCs.9 Hepatic ischemia-reperfusion injury can activate inflammatory signaling in KCs, which is mediated by JNK and NF-κB.84–86 GPR120 activation in KCs by agonists (GW9508 and Omegaven® [Fresenius Kabi, Bad Homburg, Germany], a clinical ω-3 FA-formulation) can provide protection from such reperfusion injury by inhibiting activation of JNK and NF-κB (Figure 2), which decreases induction of pro-inflammatory cytokines and upregulates expression of hepatoprotective cytokines.9
GPR120 is also involved in inhibition of inflammasome activation.7 In bone-marrow-derived macrophages and human THP-1 macrophages, ω-3 FAs suppress activation of the NLRP3/1b inflammasome, leading to inhibition of caspase-1 and IL-1β through activation of GPR120 and GPR40.7 In this process, β-arrestin-2 functions as the downstream protein of GPR120 and GPR40 to repress inflammasome activation via binding to NLRP3/1b (Figure 2).7
Insulin resistance, a characteristic feature of T2DM, is associated with inflammation in adipose, hepatic, and muscle tissues. Moreover, pancreatic islets themselves also undergo inflammation as the disease progresses.87,88 Therefore, given the role of GPR120 in anti-inflammatory effect in macrophages, it seems possible that GPR120 could produce anti-diabetic effects via an anti-inflammatory influence in the above tissues.
GPR120 and its anti-diabetic potential
It has been shown that GPR120 mutations in obese Europeans, named as R270H, are associated with obesity.4 The p.R270H variant cannot functionally transduce signals of FFAs, and thus FFA-induced Ca2+ mobilization and GLP-1 secretion have been found to be significantly suppressed in cells expressing this GPR120 variant.4 This may be one of the reasons why the p.R270H variant of GPR120 is related to obesity in humans. It is noteworthy that the R270H variant is rare in Japanese, suggestive of the ethnic viability in GPR120 variation.89 Indeed, GPR120 dysfunction is also associated with insulin resistance, the other obesity-related symptoms of metabolic disorder.
Insulin sensitivity in adipose, hepatic, and muscle tissues
Consumption of a HFD can induce upregulation of GPR120 expression in white adipose, cardiac, and EDL skeletal muscle tissues of mouse or rat.40,41 Also, GPR120 is expressed in subcutaneous and omental adipose tissues at higher levels in obese individuals than in lean controls.4 Thus, it seems that GPR120 expression becomes upregulated to support maintenance of energy metabolism homeostasis (energy uptake, storage, and utilization). If so, a GPR120 deficiency would impair metabolic balance, which could lead to insulin resistance. Indeed, compared with wild-type mice, GPR120 knockout mice exhibit more severe signs of insulin resistance when fed a HFD. Specifically, they exhibit worse glucose intolerance, larger islets, more Ki67-positive cells in the pancreas, reduced expression of insulin-signaling-related genes (IRS1, IRSβ, or IRS2) in white adipose tissue and liver, and impaired insulin-induced Akt phosphorylation in white adipose tissue, liver, and muscle.4 The notion that GPR120’s role as an FA receptor is critical in regulation of energy metabolism homeostasis is supported strongly by the observation that FA administration can enhance muscle and hepatic insulin sensitivity, increase glucose infusion rate, promote hepatic lipid metabolism, and decrease hepatic steatosis in wild-type mice but not GPR120 knockout mice.3
It has been suggested that a failure to store energy through adipogenesis when excess nutrients are ingested could cause insulin resistance related to an overload of lipids in the liver and muscles.6,90,91 Since FFAs can promote adipogenesis in adipose tissue by activating GPR120, dysfunction of this receptor may lead to a failure of adipocyte differentiation in adipose, resulting in lipid accumulation in the liver.40,78 In the absence of functional GPR120, the decreased lipogenesis in adipose with hepatic steatosis and increased lipogenesis in the liver may contribute to the development of insulin resistance.3,4 If so, then activation of normal GPR120 by FFAs may facilitate normal lipogenesis in adipose and liver, and thereby perhaps prevent hepatic steatosis and maintain insulin sensitivity.
GPR120 might also contribute to metabolic homeostasis via its important role in maintaining normal adipose–liver interactions. GPR120 dysfunction may lead to lipid metabolic disorders due to insufficient production of palmitoleate (C16:1n7), a lipid hormone that modulates metabolic homeostasis and through which adipose interacts with other distant tissues such as liver and muscle.92 In addition, GPR120 may also contribute to maintenance of metabolic balance by way of GLUT4 translocation in adipocytes.3 That is, GPR120 dysfunction may downregulate FA-stimulated glucose transmembrane transport in adipose tissues, leading to decreased glucose uptake and utilization.
Aside from any direct effects of GPR120 on lipid and glucose metabolism, GPR120 activation may temper the pro-inflammatory effects induced by a HFD in mice.3,4 GPR120 expression is increased markedly in macrophages in adipose and liver in obese mice.3 Indeed, ω-3 FA administration decreases the expression of M1 pro-inflammatory genes, decreases inflammatory macrophage infiltration in adipose tissue, and increases transcription of M2 anti-inflammatory genes in adipose tissue of HFD mice, but only if they had a functional GPR120 gene.3 Moreover, ω-3 FA administration inhibits NLRP3 inflammasome activation in HFD mice, leading to reduced caspase-1 activation as well as repressed production of IL-1β, IL-18, TNF-α, and MCP-1, ultimately preventing NLRP3 inflammasome-dependent insulin resistance, which may be due, at least partially, to FA stimulation of GPR120.7 These findings suggest that FA stimulation of GPR120 in macrophages could inhibit HFD-induced inflammation. Moreover, inflammation can interrupt normal energy metabolism, favoring the pathogenesis of insulin resistance through the inflammatory signaling cascades discussed above.93–95 Taken together, in macrophages of adipose tissue and liver (KCs), GPR120 activation by FFAs could inhibit these inflammatory signaling pathways and thereby perhaps reverse insulin resistance. In contrast, under conditions of GPR120 dysfunction, deficiency of such protective anti-inflammatory effects may leave tissue vulnerable to the development of insulin resistance. This possibility is supported by findings that mice with a GPR120 deficiency that are fed a HFD lose the benefit of FA stimulation – such as reduction of IL-1β in adipose – leading to a resultant decrease in IRS1 and leaving the mice susceptible to worsening insulin resistance (Figure 2).4,96
Oh et al3 concluded that FFAs can enhance the insulin sensitivity of skeletal muscle in HFD mice by exerting anti-inflammatory effects. However, GPR120 expression in muscle is negligible, and there has been little evidence to date indicating that GPR120 mediates direct anti-inflammatory effects in muscle macrophages. Since activation of inflammation in adipose macrophages is an inducing factor for insulin resistance in muscle tissue,97 GPR120-mediated anti-inflammation in adipose tissue might also be able to relieve insulin resistance in muscle to some extent.
Although insulin resistance is considered to be one of the T2DM features, some researchers propose that insulin resistance might be also linked with development of type 1 diabetes as well.98–100 Given the important roles of GPR120 in the prevention of insulin resistance, it appears that GPR120 activation might also be beneficial for the management of type 1 diabetes.
Pancreatic islet and β-cell function
Intestinal hormone secretion
GPR120 could affect pancreatic islet function through modulation of GLP-1, CCK, and GIP secretion from the intestinal tract. The β-cell dysfunction and loss of β-cell mass that occur as diabetes progresses could cause insufficient pancreatic insulin secretion. GPR120 activation can increase intestinal secretion of CCK and GLP-1, two incretins that promote pancreatic insulin secretion. In addition, since CCK and GLP-1 can also increase β-cell survival, stimulate proliferation of β-cells and improve β-cell function,101–104 it seems possible that induction of CCK and GLP-1 secretion by GPR120 activation could expand β-cell mass and support β-cell function. Moreover, the intestinal hormone GIP, which also can promote β-cell survival,105 may be another target for enhancement of β-cell mass through GPR120 activation.26
Resident GPR120 in pancreatic islets
Modulation of gastrointestinal hormone secretion by GPR120 activation is an indirect way of regulating pancreatic islet function. Meanwhile, GPR120 stimulation in pancreatic islets may manipulate islet function directly. Although it remains controversial whether GPR120 is expressed in rodent pancreas, some researchers have detected GPR120 in mouse pancreatic islets, as well as in MIN6 β-cells, and thus have predicted that it may be involved in FFA-induced regulation of pancreatic islet function.16,39
GPR120 activation may affect pancreatic physiology through regulating function of the pancreatic α-cell, which is best known for its glucagon-secreting function. Whalley et al observed GPR120 expression in a murine pancreatic α-cell line (αTC1-6), and they further showed that αTC1-6 cells can secrete GLP-1 and express prohormone convertase 1, which cleaves proglucagon into GLP-1.106 They demonstrated that GPR120 agonism can stimulate GLP-1 secretion and inhibit glucagon secretion in αTC1-6 cells.106 Furthermore, the same group showed that these cells secrete more GLP-1 under diabetic (high-glucose) conditions. To test whether such GLP-1 secretion is induced via GPR120 agonism, further studies are needed to determine whether genetic knockdown or knockout of GPR120 could attenuate or abolish induction of GLP-1 secretion in the presence or absence of a high concentration of glucose.
In human islets, GPR120 expression is associated positively with insulin secretion and insulin content but negatively with hemoglobin A1c percentage, and pancreatic islets from hyperglycemic or diabetic patients have reduced GPR120 expression compared with healthy individuals.15 These observations suggest that human pancreatic insulin secretion correlates with islet GPR120 expression. GPR120 expressed in pancreatic islets may be involved in FA-stimulated insulin secretion from β-cells. Although GPR40 has been demonstrated to mediate FA-induced stimulation on insulin secretion directly,48,50 it appears to account for only about half of FA-stimulated insulin secretion in mice.48 Furthermore, GPR120 can mediate FA-stimulated elevation of [Ca2+]i in intestinal cells, an important event in triggering insulin secretion.14,107 Taken together, these findings suggest that pancreatic GPR120 may have a direct role in regulating insulin secretion from β-cells.
Since GPR120 can mediate inhibition of ghrelin secretion from the stomach, it raises the possibility that GPR120 may also inhibit ghrelin secretion from epsilon cells in pancreatic islets.94,95 And it is encouraging that, Dezaki et al69 reported that blockade of pancreatic islet-derived ghrelin can enhance insulin secretion and thereby prevent glucose intolerance in mice fed a HFD.
In human islets, GPR120 activation by ω-3 FA prevents lipid-induced apoptosis and GPR120 knockdown attenuates ω-3 FA-related anti-apoptotic effects.15 Moreover, in serum-starved STC-1 cells, GPR120 has been implicated in the anti-apoptotic effect of ω-3 FAs via activation of ERK and Akt signaling. Furthermore, induction of ERK and Akt phosphorylation can protect against β-cell apoptosis and increase insulin secretion.64 Hence, there are some clues suggesting that GPR120 may be involved in the anti-apoptosis effects of ω-3 FAs on β-cells in diabetic islets. Interestingly, it has been reported that β-arrestin-2 has important roles in the regulation of insulin-Akt signaling in the liver and pancreatic islets.108,109 The potential mechanism is that β-arrestin-2 is linked to this signaling pathway through scaffolding Akt to insulin receptor.109 If GPR120 stimulation in pancreatic islets could activate Akt signaling so as to protect β-cell function, it may be plausible that β-arrestin-2 also functions as a scaffold protein to recruit Akt.
ω-3 FAs can exert anti-inflammatory and anti-diabetic effects on the pancreas. Using fat-1 transgenic mice, Bellenger et al110 showed that high levels of endogenous ω-3 FAs can prevent streptozotocin-induced diabetes via inhibition of pro-inflammatory factors (eg, TNF-α, NF-κB, IL-1β, MCP-1, and PGE2) in pancreas. Hence, as a ω-3 FA receptor, GPR120 may be involved in ω-3 FA inhibition of inflammation in pancreatic islets.
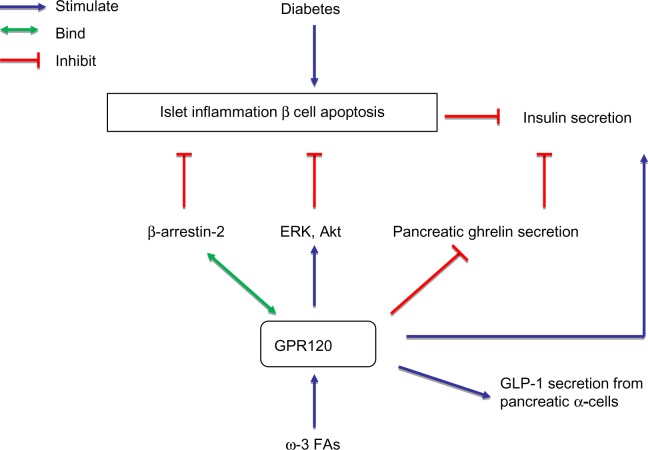
Diabetic islets have high levels of activated islet macrophages, leading to inflammation (eg, via NLRP3 inflammasome activation) and release of cytokines (eg, IL-8 and MCP-1).97,111 Pancreatic β-cells also ramp up cytokine production under diabetic conditions in a manner that leads to β-cell dysfunction and death.112 For example, IL-1β-induced production of COX-2 and PGE2 can impair glucose-stimulated insulin secretion in human and rodent pancreatic islets.113 Conversely, inhibition of IL-1β-induced NF-κB activation and COX-2/PGE2 generation could enhance pancreatic islet cell function.114 Given that GPR120 plays important roles in mediating anti-inflammation in macrophages,3,7–9 it is likely that the anti-diabetic effects of GPR120 may involve anti-inflammatory effects in pancreatic macrophages and β-cells. Figure 3 summarizes the potential mechanism by which GPR120 activation in pancreatic islets exhibits the anti-diabetic effects.
Figure 3.
Schematic overview of the potential mechanism by which GPR120 activation in pancreatic islets exerts anti-diabetic effects.
Notes: In pancreatic islets, ω-3 FA-induced GPR120 stimulation may regulate and protect islet function to prevent against diabetes via eliciting anti-apoptotic, anti-inflammatory effects, and/or regulating pancreatic ghrelin, insulin secretion, and/or α-cell GLP-1 secretion.
Abbreviations: Akt, protein kinase B; GPR120, G-protein coupled receptor 120; GLP-1, glucagon-like peptide-1; ERK, extracellular-signal-regulated kinase; FA, fatty acid.
Conclusion and perspective
Although GPR120 acts as a tumor-processing factor in CRC, it may have a positive role in the management of diabetes. GPR120 activation supports metabolic homeostasis by inhibiting inflammation in macrophages and regulating glucose and/or lipid metabolism in adipose, liver, and muscle tissues directly or indirectly. Furthermore, GPR120 stimulation can indirectly promote pancreatic islet function and β-cell proliferation through its modulatory influence on gastrointestinal hormone secretion. Therefore, GPR120 is identified as a potential target for development of anti-diabetic drugs.
Although it is yet unclear whether pancreatic GPR120 regulates islet function, the fact that GPR120 expression is associated with insulin secretion in human islets supports the possibility that GPR120 in the pancreas could be involved in regulating islet function. Given the important roles of GPR120 in the modulation of gastrointestinal hormone secretion, anti-inflammation in macrophages, and anti-apoptosis in enteroendocrine cells and human islets, it is likely that in diabetic islets, GPR120 may promote β-cell survival/proliferation and islet function through the following activities: anti-apoptotic effects in β-cells, anti-inflammatory effects in islet macrophages and/or β-cells, direct stimulation of β-cell insulin secretion, induction of GLP-1 secretion from pancreatic α-cells, and/or inhibition of pancreatic ghrelin secretion.
Acknowledgments
The work was fully supported by the General Research Fund of the Research Grants Council of Hong Kong (Ref No 470413), awarded to PS Leung.
Footnotes
Disclosure
No conflicts of interest relevant to this article are reported.
References
- 1.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult US population, 1999–2010. J Am Coll Cardiol. 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichimura A, Hirasawa A, Poulain-Godefroy O, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzaka T, Shimano H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J Diabetes Investig. 2011;2(3):170–175. doi: 10.1111/j.2040-1124.2011.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26(1):13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 7.Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38(6):1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Yu Y, Funk CD. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4) FASEB J. 2013;27(12):4987–4997. doi: 10.1096/fj.13-235333. [DOI] [PubMed] [Google Scholar]
- 9.Raptis DA, Limani P, Jang JH, et al. Gpr120 on Kupffer cells mediates hepatoprotective effects of omega3-fatty acids. J Hepatol. 2014;60(3):625–632. doi: 10.1016/j.jhep.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Zhang H, Jiang C, et al. Hyperhomocysteinemia promotes insulin resistance by inducing endoplasmic reticulum stress in adipose tissue. J Biol Chem. 2013;288(14):9583–9592. doi: 10.1074/jbc.M112.431627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Katsuma S, Adachi T, et al. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4–6):523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Yano T, Adachi T, et al. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4–6):515–522. doi: 10.1007/s00210-007-0250-y. [DOI] [PubMed] [Google Scholar]
- 14.Shah BP, Liu P, Yu T, Hansen DR, Gilbertson TA. TRPM5 is critical for linoleic acid-induced CCK secretion from the enteroendocrine cell line, STC-1. Am J Physiol Cell Physiol. 2012;302(1):C210–C219. doi: 10.1152/ajpcell.00209.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taneera J, Lang S, Sharma A, et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16(1):122–134. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab. 2009;11(Suppl 4):10–20. doi: 10.1111/j.1463-1326.2009.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns RN, Moniri NH. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem Biophys Res Commun. 2010;396(4):1030–1035. doi: 10.1016/j.bbrc.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 18.Watson SJ, Brown AJ, Holliday ND. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol. 2012;81(5):631–642. doi: 10.1124/mol.111.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore K, Zhang Q, Murgolo N, Hosted T, Duffy R. Cloning, expression, and pharmacological characterization of the GPR120 free fatty acid receptor from cynomolgus monkey: comparison with human GPR120 splice variants. Comp Biochem Physiol B Biochem Mol Biol. 2009;154(4):419–426. doi: 10.1016/j.cbpb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Shimpukade B, Hudson BD, Hovgaard CK, Milligan G, Ulven T. Discovery of a potent and selective GPR120 agonist. J Med Chem. 2012;55(9):4511–4515. doi: 10.1021/jm300215x. [DOI] [PubMed] [Google Scholar]
- 21.Holliday ND, Watson SJ, Brown AJ. Drug discovery opportunities and challenges at g protein coupled receptors for long chain free fatty acids. Front Endocrinol (Lausanne) 2012;2:112. doi: 10.3389/fendo.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki T, Igari S, Hirasawa A, et al. Identification of G protein-coupled receptor 120-selective agonists derived from PPARgamma agonists. J Med Chem. 2008;51(23):7640–7644. doi: 10.1021/jm800970b. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Hirasawa A, Hara T, et al. Structure-activity relationships of GPR120 agonists based on a docking simulation. Mol Pharmacol. 2010;78(5):804–810. doi: 10.1124/mol.110.066324. [DOI] [PubMed] [Google Scholar]
- 24.Cen B, Xiong Y, Ma L, Pei G. Direct and differential interaction of beta-arrestins with the intracellular domains of different opioid receptors. Mol Pharmacol. 2001;59(4):758–764. doi: 10.1124/mol.59.4.758. [DOI] [PubMed] [Google Scholar]
- 25.Hudson BD, Shimpukade B, Mackenzie AE, et al. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol Pharmacol. 2013;84(5):710–725. doi: 10.1124/mol.113.087783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52(2):289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q, Wang H, Zhao X, et al. Identification of G-protein-coupled receptor 120 as a tumor-promoting receptor that induces angiogenesis and migration in human colorectal carcinoma. Oncogene. 2013;32(49):5541–5550. doi: 10.1038/onc.2013.264. [DOI] [PubMed] [Google Scholar]
- 28.Iakoubov R, Izzo A, Yeung A, Whiteside CI, Brubaker PL. Protein kinase Czeta is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology. 2007;148(3):1089–1098. doi: 10.1210/en.2006-1403. [DOI] [PubMed] [Google Scholar]
- 29.Miyauchi S, Hirasawa A, Iga T, et al. Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):427–434. doi: 10.1007/s00210-008-0390-8. [DOI] [PubMed] [Google Scholar]
- 30.Janssen S, Laermans J, Iwakura H, Tack J, Depoortere I. Sensing of fatty acids for octanoylation of ghrelin involves a gustatory G-protein. PLoS One. 2012;7(6):e40168. doi: 10.1371/journal.pone.0040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X, Zhao X, Feng J, et al. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(3):G367–G376. doi: 10.1152/ajpgi.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong Z, Yoshimura M, Aizawa S, et al. G protein-coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. Am J Physiol Endocrinol Metab. 2014;306(1):E28–E35. doi: 10.1152/ajpendo.00306.2013. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura S, Mizushige T, Yoneda T, et al. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28(1):49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura S, Eguchi A, Mizushige T, et al. Colocalization of GPR120 with phospholipase-Cbeta2 and alpha-gustducin in the taste bud cells in mice. Neurosci Lett. 2009;450(2):186–190. doi: 10.1016/j.neulet.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 35.Cartoni C, Yasumatsu K, Ohkuri T, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30(25):8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin C, Passilly-Degrace P, Chevrot M, et al. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res. 2012;53(11):2256–2265. doi: 10.1194/jlr.M025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozdener MH, Subramaniam S, Sundaresan S, et al. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 2014;146(4):995–1005. doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galindo MM, Voigt N, Stein J, et al. G protein-coupled receptors in human fat taste perception. Chem Senses. 2012;37(2):123–139. doi: 10.1093/chemse/bjr069. [DOI] [PubMed] [Google Scholar]
- 39.Morgan NG, Dhayal S. G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic beta-cell. Biochem Pharmacol. 2009;78(12):1419–1427. doi: 10.1016/j.bcp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Gotoh C, Hong YH, Iga T, et al. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun. 2007;354(2):591–597. doi: 10.1016/j.bbrc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Cornall LM, Mathai ML, Hryciw DH, McAinch AJ. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem. 2011;28(5):949–958. doi: 10.1159/000335820. [DOI] [PubMed] [Google Scholar]
- 42.Katsuma S, Hatae N, Yano T, et al. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem. 2005;280(20):19507–19515. doi: 10.1074/jbc.M412385200. [DOI] [PubMed] [Google Scholar]
- 43.Hara T, Hirasawa A, Sun Q, et al. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch Pharmacol. 2009;380(3):247–255. doi: 10.1007/s00210-009-0425-9. [DOI] [PubMed] [Google Scholar]
- 44.Briscoe CP, Peat AJ, McKeown SC, et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148(5):619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roduit R, Nolan C, Alarcon C, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes. 2004;53(4):1007–1019. doi: 10.2337/diabetes.53.4.1007. [DOI] [PubMed] [Google Scholar]
- 46.Horia E, Watkins BA. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis. 2007;28(4):809–815. doi: 10.1093/carcin/bgl183. [DOI] [PubMed] [Google Scholar]
- 47.Zapata-Gonzalez F, Rueda F, Petriz J, et al. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukoc Biol. 2008;84(4):1172–1182. doi: 10.1189/jlb.1007688. [DOI] [PubMed] [Google Scholar]
- 48.Latour MG, Alquier T, Oseid E, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56(4):1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Xu M, Zhang S, et al. The role of G protein-coupled receptor 40 in lipoapoptosis in mouse beta-cell line NIT-1. J Mol Endocrinol. 2007;38(6):651–661. doi: 10.1677/JME-06-0048. [DOI] [PubMed] [Google Scholar]
- 50.Itoh Y, Kawamata Y, Harada M, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422(6928):173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 51.Stoddart LA, Brown AJ, Milligan G. Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5′-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol Pharmacol. 2007;71(4):994–1005. doi: 10.1124/mol.106.031534. [DOI] [PubMed] [Google Scholar]
- 52.Williams RH, Champagne J. Effects of cholecystokinin, secretin, and pancreatic polypeptide on secretion of gastric inhibitory polypeptide, insulin, and glucagon. Life Sci. 1979;25(11):947–956. doi: 10.1016/0024-3205(79)90500-9. [DOI] [PubMed] [Google Scholar]
- 53.Rossetti L, Shulman GI, Zawalich WS. Physiological role of cholecystokinin in meal-induced insulin secretion in conscious rats. Studies with L 364718, a specific inhibitor of CCK-receptor binding. Diabetes. 1987;36(10):1212–1215. doi: 10.2337/diab.36.10.1212. [DOI] [PubMed] [Google Scholar]
- 54.Fried M, Schwizer W, Beglinger C, et al. Physiological role of cholecystokinin on postprandial insulin secretion and gastric meal emptying in man. Studies with the cholecystokinin receptor antagonist loxiglumide. Diabetologia. 1991;34(10):721–726. doi: 10.1007/BF00401517. [DOI] [PubMed] [Google Scholar]
- 55.Luo J, Swaminath G, Brown SP, et al. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS One. 2012;7(10):e46300. doi: 10.1371/journal.pone.0046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woessmann W, Mivechi NF. Role of ERK activation in growth and erythroid differentiation of K562 cells. Exp Cell Res. 2001;264(2):193–200. doi: 10.1006/excr.2000.5124. [DOI] [PubMed] [Google Scholar]
- 57.Verheijen MH, Wolthuis RM, Defize LH, den Hertog J, Bos JL. Interdependent action of RalGEF and Erk in Ras-induced primitive endoderm differentiation of F9 embryonal carcinoma cells. Oncogene. 1999;18(31):4435–4439. doi: 10.1038/sj.onc.1202834. [DOI] [PubMed] [Google Scholar]
- 58.De Vita G, Berlingieri MT, Visconti R, et al. Akt/protein kinase B promotes survival and hormone-independent proliferation of thyroid cells in the absence of dedifferentiating and transforming effects. Cancer Res. 2000;60(14):3916–3920. [PubMed] [Google Scholar]
- 59.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60(22):6353–6358. [PubMed] [Google Scholar]
- 60.Soto-Guzman A, Robledo T, Lopez-Perez M, Salazar EP. Oleic acid induces ERK1/2 activation and AP-1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol Cell Endocrinol. 2008;294(1–2):81–91. doi: 10.1016/j.mce.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 62.Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW. Beta cell apoptosis in diabetes. Apoptosis. 2009;14(12):1389–1404. doi: 10.1007/s10495-009-0339-5. [DOI] [PubMed] [Google Scholar]
- 63.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1) J Biol Chem. 2002;277(51):49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- 64.Wijesekara N, Krishnamurthy M, Bhattacharjee A, et al. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285(44):33623–33631. doi: 10.1074/jbc.M109.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther. 2008;118(2):239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Vatansever-Ozen S, Tiryaki-Sonmez G, Bugdayci G, Ozen G. The effects of exercise on food intake and hunger: relationship with acylated ghrelin and leptin. J Sports Sci Med. 2011;10(2):283–291. [PMC free article] [PubMed] [Google Scholar]
- 67.Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91(9):3296–3302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- 68.Delhanty PJ, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides. 2011;32(11):2309–2318. doi: 10.1016/j.peptides.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55(12):3486–3493. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 70.Mela DJ, Sacchetti DA. Sensory preferences for fats: relationships with diet and body composition. Am J Clin Nutr. 1991;53(4):908–915. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- 71.Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol Behav. 1985;35(4):617–622. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 72.Cai H, Cong WN, Daimon CM, et al. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS One. 2013;8(10):e76553. doi: 10.1371/journal.pone.0076553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin YK, Martin B, Golden E, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106(1):455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stewart JE, Feinle-Bisset C, Golding M, et al. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104(1):145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 75.Chen CS, Bench EM, Allerton TD, et al. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1346–R1355. doi: 10.1152/ajpregu.00582.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Godinot N, Yasumatsu K, Barcos ME, et al. Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. Neuroscience. 2013:25020–25030. doi: 10.1016/j.neuroscience.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 77.Martin C, Passilly-Degrace P, Gaillard D, et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 2011;6(8):e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu D, Wang L, Meng Q, Kuang H, Liu X. G-protein coupled receptor 120 is involved in glucose metabolism in fat cells. Cell Mol Biol. 2012;(Suppl 58):OL1757–OL1762. [PubMed] [Google Scholar]
- 79.Auwerx J, Cock TA, Knouff C. PPAR-gamma: a thrifty transcription factor. Nucl Recept Signal. 2003;1:e006. doi: 10.1621/nrs.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoogeveen EK, Kostense PJ, Jakobs C, et al. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes: 5-year follow-up of the Hoorn Study. Circulation. 2000;101(13):1506–1511. doi: 10.1161/01.cir.101.13.1506. [DOI] [PubMed] [Google Scholar]
- 81.Martos R, Valle M, Morales R, et al. Hyperhomocysteinemia correlates with insulin resistance and low-grade systemic inflammation in obese prepubertal children. Metabolism. 2006;55(1):72–77. doi: 10.1016/j.metabol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Golbahar J, Aminzadeh MA, Kassab SE, Omrani GR. Hyperhomocysteinemia induces insulin resistance in male Sprague-Dawley rats. Diabetes Res Clin Pract. 2007;76(1):1–5. doi: 10.1016/j.diabres.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 83.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suetsugu H, Iimuro Y, Uehara T, et al. Nuclear factor {kappa}B inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut. 2005;54(6):835–842. doi: 10.1136/gut.2004.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giakoustidis DE, Giakoustidis AE, Iliadis S, et al. Attenuation of liver ischemia/reperfusion induced apoptosis by epigallocatechin-3-gallate via down-regulation of NF-kappaB and c-Jun expression. J Surg Res. 2010;159(2):720–728. doi: 10.1016/j.jss.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 86.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143(2):307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donath MY, Schumann DM, Faulenbach M, et al. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 88.Imai Y, Dobrian AD, Morris MA, Nadler JL. Islet inflammation: a unifying target for diabetes treatment? Trends Endocrinol Metab. 2013;24(7):351–360. doi: 10.1016/j.tem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waguri T, Goda T, Kasezawa N, Yamakawa-Kobayashi K. The combined effects of genetic variations in the GPR120 gene and dietary fat intake on obesity risk. Biomed Res. 2013;34(2):69–74. doi: 10.2220/biomedres.34.69. [DOI] [PubMed] [Google Scholar]
- 90.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42(2):128–138. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 91.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 92.Cao H, Gerhold K, Mayers JR, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kabayama K, Sato T, Kitamura F, et al. TNFalpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology. 2005;15(1):21–29. doi: 10.1093/glycob/cwh135. [DOI] [PubMed] [Google Scholar]
- 94.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245(6):621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 95.Gorgens SW, Eckardt K, Elsen M, Tennagels N, Eckel J. Chitinase-3-like protein 1 protects skeletal muscle from TNFalpha-induced inflammation and insulin resistance. Biochem J. 2014;459(3):479–488. doi: 10.1042/BJ20131151. [DOI] [PubMed] [Google Scholar]
- 96.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148(1):241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases – did Warburg miss inflammation? Nat Immunol. 2012;13(4):352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia. 2001;44(7):914–922. doi: 10.1007/s001250100548. [DOI] [PubMed] [Google Scholar]
- 99.Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54(Suppl 2):S32–S39. doi: 10.2337/diabetes.54.suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- 100.Greenbaum CJ. Insulin resistance in type 1 diabetes. Diabetes Metab Res Rev. 2002;18(3):192–200. doi: 10.1002/dmrr.291. [DOI] [PubMed] [Google Scholar]
- 101.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52(1):124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 102.Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4(5):391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Lavine JA, Raess PW, Stapleton DS, et al. Cholecystokinin is up-regulated in obese mouse islets and expands beta-cell mass by increasing beta-cell survival. Endocrinology. 2010;151(8):3577–3588. doi: 10.1210/en.2010-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lavine JA, Raess PW, Davis DB, et al. Overexpression of pre-pro-cholecystokinin stimulates beta-cell proliferation in mouse and human islets with retention of islet function. Mol Endocrinol. 2008;22(12):2716–2728. doi: 10.1210/me.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Kim SJ, Winter K, Nian C, et al. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem. 2005;280(23):22297–22307. doi: 10.1074/jbc.M500540200. [DOI] [PubMed] [Google Scholar]
- 106.Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic alpha-cells: is this a paracrine mechanism enabling GLP-1 to act on beta-cells? J Endocrinol. 2011;211(1):99–106. doi: 10.1530/JOE-11-0094. [DOI] [PubMed] [Google Scholar]
- 107.Ashcroft FM, Proks P, Smith PA, et al. Stimulus-secretion coupling in pancreatic beta cells. J Cell Biochem. 1994;55(Suppl):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- 108.Luan B, Zhao J, Wu H, et al. Deficiency of a beta- arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457(7233):1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- 109.Zhang M, Zhu Y, Mu K, et al. Loss of beta-arrestin2 mediates pancreatic-islet dysfunction in mice. Biochem Biophys Res Commun. 2013;435(3):345–349. doi: 10.1016/j.bbrc.2013.04.079. [DOI] [PubMed] [Google Scholar]
- 110.Bellenger J, Bellenger S, Bataille A, et al. High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: inflammatory pathway inhibition. Diabetes. 2011;60(4):1090–1099. doi: 10.2337/db10-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56(9):2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 112.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110(6):851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parazzoli S, Harmon JS, Vallerie SN, et al. Cyclooxygenase-2, not microsomal prostaglandin E synthase-1, is the mechanism for interleukin-1beta-induced prostaglandin E2 production and inhibition of insulin secretion in pancreatic islets. J Biol Chem. 2012;287(38):32246–32253. doi: 10.1074/jbc.M112.364612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tran PO, Gleason CE, Robertson RP. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes. 2002;51(6):1772–1778. doi: 10.2337/diabetes.51.6.1772. [DOI] [PubMed] [Google Scholar]