Abstract
Background
Breast cancer-related lymphedema (BCRL) is a feared complication for breast cancer patients who have undergone axillary surgery. Although clinical risk factors for BCRL are defined; data are sparse regarding common exposures that might induce incident arm swelling. The goal of this study was to quantify the association between common exposures thought to be potential risk factors, and the occurrence of incident arm swelling, among breast cancer survivors with or at-risk for BCRL.
Methods
This is a prospective sub-analysis of the PAL trial, a randomized controlled trial of 295 breast cancer survivors. Participants reported their exposure to 30 different potential risk-factors at three month intervals for one year. Incident arm swelling was defined as a ≥5% increase in inter-limb water volume difference between two consecutive time points.
Results
Twenty-seven participants (9%) experienced incident arm swelling and 268 patients (91%) did not. Sauna use was the only exposure that was significantly predictive of incident arm swelling (p=0.05). Non-White and non-Black participants had a significantly increased risk for experiencing incident arm swelling (p=0.005) for both comparisons.
Conclusions
In our patient cohort, many common exposures that have been reported to be risk factors did not prove to have a significant predictive relationship for incident arm swelling. This study supports the recommendation that breast cancer patients who have had axillary surgery should avoid sauna use. The results do not confirm the need for other restrictions that may interfere with the quality of life in women with breast cancer.
INTRODUCTION
Lymphedema is a chronic condition that results when inadequate lymphatic drainage leads to the accumulation of protein-rich fluid in the interstitial space. Lymphedema is characterized by progressive swelling that has an adverse effect on quality of life and increases utilization of healthcare resources [1]. All breast cancer patients who undergo surgical treatment in the axilla (axillary lymph node dissection (ALND) and sentinel lymph node biopsy (SLNB)) are at risk for the development of breast cancer related lymphedema (BCRL). The incidence of BCRL varies in the literature from 7% to 30% depending on the definition of BCRL used, the type of surgery performed and the length of follow-up [2-4].
Numerous studies have been published in an attempt to determine both risk factors for developing BCRL [4-8] and preventive strategies [9-11] to minimize the development of BCRL. Clinically-related risk factors, such as the type of surgery and adjuvant treatment, are the most commonly studied causes of BCRL because they are easiest to ascertain and are not subject to patient interpretation. Many of the studies that have tried to elucidate prevention strategies are small, retrospective, single-institution reports, making it difficult to draw definitive conclusions. No definitive associations have been established between potentially high-risk, modifiable behaviors and lymphedema onset or worsening. This deficit is problematic because, confronted with an information void, non-evidence based recommendations proliferate and patients may engage in needless and potentially harmful avoidance behaviors [11]. One such example is the recommendation to avoid weight lifting exercise. This recommendation has been challenged [12], now that weight lifting among breast cancer survivors has been demonstrated to be safe, and to reduce the severity of self-reported lymphedema symptoms among breast cancer survivors [10].
The primary goal of the current study was to quantify the association between common exposures and the occurrence of incident arm swelling. The secondary goals were to estimate the frequency of common exposures hypothesized to be associated with incident arm swelling as well as the frequency of incident arm swelling in a one year period in our patient cohort.
METHODS
Study participants
This study is a prospective sub-analysis of the physical activity and lymphedema (PAL) trial [13], a randomized controlled trial of 295 breast cancer survivors who were at risk for developing BCRL (n=154); [9], or who had stable BCRL (n=141); [10]. Participants with stable BCRL were 1–15 years post breast cancer diagnosis, and participants at-risk for BCRL were 1–5 years post breast cancer diagnosis. Among participants with BCRL, for the purpose of the study, BCRL was defined as ≥10% interlimb difference, or meeting any of the Common Toxicity Criteria version 3.0 for BCRL (swelling, obscuration, pitting) [14], or a prior clinical diagnosis of BCRL [13]. In addition, participants with BCRL were ineligible if they had any of the following: 1) intensive BCRL therapy within the past three months; 2) a recorded 10% change in volume or circumference of the arm that lasted for ≥7 days in the past three months; 3) more than one BCRL-related infection that required antibiotics in the past three months; 4) a BCRL exacerbation that resulted in a change in activities of daily living within the past three months [13]. The patient cohort was recruited between October 2005 and February 2007. The University of Pennsylvania Institutional Review Board approved the study protocol, and informed consent was obtained from all participants.
Participants in the PAL trial were randomized to a progressive weight lifting intervention versus no exercise. The primary aim of the PAL trial was to assess change in BCRL outcomes resulting from twice-weekly weight lifting among breast cancer survivors [13]. The primary outcomes [9, 10] and detailed methods of the PAL trial [13] have been reported previously. All participants were required to attend a one-hour educational lecture entitled the ‘lymphedema education session’ that was based on material from the National Lymphedema Network (NLN) [15-18]. Demographic characteristics, cancer treatment and medication history were obtained by self-report. Cancer staging was taken from the state cancer registries, surgical pathology reports, or self-report. The number of lymph nodes removed was obtained from surgical pathology reports. All participants with BCRL in the PAL trial were provided with a compression sleeve to wear throughout the study. Compression sleeve adherence was assessed using a self-report measure with four categories, <25%, 25-49%, 50-74%, or ≥75% adherence. This questionnaire was completed at baseline, 3-, 6- and 12-months and averaged over one year.
Incident arm swelling Questionnaire
Participants were asked to report their exposure to 30 different potential risk-factors. The list of exposures was compiled by a physical medicine and rehabilitation physician expert in lymphedema. Though non-validated, the questionnaire’s strong face validity was attested during review by therapists treating a high volume of patients with lymphedema. The questionnaire was administered at the time of entry into the study, 3-, 6-, and 12-months. Patients were queried about exposure to any of the risk-factors encountered in the time interval since the last study visit.
Objective Measures of Arm Volume
Participants completed water volume displacement measures [16] to assess arm volume at baseline, 3-, 6-, and 12-months by blinded study staff. Water volume is accurate by raters to 1% [19]. For the purpose of this analysis, incident arm swelling was diagnosed if there was an inter-limb volume of difference of ≥5% accompanied by a ≥5% increase in the inter-limb difference when compared to the last measurement time point. This method of calculation differs from previous analyses in that we compared arm volume to the most recent previous arm volume rather than to baseline arm volume. We used the modified calculation of arm volume to isolate the change in arm volume percentage over each time interval to align with the data captured in the exposure questionnaire. Therefore, the frequency of incident arm swelling reported in this paper is different from the primary outcome analysis papers [9, 10].
Statistical Analysis
Descriptive statistics reported for baseline variables include rates for categorical variables and means or medians and standard deviations for continuous variables. Baseline characteristics of women who did and did not experience incident arm swelling were compared using nonparametric methods that included Fishers exact test for categorical variables and the Kruskal-Wallis test for continuous variables. Mixed models were utilized for the longitudinal analysis of data to estimate the odds ratios of experiencing incident arm swelling and 95% confidence intervals over the three time points of interest. Model fit was assessed using the Akaike and Bayesian information criterion methods. Sensitivity analysis was conducted using generalized estimating equations with exchangeable and autoregressive correlation matrices; the study findings and conclusions did not differ among methods. Additional subgroup analysis was conducted between breast cancer survivors with BCRL and those at risk, and the results did not differ between groups. Two-sided statistical significance was p ≤ 0.05. All statistical analyses were performed with Stata SE 12.0 software (College Station, TX).
RESULTS
Table 1 summarizes baseline demographic characteristics of all 295 participants. Participants were aged 36-80 years at baseline (mean 56 years). Table 2 summarizes baseline clinical characteristics of all 295 participants. The number of lymph nodes removed ranged from 1-38 (mean 11.7). Time since breast cancer diagnosis ranged from 11-138 months (mean 62 months). Baseline BMI ranged from 17.7-48.2 kg/m2 (mean 29 kg/m2). One hundred and ninety one (65%) of the participants reported their race as white, 90 (30%) as black, and 14 (5%) as other. Five percent of participants (n=14) reported previous weight lifting experience, however no participants reported engaging in weight lifting exercise in the year prior to study entry. Participation in self-reported physical activity did not change between baseline and 12-months [9, 10]. Among the 141 participants with BCRL, 23 (16%), 35 (25%), 33 (23%), and 50 (35%) participants reported an average compliance of <25%, 25-49%, 50-74%, and ≥75% with their compression garments, respectively. Adherence to wearing the compression garment was not associated with incident arm swelling (p=0.28)
TABLE 1.
Baseline demographic characteristics of the study participantsa
| Characteristic | Total Sample (n=295) |
Incident Arm Swelling (n=27) |
No Incident Arm Swelling (n=268) |
P |
|---|---|---|---|---|
| Age — yr | 56.1±8.7 | 54.5±6.2 | 56.4±9.0 | 0.22 |
| Education — no. (%) | 0.20 | |||
| High school or less | 47 (16%) | 6 (22%) | 41 (15%) | |
| Some college | 101 (34%) | 12 (44%) | 89 (33%) | |
| College degree or more | 147 (50%) | 10 (37%) | 137 (51%) | |
| Self-reported race — no. (%) | 0.01 | |||
| White | 191 (65%) | 15 (56%) | 176 (66%) | |
| Black | 90 (30%) | 7 (26%) | 83 (31%) | |
| Other | 14 (5%) | 5 (18%) | 9 (3%) | |
| Occupation — no. (%) | 0.26 | |||
| Professional | 118 (40%) | 12 (44%) | 106 (40%) | |
| Clerical or service | 55 (19%) | 9 (33%) | 46 (17%) | |
| Homemaker, student, or unemployed | 26 (9%) | 1 (4%) | 25 (9%) | |
| Other or unknown | 27 (9%) | 1 (4%) | 26 (10%) | |
| Retired | 69 (23%) | 4 (15%) | 65 (24%) |
Plus-minus values are means ±SD. Percentage may not sum to 100% due to rounding error
TABLE 2.
Baseline clinical characteristics of study participantsa
| Characteristic | Total Sample (n=295) |
Incident Arm Swelling (n=27) |
No Incident Arm Swelling (n=268) |
P |
|---|---|---|---|---|
| BMI — kg/m2 | 29.2±6.1 | 29.0±5.9 | 29.2±6.1 | 0.74 |
| Cancer stage — no. (%) | 0.55 | |||
| Ductal in situ | 1 (1%) | 1 (4%) | 0 (0%) | |
| 1 | 143 (48%) | 6 (22%) | 137 (51%) | |
| 2 | 15 (5%) | 8 (30%) | 7 (3%) | |
| 3 | 97 (33%) | 1 (4%) | 96 (36%) | |
| Unknown | 39 (13%) | 11 (41%) | 28 (10%) | |
| No. of nodes removed | 11.7±7.9 | 9.3±8.3 | 11.9±8.0 | 0.05 |
| Chemotherapy — no. (%) | 220 (76%) | 22 (81%) | 198 (74%) | 0.58 |
| Radiation — no. (%) | 229 (78%) | 16 (59%) | 213 (79%) | 0.005 |
| Current receipt of drugs — no. % | ||||
| Tamoxifen | 69 (23%) | 3 (11%) | 66 (25%) | 0.27 |
| Aromatase inhibitor | 2 (<1%) | 0 (0%) | 2 (<1%) | 0.80 |
| Months since cancer diagnosis | 61.6±29.4 | 54.6±31.1 | 61.2±40.1 | 0.39 |
| Lymphedema Diagnosis — no. (%) | 0.09 | |||
| With lymphedema | 141 (48%) | 10 (37%) | 131 (49%) | |
| At-Risk for lymphedema | 154 (52%) | 17 (63%) | 137 (51%) | |
| Common Toxicity Criteria lymphedema grade – no. (%)b |
0.14 | |||
| 0 | 12 (9%) | 3 (30%) | 9 (7%) | |
| 1 | 30 (21%) | 2 (20%) | 28 (21%) | |
| 2 | 58 (41%) | 3 (30%) | 55 (42%) | |
| 3 | 41 (29%) | 2 (20%) | 39 (30%) | |
| Hypertension | 86 (29%) | 9 (33%) | 77 (29%) | 0.66 |
| Diabetes | 30 (10%) | 3 (11%) | 27 (10%) | 0.74 |
Plus-minus values are means ±SD. Percentage may not sum to 100% due to rounding error
Among the 141 women with lymphedema.
Over the 12 month study period, 27 (9%) participants had incident arm swelling as defined for this paper. No participant experienced more than one episode of incident arm swelling while in the study. Participants who experienced incident arm swelling differed statistically from participants without incident arm swelling with respect to race (p=0.01), number of lymph nodes removed (p=0.05) and treatment with radiation (p=0.005) (Tables 1 and 2).
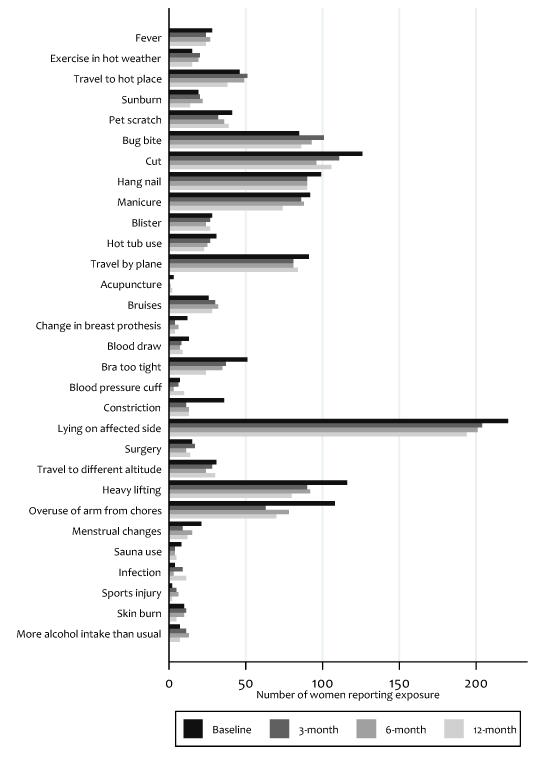
Figure 1 and Table 3 summarize the frequency of the 30 exposures for the entire cohort. The median number of exposures for each participant at each time point was 4. The prevalence of exposures varied from <1% for acupuncture to >75% for lying on the affected arm.
Figure 1.
Lymphedema risk-factor exposures over 12-months for all participants Uploaded separately.
TABLE 3.
Lymphedema risk-factor exposures over 12-months, all participants — no (%)
| Exposure | 3-month (n=271) |
6-month (n=266) |
12-month (n=253) |
OR (95% CI) | P |
|---|---|---|---|---|---|
| Fever | 24 (9%) | 27 (10%) | 24 (10%) | 1.22 (0.36–4.22) | 0.74 |
| Vigorous exercise in hot weather | 20 (7%) | 19 (7%) | 15 (6%) | 1.00 (0.11–4.17) | 0.99 |
| Travel to hot/humid place | 51 (19%) | 49 (19%) | 38 (15%) | 1.09 (0.40–2.96) | 0.87 |
| Sunburn | 20 (7%) | 22 (8%) | 14 (6%) | 1.76 (0.49–6.26) | 0.38 |
| Pet scratch | 32 (12%) | 36 (14%) | 39 (16%) | 1.49 (0.54–4.11) | 0.44 |
| Bug Bite | 101 (37%) | 93 (35%) | 86 (34%) | 1.09 (0.49–2.45) | 0.81 |
| Cut | 111 (41%) | 96 (36%) | 106 (42%) | 1.99 (0.91–4.35) | 0.08 |
| Hang nail | 90 (34%) | 90 (34%) | 90 (36%) | 0.66 (0.27–1.57) | 0.34 |
| Manicure | 86 (32%) | 88 (33%) | 74 (29%) | 1.33 (0.59–3.01) | 0.49 |
| Blister | 27 (10%) | 24 (9%) | 27 (11%) | 0.77 (0.18–3.36) | 0.73 |
| Hot tub use | 27 (10%) | 25 (9%) | 23 (9%) | 0.76 (0.17–3.31) | 0.71 |
| Travel by airplane | 81 (30%) | 81 (30%) | 84 (33%) | 0.62 (0.24–1.57) | 0.31 |
| Acupuncture | 0 (0%) | 1 (1%) | 2 (1%) | 5.16 (0.11–47.98) | 0.11 |
| Bruise | 30 (11%) | 32 (12%) | 28 (11%) | 1.98 (0.69–5.67) | 0.20 |
| Change of breast prosthesis | 4 (1%) | 6 (2%) | 4 (2%) | 2.10 (0.26–17.00) | 0.49 |
| Blood draw | 8 (3%) | 7 (3%) | 9 (4%) | 1.13 (0.15–8.74) | 0.91 |
| Bra too tight | 37 (14%) | 35 (13%) | 24 (10%) | 1.26 (0.42–3.76) | 0.67 |
| Blood pressure cuff | 6 (2%) | 3 (1%) | 10 (4%) | 1.47 (0.18–11.77) | 0.72 |
| Constriction | 11 (4%) | 13 (5%) | 13 (5%) | 0.78 (0.10–5.97) | 0.81 |
| Lying on affected arm | 204 (75%) | 201 (76%) | 194 (78%) | 0.52 (0.23–1.17) | 0.11 |
| Surgery | 17 (6%) | 11 (4%) | 14 (6%) | 1.42 (0.32–6.31) | 0.65 |
| Travel to different altitude | 28 (10%) | 24 (9%) | 30 (12%) | 0.51 (0.05–2.06) | 0.35 |
| Heavy lifting | 90 (33%) | 92 (35%) | 80 (32%) | 0.56 (0.22–1.41) | 0.22 |
| Overuse from chores | 63 (23%) | 78 (29%) | 70 (28%) | 0.47 (0.16–1.38) | 0.17 |
| Menstrual changes | 9 (3%) | 15 (6%) | 12 (5%) | 1.68 (0.37–7.49) | 0.50 |
| Sauna use | 4 (1%) | 4 (2%) | 5 (2%) | 5.77 (1.00–33.82) | 0.05 |
| Infection | 9 (3%) | 3 (1%) | 11 (4%) | 1.35 (0.17–10.60) | 0.78 |
| Sports injury | 5 (2%) | 6 (2%) | 2 (1%) | 1.82 (0.35–15.12) | 0.56 |
| Skin burn | 11 (4%) | 10 (4%) | 5 (2%) | 2.52 (0.53–11.93) | 0.24 |
| More alcohol intake than usual | 11 (4%) | 13 (5%) | 7 (3%) | 1.37 (0.15–5.79) | 0.67 |
|
| |||||
| Median # of exposures [IQR] | 4 [2–6] | 4 [2–6] | 4 [1–6] | 0.98 (0.85–1.12) | 0.73 |
| Range | 0–18 | 0–15 | 0–17 | — | — |
In univariable mixed model analysis, sauna use (n=13, OR= 5.77, CI= 1.00-33.82, p=0.05) was the only exposure associated with incident arm swelling that reached statistical significance (Table 3). In multivariable analysis, sauna use remained significantly associated with incident arm swelling (OR=6.67, 95% CI= 1.36-32.56, p=0.01); (Table 4). There was a significant interaction between sauna use and having a cut on the affected arm that was associated with an increased risk of incident arm swelling (OR=18.74, 95% CI= 1.41-249.48, p=0.027).
TABLE 4.
Risk prediction model for lymphedema exacerbation
| Exposure | Univariate Analysis | Multivariable Analysisa,b | ||
|---|---|---|---|---|
|
|
||||
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Sauna use | 5.77 (1.00–33.82) | 0.05 | 6.67 (1.36–32.56) | 0.01 |
| Cut on affected arm | 1.99 (0.91–4.35) | 0.08 | 1.98 (0.89–4.38) | 0.09 |
| Race | ||||
| White | 1 — Referent | — | 1 — Referent | — |
| Black | 1.00 (0.40–2.50) | 0.99 | 0.92 (0.36–2.32) | 0.89 |
| Other | 5.74 (1.71–19.31) | 0.005 | 5.47 (1.59–18.89) | 0.007 |
Adjusted for number of lymph nodes removed and receipt of radiation treatment. Fit statistics for multivariable model: AIC=225.71, BIC=267.12.
In separate multivariable analysis we noted an interaction with sauna use and having a cut on the affected arm [OR=18.74; 95%: 1.41–249.48), p=0.027]; other predictors in the model were statistically significant; AIC=227.56, BIC=273.57.
In post-hoc, exploratory analysis, a pattern was identified relating to the risk of incident arm swelling and race. Fourteen participants in the study were classified as other race. Five of the 14 (36%) experienced incident arm swelling; this represents a 5.7-fold increase in risk for incident arm swelling compared to White and Black participants. Three of the 6 (50%) Asian participants in the study had incident arm swelling, as did (100%) of the Pacific Islander participants.
DISCUSSION
This study evaluated the relationship between common exposures and incident arm swelling in women who have undergone axillary surgery for the treatment of breast cancer. Data pertaining to common exposures that may be related to BCRL are limited and inconclusive. This makes it difficult to give breast cancer patients definitive recommendations for risk reduction strategies. Of the 30 exposures asked about in this study, only sauna use proved to be a significant predictive factor for incident arm swelling. Multiple other exposures that are commonly considered risk-factors and are part of the NLN guidelines [15-18] for risk reduction were not associated with incident arm swelling in our study cohort.
The most consistently reported clinical risk factors found to be associated with BCRL are the number of nodes removed during surgery [1, 4, 5, 20-22], the use of adjuvant chemotherapy and radiation therapy [1, 20, 22], pathologic nodal status [5, 22], and the body mass index (BMI) of the patient [4-6, 8, 20]. Although this study was not designed to evaluate clinical risk factors, we did find that the number of nodes removed (p=0.05) and use of radiation (p=0.005) were significantly associated with incident arm swelling. In our study, BMI was not a significant predictor for incident arm swelling (p=0.74). While clinicians treating women with breast cancer do consider these clinical risk factors when formulating treatment plans, they are usually not modifiable.
Demographic-related risk factors are rarely reported to be associated with increased risk of BCRL. In post-hoc analysis, we found a relationship between Asian race and increased risk for incident arm swelling. Data are sparse regarding to the association between race and BCRL. In a prospective cohort study of breast cancer patients, Kwan et al reported an increased risk of BCRL in Black women when compared to other races [7]. In a study that focused on risk factors for lymphedema in Black and White women, Meeske et al did not find that race was associated with increased risk for BCRL [8]. The authors did find that BCRL was associated with modifiable factors such as hypertension and obesity. The possible association between demographic variables such as race and the development of a BCRL flare warrants further investigation.
Most lifestyle-related risk-factors for BCRL are elusive and hard to relate directly to the diagnosis of incident arm swelling. In this prospective study, we were able to obtain patient data pertaining to potential exposures at multiple time points and then to correlate this information precisely with documented arm measurements. Sauna use was the only exposure that was significantly associated with incident arm swelling in our patient cohort. In the current recommendations for the prevention of lymphedema, both the NLN [17] and the Lymphedema Framework [23] advise patients to avoid prolonged exposure to heat in hot tubs and saunas. There is little evidence-based literature for these recommendations as they are mostly based on clinical experience and the concept that heat may increase blood flow to the arm and subsequently increase the load on the lymphatic system [24]. In a 2011 review of preventive measures for lymphedema, Cemal et al. [11] concluded that there are not sufficient data to recommend avoidance of extreme temperatures [25-27]. Although only a small number of the participants in our study reported sauna use (n=13), the significant association found between sauna use and incident arm swelling is worth acknowledgment and future investigation. We also found a significant interaction between sauna use and having a cut on the affected arm that was associated with an increased risk of incident arm swelling. We recommend that women with or at risk for developing BCRL avoid sauna use.
The null findings with regard to potential exposures and BCRL are as relevant as the positive associations. The participants in this study were asked about 30 different exposures that have all been hypothesized to be associated with BCRL. The data to support the avoidance of ipsilateral needle sticks are conflicting. In a 2005 prospective observational study of 188 breast cancer patients, Clark reported a significant relationship between needle sticks and BCRL and concluded that needle sticks significantly increased the risk of developing BCRL; 44% of patients who had needle sticks developed lymphedema compared to 18% of those without needle sticks [4]. Of note, the authors did not comment on the timing between needle stick and the development of BCRL in their cohort. In contrast, in a 2010 self-reported questionnaire study, 88 patients reported having had intravenous procedures in the affected limb and only 4 patients (4%) developed lymphedema presumed to be in relation to venipuncture [28]. In our cohort, 17 individual participants reported having venipuncture. One participant reported three, five participants reported two, and 11 reported one, venipuncture procedure while in the one year study, respectively. Our study did not support an association between needle sticks and the development of incident arm swelling; and we therefore cannot conclude that venipuncture increases risk for the development of incident arm swelling.
An infection in the ipsilateral extremity is another patient-related exposure that has been theorized to be associated with BCRL. In a retrospective study of 923 breast cancer patients treated with ALND and followed for 20 years, Petrek et al reported that arm infection was associated with the onset and clinical course of lymphedema [29]. In addition, a 2006 case-control study reported a significant relationship between arm infection and the diagnosis of BCRL; 2% of the controls and 37% of patients with BCRL reportedly had an arm infection [6]. In contrast, arm infection was not associated with incident arm swelling in a study by Querci della Rovere et al [30], and in our study.
Recommendations to avoid limb constriction are pervasive, but are based on limited scientific evidence. Those in support of this risk hypothesize that blood pressure monitoring can increase pressure in the limb resulting in increased lymph production, fibrosis and stenosis of the lymphatic vessels [31, 32]. The results of our study do not support the recommendation to avoid blood pressure measurement. Other groups have also challenged this recommendation, stating that limb compression is often used in the management of lymphedema [33].
This study is unique in that it is a prospective analysis of a large cohort of breast cancer patients in whom exposure to various potential BCRL triggers was monitored. Incident arm swelling was diagnosed in a consistent and validated manner that did not rely on patient report of arm symptoms. The study does have weaknesses. The major limitation of this study is the lack of information relating to the type of radiation and surgery the participants received as treatment for their breast cancer. The patient cohort is not necessarily generalizable to all breast cancer patients because the participants were physically and psychologically able to tolerate a one-year exercise program. In addition, all participants received a custom-fitted compression garment to wear and attended a lymphedema education session. The increased resources may have increased BCRL awareness and management relative to patients who were not in this trial. The results of the questionnaires are subject to recall bias, although recall bias has been shown to be an acceptable risk in short-term studies [34]. Because of the rarity of some of the events and participant pre-conceived ideas of risk factors to avoid, some of the exposures queried about in this study were experienced by small numbers of the participants, and subsequent limited statistical power to detect associations between the exposures of interest and incident arm swelling. Randomized controlled trials designed to examine many of these exposures are not possible, and this study provides useful insights into the effects of these exposures.
Future work with long-term follow up (i.e., ≥3 years) will be helpful to clarify the common exposures associated with the development of BCRL. This study supports the recommendation that breast cancer patients who have had axillary surgery should avoid sauna use. The results do not confirm the need for other restrictions that may interfere with the quality of life in women with breast cancer.
REFERENCES
- 1.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27:2007–14. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 2.Francis WP, Abghari P, Du W, Rymal C, Suna M, Kosir MA. Improving surgical outcomes: standardizing the reporting of incidence and severity of acute lymphedema after sentinel lymph node biopsy and axillary lymph node dissection. Am J Surg. 2006;192:636–9. doi: 10.1016/j.amjsurg.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM. 2005;98:343–348. doi: 10.1093/qjmed/hci053. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130:981–991. doi: 10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soran A, D’Angelo G, Begovic M, et al. Breast cancer-related lymphedema--what are the significant predictors and how they affect the severity of lymphedema? Breast J. 2006;12:536–543. doi: 10.1111/j.1524-4741.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Kwan ML, Darbinian J, Schmitz KH, Citron R, Partee P, Kutner SE, Kushi LH. Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Arch Surg. 2010;145:1055–1063. doi: 10.1001/archsurg.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeske KA, Sullivan-Halley J, Smith AW, McTiernan A, Baumgartner KB, Harlan LC, Bernstein L. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat. 2009;113:383–391. doi: 10.1007/s10549-008-9940-5. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304:2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 11.Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg. 2011;213:543–551. doi: 10.1016/j.jamcollsurg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz KH. Balancing lymphedema risk: exercise versus deconditioning for breast cancer survivors. Exerc Sport Sci Rev. 2010;38:17–24. doi: 10.1097/JES.0b013e3181c5cd5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz KH, Troxel AB, Cheville A, et al. Physical Activity and Lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials. 2009;30:233–245. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheville AL, McGarvey CL, Petrek JA, Russo SA, Thiadens SR, Taylor ME. The grading of lymphedema in oncology clinical trials. Semin Radiat Oncol. 2003;13:214–225. doi: 10.1016/S1053-4296(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 15.Position Statement of the National Lymphedema Network [accessed July 1, 2012];Topic: Exercise. 2011 May; Available: http://www.lymphnet.org/pdfDocs/nlnexercise.pdf.
- 16.Position State of the National Lymphedema Network [accessed July 1, 2012];Topic: Training of Lymphedema Therapists. 2011 Available: http://www.lymphnet.org/pdfDocs/nlntraining.pdf.
- 17.Position Statement of the National Lymphedema Network [accessed July 1, 2012];Topic: Lymphedema Risk Reduction Practices. 2011 Available: http://www.lymphnet.org/pdfDocs/nlnriskreduction.pdf.
- 18.Position Statement of the National Lymphedema Network [accessed July 1, 2012];Topic: The Diagnosis and Treatment of Lymphedema. 2011 Available: http://www.lymphnet.org/pdfDocs/nlntreatment.pdf.
- 19.Waylett-Rendell J. A study of the accuracy of a commercially available volumeter. J Hand Ther. 1991;4:3. [Google Scholar]
- 20.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16:775–782. doi: 10.1158/1055-9965.EPI-06-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yen TW, Fan X, Sparapani R, Laud PW, Walker AP, Nattinger AB. A contemporary, population-based study of lymphedema risk factors in older women with breast cancer. Ann Surg Oncol. 2009;16:979–988. doi: 10.1245/s10434-009-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–1972. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 23.Lymphoedema Framework . Best Practice for the Management of Lymphoedema. MEP Ltd; 2006. [Google Scholar]
- 24.Dell DD, Doll C. Caring for a patient with lymphedema. Nursing. 2006;36:49–51. doi: 10.1097/00152193-200606000-00041. [DOI] [PubMed] [Google Scholar]
- 25.Gan JL, Li SL, Cai RX, Chang TS. Microwave heating in the management of postmastectomy upper limb lymphedema. Ann Plast Surg. 1996;36:576–80. doi: 10.1097/00000637-199606000-00003. discussion 580-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu NF, Olszewski W. The influence of local hyperthermia on lymphedema and lymphedematous skin of the human leg. Lymphology. 1993;26:28–37. [PubMed] [Google Scholar]
- 27.Hettrick H, Nof L, Ward S, Ecthernach J. Incidence and prevalence of lymphedema in patients following burn injury: a five-year retrospective and three-month prospective study. Lymphat Res Biol. 2004;2:11–24. doi: 10.1089/1539685041690472. [DOI] [PubMed] [Google Scholar]
- 28.Winge C, Mattiasson AC, Schultz I. After axillary surgery for breast cancer--is it safe to take blood samples or give intravenous infusions? J Clin Nurs. 2010;19:1270–1274. doi: 10.1111/j.1365-2702.2009.03153.x. [DOI] [PubMed] [Google Scholar]
- 29.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Querci della Rovere G, Ahmad I, Singh P, Ashley S, Daniels IR, Mortimer P. An audit of the incidence of arm lymphoedema after prophylactic level I/II axillary dissection without division of the pectoralis minor muscle. Ann R Coll Surg Engl. 2003;85:158–161. doi: 10.1308/003588403321661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loudon L, Petrek J. Lymphedema in women treated for breast cancer. Cancer Pract. 2000;8:65–71. doi: 10.1046/j.1523-5394.2000.82004.x. [DOI] [PubMed] [Google Scholar]
- 32.Petrek JA, Pressman PI, Smith RA. Lymphedema: current issues in research and management. CA Cancer J Clin. 2000;50:292–307. doi: 10.3322/canjclin.50.5.292. quiz 308-11. [DOI] [PubMed] [Google Scholar]
- 33.Greene AK, Borud L, Slavin SA. Blood pressure monitoring and venipuncture in the lymphedematous extremity. Plast Reconstr Surg. 2005;116:2058–2059. doi: 10.1097/01.prs.0000192621.97804.d4. [DOI] [PubMed] [Google Scholar]
- 34.Middel B, Goudriaan H, de Greef M, Stewart R, van Sonderen E, Bouma J, de Jongste M. Recall bias did not affect perceived magnitude of change in health-related functional status. J Clin Epidemiol. 2006;59:503–511. doi: 10.1016/j.jclinepi.2005.08.018. [DOI] [PubMed] [Google Scholar]