Abstract
Food-borne Campylobacter jejuni (Cj) is an important cause of enteritis. We showed that C57BL/6 and congenic interleukin (IL)-10−/−mice serve as models of Cj colonization and enteritis, respectively. Thus, C57BL/6 mice are resistant to Cj induced disease. Because dendritic cells (DCs) are central to regulating adaptive immune responses, we investigated the interaction of Cj with murine bone marrow-derived DCs (BM-DCs) to assess bacterial killing, DC activation, and the ability of Cj-infected BM-DCs to stimulate Campylobacter-specific T cell responses in vitro. BM-DCs challenged with Cj efficiently internalized and killed Cj 11168 and significantly upregulated surface MHC-II, CD40, CD80 and CD86 demonstrating a mature phenotype. Infected BM-DCs secreted significant amounts of tumor necrosis factor-α (TNF-α), IL-6 and IL-12p70. Formalin-killed Cj also induced maturation of BM-DCs with similar cytokine production but at a significantly lower magnitude than live bacteria. Maximal activation of murine BM-DCs required internalization of Cj; attachment alone was not sufficient to elicit significant responses. Also, various strains of Cj elicited different magnitudes of cytokine production from BM-DCs. Finally, in a coculture system, Cj-infected BM-DCs induced high level interferon-γ (INF-γ) production from CD4+T cells indicating Th1 polarization. Thus, DCs from resistant C57BL/6 mice initiate T cell responses against Cj.
Keywords: Campylobacter jejuni, Dendritic cells, Maturation, IL-12, IFN-γ, TH1 Response
1. Introduction
Campylobacter jejuni (Cj) is an exceedingly common bacterial agent of enteritis worldwide. Campylobacteriosis usually presents as self-limiting intestinal illness with the clinical spectrum ranging from mild watery to severe bloody diarrhea. However, persistent and systemic illnesses can develop in immunocompromised patients [1].
Cj invades the intestinal mucosa [2,3] and resides inside intestinal epithelial cells (IEC) [3] and in the lamina propria causing pathology predominantly in colon [3] and, in mice the cecum [2]. DCs densely populate the lamina propria, are in close proximity to the epithelium, and can form transepithelial dendrites to sample the lumen for microbes [4]. We observed Cj associated with mononuclear cells morphologically similar to DCs in the lamina propria and submucosa of infected C57BL/6 IL-10−/− mice with enteritis [2]. The strategic location of DCs in the subepithelial area and the invasive nature of Cj [2,3] together with our recent observations suggest that DCs in the lamina propria are likely candidates for capture and processing of Cj for antigen presentation. Furthermore, Johanesen and Dwinell demonstrated that infection of cultured IEC with Cj upregulates secretion of the DC chemokine CCL20, suggesting this as a mechanism of increased DC recruitment into the intestinal mucosa [5]. Therefore, it is crucial to understand DC–Cj interactions and the role of DCs in stimulating host defense against Cj.
Human monocyte-derived DCs were recently shown to undergo maturation and secrete proinflammatory cytokines subsequent to Cj 81–176 challenge in vitro [6]; but to our knowledge, the resultant antigen-presenting capacity of DCs for CD4+T cell polarization has not been investigated. We hypothesized that murine (BM-DCs) internalize Cj, which induces BM-DCs to undergo activation and initiate a Th1-type immune response. In this study, we investigated activation of BM-DCs by Cj, mechanisms mediating the activation and the ability of Cj-infected BM-DCs to stimulate T cell responses.
2. Materials and methods
2.1. Mice
C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, ME) were bred, maintained, and monitored as described [2], and used at 8–12 weeks of age. Animal protocols were approved by MSU Institutional Animal Care & Use Committee conforming to NIH guidelines.
2.2. Bacteria and inoculum preparation
Cj strains were grown on Bolton agar (BA) plates at 37 °C in 10% CO2. Bacterial growth was resuspended in R10.2 medium (RPMI 1640 Dutch modification [Sigma-Aldrich, St. Louis, MO] with 2 mm L-glutamine [Invitrogen, Grand Island, NY] and 10% fetal bovine serum [FBS; Invitrogen]) to an optical density of 0.12 at 560 nm (~2 × 108 colony forming units [CFU]/ml). Where needed, bacteria were inactivated by treatment with 2% formaldehyde in Hank’s Balanced Salt Solution (HBSS) for 30 min at 37 °C. CFU in the inocula and bacterial killing were confirmed by limiting dilution on BA plates.
2.3. Generation of BM-DCs
BM-DCs were generated as described with minor modifications [7–9]. Bone-marrow cells from femurs and tibiae were cultured in bacteriological-grade Petri-dishes in 10 ml (2.5 × 105 cells/ml) of R10 medium (R10.2 medium containing 50 μM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin [Invitrogen] and 20 ng/ml murine granulocyte-macrophage colony-stimulating factor [Peprotech, Rocky Hill, NJ]). Non-adherent cells in the culture were discarded on days 3 and 6. On day 3, 10 ml of fresh medium was added; on days 6 and 8, 10 ml of medium was exchanged for fresh medium. DCs were collected and used on day 9. The proportion of DCs in the culture was consistently >90% as determined by flow cytometric analysis of CD11c and MHC-II markers (data not shown).
2.4. Gentamicin killing assay
This assay was performed as described with minor modifications [10]. Bacteria diluted in R10.2 medium were added to 2 × 105 BM-DCs per well in a 24-well plate (BD Falcon, Bradford, MA) at multiplicity of infection (MOI) of 10 or 100 bacteria per BM-DC. After 1 h of incubation, non-phagocytosed bacteria were removed by washing the cells and resus-pending in R10 medium containing 250 μg/ml gentamicin but without penicillin and streptomycin. After 1 h, cells were washed and resuspended in R10 medium without antibiotics. Viable intracellular bacteria were enumerated at 2, 4 and 8 h post-infection (p.i.) by lysing the cells with 0.5 ml of 0.1% Triton X-100 in phosphate-buffered saline for 15 min and spreading serial dilutions of lysate on BA plates. Colonies were counted 72 h after incubation at 37 °C with 10% CO2.
2.5. Assessment of maturation and cytokine secretion by Cj-infected BM-DCs
BM-DCs (2 × 105 cells/ml) were treated with Cj (MOI 10:1 [in cytokine experiments only] or 100:1), R10.2 medium (negative control) or 0.1 μg/ml Salmonella enterica serovar Typhimurium lipopolysaccharide (LPS; positive control; Sigma-Aldrich). In some experiments, to inhibit internalization of Cj, BM-DCs were preincubated with cytochalasin D (1 μg/ml) and nocodazole (20 μM; Sigma-Aldrich) or DMSO (solvent control, 0.1%) for 1 h and treated as described above. In other experiments, BM-DCs were also treated with formalin-killed bacteria. One hour after infection, gentamicin (250 μg/ml) was added to all wells to kill extracellular bacteria. DCs were analyzed by flow cytometry at 24 h p.i. For cytokine analysis, supernatants were collected at 4, 24 and 48 h p.i. unless otherwise indicated.
2.6. Flow cytometry
Antibodies were from BD Pharmingen (San Diego, CA) unless otherwise noted, including R-PE-conjugated anti-CD11c (HL3), FITC-conjugated anti-MHC-II (I-Ab) (AF6-120.1), FITC-conjugated anti-CD40 (3/23), FITC-conjugated anti-CD80 (16-10A1), PE-Cy5-conjugated anti-CD80 (Biolegend, San Diego, CA), FITC-conjugated anti-CD86 (GL1), PE-Cy5-conjugated anti-CD86 (Biolegend) and FITC-conjugated anti-CD4 (RM4-5) along with corresponding isotype controls. Cells were incubated with Fc block (anti-mouse FcRII/III) for 10 min at 4 °C. Fluorochrome-conjugated antibodies were added to cells and incubated for 30 min at 4 °C. The cells were washed twice with HBSS containing 1% FBS and 0.1% sodium azide and analyzed with a FACS Vantage flow cytometer (BD Biosciences).
2.7. DC-CD4+T cell coculture
CD4+T cells were negatively selected from splenocytes using the BD™ IMag Mouse CD4 T Lymphocyte Enrichment Set-DM (BD Pharmingen) according to manufacturer’s instructions. The proportion of CD4+T cells was enriched to >90% as measured by flow cytometry (data not shown). BM-DCs in complete DMEM containing 25 mM HEPES, 10% FBS, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, and 1% MEM non-essential amino acids seeded in 24-well plates (105 cells/well) were treated with one of the following: Cj (MOI of 100:1), R10.2 medium, S. typhimurium LPS (0.1 μg/ml; inducer of IFN-γ producing Th1 cells), or zymosan (10 μg/ml; inducer of IL-4 production from Th cells; Sigma-Aldrich). One hour after infection, gentamicin (250 μg/ml) was added to all wells. At 24 h p.i., CD4+T cells in complete DMEM supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin were added to DCs at a DC-to-T cell ratio of 1:10. After coculturing for 72 h, supernatants were collected for measurement of IFN-γ, IL-4 and IL-10.
2.8. ELISA
Cytokines in culture supernatants were measured using Ready-SET-Go! ELISA Sets (eBioscience, San Diego, CA) according to manufacturer’s instructions. Assay detection limits (in pg/ml) were IL-12, 15; IL-10, 30; IL-4, 4; IL-6, 4; TNF-α, 8; IFN-γ, 0.8.
2.9. Statistical analysis
One-way or two-way analysis of variance followed by the Holm–Sidak correction for multiple comparisons was performed using SigmaStat 3.1 (Systat Software, San Jose, CA). P values <0.05 were considered significant.
3. Results
3.1. BM-DCs internalize and kill Cj
Within 2 h p.i., BM-DCs internalized Cj in a dose-dependent manner (Fig. 1A). Approximately 5 ± 0.8 × 103 and 7.38 ± 0.2 × 104 viable intracellular bacteria were recovered at MOIs of 10:1 and 100:1, respectively, 2 h p.i. (Fig. 1A), which decreased rapidly to 1 ± 0.18 × 103 and 1.3 ± 0.0076 × 104 for MOIs of 10:1 and 100:1, respectively, by 4 h p.i.; no viable bacteria were recovered at 8 h p.i. at either MOI. In contrast, CFU of Cj inoculated into R10 medium without antibiotics and DCs increased by several-fold at 8 h p.i. (Fig. 1B).
Fig. 1.
(A) Internalization and killing of Cj by BM-DCs. BM-DCs (2 × 105) in a 24-well plate were infected with Cj at MOIs of 10:1 (2.1 × 106 CFU; △) and 100:1 (1.91 × 107 CFU; ▲). After 1 h of infection, non-phagocytosed bacteria were removed or killed by washing followed by gentamicin treatment. Subsequently BM-DCs were lysed at 2, 4, and 8 h p.i. and lysates cultured on BA plates to enumerate viable intracellular bacteria. (B) Viability of Cj in R10.2 medium without BM-DCs. R10.2 medium without BM-DCs (as a control) was inoculated with 2.1 × 106 CFU or 1.91 × 107 CFU of Cj and the bacterial viability was assessed at 2, 4, and 8 h post-inoculation. Data are from three-wells in an experiment and are expressed as mean ± SEM. One experiment representative of three independent experiments is shown. (C) Inhibition of BM-DC internalization of Cj by cytochalasin D (CCD) and nocodazole (NCD). BM-DCs, left untreated or preincubated with 1 μg/ml CCD, 20 μM NCD, 1 μg/ml CCD and 20 μM NCD or DMSO (0.1%, vehicle control) for 1 h, were inoculated with Cj (MOI of 100:1). The internalized bacteria were determined at 2 h p.i. by using gentamicin killing assay as described above. Internalized bacteria in each treatment group were presented as the % of that observed in DC + Cj group (without inhibitors). Data are from two-wells in an experiment and are expressed as mean ± SEM. One experiment representative of two independent experiments is shown.
3.2. BM-DCs undergo maturation following Cj infection
In experiments to test BM-DC maturation, mean fluorescence intensities (MFI) indicating the levels of expression of MHC-II, CD40, CD80 and CD86 were significantly higher by 2.1-, 1.9-, 3.7-, and 2.5-fold, respectively, in Cj-infected BM-DCs compared to uninfected cells (Fig. 2A). Furthermore, infection with Cj resulted in a significant (P < 0.05) increase in the proportion of BM-DCs expressing these markers (Fig. 2A). In all cases, these levels were comparable to those induced by LPS.
Fig. 2.
Maturation and cytokine production by BM-DCs infected with Cj. Data are from three-wells in an experiment and are expressed as mean ± SEM. One experiment representative of two independent experiments is shown. (A) Maturation: BM-DCs (2 × 105 cells/ml) seeded in six-well plates (BD Falcon) were infected with Cj at a MOI of 100:1, treated with LPS (0.1 μg/ml) or R10.2 medium alone. After 24 h, cells were double-stained with R-PE-conjugated anti-CD11c and FITC-conjugated anti-MHC-II (I-Ab), anti-CD40, anti-CD80 or anti-CD86 antibodies or appropriate isotype controls and subjected to flow cytometric analysis. The mean fluorescence intensities of MHC-II, CD40, CD80, and CD86 markers on CD11c+ cells and the percentage of CD11c+ cells expressing these maturation markers are shown. Asterisks indicate P < 0.05 for 1) Cj-infected DCs or LPS-treated DCs vs. medium alone-treated DCs. (B) Cytokine production: BM-DCs (2 × 105 cells per well of a 24-well plate) in R10 medium without antibiotics were treated with Cj at MOI of 10:1 and 100:1 or LPS (0.1 μg/ml) or R10.2 medium alone. The levels of IL-12, IL-6 and TNF-α cytokines in the culture supernatants at 4, 24 and 48 h p.i. were measured by cytokine-specific sandwich ELISA. Data from LPS treatment group was included in the statistical analysis but the significance levels are not shown in figure.
3.3. Cj-infected BM-DCs secrete IL-12p70 and proinflammatory cytokines
Cj induced a dose-dependent increase in IL-12p70 secretion at 24 and 48 h p.i. (Fig. 2B). Peak levels of IL-12p70 secretion were observed with the higher dose of Cj at 24 h p.i. In contrast, the lower dose of Cj induced markedly lower amounts of IL-12p70 at 24 and 48 h p.i. Cj also induced dose-dependent production of IL-6 and TNF-α by BM-DCs at 4, 24 and 48 h p.i. (Fig. 2B). At 4 h p.i., moderate production of IL-6 was induced by the higher dose of Cj, which increased at later time points (24 and 48 h). At the lower dose of bacteria, a similar trend was observed in IL-6 secretion but at a significantly lower magnitude (Fig. 2B). BM-DCs challenged with Cj did not produce detectable quantities of IL-10 at any of the three time points studied (data not shown).
3.4. Cj viability is required for maximal activation of BM-DCs
Infection with killed Cj also resulted in upregulation of maturation markers; however, live Cj induced significantly higher (P < 0.05) levels of expression of CD80 and CD86 than formalin-killed Cj. Similarly, a significantly higher proportion (P < 0.05) of live Cj-exposed DCs expressed CD40 and CD86 than DCs treated with formalin-killed Cj (Fig. 3A). However, the level of MHC-II expression and proportion of DCs expressing MHC-II or CD80 were not significantly different (P > 0.05) between live- and formalin-killed Cj-exposed DCs (Fig. 3A).
Fig. 3.
Effect of viability of Cj on activation of DCs. BM-DCs were treated with approximately equal numbers of live or formalin-killed Cj (MOI of 100:1), LPS (0.1 μg/ml), or R10.2 medium alone. After 24 h, the expression of cell surface markers was analyzed by flow cytometry. (A) Mean fluorescence intensities of MHC-II, CD40, CD80, and CD86 markers on CD11c+ cells and the percentage of CD11c+ cells expressing these maturation markers are shown. (B) The levels of IL-12, IL-6 and TNF-α cytokines in the culture supernatants at 4, 24 and 48 h p.i. were measured by cytokine-specific sandwich ELISA. Asterisks indicate significance P < 0.05. Data are from three-wells in an experiment and are expressed as mean ± SEM. One experiment representative of two independent experiments is shown.
Bacterial viability also affected cytokine secretion. At 24 and 48 h p.i., live Cj-exposed DCs secreted 4.7- and 3.2-fold higher levels of IL-12 and 4.5- and 4.2-fold higher levels of IL-6 than DCs exposed to formalin-killed Cj (Fig. 3B). Live Cj elicited significantly greater (P < 0.05) TNF-α production from DCs than formalin-killed Cj at all time points. Like live Cj, formalin-killed Cj did not elicit detectable IL-10 secretion from DCs at 4, 24 or 48 h p.i. (data not shown).
3.5. Full activation of BM-DCs requires contact of Cj with DCs and bacterial internalization
Cj entry into IEC has been suggested to involve both actin filaments and microtubules [11]. Therefore, we used a combination of cytochalasin D (1 μg/ml) and nocodazole (20 μM), inhibitors of actin and microtubule polymerization respectively, to inhibit phagocytosis of Cj by DCs. This treatment of DCs resulted in about 80% inhibition of uptake of Cj as assessed by the gentamicin killing assay, which was markedly higher than that observed with either inhibitor alone (Fig. 1C).
Preincubation with inhibitors alone caused a significant increase in the MFI of MHC-II and CD80 and in the proportion of CD40+ and CD86+ DCs as shown previously [12]. Comparison of inhibitor-treated and non-treated DCs infected with Cj showed that pretreatment with cytochalasin D and nocodazole abolished the increase in MHC-II expression level and markedly reduced CD40, CD80 and CD86 expression levels and the proportion of DCs expressing CD40, CD80 and CD86 (Fig. 4A,B). Blocking DC phagocytosis of Cj with cytochalasin D and nocodazole completely inhibited IL-12 secretion and significantly (P < 0.05) reduced TNF-α secretion, but did not inhibit IL-6 production. The effect of inhibitors on Salmonella LPS-induced expression of surface markers and cytokines was comparable to that observed for Cj induced responses.
Fig. 4.
Requirement for contact and internalization of Cj for activation of DCs. BM-DCs, preincubated with 1 μg/ml CCD and 20 μM NCD or DMSO (0.1% vehicle control) for 1 h, were treated with Cj (MOI of 100:1), LPS (0.1 μg/ml) or R10.2 medium alone. In experiments with transwells, BM-DCs were added to wells of cell culture plates and 0.2 μM Anopore membrane cell culture inserts were placed in wells. Then, Cj at an MOI of 100:1, R10.2 medium alone or 0.1 μg/ ml of S. typhimurium LPS was added to the top chamber. After 24 h of incubation, maturation and cytokine responses of BM-DCs were analyzed as described before. (A–D) The mean fluorescence intensities of MHC-II, CD40, CD80, and CD86 markers on CD11c+ cells and the percentage of CD11c+ cells expressing these maturation markers are shown. For the experiment with actin and microtubule inhibitors, MFI and percentage of CD11c cells positive for markers in the medium alone group were considered basal values and the increase in these parameters in the other two treatment groups was expressed as fold increase over basal value. (E) The levels of cytokines secreted at 24 h p.i. Data are from three-wells in an experiment and are expressed as mean ± SEM. One experiment representative of two independent experiments is shown.
To test whether activation of DCs depends on direct contact with Cj or whether a diffusible bacterial product is sufficient to stimulate DC responses, Cj and DCs were separated by a 0.2 μM transwell insert, which allowed passage of bacterial products across the membrane but not intact bacteria. The presence of a membrane between DCs and Cj resulted in a significant (P < 0.05) reduction in MHC-II and CD80 expression levels and in the number of CD40+, CD80+, and CD86+ DCs. Furthermore, membrane presence abolished the increase in CD86 expression level and in the proportion of DCs expressing MHC-II after Cj challenge (Fig. 4C,D). Importantly, the prevention of direct contact between Cj and DCs resulted in abrogation of IL-12, TNF-α and IL-6 production (Fig. 4E). However, the presence of membrane only minimally affected DC expression of surface markers, TNF-α and IL-6 in response to Salmonella LPS. Conversely, Salmonella LPS-induced IL-12 secretion was significantly reduced by the presence of transwells (Fig. 4C–E).
3.6. Various strains of Cj differ in their ability to elicit cytokine responses from BM-DCs
We investigated whether different strains of Cj elicit different cytokine responses from BM-DCs. IL-12 production by DCs exposed to various strains ranged from 107.9 ± 1.3 to 310.5 ± 8.6 pg/ml at 24 h p.i. and 70.2 ± 2.4 to 231.7 ± 7.3 pg/ ml at 48 h p.i. (Fig. 5A). At both time-points, strain 33560 induced the highest levels whereas strain NW induced the lowest levels of IL-12. Peak levels of IL-12 secretion were observed at 24 h p.i. with all strains, consistent with previous experiments with strain 11168. All Cj strains elicited TNF-α and IL-6 secretion; maximum levels of TNF-α and IL-6 were observed with strain 33560 and minimum levels were detected with strains NW or D0835 (Fig. 5B,C). The highest levels of TNF-α and IL-6 were detected at 24 and 48 h p.i. respectively for all strains. Strain 33560 induced a minimal amount of IL-10 (36.6 ± 1.6 pg/ml) only at 24 h p.i.; no other strains induced detectable amounts of IL-10 (data not shown).
Fig. 5.
Cytokine responses of BM-DCs to various strains of Cj. BM-DCs were infected with the one of following strains of Cj: 11168, D2600, D0835, NW, D0121 and 33560 or treated with LPS (0.1 μg/ml) or R10.2 medium alone. At 24 and 48 h p.i., the concentrations of IL-12 (A), TNF-α (B) and IL-6 (C) in the culture supernatants were measured by sandwich ELISA. Data are from three-wells in an experiment and are expressed as mean ± SEM. One experiment representative of two independent experiments is shown. DCs treated with all Cj strains or LPS were significantly different (P < 0.05) from medium alone-treated DCs within each time point.
3.7. Cj-infected BM-DCs induce Th1 polarization of CD4+T cells
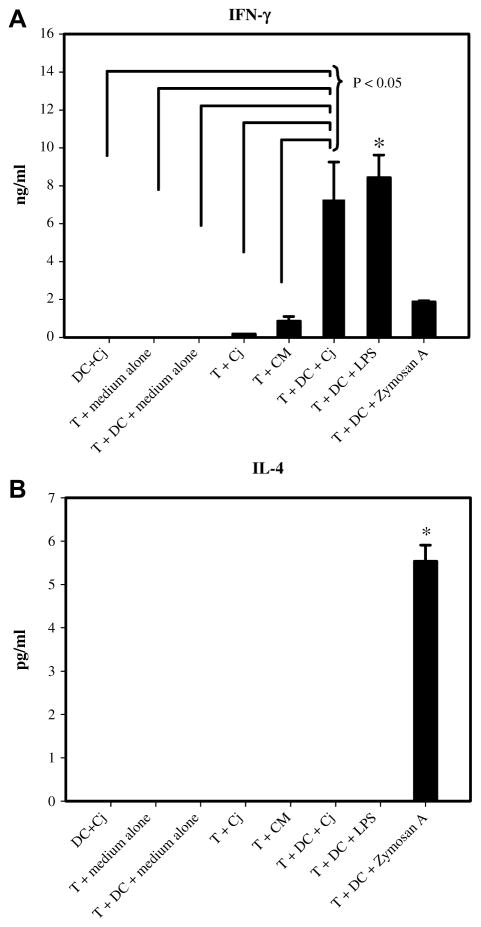
Because Th-polarizing ability of Cj-infected DCs is not known, an in vitro DC-CD4+T cell coculture system was employed to assess this function. Negative control groups (T cells + medium alone, T cells + medium alone-treated DCs, and DCs + Cj) did not produce detectable quantities of IFN-γ (Fig. 6A). However, coculture of Cj-infected BM-DCs with CD4+T cells induced marked production of IFN-γ that was significantly higher (P < 0.05) than observed with all negative controls and comparable to that induced by S. typhimurium LPS-treated BM-DCs. In contrast, Cj-infected BM-DCs did not elicit detectable IL-4 (Fig. 6B) or IL-10 (data not shown) secretion by CD4+T cells. It is evident from this experiment that BM-DCs infected with Cj induce Th1 polarization of CD4+T cells in vitro.
Fig. 6.
Polarization of CD4+T cells by Cj-infected BM-DCs. BM-DCs treated with Cj (MOI of 100:1) or LPS (0.1 μg/ml) or R10.2 medium alone or zymosan (10 μg/ml) were cocultured with CD4+T cells isolated from spleen for 72 h. The levels of IFN-γ (A) and IL-4 (B) in the supernatants were determined by a sandwich ELISA. Asterisks indicate P < 0.05: (A) T + DC + LPS was significantly different from all the negative control groups; (B) T + DC + zymosan was significantly different from all other treatments. Data are from three-wells in an experiment and are expressed as mean ± SEM. One experiment representative of two independent experiments is shown. CM (conditioned medium) is 0.22 μM filtered supernatant from DC + Cj culture.
4. Discussion
Pathogen-host cell interactions in Cj infection remain largely unknown. We demonstrate here that Cj induces phenotypic and functional activation of DCs from resistant C57BL/6 mice. Cj viability and internalization were necessary for maximal activation of DCs. Furthermore, murine DCs primed Cj-specific Th1-effector responses in vitro, consistent with this murine model where Cj was limited to colonization of the gastrointestinal tract [2]. Together these findings support a role for DCs in regulating innate and adaptive immune responses during Cj infection.
Our data show that murine DCs efficiently internalize and kill Cj within 8 h p.i. and that Cj internalization is mediated by both actin filaments and microtubules as suggested previously [11]. Wassenaar et al. [10] reported failure of Cj to survive inside activated human macrophages and suggested that “intra-phagocytic survival is not a common phenomenon” in Cj infection. While Cj persists within cultured IEC [13], our data and that of Hu et al. [6] suggest that near complete killing of Cj by DCs likely rules out the possibility that DCs serve as transporters of Cj for extra-intestinal dissemination.
In our study, immature murine DCs infected with Cj undergo maturation by upregulating surface expression of MHC-II, CD40, and costimulatory molecules. This implies that Cj-infected murine DCs are capable of engaging both T-cell receptors and CD28 molecules on naive Th cells, thereby delivering essential signals for induction of anti-microbial T cell responses. In our studies, most of the unstimulated immature DCs (>70%) expressed CD80 but only a small fraction of unstimulated DCs (<20–35%) expressed CD86. As a result of this basal difference, the magnitude of change due to Cj stimulation and/or other treatment conditions, like presence of transwells, is distinct for CD80 and CD86. That is, the magnitude of increase in proportion of cells expressing CD80 following Cj infection is lower than that observed with CD86 (1.1–1.2-fold increase for CD80 vs. 2.5–3-fold increase for CD86 in our studies). Thus, we show for the first time the effect of Cj viability on DC maturation. When compared to live Cj, killed Cj induced comparable levels of MHC-II expression but significantly lower levels of costimulatory molecule expression, which may be due in part to very low amounts of TNF-α—a known stimulus inducing DC maturation—elicited by non-viable Cj.
We observed a Cj dose- and time-dependent secretion of IL-12 by DCs. IL-12 secreted by DCs enhances CD4+T cell proliferation and IFN-γ production. Furthermore, recent studies demonstrated that IL-12 mediates DC activation of natural killer (NK) cells, contributing to initial resistance to bacterial pathogens [14,15]. This ability to elicit IL-12 suggests that Cj may also stimulate NK cells, leading to mucosal resistance.
Cj-infected DCs secreted TNF-α and IL-6 suggesting that DCs may initiate or amplify the proinflammatory reaction to Cj enhancing recruitment of neutrophils and macrophages to the intestinal mucosa. Indeed, neutrophilic exudates are a prominent feature of Cj induced disease in humans and animals [2,16,17]. DC-mediated recruitment of neutrophils to intestinal mucosa may contribute to Cj killing as recently suggested in Aspergillus fumigatus infection [18]. Additionally, killed Cj elicited significantly lower levels of IL-12 and proinflammatory cytokines than live Cj. Our data taken together with previous reports [6,19,20] demonstrate that, in contrast to IEC, phagocytes such as DCs and THP-1 monocytic cells do not require live Cj to secrete proinflammatory cytokines. However, Cj viability is necessary for maximal induction of cytokine secretion by murine DCs, unlike human DCs and monocytes. We also observed that the maturation and cytokine responses of DCs from C3H/HeOuJ mice to Cj were similar to that observed with DCs from C57BL/6 mice (V. Rathinam and L. Mansfield, unpublished data).
Blocking phagocytosis of Cj resulted in inhibition of IL-12 and TNF-α but not IL-6 production by murine DCs, consistent with previous reports on Neisseria meningitidis and Streptococcus pneumoniae [21,22]. Helicobacter pylori-induced DC secretion of IL-12 but not IL-8 also depended on phagocytosis [14]. Cj internalization was also essential for optimal surface expression of DC maturation markers. Transwell experiments showed that diffusible products secreted by Cj induced only partial upregulation of maturation markers and did not elicit secretion of proinflammatory cytokines. Collectively, these findings show that internalization of Cj was necessary for complete maturation of DCs and secretion of IL-12 and TNF-α but was dispensable for IL-6 production. A possible explanation for this requirement is that, following Cj uptake, additional intracellular signaling pathways are activated from phagosomes that are required for complete activation of DCs. As shown previously, our data also suggest that the Salmonella LPS-induced responses of DCs depend on internalization of LPS [23]. To the best of our knowledge, this is the first report describing the requirements of Cj contact and internalization for DC activation.
We show that murine DC secretion of IL-12, TNF-α, and IL-6 varies among Cj strains. This result is likely due to variation in antigenic surface structures among these strains of Cj [24]. Similar variation in induced levels of IL-8 and CCL20 secretion was observed in IEC challenged with different clinical isolates of Cj [5,20]. Moreover, separate studies in our lab showed that these Cj strains exhibited varying colonization and disease phenotypes in C57BL/6 IL-10−/− mice (Bell et al., unpublished data). No detectable IL-10 production was observed from Cj 11168-infected DCs. We suspect that IL-10 secretion by Cj-infected DCs may require, along with a microbial stimulus, ligation of CD40 on DCs with CD40L that occurs during encounter with NK cells and T cells [25].
We show for the first time that Cj-infected DCs elicit high levels of IFN-γ secretion from CD4+T cells demonstrating a Th1 polarization. This finding correlates with the secretion of IL-12 from Cj-infected DCs observed here and is further strengthened by in vivo studies demonstrating Th1-associated IgG2b antibody responses in IL-10+/+ and IL-10−/− mice of C57BL/6 [2], C3H and non-obese diabetic (NOD) genetic backgrounds [26] and in C57BL/129 mice [16] challenged orally with Cj. Consistent with our data, two earlier studies documented elevated plasma levels of IFN-γ in a naturally infected human patient and in C57BL/6 mice intraperitoneally inoculated with Cj [27,28]. IFN-γ secreted by effector CD4+T cells could activate infected macrophages to augment microbicidal activities for killing of ingested Cj. Wassenaar et al. [10] and Iovine et al. [29] showed that IFN-γ treated human or murine macrophages were enhanced for killing clinical isolates of Cj. Similarly, DC-induction of IFN-γ from CD4+T cells has been reported for infections with various pathogens including H. pylori and A. fumigatus [14,30].
Thus, adaptive immunity plays a role in clearing Cj infection in mouse models [31]. Th1-effector responses initiated by DCs may contribute to mucosal resistance to Cj infection in C57BL/6 mice. However, excessive innate or T-cell mediated inflammatory responses in the intestine triggered by DCs in the absence of immunoregulatory elements, like IL-10, presumably contribute to immune pathology as evident in our C57BL/6 IL-10−/− enteritis model [2]. Further investigations are needed to establish these mechanisms in vivo. Currently, we are investigating the role of toll-like receptor signaling in the activation of DCs in response to Cj.
Acknowledgments
We thank Alice Murphy for breeding mice and Julia Bell for critical manuscript review. This project was funded in part with federal funds from NIAID, NIH, Department of Health and Human Services, under Contract No. NO1-AI-30058 and Grant No. K26 RR023080-01. Dr. Rathinam was supported by funds from the MSU-CVM.
References
- 1.Wassenaar TM, Blaser MJ. Pathophysiology of Campylobacter jejuni infections of humans. Microb Infect. 1999;1:1023–1033. doi: 10.1016/s1286-4579(99)80520-6. [DOI] [PubMed] [Google Scholar]
- 2.Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, Rathinam VA, Fierro BR, Linz JE, Young VB. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun. 2007;75:1099– 1115. doi: 10.1128/IAI.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985;26:945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 5.Johanesen PA, Dwinell MB. Flagellin-independent regulation of che-mokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect Immun. 2006;74:3437–3447. doi: 10.1128/IAI.01740-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu L, Bray MD, Osorio M, Kopecko DJ. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect Immun. 2006;74:2697–2705. doi: 10.1128/IAI.74.5.2697-2705.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 8.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao C, Wood MW, Galyov EE, Hopken UE, Lipp M, Bodmer HC, Tough DF, Carter RW. Salmonella typhimurium infection triggers dendritic cells and macrophages to adopt distinct migration patterns in vivo. Eur J Immunol. 2006;36:2939–2950. doi: 10.1002/eji.200636179. [DOI] [PubMed] [Google Scholar]
- 10.Wassenaar TM, Engelskirchen M, Park S, Lastovica A. Differential uptake and killing potential of Campylobacter jejuni by human peripheral monocytes/macrophages. Med Microbiol Immunol (Berl) 1997;186:139–144. doi: 10.1007/s004300050056. [DOI] [PubMed] [Google Scholar]
- 11.Oelschlaeger TA, Guerry P, Kopecko DJ. Unusual microtuble-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizumoto N, Gao J, Matsushima H, Ogawa Y, Tanaka H, Takashima A. Discovery of novel immunostimulants by dendritic-cell-based functional screening. Blood. 2005;106:3082–3089. doi: 10.1182/blood-2005-03-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson RO, Galan JE. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 2008;4:e14. doi: 10.1371/journal.ppat.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafsi N, Voland P, Schwendy S, Rad R, Reindl W, Gerhard M, Prinz C. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J Immunol. 2004;173:1249–1257. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi T, Hahn CL, Tanaka S, Barbour SE, Schenkein HA, Tew JG. Dendritic cells stimulated with Actinobacillus actino-mycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect Immun. 2004;72:5089–5096. doi: 10.1128/IAI.72.9.5089-5096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JG, Rogers AB, Whary MT, Ge Z, Taylor NS, Xu S, Horwitz BH, Erdman SE. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun. 2004;72:1116–1125. doi: 10.1128/IAI.72.2.1116-1125.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skirrow MB, Blaser MJ. Clinical aspects of Campylobacter infection. In: Nachamkin I, Blaser MJ, editors. Campylobacter. 2. ASM Press; Washington, D.C: 2000. pp. 69–88. [Google Scholar]
- 18.Gafa V, Remoli ME, Giacomini E, Gagliardi MC, Lande R, Severa M, Grillot R, Coccia EM. In vitro infection of human dendritic cells by Aspergillus fumigatus conidia triggers the secretion of chemokines for neutrophil and Th1 lymphocyte recruitment. Microb Infect. 2007;9:971–980. doi: 10.1016/j.micinf.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Jones MA, Totemeyer S, Maskell DJ, Bryant CE, Barrow PA. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect Immun. 2003;71:2626–2633. doi: 10.1128/IAI.71.5.2626-2633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey TE, Baqar S, Bourgeois AL, Ewing CP, Guerry P. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect Immun. 1999;67:88–93. doi: 10.1128/iai.67.1.88-93.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uronen-Hansson H, Steeghs L, Allen J, Dixon GLJ, Osman M, van der Ley P, Wong SYC, Callard R, Klein N. Human dendritic cell activation by Neisseria meningitidis: phagocytosis depends on expression of lipooligosaccharide (LOS) by the bacteria and is required for optimal cytokine production. Cell Microbiol. 2004;6:625. doi: 10.1111/j.1462-5822.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- 22.Colino J, Snapper CM. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J Immunol. 2003;171:2354–2365. doi: 10.4049/jimmunol.171.5.2354. [DOI] [PubMed] [Google Scholar]
- 23.Cowan DB, Noria S, Stamm C, Garcia LM, Poutias DN, del Nido PJ, McGowan FX., Jr Lipopolysaccharide internalization activates endotoxin-dependent signal transduction in cardiomyocytes. Circ Res. 2001;88:491–498. doi: 10.1161/01.res.88.5.491. [DOI] [PubMed] [Google Scholar]
- 24.Pearson BM, Pin C, Wright J, I’Anson K, Humphrey T, Wells JM. Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett. 2003;554:224–230. doi: 10.1016/s0014-5793(03)01164-5. [DOI] [PubMed] [Google Scholar]
- 25.Scott K, Manunta M, Germain C, Smith P, Jones M, Mitchell P, Dessi D, Branigan Bamford K, Lechler RI, Fiori PL, Foster GR, Lombardi G. Qualitatively distinct patterns of cytokines are released by human dendritic cells in response to different pathogens. Immunology. 2005;116:245–254. doi: 10.1111/j.1365-2567.2005.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield LS, Patterson JS, Fierro BR, Murphy AJ, Rathinam VA, Kopper JJ, Barbu NI, Onifade TJ, Bell JA. Genetic background of IL-10−/− mice alters host-pathogen interactions with Campylobacter jejuni and influences disease phenotype. Microbial Pathogenesis. doi: 10.1016/j.micpath.2008.05.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baqar S, Rice B, Lee L, Bourgeois AL, El Din AN, Tribble DR, Heresi GP, Mourad AS, Murphy JR. Campylobacter jejuni enteritis. Clin Infect Dis. 2001;33:901–905. doi: 10.1086/322594. [DOI] [PubMed] [Google Scholar]
- 28.Abram M, Vukovic D, Wraber B, Doric M. Plasma cytokine response in mice with bacterial infection. Mediators Inflamm. 2000;9:229–234. doi: 10.1080/09629350020025746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iovine NM, Pursnani S, Voldman A, Wasserman G, Blaser MJ, Weinrauch Y. Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni. Infect Immun. 2008 doi: 10.1128/IAI.01063-07. IAI.01063–01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gafa V, Lande R, Gagliardi MC, Severa M, Giacomini E, Remoli ME, Nisini R, Ramoni C, Di Francesco P, Aldebert D, Grillot R, Coccia EM. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect Immun. 2006;74:1480–1489. doi: 10.1128/IAI.74.3.1480-1489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang C, Miller JF. Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun. 2006;74:5261–5271. doi: 10.1128/IAI.01094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]