Abstract
This review examines the relationship between physical activity and cancer along the cancer continuum, and serves as a synthesis of systematic and meta-analytic reviews conducted to date. There exists a large body of epidemiologic evidence that conclude those who participate in higher levels of physical activity have a reduced likelihood of developing a variety of cancers compared to those who engage in lower levels of physical activity. Despite this observational evidence, the causal pathway underling the association between participation in physical activity and cancer risk reduction remains unclear. Physical activity is also a useful adjunct to improve the deleterious sequelae experienced during cancer treatment. These deleterious sequelae may include fatigue, muscular weakness, deteriorated functional capacity, including many others. The benefits of physical activity during cancer treatment are similar to those experienced after treatment. Despite the growing volume of literature examining physical activity and cancer across the cancer continuum, a number of research gaps exist. There is little evidence on the safety of physical activity among all cancer survivors, as most trials have selectively recruited participants. It is also unclear the specific dose of exercise needed that is optimal for primary cancer prevention or symptom control during and after cancer treatment.
Introduction
Cancer is the leading cause of death in high-income countries and the second leading cause of death in low- and middle-income countries. Approximately 1 in 4 deaths in the United States are due to cancer (75). The International Agency for Research on Cancer (IARC) estimates approximately 12.8 million new diagnoses worldwide, and 7.6 million deaths worldwide resulting from cancer occurred in 2008. This growing differential ratio of cancer diagnoses to cancer-related deaths has resulted in a population of “cancer survivors”—those diagnosed with cancer—which exceeds 28 million worldwide (75). This growing population of cancer survivors has emerged from improvements in screening and detection, as well as improvements in a variety of treatment modalities, including, surgery, chemotherapy, and radiation therapy (128).
Despite the success of procedures to identify and control cancer once detected, primary prevention of cancer is an area of great interest on many levels including scientific, economic, and political. Despite the genetic hallmarks of cancer, lifestyle and environmental variables are pivotal influences in the development of cancer (39). Identifying lifestyle and environmental risk factors associated with developing cancer, educating the public about these risk factors, and providing interventions to modify the exposure to these risk factors may provide a viable route to decrease the burden of cancer. Numerous risk factors associated with developing cancer have emerged including, sexual behavior, addictive substances, and factors including overweight and obesity, low fruit and vegetable intake, and physical inactivity.
Overweight, obesity, and physical inactivity contribute to the risk of developing a number of cancers. Though overweight and obesity may appear to be separate from physical activity, both constructs relate to energy balance (63). Maintaining an optimal level of energy balance—caloric expenditure relative to caloric intake—is associated with primary prevention of cancer, survival after diagnosis and recurrence of primary cancer (97, 134). Therefore, it is necessary to acknowledge the synergistic relationship between overweight or obesity and physical inactivity along the spectrum of cancer prevention and survivorship (68, 121).
For example, being overweight, obese, or physically inactive contributed 26% of total risk of developing colorectal cancer, and has been attributed to 159,000 colorectal cancer related deaths worldwide in 2001. More generally, of the 7-million deaths that occurred from cancer worldwide in 2001, an estimated 2.43-million (~35%) were attributable to modifiable risk factors (39). In a large prospective cohort study of >900,000 American adults, increased body mass indices were associated with increased death rates for all types of cancer combined and at numerous cancer-specific sites, among both men and women (19). Men and women in the highest quintile of body mass index (BMI ≥ 40), had a 52% and 62% higher death rate from cancer, compared to men and women in the lowest quintile of body mass index (BMI<24.9), respectively (19).
There also exists a depth and breadth of literature examining the independent effects of physical activity along the cancer continuum. To this end, we acknowledge the synergistic relationship among obesity, physical activity, and energy balance; however, we choose to focus our review on the independent effects of physical activity, rather than the overarching influence of energy balance.
Physical Activity
To critically analyze and interpret the results from any scientific study, it is important to understand the methodological characteristics associated with a particular investigation, including study design, methods of assessment and quantification of physical activity, and appropriate conduct and interpretation of statistical analyses. In the ensuing paragraphs, we define physical activity, how to measure and quantify physical activity, and we briefly review two common study designs used in the assessment of physical activity and the risk of cancer.
What is physical activity?
Physical activity is any movement using skeletal muscles (24). Physical activity can be categorized into four major subgroups. These subgroups include occupational (activity done at work), household (activity done at home), transport (activity done to commute), and recreational or leisure-time (activity done for enjoyment and/or pleasure); (23). Physical activity can also be of varying intensities, including light, moderate, and vigorous intensity (164). Examples of activities with light, moderate, and vigorous intensities include housework, brisk walking, and running, respectively (2).
Measurement of Physical Activity
The most common method of ascertainment of physical activity is through the use of self-report measures. The popular method of subjective physical activity estimation is with the use of physical activity questionnaires (89). There are over two-dozen physical activity questionnaires that demonstrate validity and reliability—the ability to quantify a variable of interest and to quantify the variable repeatedly among a large sample of participants. Physical activity questionnaires vary in the complexity of questions asked, time needed to complete, and the type and dose of physical activity measured. For a compendium of over 100 physical activity questionnaires, we refer the reader to the National Cancer Institute physical activity questionnaire website (113).
Introduction to the quantification of physical activity dose – The FITT principle
To assess physical activity, there are four parameters that may be estimated: frequency, intensity, time, and type. Frequency is the number of days per week dedicated to engaging in physical activity (d·wk−1). Intensity is how strenuous or how physically demanding a single bout of physical activity is. Most epidemiologic studies measure intensity with METs, metabolic equivalents of energy expenditure, where 1-MET is sitting quietly, and 18-METs is running a <5 min·mile−1 pace (2). Time is the length of a single bout of physical activity, measured in minutes or hours (min·d−1 or hr·d−1). Type is the modality of physical activity, and frequently includes aerobic, strength and flexibility activities. However, from an epidemiologic perspective, modality includes broad categories such as occupational, leisure-time, or personal-care physical activities. It is these four components, frequency, intensity, time, and type that form the foundation of physical activity or exercise prescription, referred to as the FITT principle (frequency, intensity, time, type); (164).
Physical Activity Study Design & Challenges
The two most common study designs used to examine the association between physical activity and risk of cancer are the cohort and case-control study. Each of these observational study designs is subject to methodological strengths and weaknesses. We refer the reader to two excellent reviews comparing cohort and case-control studies and the interpretation of statistical analyses and conclusions from each study design (28, 100).
Despite this exciting time, the etiology of cancer has provided numerous challenges to conduct high-quality research. For example, the growth and development of cancer may take decades to occur. This long latent period makes the study of physical activity and cancer research difficult. The spectrum from cancer prevention to palliative end-of-life care encapsulates many decades of life. The population of cancer survivors has grown to include approximately 13-million in the United States, and 28-million worldwide (75, 76). Approximately 4% of the U.S. population is cancer survivors (76). This makes the identification and assembly of a population of cancer survivors difficult for research endeavors.
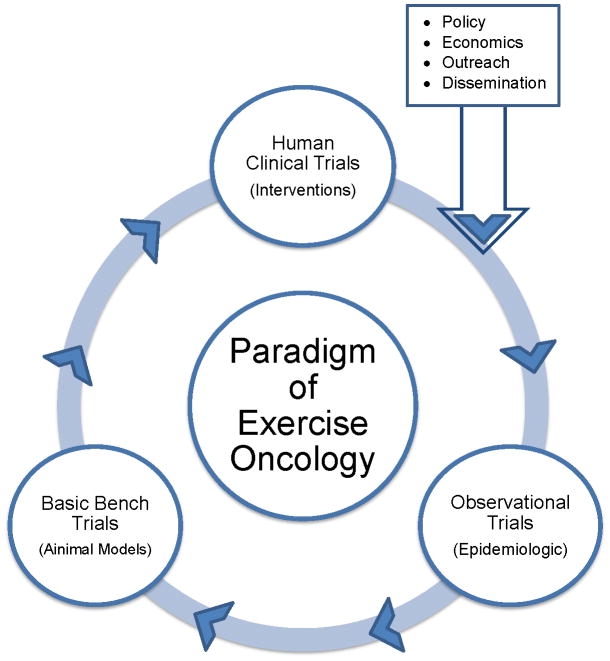
The multifactorial origins of cancer, long latent period, and relatively small population influence the research design used in physical activity or exercise and cancer (what we refer to as., “exercise oncology”) research. For continued success in exercise oncology research, three corner stones must exist (Figure 1). The paradigm of exercise oncology research is similar to the paradigm originally proposed by Henry Blackburn much more generally for multiple areas of biomedical research (9), and posits that human clinical trials, observational trials, and basic bench science trials should not be considered discrete components of research, but more as parts of a continuum. The paradigm of exercise oncology suggests observational trials may generate hypotheses to be tested, manipulated, and explored in animal models, and then translated into human clinical trials. We remind the reader this example, is exactly that, an example. One might begin with an animal model or human clinical trial, and traverse the paradigm. Another critical component to the success of physical activity and cancer research is the integration of policy reform, economic analysis, outreach, and dissemination. One or more of these components may occur after conduct of a methodologically rigorous human clinical trial.
Figure 1.
A paradigm of physical activity and cancer research.
Physical Activity Research along the Cancer Continuum
According to Sporn et al. (155), in contemporary medicine more focus is devoted to curing cancer in advanced stages rather than to the primary prevention of cancer. Despite the importance of physical activity in the primary prevention of cancer, physical activity is also a modality with the capacity to provide health-benefits after diagnosis of cancer. Participation in physical activity has emerged as a potent rehabilitative modality for cancer survivors in the past 20 years (31). Physical activity has numerous documented health-benefits among cancer survivors, including improved disease-free survival, muscular strength, aerobic capacity, and quality of life.
An organizational model exists to delineate the role of physical activity across the continuum of cancer control, including the major subsets prior to and after diagnosis of cancer (Figure 2); (32). Within the two major subsets of diagnosis, there exist six distinct periods including eight total outcomes that are applicable to physical activity and cancer (32). We will use this conceptual model to guide us through the role of physical activity and the cancer survivorship continuum.
Figure 2.
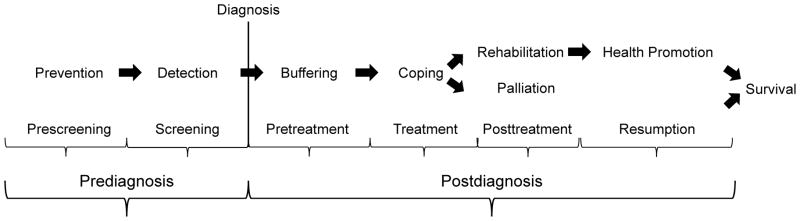
A conceptual model to guide and organize the role of physical activity across the cancer control continuum. Reproduced with permission from (32).
A Brief Primer for Oncology Care Clinicians or Exercise Physiologists
A brief primer for oncology care clinicians
Over the previous two decades, major improvements have been made in our ability to detect, diagnose, treat, and in some cases, cure cancer. Given this evolution, patients may die with, rather than from some forms of cancer. Despite this, the ultimate goal of all oncology care clinicians and cancer centers around the world is to eradicate cancer as a cause for human suffering—a goal we have not yet reached. Nonetheless, our investment in working towards this goal has uncovered encouraging research relating to behavioral components of health that may influence the continuum of cancer. Evidence of physical activity has emerged in a variety of forms—animal models, observational, and randomized controlled trials—to influence an array of cancer outcomes. However, providing patient care includes numerous competing demands. Therefore, it is unrealistic to expect cancer care clinicians to stay abreast of all the rapidly developing literature relating to cancer pathology, treatment modalities, and physical activity and rehabilitation. Therefore, the goal of this review is to provide an overview of the benefits associated with physical activity across the cancer continuum. We aim to delineate the efficacy of physical activity and identify the need for exercise physiologists trained with the knowledge, skills, and abilities necessary to provide safe and efficacious exercise prescriptions to cancer survivors. This will allow the oncology care clinicians to focus their time on patient care and treatment, and facilitate referral to available exercise physiologists if such care is necessary.
A brief primer for exercise physiologists
The interest in pursuing careers in exercise physiology has grown dramatically in the previous 30-years (49). The interest in clinical exercise physiology was instrumental in the late 1970’s and 1980’s when supervised physical activity or exercise after a myocardial infarction was realized to have provided numerous health benefits including improved physical function, and reduced likelihood for experiencing a second myocardial infarction. Moreover, an evidence base of great depth and breadth has emerged that suggests physical activity provides numerous heart healthy benefits. Analogous to the spectrum of cardiovascular physiology and cardiac rehabilitation, cancer physiology has emerged along a similar paradigm. Despite a considerably smaller foundation of evidence, the current depth and breadth of evidence is promising—supporting the role of physical activity along the cancer continuum. Though similarities between cardiac rehabilitation and cancer rehabilitation exist, there are also many differences. Academic training in exercise physiology foci include emphasis on cardiopulmonary parameters of rehabilitation and exercise training (i.e. V02, HRmax, etc.); (3, 164). However, cancer rehabilitation requires knowledge beyond that of cardiovascular physiology. Cancer and cancer treatment affects musculoskeletal, nervous, immune, endocrine, and cognitive systems, in addition to the cardiopulmonary systems described above (138). It is our hope that this review will serve as the bridge from basic exercise physiology to clinical exercise physiology by providing a foundation of knowledge to build upon, for those interested in learning more about the role of physical activity along the cancer continuum.
Physical Activity and Primary Cancer Prevention
This section provides a summary of the epidemiologic evidence of physical activity and primary cancer prevention. Due to the extensive depth and breadth of the epidemiologic evidence concerning primary cancer prevention, this section cannot provide an exhaustive review of all relevant literature. This section will serve as a brief synthesis of the most recent published systematic or meta-analytic reviews (36, 51, 52, 62, 118, 119, 163, 173, 182).
Why study physical activity and cancer prevention?
It is estimated that 35% or 2.43 million of the 7-million cancer deaths worldwide each year are attributable to the joint effect of preventable lifestyle-related risk factors (176). Population-attributable-fraction is an estimate of the proportion of reduction in cancer that would occur if exposure to a certain risk factor (i.e., physical activity or lack thereof) were reduced to the optimal exposure distribution (i.e., everyone in the world engaging in 2.5 h·wk−1 of moderate-intensity physical activity); (39). Worldwide, the population-attributable-fraction of physical inactivity and all cancer is 2% (Figure 3); (176). A population-attributable-fraction of 2% equates to approximately 135,000 cancer deaths each year, and can be projected to directly affect 560,000 of the 28-million cancer survivors worldwide (125). It is of interest to modify unfavorable risk factors that increase ones risk for developing cancer. Primary prevention through behavioral and environmental modification is a cost-effective means of preventing the large burden cancer has on societies worldwide (176).
Figure 3.
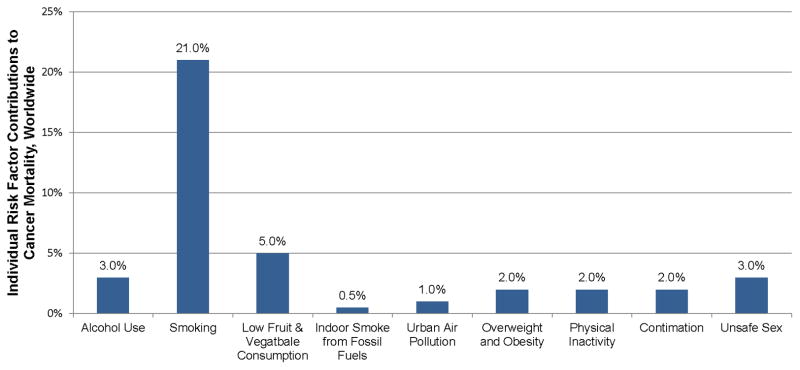
Individual risk factor contributions to mortality from all cancers, worldwide. Data from (176).
Breast Cancer
Background
Worldwide, approximately 1.4 million women are diagnosed with breast cancer each year (23% of all female cases of cancer); (75). Use of mammography and the promotion of clinical breast examination strategies have improved the 5-year survival rate across all stages of breast cancer to exceed 90% in Europe (60, 82) and the United States (76). Therefore, despite the 500,000 who die from breast cancer each year (14% of all female cancer deaths), the population of breast cancer survivors continues to grow at a rapid pace (75). Breast cancer incidence varies geographically; likely related to the numerous risk factors, such as the reproductive and hormonal milieu, alcohol consumption, obesity, and physical inactivity; factors contingent on culture, lifestyle, and environmental determinants (45).
Overall Association
There have been over 73 observational studies that examine the association between breast cancer and physical activity (51, 52). These studies (k) have been either cohort (k=33), and case-control (k=40) epidemiologic studies. The proportion of studies observing a statistically significant association in risk reduction in breast cancer was similar between study designs, 39% and 40% of cohort and case-control studies, respectively. However, the magnitude of the reduction in breast cancer risk was different between study designs. Cohort studies estimated a 20% absolute risk reduction, whereas case-control studies estimated a 30% absolute risk reduction.
Physical Activity Type and Dose
Among studies examining a dose-response relationship between breast cancer risk and physical activity, 80% of studies (33 out of 41), observed evidence of a dose-response relationship (52). The type of physical activity that provided the largest reductions in breast cancer risk were recreational, household, and occupational physical activity, with associated risk reductions of 21%, 21%, and 18%, respectively (51, 52). Interestingly, activities such as walking or cycling, used for transport, provided a more modest, 13% risk reduction in breast cancer (51, 52). When considering the intensity of physical activity needed to provide a reduction in breast cancer risk, both moderate and vigorous intensity physical activity provide significant reductions in risk, in the order of 15%, and 18%, respectively (52). Volume of physical activity (min·wk−1) is the product of frequency (d·wk−1) and time of physical activity (min·d−1). The magnitude of breast cancer risk-reduction does not increase proportionally with larger volumes of physical activity. Volume of physical activity with levels including 2.0–3.0 h·wk−1, 3.25–4.25 h·wk−1, 4.5–5.5 h·wk−1, and ≥6.5 h·wk−1, provided risk reductions of 7%, 14%, 6%, and 28%, respectively. It is unclear why 4.5–5.5 h·wk−1, provides only one-fifth of the risk reduction when compared to ≥6.5 h·wk−1 of physical activity (52). It is plausible that an insufficient number of studies have examined a dose of physical activity falling in the range of 4.5–5.5 h·wk−1. Most studies have favored examining extremes of physical activity dose in attempt to answer the questions: does there exist a minimal dose of physical activity needed to reduce risk of breast cancer (i.e., a floor effect), and what is the maximum dose of physical activity that provides the largest reductions in risk of breast cancer (i.e., a ceiling effect)?
Subgroups
Numerous subgroups have been examined when comparing the association between breast cancer risk and physical activity. These subgroups have included menopausal status, race, BMI, family history of breast cancer, parity, and breast cancer tumor-characteristics (52). When comparing menopausal status of women the reductions in breast cancer risk associated with physical activity are similar. Premenopausal and postmenopausal women have an estimated risk reduction of 27%, and 31%, when comparing the highest versus lowest levels of physical activity (52). Race has also been explored in subgroup analysis comparing the risk of breast cancer and associations with physical activity. Asian and Black women have the largest reductions in breast cancer risk from physical activity, 41% for both groups (52). Indian, and Hispanic women have slightly lower reductions in risk of breast cancer from physical activity, 38% and 28%, respectively (52). The smallest reductions in breast cancer risk are in Caucasian women, 20% (52). To date, it is unclear as to why Asian and African American women have a two-fold higher reduction in risk of breast cancer compared to Caucasian women when comparing the highest versus lowest levels of physical activity, respectively.
Body mass index (BMI) has also been examined in subgroup analysis examining the association between breast cancer risk and physical activity. There appears to be a linear dose-response relationship between BMI and breast cancer risk-reduction from physical activity, with larger risk reductions occurring among women with lower BMI’s. The risk reduction of breast cancer among from being physically active among four BMI groups, <22, 22.1–24.9, 25.0–29.9, and ≥30, were 27%, 24%, 18%, and 0.4%, respectively (51, 52). It is plausible the dose response relationship between BMI and risk reduction of breast cancer exists because of a mechanistic underpinning associated with physical activity and the associated reductions in breast cancer risk (see biologic mechanisms for discussion).
When comparing women with a family history of breast cancer the risk reduction associated with physical activity is in the order of 1%, whereas the risk reduction among women without a family history of breast cancer is 21% (51, 52). Among women who have had a child, the reduction in breast cancer risk associated with physical activity is 38%, whereas among women who have not had a child, the breast cancer risk associated with physical activity is 18% (51, 52). There have been subgroup analyses that examine hormone receptor status. The largest risk reductions in developing breast cancer through the use of physical activity were among women with estrogen and progesterone negative breast cancer, with a risk reduction of 27% (52). All other combinations of hormone receptor status provide more modest reductions in breast cancer risk; these combinations include estrogen receptor positive, estrogen receptor negative, progesterone receptor positive, progesterone receptor negative, and estrogen receptor positive/progesterone receptor positive breast cancer, which have associated risk reductions of 20%, 21%, 21%, 14%, and 14%, respectively (52).
Summary
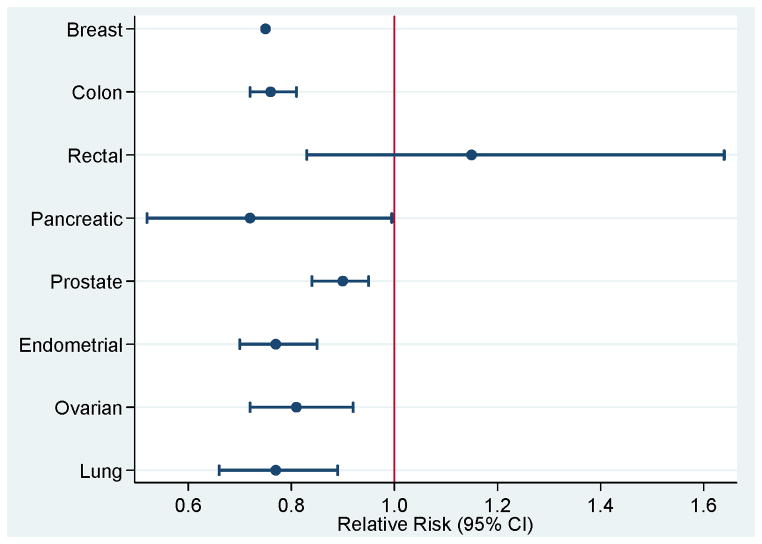
There exists a large depth and breadth of epidemiologic evidence supporting the association between physical activity and breast cancer risk. The average risk reduction when comparing the highest versus lowest levels of physical activity is 25% (Figure 4). The International Agency for Research on Cancer (IARC) has categorized the association between physical activity and risk of breast cancer convincing (72). Numerous subgroups have been examined, including the types and doses of physical activity optimal for breast cancer risk-reduction as well as demographic characteristics that influence the association between physical activity and breast cancer risk.
Figure 4.
Relative risk and 95% confidence intervals (95% CI) comparing highest versus lowest levels of physical activity and cancer risk reduction.
Colon and Rectal Cancer
Background
Worldwide, approximately 1.2 million people are diagnosed with colon cancer each year (75). Use of colonoscopy and improved chemotherapy treatments has improved the 5-year survival rate across stages I–III colon cancer to exceed 55% (60, 82). Therefore, despite the 600,000 who die from colon cancer each year, the population of colon cancer survivors continues to grow at a rapid pace (75). Numerous risk factors for colon cancer have been identified, including older age (>50 yr.), African-American race, history of colon polyps, family history of colon cancer, low-fiber and high-fat diet, sedentary lifestyle, diabetes, obesity, and smoking (59).
Overall Association
There have been over 50 studies examining the association between colon cancer and physical activity (62, 182). In a considerably smaller, but informative literature, seven prospective cohort studies have examined the association between rectal cancer and physical activity (62). There is strong and consistent evidence from multiple meta-analyses that physical activity is associated with a significant reduction in risk of colon cancer, in the order of 24%, Risk-Ratio (RR)=0.76, and 95% confidence interval ((95% CI) of 0.72–0.81); (62, 182). Consistent with observational evidence among physical activity and breast cancer risk, cohort studies estimate a 17% risk reduction in colon cancer when comparing the highest versus lowest levels of physical activity, RR=0.83 (95% CI: 0.78–0.88). Whereas case-control studies estimate a 31% reduction in colon cancer when comparing the highest versus lowest levels of physical activity, RR=0.69 (95% CI: 0.65–0.74); (182).
Contrastingly, a meta-analysis of seven cohort studies examining physical activity and rectal cancer concluded there is a null association between physical activity and risk of rectal cancer, RR=1.15 (95% CI: 0.83–1.64); (62).
Physical Activity Type and Dose
The type of physical activity performed has been examined in subgroup analysis when comparing the association between colon cancer and physical activity. Occupational and leisure-time physical activity are both associated with significant reductions in colon cancer risk of 22%, RR=0.78 (95% CI: 0.74–0.83), and 23%, RR=0.77 (95% CI: 0.72–0.82), respectively (182). When controlling for study design (case-control versus cohort), the results were attenuated among cohort designs, but the results did not differ substantively compared to the unadjusted risk reduction estimates (182). Evidence among a meta-analysis of 48 studies including 40,674 men and women with colon cancer suggest a dose-response effect does exist, such that increasing doses of physical activity provide larger reduction in risk of colon cancer, particularly when physical activity is of moderate or vigorous intensity, yielding 13–41% reductions in colorectal cancer risk (167).
Subgroups
The association between colon cancer and physical activity has been examined between men and women, resulting in similar statistically significant risk reductions 24%, RR=0.76 (95% CI: 0.71–0.82) and 21%, RR=0.79 (95% CI: 0.71–0.88), respectively (182). When stratified by gender and study design, case-control versus cohort, results do not differ substantively (182). Some have speculated that the magnitude of colon cancer risk-reduction associated with physical activity would be attenuated over time due to increased surveillance through the promotion of colonoscopies at regular time intervals. However the results over time did not change when comparing studies conducted prior to 1993 versus after 1993 (182). Other evidence suggest physical activity may be more potent in reducing risk of left versus right-sided colon cancer, particularly among those with a healthier BMI (182).
The association between rectal cancer and physical activity has been examined between men and women, resulting in similar, non-significant, risk reductions, RR=1.02 (95% CI: 0.83–1.26), and RR=1.29 (95% CI: 0.82–2.01), respectively (62). The results from these analyses did not differ when controlling known confounders such as BMI, family history, smoking and alcohol consumption. Too few studies (k=7) existed to conduct a meaningful subgroup analysis beyond those described above (62).
Summary
There exists a consistent depth and breadth of observational evidence that suggests physical activity reduces risk of colon cancer (Figure 4). Evidence suggests that larger volumes or more vigorous-intensity physical activity provide the largest reductions in risk of colon cancer. Conversely, there appears to be evidence of a null association between physical activity and risk of rectal cancer (Figure 4).
Pancreatic Cancer
Background
Worldwide, approximately 217,000 people are diagnosed with pancreatic cancer each year (61). Despite improvements in 5-year survival among other gastrointestinal cancers (colon, rectum), pancreatic cancer 5-year survival remains at 4% across all stages (60, 82). Approximately 213,000 deaths occur from pancreatic cancer worldwide, each year (61). Similar to colon cancer, numerous risk factors for pancreatic cancer have been identified, including cigarette smoking, older age (>60 yr.), African-American race, male gender, diabetes, and high fat diet (93, 152).
Overall Association
There have been 28 observational studies conducted examining the association between pancreatic cancer and physical activity (119). Evidence suggests physical activity is associated with a statistically significant reduced risk of pancreatic cancer of 28%, RR=0.72 (95% CI: 0.52–0.99) when comparing the highest versus lowest levels of physical activity (119). Using sensitivity analyses, the removal of one outlier study enlarged the risk reduction to 37%, RR=0.63 (95% CI: 0.45–0.88); (119).
Physical Activity Type and Dose
Recreational physical activity has been examined in 16 prospective cohort and case-control studies (119). The pooled risk reduction among these 16 studies was a non-significant risk reduction of 6%, RR=0.94 (95% CI: 0.88–1.01) when comparing the highest versus lowest levels of recreational physical activity, respectively. Among three retrospective studies, the pooled risk reduction was 26%, RR=0.74 (95% CI: 0.59–0.94). Occupational physical activity has been examined in five prospective studies, with evidence of no modification in risk reduction, RR=1.00 (95% CI: 0.57–1.76) comparing the highest versus lowest levels of occupational physical activity, respectively. Using sensitivity analysis by eliminating one study, the risk reduction significantly increased to 25%, RR=0.75 (95% CI: 0.59–0.96). Physical activity used for transportation has been examined in five prospective studies, with a pooled non-significant risk reduction of 23%, RR=0.77 (95% CI: 0.55–1.10), when comparing the highest versus lowest levels of transport physical activity levels, respectively. Therefore, it remains unclear as to the specific type of physical activity necessary to consistently reduce risk of prostate cancer.
Intensity of physical activity has also been examined in prospective observational studies. Low intensity physical activity has been explored by two prospective studies, yielding a non-significant increase in pancreatic cancer risk of 1%, RR=1.01 (95% CI: 0.77–1.34); (119). Moderate intensity physical activity has been explored by six prospective studies, yielding a non-significant risk reduction of 21%, RR=0.79 (95% CI: 0.52–1.20); (119). When restricting the analysis to only prospective studies that assessed physical activity using a validated measure of physical activity, the risk reduction was augmented to a significant reduction in risk of 65%, RR=0.45 (95% CI: 0.29–0.69), however this pooled analysis was limited to two studies (119). Vigorous intensity physical activity has been explored by nine prospective studies, yielding a non-significant risk reduction of 3%, RR=0.97 (95% CI: 0.88–1.07); (119).
Subgroups
The above summarized meta-analyses, along with others, have examined potential subgroups and known confounders, study region, sex, follow-up duration, BMI, and diabetes, and have elucidated no evidence of subgroup differences (119, 181).
Summary
There exists a small body of evidence that suggests physical activity reduces the risk of pancreatic cancer (Figure 4). The type of physical activity necessary to elicit the largest reductions in pancreatic cancer risk remains to be elucidated. Based on two studies using validated measures of physical activity, moderate intensity physical activity appears to garner the largest reductions in prostate cancer risk. Other characteristics relating to the dose of physical activity and other population subgroups remain unknown.
Prostate Cancer
Background
Worldwide, approximately 903,500 men are diagnosed with prostate cancer each year (75). Through the use of screening and population awareness strategies, the 5-year survival rate for non-metastatic prostate cancer is >99%, with metastatic prostate cancer survival rates near 30% (60, 82). Approximately 258,000 men die each year from prostate cancer, worldwide (75), yet many will die with prostate cancer rather than from prostate cancer. Risk factors for prostate cancer include age (> 50 yr.), African-American race, family history, genetics, diets high in red meat and high-fat dairy, obesity, sedentary lifestyle, smoking, and previous prostatitis (126).
Overall Association
There have been 43 observational studies conducted that examine the risk of prostate cancer and physical activity that have included 2,198,786 participants and 88,294 cases (92). The 43 studies have consisted of 19 cohort studies (44%), and 24 case-control studies (66%); (92). Evidence suggests physical activity is associated with a statistically significant 10% risk reduction of prostate cancer, RR=0.90 (95% CI: 0.84–0.95); (92). Consistent with breast and colon cancer, case-control studies estimate a larger risk reduction, 14%, RR=0.86 (95% CI: 0.75–0.97) in prostate cancer. When comparing the highest versus lowest levels of physical activity compared to the estimate of risk reduction from cohort studies, there is a more modest, yet statistically significant 6% reduction in risk, RR=0.94 (95% CI: 0.91–0.98); (52, 92, 182).
Physical Activity Type and Dose
Occupational physical activity was associated with a significant risk reduction in prostate cancer of 19%, RR=0.81 (95% CI: 0.73–0.91); (92). Subgroup analysis stratified by study design (cohort versus case-control) risk reductions did not differ substantively, though the cohort RR was attenuated; RR=0.91 (95% CI: 0.87–0.95) compared to case-control RR=0.73 (95% CI: 0.62–0.89); (92). Recreational physical activity was associated with a reduction in risk of 5%, RR=0.95 (95% CI: 0.89–1.00); (90, 92). Subgroup analysis stratified by study design (cohort by case-control) risk reductions in prostate cancer were only significant among cohort studies, RR=0.95 (95% CI: 0.90–1.00), but not significant among case-control studies, RR=0.98 (95% CI: 0.85–1.14); (92).
Subgroups
The association between physical activity and prostate cancer risk has been examined among varying geographic and ethnic groups including Europeans, North Americans, and African Americans.. Comparing the highest to lowest levels of total physical activity and prostate cancer risk, Europeans, North Americans, and African Americans observe significant reductions in prostate cancer risk, RR=0.91 (95% CI: 0.87–0.95), RR=0.85 (95% CI: 0.78–0.94), RR=0.74 (95% CI: 0.57–0.95), respectively (92). These patterns of physical activity and risk reduction of prostate cancer are generally similar when comparing subgroups of occupational physical activity, and risk reductions attenuate when comparing cohort versus case-control study designs (90, 92).
The association between physical activity and prostate cancer risk has been examined among varying life-periods of age, stage of prostate cancer, presence or absence of prostate-specific antigen testing, and BMI. Within the age groups of <20, 20–45, 45–65, and ≥ 65, the largest statistically significant reductions in prostate cancer risk were observed among those 20–45, and 45–65, with RR=0.93 (95% CI: 0.89–0.97) and RR=0.91 (95% CI: 0.86–0.97), respectively (92). The other age groups, <20, and ≥65 did not observe significant reductions in prostate cancer risk. Stage of prostate cancer, localized versus advanced, does not appear to moderate the association between physical activity and prostate cancer risk with RR=0.96 (95% CI: 0.86–1.05), and RR=0.94 (95% CI: 0.80–1.10), respectively. The use of prostate-specific antigen testing also does not appear to moderate the association between physical activity and prostate cancer risk with RR=1.05 (95% CI: 0.92–1.20), and RR=0.83 (95% CI: 0.63–1.11), respectively (92). Lastly, BMI does not appear to be a moderator of the association between physical activity and prostate cancer risk comparing BMI<25 to BMI ≥25 yields RR=0.98 (95% CI: 0.81–1.20), and 0.95 (0.82–1.11), respectively (90, 92).
Summary
Among cohort and case-control study designs, evidence suggests physical activity provides a reduction in the risk of prostate cancer (Figure 4). It appears occupational physical activity provides reductions in prostate cancer risk; however it is unclear if recreational physical activity garners the same reductions in physical activity. The largest reductions in prostate cancer risk appear in physically active African American and Asian men. Among various subgroup analyses, age is the only potential effect modifier of the association between physical activity and prostate cancer risk, where younger men seem better protected by physical activity than older men.
Endometrial Cancer
Background
Worldwide, approximately 275,000 women are diagnosed with endometrial cancer each year (75). The 5-year survival rate of non-metastatic endometrial cancer is approximately 68%, with metastatic endometrial cancer 5-year survival rates around 16% (60, 82). Approximately 75,000 women will die each year from endometrial cancer (75). Risk factors for endometrial cancer include hormone imbalance, menstruation patterns, nulliparity, older age, hormone therapy for breast cancer, and family history (17, 99).
Overall Association
There have been 20 observational studies examining the risk of endometrial cancer and physical activity (36, 173). Of the 20 studies completed to date, seven have been cohort designs, and the remaining 13 have been case-control designs. The pooled risk reduction of endometrial cancer comparing highest versus lowest levels of physical activity among cohort studies has yielded a risk reduction of 23%, RR=0.77 (95% CI: 0.70–0.85). Among case-control studies, the pooled risk reduction is 29%, RR=0.71 (95% CI: 0.63–0.80), however this risk reduction included significant heterogeneity as studies varied between 2-fold increases to 2-fold decreases in risk, moreover these case-control studies were noted to be of poor methodological quality (36, 173).
Physical Activity Type and Dose
Leisure-time and occupational types of physical activity have been associated with reductions in endometrial cancer risk. Comparing the highest versus lowest levels of leisure-time physical activity, there was a statistically significant 27% risk reduction in endometrial cancer, RR=0.73 (95% CI: 0.62–0.86); (173). Similarly, comparing the highest versus lowest levels of occupational physical activity, there was a statistically significant 20% risk reduction in endometrial cancer, RR=0.80 (95% CI: 0.66–0.96); (173).
Subgroups
Four of seven cohort studies included in this meta-analysis examined BMI as a modifier of the association between physical activity and endometrial cancer risk, and did not find any evidence of effect modification (173). Similarly, six case-control studies examined BMI as a modifier of the association between physical activity and endometrial cancer risk, and two (33%), found evidence of effect modification (173). Few studies have examined the potential for menopausal status as an effect modifier (36, 173). Yet among those that have, it has been noted that there does not appear to be evidence of effect modification by menopausal status on endometrial cancer risk and physical activity (173).
Summary
A small, growing body of evidence suggests physical activity is associated with a risk reduction in endometrial cancer (Figure 4). Leisure-time and occupational physical activity provide a significant risk-reduction in endometrial cancer. There is insufficient evidence to identify other physical activity characteristics associated with endometrial cancer risk reduction. BMI has not been clearly and consistently demonstrated as an effect modifier of the physical activity and endometrial risk relationship among cohort and case-control studies.
Ovarian Cancer
Background
Worldwide, approximately 204,000 women are diagnosed with ovarian cancer each year (75). The overall 5-year survival of ovarian cancer is 47% (>90% for early stage diagnoses, <20% for metastatic disease); (60, 82). Approximately 125,000 women die each year from ovarian cancer, worldwide (75). Risk factors for ovarian cancer include genetic mutations, family history of ovarian cancer, previous breast, colon, rectum or uterine cancer diagnosis, nulliparity, and hormone replacement therapy for menopause (13, 103).
Overall Association
There have been 12 studies examining recreational physical activity and ovarian cancer risk (36, 118). Among the 12 studies, six cohort and six case-control studies have examined the association between recreational physical activity and ovarian cancer risk. The pooled risk reduction among the 12 studies comparing the highest versus lowest levels of recreational physical activity yielded a significant 19% risk reduction, RR=0.81 (95% CI: 0.72–0.92); (118). When stratifying by study design, the risk reductions were similar between cohort, and case-control study designs of 19%, RR=0.81 (95% CI: 0.57–1.17) and 21%, RR=0.79 (95% CI: 0.70–0.85), respectively (118).
Physical Activity Type and Dose
There has been no quantitative review examining various doses of physical activity on the magnitude of risk reduction for ovarian cancer (36, 118).
Subgroups
All studies included in this meta-analysis adjusted for age and parity, and most adjusted for BMI and oral contraceptive use. In subgroup analysis, when excluding four studies that did not adjust for oral contraceptive use, the risk reduction was attenuated to 17%, RR=0.83 (95% CI: 0.80–0.86); such that the risk reduction of developing ovarian cancer associated with physical activity may be confounded by prior oral contraceptive use. (118). In separate subgroup analysis, when excluding four studies that did not adjust for BMI, the risk reduction was attenuated to 19%, RR=0.81 (95% CI: 0.76–0.86); such that the risk reduction of developing ovarian cancer associated with physical activity may be confounded by BMI (118).
Summary
A small body of evidence suggests physical activity associates with a risk reduction in endometrial cancer (Figure 4). Despite this reduction in risk, there is insufficient evidence to identify the optimal dose of exercise and population subgroups that may or may not respond to physical activity to reduce their risk of ovarian cancer.
Lung Cancer
Background
Worldwide, approximately, 1.2 million people are diagnosed with lung cancer each year (75). The overall 5-year survival rate for lung cancer is 15% (75). Approximately 950,000 people die each year from lung cancer (75). Risk factors for lung cancer include smoking and secondhand smoke, exposure to carcinogenic chemicals (i.e., radon, asbestos, arsenic), prior radiation to the chest, and a prior family history of lung cancer (46).
Overall Association
There have been over 16 studies examining physical activity and lung cancer risk (46). Among these 16 studies, 12 cohort and four case-control studies have examined the association between physical activity and lung cancer. When stratifying by study design, the pooled risk reduction among the 12 cohort studies is 23%, and among 4 case-control studies, a pooled risk reduction of 38% (46).
Physical Activity Type and Dose
Among a meta-analysis of 11 studies comparing highest versus lowest levels of leisure-time physical activity, including odds ratios from studies in which the association between physical activity and cancer prevention was adjusted for smoking (163) intensity of leisure time moderated the relationship between physical activity and lung cancer risk. Moderate-intensity physical activity was associated with a statistically significant risk reduction in lung cancer, OR=0.87 (95% CI: 0.79–0.95), and vigorous-intensity physical activity was associated with a statistically significant risk reduction in lung cancer, OR=0.70 (95% CI: 0.62–0.79). The test for trend across these categories was significant ptrend<0.01, furthering supporting the dose-response relationship between intensity of physical activity and risk of lung cancer. Restricting the analysis to the subset of studies that implemented a previously validated physical activity questionnaire did not change the conclusions outlined above.
Subgroups
There have been seven studies that have examined the smoking-adjusted relationship between physical activity and risk of lung cancer among men, and four among women (163). The magnitude of risk reduction appears to be larger among women for moderate intensity of physical activity, OR=0.77 (95% CI: 0.66–0.89), compared to men, OR=0.93 (95% CI: 0.85–1.00). A similar relationship exists among vigorous intensity physical activity as well, among women, OR=0.62 (95% CI: 0.48–0.79), and among men, OR=0.75 (95% CI: 0.66–0.86).
Summary
A small body of evidence suggests physical activity associates with a risk reduction in lung cancer (Figure 4). There appears to be a dose-response relationship between intensity of physical activity and magnitude of risk reduction among both men and women. However, there is little evidence to suggest that risk reduction in lung cancer accomplished through physical activity is limited to current or former smokers. Moreover, there are limited known clinical characteristics that modify the relationship between physical activity and reductions in lung cancer risk.
Physical Activity and Primary Cancer Prevention Section Summary
As reviewed above, there is an abundant volume of epidemiologic observational literature examining the relationship between physical activity and risk reduction for a variety of cancer sites. A large proportion of this literature supports the association between physical activity and risk reduction for breast and colon cancers. A smaller proportion of this literature supports the association between physical activity and pancreatic, prostate, endometrial, ovarian, and lung cancer. There is little data on the association between physical activity and risk reduction in other cancer sites, particularly hematologic cancers, such as multiple myeloma, leukemia, and lymphoma. Beyond the overall association between physical activity and site-specific cancer risk (i.e., the main effect), there is limited evidence to support a specific dose of physical activity among a specific population subgroup, tailored for site-specific cancer risk reductions.
Physical Activity and Primary Cancer Prevention: Biologic Mechanisms
The previous section of this review examined the state of observational evidence on cancer prevention and physical activity. Though the depth and breadth of epidemiologic literature on physical activity and cancer prevention is rich, the molecular mechanisms that are associated with the observed reductions in cancer risk are less abundant, and thus less well understood. It has been suggested that an understanding of the molecular underpinnings would be useful to understand the associations between physical activity and cancer prevention, and provide more specific recommendations to engage in an adequate dose of physical activity (105).
There exist numerous mechanistic models hypothesized that include pathways relating to sex hormones, metabolic hormones, inflammation and adiposity, immune function, oxidative stress, DNA repair, and xenobiotic enzyme systems, to name a few (130). Given the numerous hypothesized mechanistic pathways posited, the literature to support any one of these pathways is limited. Cancer requires a long latent period to develop, and as seen in the primary prevention section of this review, it is desirable to follow a large number of persons asking them about their physical activity at pre-specified time-points, and waiting until a subset develop cancer (viz., a cohort study). To remedy the logistic issues associated with following a group of people until they develop the outcome of interest (i.e., cancer), an alternative is to identify and measure surrogate biomarkers for the molecular pathways that are assumed to underlie the purported causal relationship of physical activity and cancer risk (133). Despite the wide deployment of biomarkers as surrogates for the development of cancer, any one biomarker may provide a small fraction of the complete causal pathway linking physical activity with cancer risk reduction.
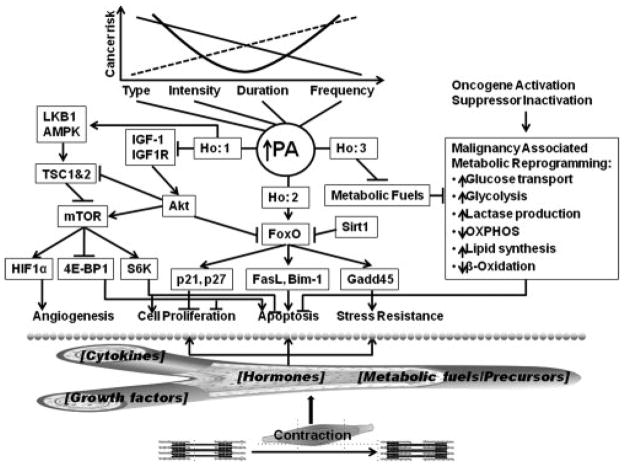
In a review by Thompson et al. (165) numerous candidate pathways have been identified to associate with varying doses of physical activity in animal models of mammary carcinogenesis (Figure 5). Through this example, we highlight the complexity of these biologic systems, and the synergistic relationship between each biologic pathway and physical activity. This complexity warrants further examination of the complete causal pathway, in attempt to delineate the complex mechanistic underpinning of physical activity and cancer risk reduction, such as that used in animal models of carcinogenesis.
Figure 5.
Candidate mechanistic pathways linking physical activity and breast cancer. Reproduced with permission from (165).
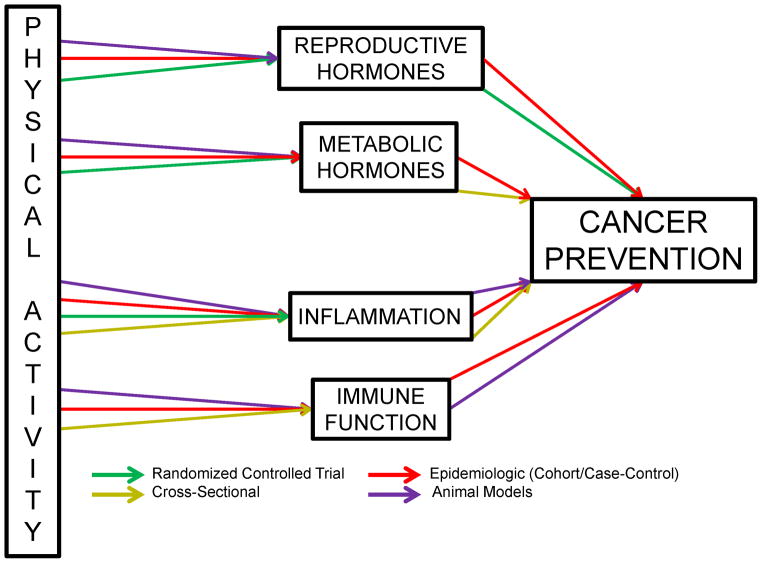
In this section we review the most commonly hypothesized, and well-supported pathways associated with physical activity and cancer prevention sex hormones, metabolic hormones, inflammation and adiposity, and immune function (Figure 6) (20, 52, 105, 130).
Figure 6.
Strength of evidence linking physical activity and hypothesized cancer prevention mechanistic pathways.
Sex Hormones
Elevated levels of estradiol and estrone have been associated with an increased risk of postmenopausal breast cancer (51, 84, 105). This association has been reproduced in both animal models of mammary carcinogenesis and in human trials (130). In a preclinical study, 120 rats were injected with 1-methly-1-nitrosourea, and randomized to physical activity or sedentary control (188). Rats in the physical activity group had access to a non-motorized activity wheel, and running behavior was rewarded with food. Rats in the sedentary control received the same volume of food as their physically active paired counterpart. Rats in the physical activity group had reduced mammary cancer incidence (p=0.015), and multiplicity (p=0.01). Physically active rats had more favorable levels of insulin, insulin growth factor-1, c-reactive protein, leptin, and estradiol, compared to sedentary rats (165, 188).
Among a reanalysis of nine prospective studies including 2,428 postmenopausal women not taking exogenous sex hormones, 663 women developed breast cancer, and 1,765 did not (84). Women in the highest quintile of estradiol concentration compared to the lowest quintile were at elevated risk for developing breast cancer, RR=2.00 (95% CI: 1.47–2.71); (84). The pattern of increased estradiol concentration and increased risk of breast cancer occurred in dose-response fashion, ptrend<0.001; (84). A similar pattern emerged for free estradiol as well, with the highest versus lowest quintile of free estradiol associated with an increased risk of breast cancer, RR=2.58 (95% CI: 1.76–3.86), demonstrating a dose-response pattern of increased breast cancer risk with increasing quintiles of free estradiol, ptrend<0.001; (84). Similarly, dose-response patterns were observed for estrone, and testosterone, RR=2.19 (95% CI: 1.48–3.22), ptrend<0.001; RR=2.22 (95% CI: 1.59–3.10), ptrend<0.001, respectively (84). Conversely, sex hormone-binding globulin was negatively associated with breast cancer risk, RR=0.66 (95% CI: 0.43–1.00), ptrend=0.041; (84). This negative association provides further evidence for the association between sex hormones and breast cancer risk, as sex hormone-binding globulin reduces circulating levels of estradiol and testosterone.
Evidence has emerged from randomized controlled trials examining biomarkers associated with increased cancer risk (53, 106, 107). A prospective randomized trial among 173 sedentary, overweight, postmenopausal women aged 50–75 years old, examined the effects of an aerobic exercise intervention on serum androgens and estrogens after 12-months of exercise (106, 107). Women performed moderate-intensity aerobic exercise, 5 d·wk−1, 45-min·d−1. After the 12-month intervention there existed no differences, on average, between groups in serum androgens and estrogens. However, among women who lost >2% of their baseline body fat, testosterone and free testosterone significantly decreased in the exercise group by 10.1% (p=0.02) and 12.2% (p=0.03), respectively (106). In addition, among women who lost >2% of their baseline body fat, serum estrone, estradiol, and free estradiol were reduced by 11.9%, 13.7%, and 16.7%, respectively (all p<0.05); (107).
The Alberta physical activity and breast cancer prevention (ALPHA) trial was a prospective randomized controlled intervention among 320 postmenopausal women aged 50–74 years old (53). The ALPHA trial was a one-year exercise intervention consisting of moderate-intensity aerobic exercise performed 5 d·wk−1, 45-min·d−1. After 12-months, the aerobic exercise group demonstrated significant improvements in estradiol, sex hormone-binding globulin, and free estradiol with treatment effect ratios (TER) of TER=0.93 (95% CI: 0.88–0.98), TER=1.04 (95% CI: 1.02–1.07), TER=0.91 (95% CI: 0.87–0.96); (53). However, when controlling for change in weight over one-year, the TER of sex hormone-binding globulin was attenuated to non-significance between intervention groups, estradiol and free estradiol TER’s remained significantly different between intervention groups (Figure 7); (53). There were no between group differences in estrone and testosterone at 12-months.
Figure 7.
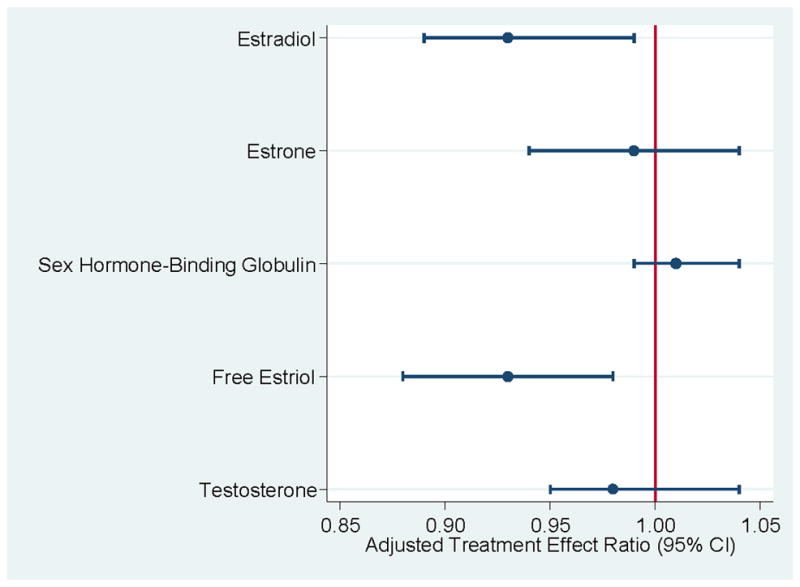
Weight adjusted treatment effect ratio of exercise to control on sex hormone concentrations after 12-months. Data from (53).
The results from the above-described trials (53, 106, 107) begin to shed light on specific sex hormones that may be mediated not by physical activity, but reductions in adiposity. In a quasi-experimental design, 7 women at elevated risk for developing breast cancer engaged in a moderate intensity aerobic exercise program. After five menstrual cycles, significant reductions in estrogen and progesterone were observed, with changes of −18.9% and −23.7%, respectively (88). A randomized controlled is underway to confirm the favorable changes in estrogen and progesterone through the use of moderate intensity aerobic exercise among women at elevated risk for developing breast cancer (26).
Metabolic Hormones
Insulin pathways have been linked to numerous cancers including breast (80), colon (59), endometrial (81), ovarian (94), multiple myeloma (108), Hodgkin’s-lymphoma (85), and acute lymphocytic and myeloblastic leukemia (85).
Basic science experiments have demonstrated insulin and insulin growth factor-1 elicit cell growth and inhibit apoptosis (97). Several cancer types have been identified to carry insulin receptors including colon (98), and hematopoietic cancer cells (85), that when stimulated by insulin, grow in size (122). This has been supported by animal models with chemically induced mammary carcinogenesis experiencing accelerated mammary growth when exposed to insulin (187). In a preclinical trial, 36 rats were given a single injection of the carcinogenic agent azoxymethane (20mg/kg) (30). After one week, rats were randomized into saline or insulin injection groups. After 100 days the rats injected with insulin had a greater multiplicity of aberrant crypt foci (p=0.007); (30). The authors of this study concluded exogenous insulin can promote colon carcinogenesis in rats, and lifestyle modification such as diet and exercise might protect humans against developing colon cancer (30).
Supporting these hypotheses, observational research has noted those in the highest quartile of insulin had an increased risk of colorectal cancer, hazard ratio (HR)=1.84 (95% CI: 1.03–3.30); (91, 142). A similar relationship was demonstrated among C-peptide, a marker of insulin secretion, comparing those in the highest versus lowest quartiles was associated with an increased risk of colorectal cancer, RR=1.63 (95% CI: 1.01–2.66), controlling for BMI and level of physical activity (175). Conversely, the relationship between insulin and breast cancer risk is less clear (114).
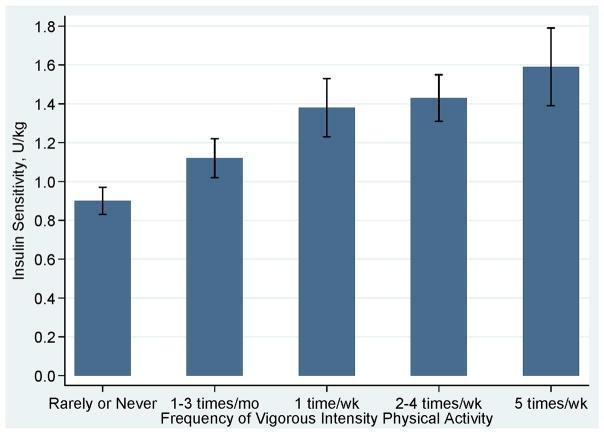
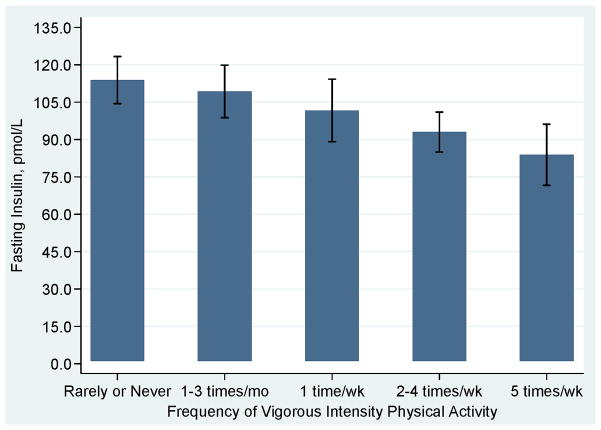
Exercise and weight loss is an effective intervention to improve insulin sensitivity, and potentially reduce risk of insulin-mediated cancers (177, 178). Interestingly, a single bout of exercise has been observed to increase insulin sensitivity for up to 60 hours (18). Chronic bouts of moderate-intensity exercise associate with reductions in insulin growth factor-1 and increased insulin growth factor binding protein (36, 50). In a study of 1,467 men and women aged 40–69 years old, insulin sensitivity and fasting insulin were moderated in dose-response fashion with more frequent bouts of vigorous physical activity producing more favorable insulin responses (Figures 8 and 9) (102).
Figure 8.
Adjusted insulin sensitivity according to frequency of participation in vigorous intensity physical Activity. Data from (102).
Figure 9.
Adjusted fasting glucose according to frequency of participation in vigorous intensity physical Activity. Data from (102).
Inflammation & Adiposity
Inflammation is linked to a variety of chronic diseases including arthritis, diabetes, heart disease, and cancer (20). Chronic states of inflammation are hypothesized to increase risk of cancer development by degrading healthy cell growth, thereby promoting the progression of damaged cellular growth, and increased risk of tumor development.
Preclinical trials have linked inflammation and adiposity to cancers in animal models. For example, over 23 studies have examined chemically induced colon carcinogenesis in rat or mouse models and concluded that inflammation is closely linked to polyp formation and progression. In particular the cyclooxygenase isoenzymes (COX-1 and COX-2) have been identified in animal models to play an important role in intestinal tumor formation (120).
Biomarkers associated with inflammatory states such as interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) have been associated with increased risk of cancer in humans (70). Among a cohort of 2,438 older adults aged 70–79 years, IL-6 was associated with a 13% increased risk of developing cancer, HR=1.13 (95% CI: 0.94–1.37); (70). CRP was associated with a 25% increased risk of developing cancer, HR=1.25 (95% CI: 1.09–1.43), and TNF-α was associated with a 28% increased risk of developing cancer, HR=1.28 (95% CI: 0.96–1.70). These biomarkers of inflammation are associated with cancer-related death, with IL-6, CRP, and TNF-α, HR’s of 1.63 (95% CI: 1.19–2.23), 1.64 (95% CI: 1.20–2.24), and 1.82 (95% CI: 1.14–2.92), respectively (70). The hypothesis of low-grade inflammation is further supported by the chemo-preventive consumption of non-steroidal anti-inflammatory medications and reduced risk of colon cancer among both men and women with RR=0.60 (95% CI: 0.40–0.89), and 0.58 (95% CI: 0.37–0.90), respectively (166).
Exercise intervention studies have reported mixed results on the reduction of biomarkers associated with physical activity including CRP, and IL-6. For example, among 120 premenopausal obese women ages 20–46 years old who were randomized to diet, physical activity, and weight loss, or to the control group, CRP and IL-6 decreased significantly in the intervention group compared to the standard care group after two years, with changes of −1.6mg·L−1, p=0.008, and −1.1pg·mL−1, p=0.009 (47). Moreover, cross-sectional descriptive studies suggest body composition may serve as a mediator to the relationship between physical activity and biomarkers of inflammation; which is similar to the sex hormone hypothesis (52, 53). However, the independent associations of physical activity and these select biomarkers is mixed; improvements in body composition, predominately the reduction in adipose tissue may mediate the observed associations (20, 51, 52, 105).
Immune Function
The immune system has been a recently hypothesized pathway in the reduction of cancer risk in animal models and humans (105, 151). This pathway is charged with identifying and destroying abnormal cells through acquired immune components (20, 105). A systemically impaired immune system such as that with the acquired immunodeficiency syndrome (AIDS) has been associated with increased incidence of cancer (i.e., Kaposi’s sarcoma); (12, 66). The immune system and cancer risk has largely evolved around interest in improving the number of natural killer (NK) cells, which influence tumor suppression (20, 51, 52, 105).
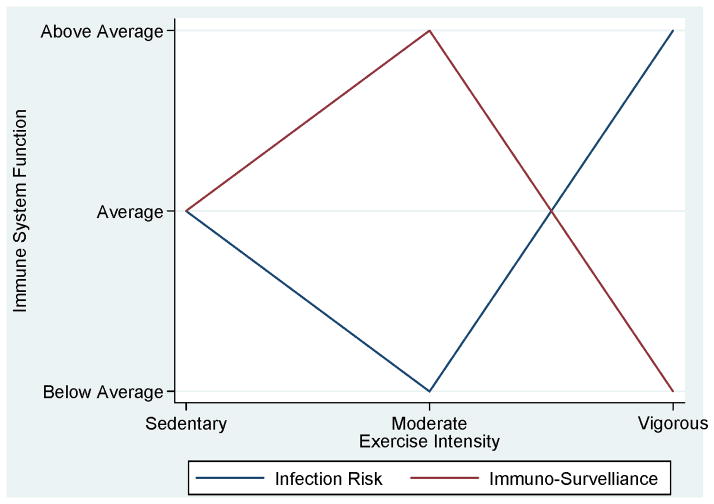
During exercise, particularly during moderate-intensity aerobic exercise T-cell populations transiently rise, NK cell populations and activity transiently rise, and neutrophil quantity and activity also transiently rise (151). Although these effects are transient during an acute bout of exercise, the repetitive effects may produce a cumulative (training) effect (151). Chronic bouts of physical activity have been associated with an inverted ‘J-curve’ such that optimal immune function is achieved with moderate-intensity physical activity and sedentary and vigorous-intensity below optimal immune-system function (Figure 10); (116). There is limited, inconclusive evidence to support the role of immune function as a pathway for cancer prevention (105). Evidence to date suggests cancers associated with an impaired immune system are different than the cancers associated with lack of physical activity (122). The cancers associated with lack of physical activity are generally hormonal in nature, such as breast and colon cancer.
Figure 10.
Exercise intensity and optimal states of infection risk and immuno-surveillance. Reproduced with permission from (115).
Conclusion
In summary, there is growing observational evidence supporting mechanistic hypothesis relating to physical activity and cancer prevention (Table 1). A scant portion of the data from these observational trials is supported by data from randomized controlled trials (130). However, there are numerous randomized intervention trials underway that may shed light on the above-described associations established by the few prospective randomized interventions conducted to date (53, 106, 107). Elucidating the—likely very complex—mechanistic pathway underling the association between participating in physical activity and cancer prevention will lend credibility to engaging in regular physical activity and may provide an impetus for policy change and reform given the large burden of cancer on the population (105, 130).
Table 1.
Physiologic pathway and biologic markers associated with cancer prevention and physical activity
| Physiologic Pathway | Biologic Marker in Physiologic Pathway | Cancer Risk |
|---|---|---|
| Sex Hormones | Estrogen | ↑ |
| Testosterone | ↑ | |
| Sex Hormone Binding Globulin | ↓ | |
| Metabolic Hormones | Insulin Resistance | ↑ |
| Insulin Levels | ↑ | |
| Insulin Growth Factors I–III | ↑ | |
| Insulin Growth Factor Binding Protein I–III | ↓ | |
| Inflammation & Adiposity | Excess Adiposity | ↑ |
| Tumor Necrosis Factor-alpha | ↑ | |
| Interleukin-6 | ↑ | |
| C-Reactive Protein | ↑ | |
| Immune Function | Natural Killer (NK) cell activity | ↓ |
| Lymphocyte Production | ↓ |
Deleterious Sequelae from Cancer Treatment
Dependent on type and stage of cancer, treatment modalities such as surgery, chemotherapy, radiation, endocrine, transplant, and targeted therapies are to achieve varying goals. Those goals may be to cure the cancer, control the cancer, relieve symptoms, or prevent recurrence (128). Given the complex nature of cancer treatment modalities are often combined making the toxicities unique to individual modalities difficult to elucidate (158, 159). Treatment modalities may act synergistically resulting in deleterious sequelae from two or more cancer treatments (159). Two examples discussed later in this section are the use of surgery and radiation therapy and the associated risk of developing upper or lower limb lymphedema. Another example includes the use of anthracycline-based chemotherapy and radiation, and the associated risk of developing cardiotoxic complications.
To this end, the focus of this section is not to describe all possible complications that may result from cancer treatment, but to highlight the most common toxicities, particularly the physiologic toxicities that are—or hypothesized to be—amendable to physical rehabilitation. Accordingly, we review surgery, chemotherapy, radiation, endocrine, transplant, and targeted therapies and their associated acute and late toxicities.
Surgical Oncology
Surgery was the first effective treatment for cancer, and is a cornerstone for the contemporary treatment of many solid tumors (128). Modern practices of surgical oncology integrate clinicians who are experts in pathology, medical oncology, radiation oncology, and other specialty disciplines. Early examples of surgery in oncology date back a century or more—1904 with radical prostatectomy, 1906 with radical hysterectomy, and 1908 with abdominoperineal resection for rectal cancer (128). The goals of surgery may be curative, palliative, for prolongation, or for prevention of complications through local control (128).
Surgical Oncology Sequealae
As noted above, we acknowledge there are an abundant number of symptoms and side effects that occur as a result of cancer surgery. Despite these numerous symptoms and side effects, there exists limited evidence to support the efficacy of exercise to improve these symptoms and side effects. As such, we focus our review on the most studied surgical complications, lymphedema of the upper and lower limbs, and general functional impairments occurring after surgery.
A potential side effect for cancer survivors with breast cancer is the risk of developing upper limb lymphedema. Among a meta-analysis of 98 studies examining secondary upper limb lymphedema after breast cancer surgery, five surgical procedures were identified as increasing the risk of developing upper limb lymphedema (168). Surgical risk factors included having a mastectomy compared to lumpectomy, RR=1.42 (95% CI: 1.08–1.87); radical mastectomy compared to any other mastectomy, RR=2.66 (95% CI: 2.01–3.52); axillary dissection compared to sentinel lymph node biopsy, RR=2.99 (95% CI: 1.89–4.74); axillary dissection compared to no axillary dissection, RR=3.19 (95% CI: 1.99–5.10); and having positive lymph nodes compared to no positive lymph nodes, RR=1.59 (95% CI: 1.35–1.86); (168). These risk factors are consistent with the understanding that treatments which disrupt lymphatic flow through the axilla could lead to development of lymphedema (136). Treatment choices for cancer patients are generally dictated by tumor characteristics and surgical needs and expertise to maximize disease control and patient outcomes, and not the risk of developing post-surgical complications (128).
Women undergoing the procedures listed above are at heightened risk for developing lymphedema. Other risk factors associated with developing lymphedema that are not directly related to surgical procedures exist (136). Non-surgical procedures that increased risk for lymphedema included receiving radiation therapy, compared to no radiation therapy, RR=1.91 (95% CI: 1.54–2.37) and radiation to the axilla, compared to no radiation to the axilla, RR=3.06 (95% CI: 2.02–4.63) (168). Receipt of chemotherapy, however, was not a risk factor for the development of lymphedema, RR=1.10 (95% CI: 0.90–1.35); (168).
Women may experience symptoms and side effects from breast surgery immediately after surgery or years after surgery (157). These side effects are termed acute and late side effects. Among a cohort of 191 women who underwent breast-surgery, upper limb dysfunction was measured at time points of 3-, 6-, and 12-months post-surgery (185). Upper limb dysfunction included signs and symptoms of any one or more of the following: pectoralis tightness, lymphedema, myofascial pain syndrome, rotator cuff disease, adhesive capsulitis, post-mastectomy pain syndrome, or axillary web syndrome (185). Among the 191 women, 24.6%, 20.9%, and 26.8%, had upper limb dysfunction at 3-, 6-, and 12-months, respectively. The prevalence of upper limb dysfunction was higher among 62 women who underwent axillary dissection compared to 125 women who underwent sentinel lymph node biopsy with 3-, 6-, and 12-month prevalence as 38.7% versus 18.4%, 40.0% versus 12.2%, and 44.1% versus 19.2%, respectively (Figure 11); (185). After adjusting for patient characteristics, factors that associated with risk of pectoralis tightness at 12-months were age, OR=0.89 (95% CI: 0.80–0.99), receipt of radiation therapy, OR=0.08 (95% CI: 0.01–0.59), and having a mastectomy, OR=18.29 (95% CI: 1.16–207.78); (185). After adjusting for patient level characteristics, the lone factor that increased risk of lymphedema at 12-months was axillary lymph node dissection, OR=4.55 (95% CI: 1.38–14.97); (185).
Figure 11.
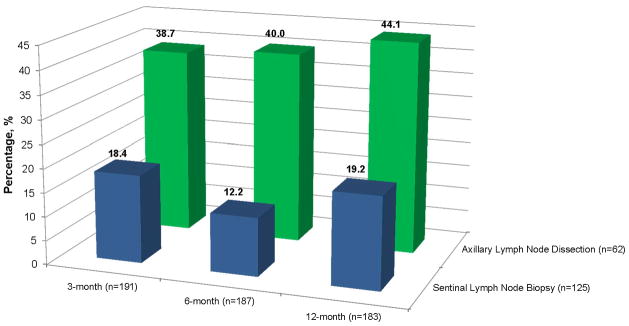
Prevalence of upper limb dysfunction among breast cancer survivors. Data from (185).
In addition to lymphedema and upper limb dysfunction, upper extremity pain disorders are common among breast cancer survivors. As many as 88% breast cancer survivors report some form of arm morbidity and associated pain (160). The most commonly diagnosed disorders include edema, upper limb tightness (referred to as postmastectomy syndrome), lateral epicondylitis, adhesive capsulitis, and rotator cuff tendonitis (160). In a retrospective case-series of eight women with breast cancer related lymphedema and rotator cuff tendonitis, seven out of eight reported subjective increases in shoulder pain at four to six week follow-up (67). It is hypothesized the complications of rotator cuff tendonitis and lymphedema is caused by derangement of tendon fibers in the shoulder, which are then subject to impingement, functional overload, and tendinopathy, which result in pain, and limited functional mobility of the shoulder (67, 157). Currently, the recommended clinical management of rotator cuff complications among breast cancer survivors with lymphedema is with non-steroidal anti-inflammatory drugs and physical rehabilitation exercises (67, 157).
The discussion up to this point has focused on surgical complications among breast cancer survivors. Nevertheless, any cancer patient who undergoes surgery is subject to deleterious surgical sequelae, particularly lymphedema if there is disruption of the lymphatic system in the upper limbs (i.e., breast cancer), or in the lower limbs (i.e., gynecologic and genitourinary cancer, and melamonas). In a meta-analysis among 47 studies including 7,779 cancer survivors, the overall incidence of lower limb lymphedema among all cancers was 15.5% (95% CI: 11.0–21.0), and varied (p<0.001) by type of malignancy (29). Among gynecologic and genitourinary cancers, and melanoma, the incidence of lower limb lymphedema was 20%, 10%, and 28%, respectively (29). Similar to upper limb lymphedema among breast cancer survivors, in the 7,779 cancer survivors with or at-risk for lower limb lymphedema, risk increased with pelvic lymph node dissection, and receipt of radiation therapy with incidence estimates of 22%, and 31%, respectively (29).
As a result of the deleterious side effects from surgery, various surgical procedures have been examined to identify techniques that reduce the risk of developing lower limb lymphedema. In a meta-analysis of four studies, preservation of the saphenous vein was associated with a reduced likelihood of developing lower limb lymphedema, OR=0.24 (95% CI: 0.11–0.53); (1). Additionally, vein sparing inguinal node dissection reduced the likelihood of developing cellulitis (a bacterial infection treated with broad-spectrum antibiotics), OR=0.40 (95% CI: 0.16–0.96) and reduced likelihood of wound breakdown, OR=0.34 (95% CI: 0.19–0.59); (1). However, there exists no evidence from randomized controlled trials to support these associations.
Chemotherapy (Medical Oncology)
Chemotherapy was first used in 1895 for breast cancer by Beatson (128). Since that time, the growing knowledge of tumor cell biology has provided the infrastructure needed for the development of a variety of chemotherapeutic agents. Varying in mechanism, we review three of the most common forms of chemotherapy, antracyclines, taxanes, and platinum-based chemotherapies that exercise specialists may encounter (149). Anthracyclines, in particular doxorubicin and daunoribicin, are classified as topoisomerase inhibitors (128). The antineoplastic behavior of anthracyclines is broad, as these drugs interact with a variety of biochemical systems in tumor cells inhibiting DNA and RNA synthesis, blocking DNA transcription and replication, and damaging cell membranes. Taxanes, commonly paclitaxel and docetaxel, impair cell function by affecting cell microtubules, thereby preventing tumor cells from successfully replicating (149). Platinum based chemotherapies, commonly cisplatin and oxaliplatin, induce cell death by interfering with DNA replication and transcription.
This section provides an overview of some of the widely studied chemotherapeutic agents and their associated late-side effects, which may be amendable to exercise rehabilitation. Chemotherapy is associated with a variety of cardiovascular complications including cardiomyopathy and potential heart failure, ischemia, hyper- or hypotension from endothelial dysfunction, thromboembolism, bradycardia, QT wave prolongation, and neurotoxicities (22, 149, 186).
Chemotherapy Sequelae
Anthracyclines
The incidence of left ventricular dysfunction among patients treated with anthrcyclines ranges from 0.9–26%; (186). The effect of dose-dependent anthracycline toxicity appears to be independent of the type of cancer being treated (22). A consensus panel identified patient and treatment risk factors for anthracycline-associated cardiomyopathy. Patient level characteristics include young (<18) or old (>65) age patients, pre-existing cardiac disease, pregnancy, and extreme or vigorous-intensity sport participation. Treatment risk factors include high cumulative dose of anthracycline, associated radiation treatment, treatment with combination therapy (i.e., targeted therapies), and longer duration of survival (22).
In a meta-analysis of 55 published studies reporting acute and late cardiotoxic factors associated with anthracycline-based chemotherapy among patients treated for breast or ovarian cancer, lymphoma, myeloma or sarcoma (153), the authors identified anthracycline-based chemotherapy associated with an increased risk of clinical cardiotoxicity compared to non-anthracycline-based chemotherapy agents, OR=5.43 (95% CI: 2.34–12.62). This association was observed when anthracyclines were administered in bolus form compared to continuous infusions, OR=4.13 (95% CI: 1.75–9.72). Sub-clinical cardiotoxicity also increased, OR=6.25 (95% CI: 2.58–15.13). As such, anthracyclines are associated with an increased likelihood of cardiac death, OR=4.94 (95% CI: 1.23–19.87); (153).
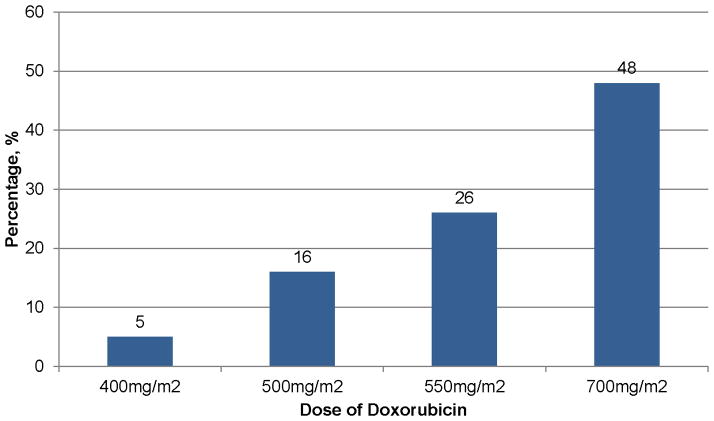
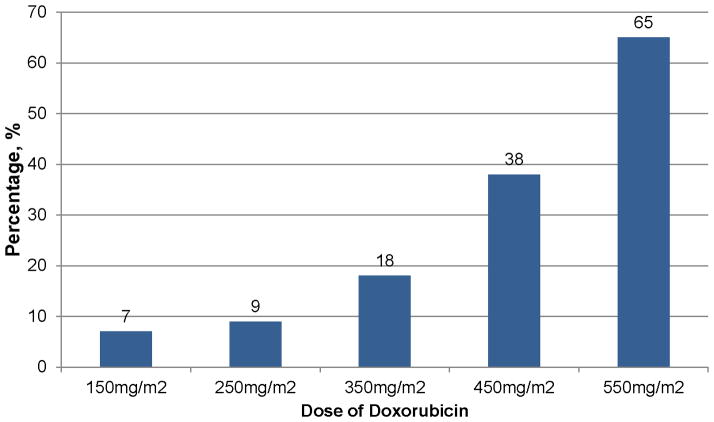
In a retrospective analysis of three treatment trials, the estimated cumulative percentage of patients with congestive heart failure was dose-dependent among 630 breast and small-cell lung cancer patients randomized to doxorubicin-plus-placebo (161). Congestive heart failure was 5%, 16%, 26%, and 48%, at cumulative doses of 400mg/m2, 500 mg/m2, 550 mg/m2, and 700 mg/m2, respectively (Figure 12); (161). Older age (> 65 yrs) was a risk factor for developing congestive heart failure while being treated with higher doses of doxorubicin (i.e., >400 mg/m2), HR=3.28 (95% CI: 1.40–7.65); (161). Among the 630 patients treated with doxorubicin, 149 (23.6%), experienced a cardiac event (161). The cumulative percentage of occurrence of any cardiac event was also doxorubicin dose-dependent with 7%, 9%, 18%, 38%, and 65% of patients treated with 150 mg/m2, 250 mg/m2, 350 mg/m2, 450 mg/m2, and 550 mg/m2, respectively (Figure 13); (161).
Figure 12.
Prevalence of congestive heart failure at varying doses of anthracyline. Data from (161).
Figure 13.
Prevalence of any cardiac event at varying doses of anthracycline. Data from (161).
The risk of cardiotoxicity from anthracyclines appears to be dose-dependent, in a non-linear fashion. This sharp increase in risk of cardiotoxicity with concurrent increases in anthracycline dose has spurred a consensus panel to state there is likely no such safe dose of doxorubicin—as an increasing number of emerging studies are finding a greater proportion of cardiotoxicity at lower cumulative doses than previously thought (22). A review was recently published that examined the evidence of cardiotoxicitity among breast cancer survivors (139). This review concluded up to 33% of breast cancer survivors may experience cardiotoxicity from cancer treatment. Despite the high prevalence of cardiotoxicity, there is limited data to help exercise physiologists develop and tailor exercise interventions for cancer survivors who may have cardiotoxicities. Current recommendations salient to exercise include maximal exercise stress testing with echocardiogram to identify heart abnormalities with physical exertion (22). This paucity of data is a major research gap given the prevalence of cardiotoxicities in the population and the potential for exercise to be an efficacious means to improve cardiovascular health.
Taxanes and Platinum based Chemotherapies
In meta-analysis of 13 studies including 22,903 patients, the addition of taxane-based chemotherapy into anthracycline-based chemotherapy regimens improved disease-free survival as well as overall survival, HR=0.83 (95% CI: 0.79–0.87), and HR=0.85 (0.79–0.91), respectively, compared to anthracycline-based chemotherapy alone (40). A meta-analysis of 37 trials including 7,633 non-small-cell lung cancer survivors reported the use of platinum-based chemotherapy increased the odds of responding to chemotherapy, OR=1.62 (95% CI: 1.48–1.80); (38). Despite the improvements in survival, numerous side effects were reported among platinum-based chemotherapies, including neutropenia, OR=1.23 (95% CI: 1.01–1.49), thrombocytopenia, OR=3.10 (95% CI: 2.29–4.19), nausea and vomiting, OR=1.92 (95% CI: 1.35–2.73), and increased risk of toxic death, OR=3.34 (95% CI: 1.03–10.8); (38). Peripheral neuropathies may also result from taxane or platinum-based chemotherapies (149), however statistically significant increases have not been consistently observed, OR=1.42 (95% CI: 0.90–2.25); (38).
Radiation Oncology
Radiation therapy is used to treat approximately of 50% of cancer patients (128). Radiation can be described as a form of energy, particularly ionizing energy. Radiation energy is used to damage the molecular structure leading to injury of DNA and other structures critical for cell survival and replication (128). Technological advances have allowed radiation oncologists to optimize the delivery of radiation to a tumor site, allowing the treatment of the tumor while minimizing any residual radiation exposure to surrounding tissues. These improvements are evidenced in the subsequent paragraphs, describing the temporal sequence of radiation-induced cardiac-related toxicity, and where research focus may be needed, given improvements in the delivery of radiation therapy treatment.
Radiation Oncology Sequealae
Patient and treatment-related factors may increase risk of cardiotoxicity after radiation to the chest wall (22). Patient-related factors for radiation-related cardiotoxicity include treatment with anthracyclines, tumor proximity to the heart border, age (<18), pre-existing cardiac disease and known cardiac risk factors, and >10 yr. post-radiation treatment (22). Radiation treatment related factors include older radiation technology (orthovoltage), volume of irradiated heart, radiation dose to the heart (>30 Gray), daily dose fraction (>2 Gray·day−1), and absence of subcranial blocking (22).
Radiation therapy is associated with coronary artery disease, valvular disease, chronic pericardial disease, cardiac abnormalities including arrhythmias and conduction, cardiomyopathy, and stenosis of the carotid artery (22). However, with newer, more precise radiation techniques, the cardiotoxic effects of radiation therapy are less frequent and severe than previous research has predicted.
For example, an analysis of 27,283 women treated with adjuvant radiation for breast cancers from 1973–1989 were reviewed to examine the temporal pattern of risk of death from ischemic heart disease (58). Among three time periods, 1973–1979, 1980–1984, and 1985–1989, the risk of ischemic heart disease decreased approximately 6% with each succeeding treatment year compared to the 1973–1979 group of women, HR=1.50 (95% CI: 1.19–1.87) compared to HR=0.94 (95% CI: 0.91–0.98), respectively (58). Additionally, in an analysis of 16,269 women diagnosed with left or right side breast cancer, followed for a mean of 9.5 years, the prevalence of any cardiac event was 3,448 (21.1%); (124). More specifically, the prevalence of ischemic heart disease was 1,594 (9.8%), valvular heart disease was 465 (2.9%), cardiac conduction abnormalities were 1,571 (9.7%), and heart failure and cardiomyopathies were 1,572 (9.6%); (124). Moreover, there exist no differences between women diagnosed with left- versus right-sided breast cancer, as radiation to the left breast is associated with cardiac damage (124).
Although the frequency and severity of cardiac-related toxicities from radiation treatment are decreasing over time, there exists an evolving burden of neuromuscular and musculoskeletal complications from radiation treatment. Case-reports of radiation-induced neuromuscular fibrosis exist from survivors of Hodgkin’s lymphoma are emerging (159). Radiation exposure may result in fibrotic sclerosis on exposed nervous tissues, resulting in pain, sensory loss, and weakness (157, 159). Radiation exposure to muscular tissue may result in focal myopathies, prone to painful spasm, weakness, and fatigue (159). It is suggested muscular spasm coupled with nerve damage results in continuous muscle contraction, subsequent acidic environmental conditions in the muscle, impaired blood flow, and muscular hypoxia (159). The hypoxic environment in the muscle elicits a cascade of inflammatory markers released into the vasculature, thereby perpetuating the above-described cycle (148). Clinical observations further suggest that radiation impairs tendon and ligaments, and deteriorates bone structure (157, 159).
Endocrine Therapy
In 1895 Beatson demonstrated that oophorectomy would slow the progression of breast cancer (128). In succeeding years, varieties of endocrine-based treatments have been developed to treat cancer (128). Three of the most common endocrine therapies include tamoxifen and aromatase inhibitors to treat breast cancer, and androgen deprivation therapy to treat prostate cancer (128). It is likely that exercise professionals working with breast and prostate cancer survivors will encounter cancer survivors treated with a variety of endocrine therapies. A broad understanding of the side effects of each of these endocrine therapies is important to enable exercise professionals to tailor exercise prescriptions to maximize outcomes and ensure safety.
Tamoxifen is a non-steroidal anti-estrogen, which alters estrogen receptor genes, blocks progesterone receptor synthesis, and stimulates gene regulation potentials. Given the diverse actions, tamoxfien may reduce the risk of breast cancer, but increase the risk of other cancers, particularly endometrial cancer (128). Aromatase inhibitors act by inhibiting aromatization of androgens to estrogens, thereby reducing the risk of breast cancer recurrence. Aromatase inhibitors produce undesirable side effects including muscle pain, and weakened bone mineral integrity (101, 132). Androgen deprivation therapy is commonly used as an adjuvant to treatment for prostate cancer (150). Androgen deprivation has many deleterious side effects including decreased libido, impotence, hot flashes, and osteopenia (150). Nevertheless, endocrine therapies have become a standard component to the management of breast and prostate cancer (128, 150).
Among 6,241 women prescribed tamoxifen or the aromatase inhibitor anastrozole at 100-month follow-up, disease-free survival, time to recurrence, time to distance recurrence, and contralateral breast cancer favored use of anastrozole, with HR’s of 0.90 (95% CI: 0.82–0.99), 0.81 (95% CI:0.73–0.91), and 0.86 (95% CI: 0.75–0.98), and 0.68 (95% CI: 0.49–0.94), respectively (7). Restricting the analyses to women with hormone-receptor-positive breast cancer produced similar outcomes (7). There were no differences between tamoxifen and anastrozole with respect to death from all-cause, death after recurrence, and death without recurrence, HR=1.00 (95% CI: 0.89–1.12), 0.91 (95% CI: 0.79–1.05), and 1.12 (0.94–1.33), respectively (7). In similar subgroup analysis among hormone-receptor positive women, the results did not differ substantively.
Endocrine Therapy Sequelae
A common complaint among breast cancer survivors on endocrine therapy, particularly aromatase inhibitors, is musculoskeletal pain (42). In a cross sectional sample of 300 consecutive breast cancer survivors, 47% reported one or more symptoms consistent with aromatase inhibitor associated pain (101). Over 50% of those surveyed with aromatase inhibitor associated pain reported experiencing symptoms within one month of starting treatment (101). This pain may impair activities of daily living, as 51.8% of women reported pain in their feet, 59.7% in their knees, 60.4% in their wrist/hand, and 54.0% in their lower back (101).
Women with hormone-receptor positive breast cancer who reported musculoskeletal symptoms and side effects from the tamoxifen or anastrozole were compared to women who did not report these symptoms to examine the relationship between musculoskeletal symptoms and cancer recurrence. Among 3,964 eligible women, 1,486 (37.5%) reported musculoskeletal symptoms at 3-month follow up (37). Women reporting musculoskeletal symptoms from either tamoxifen or anastrozole at 3-months had a lower risk of recurrence compared to women who did not report joint or musculoskeletal symptoms, HR=0.72 (95% CI: 0.60–0.85), controlling for age, BMI, previous hormone-replacement therapy, nodal status, and tumor grade (37).
In a meta-analysis of seven randomized controlled trials that included 30,023 participants, the use of aromatase inhibitors were associated with an increased risk of cardiovascular disease compared to tamoxifen, OR=1.26 (05% CI: 1.10–1.43); (5). The use of aromatase inhibitors compared to tamoxifen were associated with a decreased risk of venous thrombosis, OR=0.55 (95% CI: 0.46–0.64), and reduced risk of endometrial cancer, OR=0.34 (95% CI: 0.22–0.53); (5). Conversely, the use of aromatase inhibitors compared to tomxifen were associated with an increased risk of bone fracture, OR=1.45 (95% CI: 1.33–1.60), and an increased risk of hypercholesterolemia, OR=2.36 (95% CI: 2.15–2.60); (5). There existed no difference in developing other secondary cancers between aromatase inhibitors and tamoxifen, OR=0.98 (95% CI: 0.85–1.14); (5).
In a clinical trial, 456 prostate cancer survivors were randomized to receive radiation therapy, or radiation therapy and androgen deprivation therapy (127). At follow-up, androgen deprivation was associated with improvement in local control (p=0.016), reduction in distant metastases (p=0.04), disease free survival (p<0.0001), and cause-specific mortality (p=0.05); (127). Despite these favorable benefits in survival, many of those undergoing androgen deprivation therapy experience musculoskeletal deficits including losses in muscle strength and osteoporosis (150) which may be amendable to exercise intervention. Among 155 prostate cancer survivors undergoing androgen deprivation therapy, those randomized to participate in 12 weeks of resistance exercise demonstrated favorable improvements in upper and lower body strength, fatigue, and quality of life compared to those randomized to receive standard care (146). Improvements in lower body strength, 6-meter forward and backward walk time have also been observed among prostate cancer survivors engaging in exercise training (56).
Hematopoietic Stem Cell Transplantation
Transplantation of stem cells is a relatively new treatment modality commonly reserved for patients with hematologic malignancies. Hematopoietic stem cell transplantation (HSCT) can be divided into several types, including autologous and allogeneic (128). Autologous transplantation extracts the patient’s own cells which are then stored for future use. Conversely, allogeneic transplantation uses healthy matched donor cells. Despite the success of both techniques, each carries its own set of toxicities, particularly graft-versus-host-disease, that requires immunosuppressive medications to treat (128). Relative to the depth of literature on chemotherapy, radiation, and endocrine therapy, there is scant literature on the late effects of stem cell transplantation. Therefore, we discuss two relatively larger observational studies that have identified late effects of hematopoietic stem cell transplantation.
Hematopoietic Stem Cell Transplantation Sequelae
A case control study (162), compared the late effects of 137 patients who underwent hematopoietic cell transplantation with non-transplant controls matched on age, sex, race, self-reported medical problems, and health-related quality of life. At 10-year follow-up, more cancer survivors reported having a history of cataract surgery compared to controls, 38% vs. 1%; p<0.001 and more frequent on going moderate or severe medical problems, 3.5±2.8 versus 1.7±2.0; p<0.001 (162). Medical problems included frequent musculoskeletal issues, leg cramps, stiffness, urinary incontinence, impaired memory, ankle swelling, and skin problems (162). Among a possible total of 27 symptoms, cancer survivors had significantly more moderate or severe medical problems compared to matched controls. Cancer survivors also reported more frequent use of antidepressant or antianxiety medications 21% versus 10%, p=0.009; and poorer self-reported appearance 31% versus 3%, p<0.001, respectively (162).
Among a cohort of 1,087 cancer survivors treated with hematopoietic cell transplant, the prevalence of nonmalignant late-effects at 37-months follow-up was 44.8%, and 79%, among autologous, and allogeneic transplant patients, respectively (86). Frequent new comorbidities post-transplant are highlighted (Figure 14), for allogeneic and autologous transplant types. In multivariable analysis, among 15 potential risk factors associated with developing a new post-transplant late-effects among autologous transplant, age, multiple myeloma, and total body irradiation were risk factors with HR’s of 1.5 (95% CI: 1.1–2.3), 0.7 (95% CI: 0.5–1.0), and 0.4 (95% CI: 0.2–0.7), respectively (86). Among allogeneic transplant, age, female gender, and having an unrelated donor were associated with developing a new post-transplant late-effect with HR’s of 1.3 (95% CI: 1.0–1.6), 1.5 (95% CI: 1.2–1.9), and 1.3 (95% CI: 1.1–1.6), respectively (86).
Figure 14.
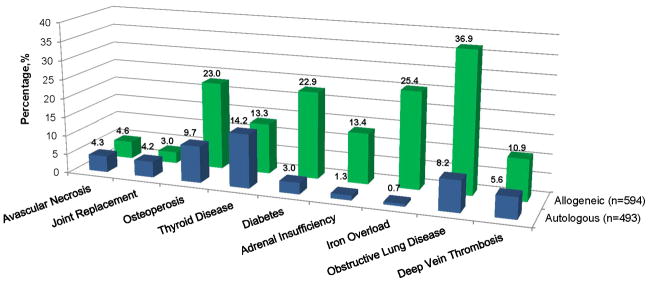
New comorbidities acquired post-transplant at 37-month follow-up. Data from (86).
Targeted Therapies
Targeted cancer therapies, referred to more specifically as molecularly targeted therapies, have recently emerged as a tumor-specific method to block cell growth and impair the spread of cancer in the body. Targeted therapies interfere with cell signals to prohibit cellular and blood vessel growth, induce cell death, and stimulate immune function (128). Over two dozen varieties of targeted therapies exist, each with a specific target and mechanism of action (128). The use of targeted therapies is a rapidly evolving science. As such, exercise specialists working with cancer survivors on targeted therapies must be informed of commonly used targeted therapies and their subsequent side effects will be described. Trastuzumab is a monoclonal antibody that impairs breast cancer cell-reproduction by binding to the HER2 protein. Bevacizumab is an angiogenic inhibitor, which acts by preventing the growth of new blood vessels through vascular endothelial growth factor A.
Targeted Therapy Sequelae
Trastuzumab
Trastuzumab is a monoclonal antibody targeting p185HER2, a receptor kinase of the epidermal growth factor family (184). Approximately 20–30% of all breast cancers overexpress this receptor protein, so trastuzumab is used among women diagnosed with HER2-positive breast cancer. Trastuzumab is associated with a variety of survival and recurrence benefits and is well studied (8, 16, 110, 156, 172, 183). However, evidence is emerging for cardiotoxic, and brain metastatic risks from treatment. Trastuzumab cardiotoxicity differs from anthracycline-based cardiotoxicity because it is not dose-related, is frequently reversible, and rarely results in death (22). It has been recommended trastuzumab not be used concurrently with an anthracycline, because the two act synergistically to increase the risk of congestive heart failure (22). Patient level risk factors for trastuzumab-related cardiotoxicity include older age, and decreased left ventricular ejection fraction associated with anthracycline treatment (22). The survival benefit associated with trastuzumab use outweighs the risk of cardiac toxicity (22).
In a meta-analysis of five randomized trials, trastuzumab significantly reduced mortality, OR=0.52 (95% CI: 0.44–0.62), recurrence, OR=0.53 (95% CI: 0.46–0.60), metastases rates, OR=0.53 (95% CI: 0.45–0.61), and other second tumors, OR=0.33 (95% CI: 0.15–0.74) among women with HER2-positive breast cancer (172). Despite the clinical benefits associated with trastuzumab, risks do exist. Trastuzumab was associated with an increased risk of cardiac toxicity, OR=2.45 (95% CI: 1.89–3.16), and brain metastases, OR=1.82 (95% CI: 1.16–2.85); (172). In a similar meta-analysis of 10,955 women with HER2-postivie breast cancer, trastuzumab was associated with an increased risk of symptomatic cardiotoxicity, and grades III–IV congestive heart failure, RR=7.05 (95% CI: 3.88–12.83) with 62 patients needing to be treated in order to harm one (16). Asymptomatic cardiotoxicity, including a significant left ventricular ejection volume reduction was amplified with trastuzumab use, RR=1.88 (95% CI: 1.66–2.13), with 14 patients needing to be treated in order to harm one (16).
Bevacizumab
Bevacizumab is an angiogenesis growth inhibitor preventing the growth of new blood vessels to solid tumors. Bevicizumab is used to treat metastatic colon, lung, and kidney cancer, as well as glioblastomas (a type of brain tumor). The primary cardiotoxicity associated with bevicizumab use is hypertension (129). However, there is emerging evidence of other forms of cardiotoxicities, and brain metastases associated with Bevacizumab treatment.
Among metastatic colorectal cancer patients, bevicizumab was associated with improvements in progression-free survival, HR=0.66 (95% CI: 0.56–0.77), and median overall survival, HR=0.77 (95% CI: 0.67–0.89); (21). Among metastatic colorectal cancer patients, chemotherapy plus bevicizumab was associated with increased risk of grade III–IV hypertenstion, OR=4.19 (95% CI: 2.76–6.36), thrombolytic events, OR=1.75 (95 % CI: 1.21–2.53), bleeding, OR=1.87 (95% CI: 1.10–3.16), and gastrointestinal perforation, OR 4.81 (95% CI: 1.52–15.3); (21).
While these examples were among metastatic colorectal cancer patients, the cardiotoxic side effects associated with bevacizumab appear to be independent of cancer tumor type. Among 12,656 varied solid tumor patients, the incidence of hypertension with bevacizumab was 23.6% (95% CI: 20.5–27.1), with 7.9% being grade III–IV hypertension (129). The relative risk of developing hypertension when treated with bevicizumab compared with cancer survivors not receiving bevacizumab was 5.28 (95% CI: 4.15–6.71); (129). This risk was observed at varying weekly doses of bevacizumab, RR=4.78 (95% CI: 3.59–6.36), and 5.39 (95% CI: 3.68–7.90) at 2.5mg/kg/week, and 5.0mg/kg/week, respectively (129).
Effects of Exercise during Cancer Treatment
Five-year survival after a diagnosis of cancer has improved steadily over the past 40 years (76). These improvements in 5-year survival are a result of earlier cancer detection, and aggressive and successful treatments, now individualized to each patient (76, 128). As discussed in earlier sections, the goals for cancer treatment may vary from patient to patient. A hierarchy of priorities must exist between the cancer patient, family, and his or her healthcare providers. Given the complex state of cancer care, it is understandable that cancer survivors may feel overwhelmed during cancer treatment. We acknowledge that exercise may not serve as a useful adjunct to all cancer survivors; however, there is a growing base of evidence that suggests engaging in exercise, such as brisk walking, yields fewer symptoms and side effects during treatment and retards the rate at which physiologic systems are affected (138).
Throughout this section examining the role of exercise during cancer treatment the reader must remember the treatment stage of cancer survivorship includes numerous tasks that must be addressed; communication with family and friends, communication with healthcare providers, and transportation to receive cancer therapy, among other physical and emotional decision-making processes (170). As such, the context of exercise during treatment must serve a variety of purposes to yield itself valuable for use by patients.
Despite the success of recent cancer treatments—as illustrated by improvements in 5-year survival rate, survivors may experience persistent symptoms and side effects of either their cancer, or the treatment used to fight their cancer (138). Some of these symptoms and side effects occur immediately after initiating cancer treatment, and resolve over days or weeks, or stop when cancer treatment is complete (138). However, some of these symptoms and side effects may persist beyond completion of treatment or manifest months or years after treatment is complete. The dynamic and multifactorial behavior of cancer or treatment-related symptoms makes the study of these symptoms difficult (159). In attempt to structure the next two sections of this review, we examine some of the physiologic and psychosocial responses to exercise rehabilitation during and after cancer treatment.
During cancer treatment, cancer survivors may experience a variety of physiologic and psychosocial symptoms and side effects. Many of these symptoms and side effects are not unique to one specific type of treatment (Table 2). Some of the most common symptoms and side effects experienced during treatment include fatigue, pain, cardiovascular and pulmonary complications, endocrine changes, musculoskeletal fatigue and weakness, immune alterations, gastrointestinal upset and skin changes (138). Throughout this section and the next section, exercise after treatment completion, the American College of Sports Medicine has graded the literature examining exercise and cancer survivors using the evidence grading criteria set forth by the National Heart, Lung, and Blood Institute (138). The grades are as follows: A (overwhelming data from randomized controlled trials), B (few randomized controlled trial data exist, or they are inconsistent, C (results from uncontrolled, non-randomized, and/or observational studies, and D (evidence insufficient for categories A to C).
Table 2.
Physiologic systems impacted as a result from various cancer treatment modalities. Adapted from Schmitz (138).
| Physiologic Symptom Affected | Treatment Modality | |||||
|---|---|---|---|---|---|---|
| Surgery | Chemotherapy | Radiation | Endocrine Therapy | Transplant | Targeted Therapy | |
| Cardiovascular | X | X | X | X | X | X |
| Endocrine | X | X | X | X | X | X |
| Musculoskeletal | X | X | X | X | X | X |
| Neurologic | X | X | X | X | X | X |
| Immune | X | X | X | X | X | X |
| Gastrointestinal | X | X | X | X | X | X |
Physiologic Outcomes
During cancer treatment, deconditioning of the cardiovascular and pulmonary system is common and is associated with diminished levels of physical activity. However, it appears that the adaptive capacity of the cardiorespiratory system to exercise training remains intact during treatment. Among a meta-analysis of 17 high-quality studies, aerobic fitness—a marker of cardiorespiratory function—improved significantly in cancer survivors during treatment over the exercise intervention period (Figure 15). The standardized mean difference (d) of aerobic fitness was of moderate size d=0.33 (95% CI: 0.08–0.57); p=0.009; (154). In a similar meta-analysis of three studies, V02peak significantly increased in the magnitude of 1.21 ml·kg−1·min−1 (95% CI: 0.50–1.92 ml·kg−1·min−1); (78). Interestingly, this 1.12 ml·kg−1·min−1 may confer clinical benefits to cancer survivors. In a separate study, the same group reported that a 1.0 ml·kg−1·min−1 decrease in V02peak is associated with a 4% greater mortality rate (77). The American College of Sports Medicine consensus statement on cancer and exercise, concluded that a grade A level of evidence existed for cardiorespiratory benefits from exercise during cancer treatment (138).
Figure 15.
Physiologic effects occurring as a result of exercise training during cancer treatment. Data from (154).
Despite the known cardiac toxicities associated with cancer treatment, particularly anthracycline-based chemotherapy, scant literature has examined the effect of exercise training on clinical measures of cardiac function. A recent review has been published by Scott et al., (145) reviewing the mechanistic underpinnings associated with the modulation of anthracycline-induced cardiotoxicity via aerobic exercise training. Most studies conducted to date have been in the form of an animal model and have demonstrated promised for the use of aerobic exercise in the prevention and treatment of anthracycline-induced cardiotoxicity (145). Among a meta-analysis of 14 trials in heart failure survivors without cancer, aerobic training significantly improved ejection fraction by 2.59% (95% CI: 1.44–3.74%), end diastolic volume by −11.49 ml (95% CI: −19.95– −3.02 ml), and end systolic volume by −12.87 ml (95% CI: −17.80– −7.93); (65). Strength training, and combined strength and aerobic training were not associated with such improvements in ejection fraction and end systolic or diastolic volumes. However, this extrapolation to cancer survivors should be interpreted cautiously. The etiology of heart failure in the cancer survivor may differ from that of a person without a history of cancer.
Muscle fatigue and muscle weakness are also common sequelae of cancer treatment, but may be amenable to exercise training. For example, among 121 prostate cancer survivors undergoing radiation therapy, chest and leg strength (measured via eight-repetition maximum testing) improved by 13.7, and 25.2 kg, respectively over 24-weeks of exercise training (147). Similarly, among 242 breast cancer survivors undergoing adjuvant chemotherapy chest and leg strength (measures via one-repetition maximum testing) improved by 6.8, and 5.2 kg, respectively after a median of 17 weeks of exercise training (95% CI: 9–24 weeks); (34). A meta-analysis of randomized controlled trials concluded that both upper body and lower body strength improve as a result of exercise training during cancer treatment, with d=0.39 (95% CI: 0.12–0.65), and d=0.24 (95% CI: 0.07–0.41), respectively (154). Similar to aerobic fitness, the American College of Sports Medicine expert consensus panel graded the effect of exercise on muscular strength during treatment for breast and prostate cancer survivors as level ‘A’—with all studies showing marked improvements in muscular strength (138).
Despite the large volume of studies examining muscular strength among cancer survivors during treatment, few studies have examined the role of strength training among those with cancer cachexia (14). Cancer cachexia is a multifactorial clinical manifestation that includes loss of body weight, adipose tissue, and skeletal muscle mass (6, 14, 111). The prevalence of cancer cachexia varies from 2.4–50%, depending on the definition, and tumor-site under investigation (14). The approved interventions for cancer cachexia are in the form of suspensions which include megestrol acetate, dronabinol, and the oral supplement eicosapentaenoci acid, which result in a variety of effects including improving appetite, preventing weight loss, and increasing weight gain (14). It is interesting, given the success of resistance training among cancer survivors to increase upper and lower body strength, that use of this modality among cancer survivors with cachexia is not more commonly studied.
Changes in body composition are also common among cancer survivors during treatment. A previously published meta-analysis observed significant reductions in body weight, d=−0.25 (95% CI: −0.49–0.00), and percentage body fat, d=−0.25 (95% CI: −0.48– −0.02), respectively (154). However, significant improvements in fat mass, lean mass, BMI, and waist circumference from exercise during treatment were not detected. This may be due in part by a limited number of studies reporting data on individual soft tissue compartments or anthropometric indices of obesity (k=5); (154). In general, exercise intervention studies conducted to date have not focused on weight loss or fat loss. Exercise in the absence of dietary modification may not be effective as the combination of exercise and diet when the goal is to achieve a substantive amount of weight or fat loss. The American College of Sports Medicine consensus panel on exercise and cancer graded body size and body composition for breast cancer and prostate cancer survivors during treatment as evidence category ‘B’—defined as few randomized controlled data exist or data are small (underpowered) and inconsistent (138).
Bone loss is a common concern among cancer patients whose treatment aims to reduce circulating sex hormone levels (e.g., breast and prostate cancer treatment). Based on a very limited number of trials, moderate-intensity exercise may preserve bone health during cancer treatment but probably has limited skeletal benefits over and above pharmacologic treatment for bone loss. Exercise should not be ignored, though, for cancer patients on anti-resorptive medications, though, because it has multiple health benefits may lower fall risk, which further reduces risk of fracture. Schwartz reported that moderate-intensity aerobic exercise, including mostly walking, prevents bone loss at the spine during chemotherapy in female cancer patients; however, a similar study with a less intense walking-only intervention failed to find that walking could prevent spine bone loss among breast cancer patients in chemotherapy (144). So far, there are no trials that have reported on hip bone mineral density as an outcome, even though hip fractures are considered the most deadliest and costliest of all skeletal fractures. Consistent with this evidence, the American College of Sports Medicine expert panel suggested exercise may be beneficial for bone health among breast cancer survivors, but due to limited evidence, did not provide an evidence category rating (138).
Psychosocial Outcomes
In addition to the various physiologic side effects from cancer treatment that may be attenuated by exercise, psychosocial outcomes are also relevant to cancer survivors while undergoing cancer treatment. There exists evidence that exercise improves a variety of mental health outcomes including, fatigue, anxiety, quality of life, and mood, (Figure 16); (138, 154). A large portion of this evidence exists among breast cancer, with a smaller portion distributed among prostate, colon, and hematologic cancer.
Figure 16.
Psychosocial effects occurring as a result of exercise training during cancer treatment. Data from (35, 154).
Cancer-related fatigue is the most commonly studied psychosocial symptom associated with cancer treatment (10, 15, 43, 104, 154). In a meta-analysis of 18 randomized controlled trials, including 12 trials in breast, 4 in prostate, and 2 in mixed populations of cancer survivors examined exercise and the relationship with cancer-related fatigue (171). Among breast cancer survivors, supervised aerobic exercise yielded a moderate sized reduction in cancer-related fatigue, d=0.30 (95% CI: 0.09–0.51); (171). However, unsupervised home-based aerobic exercise was not successful in reducing cancer-related fatigue among breast cancer survivors, d=0.10 (95% CI: −0.25–0.45); (171). Among prostate cancer survivors, no significant reductions in cancer-related fatigue were observed. Due to the mixed findings from this meta-analysis, the American College of Sports Medicine expert consensus rated cancer-related fatigue as categories ‘B’, ‘A’, and ‘B’ for breast, prostate, and hematologic cancers. For example, a group of 59 varied cancer survivors receiving high dose chemotherapy, followed by stem cell transplantation were randomized to supine cycle ergometery or to standard care for the duration of their cancer treatment (41). The exercise training group was able to attenuate increases in fatigue over the course of exercise training whereas the control group experienced a significant increase in fatigue.
Anxiety is another commonly studied psychosocial outcome among cancer survivors during treatment (138, 154). Among a meta-analysis of six studies exercise was associated with a small reduction in anxiety symptoms, d=−0.21 (95% CI: −0.39– −0.03); (154). This is consistent with the American College of Sports Medicine expert consensus, which suggested randomized controlled trial data exist for breast cancer, but the evidence is inconsistent, viz, evidence category ‘B’ (138). Limited or no evidence exists for other cancer types including colon, prostate, hematologic, and gynecologic cancers (138). In the above described cancer-related fatigue example (41), the exercise training group significantly reduced its anxiety compared to baseline, whereas the control group experienced no change in anxiety levels.
Quality of life is a commonly studied psychosocial outcome of cancer treatment (138, 154). Quality of life measures often include assessment of fatigue, depression, anxiety, and mood, thus most of the findings on quality of life are similar to those of the above-described psychosocial constructs. In a meta-analysis of ten studies (154), quality of life during treatment improved with exercise training, d =0.13 (95% CI: −0.005–0.26). The functional subscale of quality of life also demonstrated marked improvement, d=0.28 (95% CI: 0.02–0.54), and mood also increased, d=0.39 (95% CI: 0.14–0.63); (154). Conversely, the quality of life subscales, mental, physical, social, and emotional, did not demonstrate improvements. This may be the result of limited studies (k<5) and subsequent limited statistical power (154). The American College of Sports Medicine expert consensus provided evidence category ‘B’ to both breast and prostate cancers—inconsistent or small (underpowered) randomized controlled data (138).
To illustrate, 167 women were retrospectively queried about their levels of physical activity during cancer treatment (33). Women who engaged in at least one session of strenuous (vigorous) intensity exercise per week during their cancer treatment reported more favorable post-treatment levels of quality of life with respect to all quality of life subscales, including physical, functional, emotional, social, general concerns with life, overall quality of life, and satisfaction with life, with moderate to large effect sizes for all domains ranging from 0.30 (social) to 0.67 (general concerns with life) compared to women who did not perform vigorous intensity exercise. When this analysis included women that performed moderate or vigorous intensity exercise during cancer treatment, the results did not differ substantively to women who only performed vigorous intensity exercise, with the lone exception of social quality of life, which became non-significant (33).
Conclusion
Numerous physiologic and psychosocial can be achieved from exercise during cancer treatment. We are now arriving at the stage of clinical research where the successful translation and implementation of these results into standard clinical practice is needed (135). This next phase of translation and implementation will require the participation of experts in a variety of domains, including medical care and planning, exercise physiology, behavioral science, and implementation science. Implementation of these interventions will likely occur after cost-effective analyses have deemed them to be more cost-effective compared to more traditional methods (i.e., “bed rest is best”) to manage symptoms associated with cancer treatment. Another major challenge will be that, unlike for radiation, chemotherapy, or surgery, there is no infrastructure to implement exercise interventions during cancer treatment.
Exercise after Cancer Treatment Completion
Exercise has been used to help rehabilitate persons recovering from chronic illness as a means to alleviate side effects and symptoms from the disease and treatments and even to limit disease progression and mortality (74). Exercise can directly benefit persons after cancer treatment through physiologic adaptations to chronic exercise training and indirectly by reversing deconditioning that can begin around diagnosis and persist long into recovery. Physical activity levels have been shown to decrease by an average of 2 hours per week from pre-diagnosis to post-treatment in women with breast cancer (74).
Perhaps the best illustration of the role that exercise has in disease management is cardiac rehabilitation. Heart disease encompasses a cluster of pathophysiologic states that increase the risk of an adverse cardiac event, such as a heart attack or stroke. Sustained rhythmic exercise stimulates the cardiorespiratory system and results in neurologic and cellular adaptations that improve the efficiency of the heart and circulation. These structural and functional improvements ultimately reduce the workload on the heart, thereby improving functional capacity of the patient and reducing the risk of cardiac events. Likewise, exercise adaptations to regular exercise training could alleviate or reverse the physiologic insults resulting from cancer treatment (Table 3) and possibly alter disease progression. Complicating the rehabilitation of cancer survivors is the synergistic interaction of deleterious cancer treatment sequelae and the functional and psychosocial declines associated with aging (137). As previously reviewed, cancer therapies are associated with a variety of comorbidities, which may accelerate the aging process resulting in amplified physical limitations, and subsequent disablement, if not remedied through intervention (Figure 17); (137). Therefore the efficacy exercise after treatment to improve physiologic and psychosocial limitations is of significance to the longevity of cancer survivors (27).
Table 3.
Systemic exercise adaptations that could have therapeutic potential to alleviate cancer-treatment related symptoms and side effects
| Physiologic System | Normal adaptation(s) to exercise training | Side effects/Symptoms of cancer treatment |
|---|---|---|
| Cardiovascular | ↑VO2max; ↓Resting and submaximal HR; ↓BP; ↑Plasma volume; ↑Hb | ↓VO2max; reduced exercise tolerance; ↓Hb; ↑cardiomyopathy; ↑heart disease |
| Respiratory | ↓ work of breathing; ↓submaximal Ve | ↓lung capacity; ↑work of breathing; dyspnea |
| Musculoskeletal | ↑ or preserve muscle CSA; ↑ muscular strength, endurance and power; ↑ or preserve BMD; ↓ bone turnover; ↑ joint ROM and lubrication | Cachexia; ↓muscle strength, endurance, power; bone loss; arthralgia; myalgia; rhabdomyolosis |
| Neurologic | SNS withdrawal; improved muscle fiber recruitment; preservation of large alpha-motor neurons; improved gait and balance | Peripheral and central neuropathy; cognitive changes; loss of coordination; balance problems |
| Metabolic | ↑ oxidative capacity; ↑ RMR; weight management; ↑ HDL-C; ↓LDL-C; ↓TG | Weight gain; dyslipidemia; ↓RMR |
| Endocrine | ↑ insulin sensitivity; ↓ fasting BG, ↓cortisol; ↓estrogens; | Hyperinsulinemia; ↑ diabetes risk; ↓estrogens; ↓ or ↑ androgens |
| Immune | Promote anti-inflammatory state | ↑IL-6, ↑IL-10; ↑CRP |
VO2max; HR; BP; Ve; CSA; BMD; ROM; SNS; RMR; HDL-C; LDL-C; TG; BG; IL-6; IL-10; CRP
Figure 17.
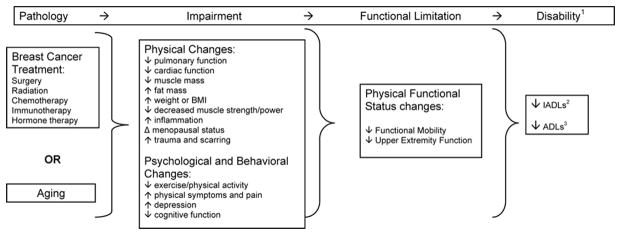
The intersection of Cancer and Aging. Reproduced with permission from (137).
Benefits of exercise after cancer treatment
Post-treatment, exercise may reverse losses that occurred during treatment, manage long-term, late effects of treatment and/or promote long-term function, health and survival. Cancer results not only in physical distress, but emotional concerns as well. The psychosocial challenges that face cancer survivors are significant. Exercise may provide relief from the emotional stress of cancer, but that topic is beyond the scope of this review and the reader is referred elsewhere (143).
To date, the majority of evidence for exercise benefits comes from studies of breast cancer survivors (154), though research in other cancer populations is steadily increasing. Numerous systematic reviews and meta-analysis have been published (71, 87, 154, 171) and exercise recommendations have been issued (138). A synopsis of the current information on exercise benefits after cancer treatment is described below with an emphasis on the physiologic adaptations that directly benefit cancer survivors.
Addressing physiologic sequelae from cancer treatment
Cardiovascular and circulatory system
Aerobic capacity, estimated by submaximal walking tests or measured by maximal oxygen consumption, improves with exercise training after cancer treatment (154). Despite the typical declines in aerobic fitness that accompany treatment, regular exercise not only prevents deconditioning but can actually increase fitness. Improved maximal aerobic capacity translates to lower demand for activities requiring submaximal effort, such as activities of daily living and recreational pursuits. Hematologic changes may, in part, underlie improvements in fitness. Though some cancer survivors (i.e., breast cancer survivors and prostate cancer survivors on androgen deprivation therapy) are at increased risk for cardiovascular disease after treatment, this is a difficult outcome to study in exercise trials. Exercise is known to lower risk factors for cardiovascular disease, such as adiposity, blood pressure and cholesterol in the general population and has the same potential for cancer survivors. Though few exercise studies include blood pressure in their outcome measures, reductions may occur both during and after treatment (140).
Respiratory system
Few exercise studies have included outcome measures of pulmonary function despite the fact that some cancers (i.e., lung) and some treatments (i.e, chest irradiation or chemotherapy) can impair lung function at rest and during exercise. In two sets of uncontrolled studies, Schneider et al reported significant improvements in the % of predicted forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) in post-treatment female cancer survivors after individually tailored 6-month multi-component exercise programs (140, 141). In the same study, pulmonary function did not change in male cancer survivors post-treatment, though it’s possible that stable values over treatment reflect a protective effect of exercise. For lung cancer patients, a few small studies suggest that pre-operative cardiopulmonary exercise training can buffer the deconditioning that occurs post-surgery (11, 79) and may shift the clinical status of patients from surgery ineligible due to poor functional status to surgery eligible (25). Studies are underway to determine exercise benefits for lung cancer patients post-treatment. Given the depth of knowledge on the respiratory response to exercise after cancer treratment, the American College of Sports Medicine assigned an evidence grade of A—overwhelming data from randomized controlled trials.
Musculoskeletal system
Keeping bones and muscles strong, yet limber, is central to optimal physical functioning after cancer treatment and also for avoiding injuries such as falls and fractures. Exercise studies must use techniques that can measure each fat, muscle and bone compartments (i.e, dual energy x-ray absorptiometry) in order to quantify training-related changes in each. Unfortunately, studies that only report % body fat are unable to decipher whether shifts in body composition are the result of changes in lean and/or fat mass. Accordingly, the evidence is mixed with regard to the ability of exercise to increase lean mass after treatment (154). The majority of studies reporting preservation or increases in lean mass used resistance exercise as the training mode, either exclusively or along with aerobic activity (154). Despite the mixed evidence for lean mass, improvements in upper and lower body strength are consistently observed across cancer types, timing relative to treatment, and exercise type (138), though resistance exercise produces superior results compared to aerobic exercise (34, 147). Strength improvements in the absence of muscle hypertrophy suggest that the adaptations resulting from strength training may be largely attributable to neural adaptations from better motor unit activation (recruitment, discharge rate), synchronization, and cross education. Neural adaptations occur early on in a strength training program and may explain strength improvements in most short-term training studies where time required for muscle hypertrophy is insufficient. The known benefits of strength training on the musculoskeletal system after cancer treatment is supported by a number of randomized trials, with an evidence grade of A from the American College of Sports Medicine.
Since certain cancer treatments, such as chemotherapy and/or sex hormone ablation therapy can cause bone loss, the osteogenic nature of exercise make it a reasonable strategy to mitigate or reverse bone loss in cancer survivors. Based on a very limited number of trials, moderate-intensity aerobic or resistance exercise may preserve bone health after cancer treatment but probably has limited skeletal benefits over and above bisphosphonate treatment (179). Saarto et al studied impact loading, via vigorous aerobic + jump exercise, in post-treatment breast cancer survivors and reported maintenance of femoral neck bone mineral denisty in premenopausal patients but no effect at the spine (131). No changes in bone mineral density among exercising postmenopausal patients was found. Combined these studies suggest that mixed loading regimens that include impact training plus moderate-vigorous resistance or aerobic training may reduce bone loss at the hip and spine. The exercise prescription that best targets both skeletal sites and is consistently effective across cancer types and subgroups, however, remains to be determined.
Given the contribution of flexibility to physical functioning and the impact of scarring from radiation and/or surgery and inactivity on joint mobility, improving range of motion is an important goal of activity programs for cancer survivors. Based mainly on evidence from studies in breast cancer survivors, upper and lower body flexibility improves with activity and particularly from dynamic exercise that emphasizes whole body range of motion, such as Tai Chi (112).
Nervous system
Peripheral neuropathy is the most prevalent indicator of the neurotoxic effect that cancer treatment can have. Neuropathy may contribute to difficulties with mobility (i.e., gait and balance). Currently, there is little information on whether exercise can mitigate neurologic side effects, like neuropathy, though increases in muscular strength could translate to better gait and stability (180). Recent studies demonstrated improved mobility, measured by better tandem backward walk scores, among breast cancer survivors participating in resistance training (169). Exercise has been explored for reducing peripheral neuropathy in non-cancer populations and may be a plausible strategy to manage cancer treatment-related neuropathy, yet remains to be tested.
Endocrine system
The classes of hormones of most interest for cancer survivorship studies tend to be sex hormones and growth factors since they are implicated in disease progression. Growth factors, including insulin, insulin-like growth factors (i.e., IGF-I, IGF-II, IGF-III) and their binding proteins are anabolic hormones that promote cell division and tissue growth. Because of its tumor-promoting potential exercise studies in cancer survivors aim to reduce insulin and related growth factors. The literature is slim on this outcome, though, and results are mixed (154). For example, Irwin and colleagues report significant reductions in circulating insulin, IGF-I and IGFBP-3 levels in breast cancer survivors participating in 150 minutes per week of moderate-intensity aerobic exercise for six months (73). On the other hand, Fairey et al. (48), reported no change in insulin, reductions in IGF-I and increases in IGFBP-3 among breast cancer survivors who cycled three times per week for 15 weeks. Discrepancies among studies could be explained by differing age, body composition and fitness levels among study cohorts as well as varying exercise prescriptions since these factors can moderate the hormone-lowering effect of exercise. It is also important to recognize a potential paradox with respect to the IGF-1 system. That is that while increases in bioavailable IGF-I are associated with tumor progression they are also a humoral driver for muscle and bone growth. In cancer populations at risk for treatment-induced bone loss, an effective bone promoting exercise program might increase IGF-I, which is an unfavorable outcome for disease progression. The implications of these contradictory roles for IGFs in survivorship are beyond the scope of this review and the reader is referred to an insightful review by Nindl and Pierce for further discussion (117).
Sex-hormone lowering effects of exercise are difficult to study in cancer survivor populations with hormone-dependent tumors because of the confounding from hormone ablation therapy. In the case of prostate cancer, however, it has been important to demonstrate that exercise does not increase androgen levels which has been shown to occur with heavy resistance training in men (55). Moderate-intensity aerobic exercise has been shown to reduce circulating estrogens in postmenopausal women without cancer (53) and may have implications for reducing disease progression in breast cancer survivors.
Metabolic system
Due to the strong role of adiposity in cancer risk and survival, as well as in numerous other disease processes, weight maintenance and body fat reduction are a central goal of exercise intervention after treatment. Though the majority of evidence comes from studies in breast and prostate cancer survivors, there is consistent modest evidence that exercise can reduce body weight and percent body fat after cancer treatment; (154) however, some trials still fail to improve body size outcomes and it is unclear what limits efficacy of these studies. Using data from the National Health and Nutrition Examination Survey (NHANES) cohort, Lynch et al (2010) reported an inverse association between time spent in moderate-vigorous physical activity measured by accelerometry and waist circumference, a measure of central adiposity (95, 96). No association was found between waist circumference and time spent in light intensity physical activities or sedentary time suggesting that greater energy expenditure is necessary to impact central adiposity.
The American College of Sports Medicine recommends that 150–250 minutes of moderate intensity aerobic exercise are necessary to prevent weight gain and cause weight loss (44). Exercise can also improve blood lipid profiles (e.g., total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides), either alone or in conjunction with weight loss. Though understudied specifically in cancer survivors, preventing dyslipidemia is a relevant health goal for this population, particularly given that prostate cancer survivors are more likely to die of heart disease than prostate cancer and breast cancer survivors are equally likely to die of heart disease as breast cancer (83, 123). Resistance training can also favorably shift body composition, reduce visceral and subcutaneous adiposity and decrease blood lipids as effectively as aerobic training.
Immune system
Depending on the nature of the exercise type and volume, exercise can boost or compromise the immune system (116). Inactivity and extreme physical activity may decrease immune function and increase inflammation whereas moderate intensity activity may optimize immune activity and promote an anti-inflammatory state (105). Several biomarkers of immunologic function and inflammation exist including neutrophil and lymphocyte counts, natural killer cell activity, C-reactive protein, IL-6, IL-10, and TNF-alpha (54, 109). It remains unclear what benefit exercise may have on immune system function after cancer treatment.
Clinical Outcomes and Translation to Practice
Exercise can clearly benefit cancer survivors in terms of better daily functioning and overall health; however, evaluating exercise effects on disease-free survival and mortality poses a greater challenge. Several epidemiologic studies now suggest that regular, moderate to vigorous physical activity can reduce the risk of cancer recurrence and cancer-specific mortality in breast cancer and colorectal cancer survivors (69, 174). These promising findings must be confirmed by large, randomized controlled trials. Another important clinical benefit of exercise improved treatment tolerance. Exercise can manage debilitating symptoms that onset with treatment (i.e., fatigue, nausea, diarrhea) and may also translate to better compliance to treatment. In a randomized, controlled trial of over 250 breast cancer patients undergoing chemotherapy, Courneya and colleagues (2007) reported significantly better treatment completion rates among resistance trained women compared to aerobic exercisers or controls (34).
Safety of exercise training and contraindications
Cancer treatment is debilitating and poses a threat to normal homeostatic function both during therapy and into recovery. Since exercise is also a physiologic stressor, it is conceivable that exercise could exacerbate treatment-related side effects and symptoms – a perception that promoted rest as the best strategy for symptom management in cancer patients until recent evidence was available to challenge that recommendation. A critical adjunct to establishing the efficacy of exercise for cancer survivors is to establish the safety of exercise in this population. For example, progressive, moderate-intensity, upper body resistance exercise is now regarded as safe for breast cancer survivors with or at risk for upper extremity lymphedema (136). Up until evidence was available to point to the contrary, recommendations for women with lymph node dissection were to limit or completely avoid lifting and carrying heavy objects with the affected or at risk arm. As in other clinical populations (i.e., patients with heart disease, diabetes, arthritis, neurologic disorders), moderate-intensity exercise is regarded as safe both during and after cancer treatment and the benefits of regular exercise for cancer survivors far outweigh the risks (138). Nonetheless, as noted in this review the side effects of cancer treatment can impact exercise ability and these limitations should be considered when prescribing exercise for individual cancer survivors. Such considerations are outlined in the ACSM exercise guidelines for cancer survivors (138).
Translation to Clinical Practice
The depth and breadth of high-quality evidence supporting the role for exercise in cancer rehabilitation is strong (138). A model of cancer rehabilitation has been proposed to better identify, and delineate the physical rehabilitation needs particularly among breast cancer survivors (Figure 18); (64). This proposed model posits that education, prospective surveillance of cancer therapy sequelae, and exercise prescription are necessary to prepare breast cancer survivors for long-term survivorship. Within each strata of education, prospective surveillance, and exercise prescription, exists a spectrum ranging from intensive education, prospective surveillance, and exercise prescription to less intense education, prospective surveillance, and exercise prescription (64). Each of these strata may be customized to the needs of a specific breast cancer survivor based on her current knowledge of healthy lifestyle behavior, treatment history and subsequent deleterious sequelae which may be amendable to intervention, and an exercise prescription that is tailored to the specific rehabilitative needs unique to each breast cancer survivor. The information needed to address the specific educational, prospective surveillance, and exercise prescription needs as proposed in this model, could come from cancer survivorship care plans provided to each cancer survivor (27). The Institute of Medicine (27) suggested all cancer survivors receive a cancer survivorship care plan that outlines surgical history, pathology findings, chemotherapy and radiation therapy history, as well as treatment with other therapies (57). Supporting the Institute of Medicine, use of treatment summaries has been mandated by all NCI-designated comprehensive cancer centers by the year 2015 (4). The utilization of cancer survivorship care plans, coupled with the professionals who are expert in cancer survivorship, would provide the resources necessary implement this model of rehabilitation into clinical practice. However, this model has yet to be integrated into clinical practice, and therefore, weaknesses to the proposed model of rehabilitation remain to be uncovered.
Figure 18.
Breast cancer surveillance and rehabilitation model. Reproduced with permission from (64).
Conclusion
Evidence to date suggests physical activity or exercise may play a pivotal role at all points of interest on the cancer survivorship trajectory. Participation in physical activity is associated with a reduced likelihood of developing cancer. Through the causal pathway underlying this association remains to be elucidated, the observational evidence is strong and convincing, particularly among breast and colon cancer. Despite the success of a variety of cancer treatments, many result in an array of deleterious symptoms and side effects. However, many of these symptoms and side effects appear to be amendable to physical activity. During and after cancer treatment, engaging in physical activity improves a number of physiologic systems, resulting in improved physiologic and psychosocial outcomes. Despite the favorable profile of physical activity along the cancer continuum, many research gaps still exist. Elucidating the optimal dose of physical activity necessary to maximize the reduction in risk of cancer is one such question. Similarly, elucidating the optimal dose of physical activity necessary to improve specific physiologic systems, or treatment-specific side effects is another research gap. If cancer rehabilitation is to become a standard part of cancer care with third party coverage, it will be necessary to justify the safety and cost-effectiveness of these physical medicine and rehabilitation intervention.
Acknowledgments
We kindly thank Gabriella John, University of Pennsylvania, for reviewing this manuscript and providing valuable feedback.
References
- 1.Abbas S, Seitz M. Systematic review and meta-analysis of the used surgical techniques to reduce leg lymphedema following radical inguinal nodes dissection. Surg Oncol. 2011;20(2):88–96. doi: 10.1016/j.suronc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 )(Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine. American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30(6):992–1008. [PubMed] [Google Scholar]
- 4.American College of Surgeons. Cancer Program Standards. Ensuring Patient-Centered Care. 2012 [Online]. www.facs.org/cancer/clp/cpstandards.pdf.
- 5.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 6.Argiles JM, Busquets S, Garcia-Martinez C, Lopez-Soriano FJ. Mediators involved in the cancer anorexia-cachexia syndrome: past, present, and future. Nutrition. 2005;21(9):977–985. doi: 10.1016/j.nut.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 8.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14(1):14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn H. Ancel Keys Lecture. The three beauties. Bench, clinical, and population research. Circulation. 1992;86(4):1323–1331. doi: 10.1161/01.cir.86.4.1323. [DOI] [PubMed] [Google Scholar]
- 10.Blaney J, Lowe-Strong A, Rankin J, Campbell A, Allen J, Gracey J. The Cancer Rehabilitation Journey: Barriers to and Facilitators of Exercise Among Patients With Cancer-Related Fatigue. Phys Ther. 2010 doi: 10.2522/ptj.20090278. [DOI] [PubMed] [Google Scholar]
- 11.Bobbio A, Chetta A, Ampollini L, Primomo GL, Internullo E, Carbognani P, Rusca M, Olivieri D. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95–98. doi: 10.1016/j.ejcts.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, Costagliola D, Salmon D, Chene G, Morlat P. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 13.Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. 1989;60(4):592–598. doi: 10.1038/bjc.1989.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossola M, Pacelli F, Tortorelli A, Doglietto GB. Cancer cachexia: it’s time for more clinical trials. Ann Surg Oncol. 2007;14(2):276–285. doi: 10.1245/s10434-006-9179-5. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bria E, Cuppone F, Fornier M, Nistico C, Carlini P, Milella M, Sperduti I, Terzoli E, Cognetti F, Giannarelli D. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109(2):231–239. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]
- 17.Brinton LA, Berman ML, Mortel R, Twiggs LB, Barrett RJ, Wilbanks GD, Lannom L, Hoover RN. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992;167(5):1317–1325. doi: 10.1016/s0002-9378(11)91709-8. [DOI] [PubMed] [Google Scholar]
- 18.Burstein R, Polychronakos C, Toews CJ, MacDougall JD, Guyda HJ, Posner BI. Acute reversal of the enhanced insulin action in trained athletes. Association with insulin receptor changes. Diabetes. 1985;34(8):756–760. doi: 10.2337/diab.34.8.756. [DOI] [PubMed] [Google Scholar]
- 19.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 20.Campbell KL, McTiernan A. Exercise and biomarkers for cancer prevention studies. J Nutr. 2007;137(1 )(Suppl):161S–169S. doi: 10.1093/jn/137.1.161S. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y, Tan A, Gao F, Liu L, Liao C, Mo Z. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009;24(6):677–685. doi: 10.1007/s00384-009-0655-9. [DOI] [PubMed] [Google Scholar]
- 22.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 23.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 24.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 25.Cesario A, Ferri L, Galetta D, Cardaci V, Biscione G, Pasqua F, Piraino A, Bonassi S, Russo P, Sterzi S, Margaritora S, Granone P. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer. 2007;57(1):118–119. doi: 10.1016/j.lungcan.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov. Exercise Programs in Healthy Young Women at Increased Risk of Developing Breast Cancer. [Online]. http://clinicaltrials.gov/ct2/show/NCT00892515?term=wiser+sister&rank=2.
- 27.Committee on Cancer Survivorship: Institute of Medicine and national Research Board, editor. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academic Press; 2006. [Google Scholar]
- 28.Concato J. Observational versus experimental studies: what’s the evidence for a hierarchy? NeuroRx. 2004;1(3):341–347. doi: 10.1602/neurorx.1.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116(22):5138–5149. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- 30.Corpet DE, Jacquinet C, Peiffer G, Tache S. Insulin injections promote the growth of aberrant crypt foci in the colon of rats. Nutr Cancer. 1997;27(3):316–320. doi: 10.1080/01635589709514543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 32.Courneya KS, Friedenreich CM. Framework PEACE: an organizational model for examining physical exercise across the cancer experience. Ann Behav Med. 2001;23(4):263–272. doi: 10.1207/S15324796ABM2304_5. [DOI] [PubMed] [Google Scholar]
- 33.Courneya KS, Friedenreich CM. Relationship between exercise during treatment and current quality of life among survivors of breast cancer. J Psychosoc Oncol. 1997;15(3–4):35–57. [Google Scholar]
- 34.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, McKenzie DC. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. 2007;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 35.Craft L, Vaniterson EH, Helenowski IB, Rademaker A, Courneya KS. Exercise Effects on Depressive Symptoms in Cancer Survivors: A Systematic Review and Meta-Analysis. Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-11-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cust AE. Physical activity and gynecologic cancer prevention. Recent Results Cancer Res. 2011;186:159–185. doi: 10.1007/978-3-642-04231-7_7. [DOI] [PubMed] [Google Scholar]
- 37.Cuzick J, Sestak I, Cella D, Fallowfield L ATAC Trialists’ Group. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9(12):1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 38.D’Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, Shepherd FA. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol. 2005;23(13):2926–2936. doi: 10.1200/JCO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 39.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M Comparative Risk Assessment collaborating group (Cancers) Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 40.De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V, Esposito A, Silvestro L, Pennacchio R, Criscitiello C, Montanino A, Limite G, Bianco AR, De Placido S. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26(1):44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 41.Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85(10):2273–2277. [PubMed] [Google Scholar]
- 42.Din OS, Dodwell D, Wakefield RJ, Coleman RE. Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat. 2010;120(3):525–538. doi: 10.1007/s10549-010-0757-7. [DOI] [PubMed] [Google Scholar]
- 43.Dodd MJ, Cho MH, Miaskowski C, Painter PL, Paul SM, Cooper BA, Duda J, Krasnoff J, Bank KA. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs. 2010;33(4):245–257. doi: 10.1097/NCC.0b013e3181ddc58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 45.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 46.Emaus A, Thune I. Physical activity and lung cancer prevention. Recent Results Cancer Res. 2011;186:101–133. doi: 10.1007/978-3-642-04231-7_5. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 48.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12(8):721–727. [PubMed] [Google Scholar]
- 49.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1995;91(2):580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 50.Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, Irwin ML, Rudolph RE, Rajan KB, Stanczyk F, Bowen D, Weigle DS, Potter JD, McTiernan A. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13(3):615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 51.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 52.Friedenreich CM. The role of physical activity in breast cancer etiology. Semin Oncol. 2010;37(3):297–302. doi: 10.1053/j.seminoncol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, Terry T, Boyd NF, Yaffe MJ, Irwin ML, Jones CA, Yasui Y, Campbell KL, McNeely ML, Karvinen KH, Wang Q, Courneya KS. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28(9):1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galvao DA, Nosaka K, Taaffe DR, Peake J, Spry N, Suzuki K, Yamaya K, McGuigan MR, Kristjanson LJ, Newton RU. Endocrine and immune responses to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis. 2008;11(2):160–165. doi: 10.1038/sj.pcan.4500991. [DOI] [PubMed] [Google Scholar]
- 55.Galvao DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, Suzuki K, Yamaya K, Newton RU. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–2052. doi: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 56.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 57.Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26(5):759–767. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- 58.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97(6):419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31(4):925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 60.Gondos A, Bray F, Brewster DH, Coebergh JW, Hakulinen T, Janssen-Heijnen ML, Kurtinaitis J, Brenner H EUNICE Survival Working Group. Recent trends in cancer survival across Europe between 2000 and 2004: a model-based period analysis from 12 cancer registries. Eur J Cancer. 2008;44(10):1463–1475. doi: 10.1016/j.ejca.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10(1):58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, Haboubi N, Renehan AG Colorectal Cancer, Lifestyle, Exercise And Research Group. . Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal Dis. 2009;11(7):689–701. doi: 10.1111/j.1463-1318.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 63.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 64.Hayes S, Johansson K, Alfano C, Schmitz K. Exercise for breast cancer survivors: bridging the gap between evidence and practice. Trans Behav Med. 2011;4:539–544. doi: 10.1007/s13142-011-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49(24):2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 66.Herida M, Mary-Krause M, Kaphan R, Cadranel J, Poizot-Martin I, Rabaud C, Plaisance N, Tissot-Dupont H, Boue F, Lang JM, Costagliola D. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21(18):3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 67.Herrera JE, Stubblefield MD. Rotator cuff tendonitis in lymphedema: a retrospective case series. Arch Phys Med Rehabil. 2004;85(12):1939–1942. doi: 10.1016/j.apmr.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 68.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 70.Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 71.Ingram C, Courneya KS, Kingston D. The effects of exercise on body weight and composition in breast cancer survivors: an integrative systematic review. Oncol Nurs Forum. 2006;33(5):937–47. doi: 10.1188/06.ONF.937-950. quiz 948–50. [DOI] [PubMed] [Google Scholar]
- 72.International Agency for Research on Cancer. Weight Control and Physical Activity. [Online]. http://www.iarc.fr/en/publications/pdfs-online/prev/handbook6/index.php.
- 73.Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, Chung GG, Jones B, Knobf MT, DiPietro L. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring) 2009;17(8):1534–1541. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 76.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 77.Jones LW, Eves ND, Kraus WE, Potti A, Crawford J, Blumenthal JA, Peterson BL, Douglas PS. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer. 2010;10:155. doi: 10.1186/1471-2407-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, Joy AA, Kumar V, Winton TW, Reiman T. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110(3):590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 80.Kaaks R. Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control. 1996;7(6):605–625. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 81.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 82.Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44(10):1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 83.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 84.Key T, Appleby P, Barnes I, Reeves G Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 85.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21(3):215–244. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 86.Khera N, Storer B, Flowers ME, Carpenter PA, Inamoto Y, Sandmaier BM, Martin PJ, Lee SJ. Nonmalignant Late Effects and Compromised Functional Status in Survivors of Hematopoietic Cell Transplantation. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.38.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim CJ, Kang DH, Park JW. A meta-analysis of aerobic exercise interventions for women with breast cancer. West J Nurs Res. 2009;31(4):437–461. doi: 10.1177/0193945908328473. [DOI] [PubMed] [Google Scholar]
- 88.Kossman DA, Williams NI, Domchek SM, Kurzer MS, Stopfer JE, Schmitz KH. Exercise lowers estrogen and progesterone levels in premenopausal women at high risk of breast cancer. J Appl Physiol. 2011;111(6):1687–1693. doi: 10.1152/japplphysiol.00319.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee I, editor. Epidemiologic Methods in Physical Activity Studies. New York, New York: Oxford University Press, Inc; 2008. [Google Scholar]
- 90.Leitzmann MF. Physical activity and genitourinary cancer prevention. Recent Results Cancer Res. 2011;186:43–71. doi: 10.1007/978-3-642-04231-7_3. [DOI] [PubMed] [Google Scholar]
- 91.Limburg PJ, Stolzenberg-Solomon RZ, Vierkant RA, Roberts K, Sellers TA, Taylor PR, Virtamo J, Cerhan JR, Albanes D. Insulin, glucose, insulin resistance, and incident colorectal cancer in male smokers. Clin Gastroenterol Hepatol. 2006;4(12):1514–1521. doi: 10.1016/j.cgh.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y, Hu F, Li D, Wang F, Zhu L, Chen W, Ge J, An R, Zhao Y. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011;60(5):1029–1044. doi: 10.1016/j.eururo.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 93.Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95(4):649–656. doi: 10.1002/jcb.20461. [DOI] [PubMed] [Google Scholar]
- 94.Lukanova A, Lundin E, Toniolo P, Micheli A, Akhmedkhanov A, Rinaldi S, Muti P, Lenner P, Biessy C, Krogh V, Zeleniuch-Jacquotte A, Berrino F, Hallmans G, Riboli E, Kaaks R. Circulating levels of insulin-like growth factor-I and risk of ovarian cancer. Int J Cancer. 2002;101(6):549–554. doi: 10.1002/ijc.10613. [DOI] [PubMed] [Google Scholar]
- 95.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006) Cancer Causes Control. 2010;21(2):283–288. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 96.Lynch BM, Dunstan DW, Winkler E, Healy GN, Eakin E, Owen N. Objectively assessed physical activity, sedentary time and waist circumference among prostate cancer survivors: findings from the National Health and Nutrition Examination Survey (2003–2006) Eur J Cancer Care (Engl) 2011;20(4):514–519. doi: 10.1111/j.1365-2354.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 97.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 98.MacDonald RS, Thornton WH, Jr, Bean TL. Insulin and IGE-1 receptors in a human intestinal adenocarcinoma cell line (CACO-2): regulation of Na+ glucose transport across the brush border. J Recept Res. 1993;13(7):1093–1113. doi: 10.3109/10799899309063266. [DOI] [PubMed] [Google Scholar]
- 99.MacMahon B. Risk factors for endometrial cancer. Gynecol Oncol. 1974;2(2–3):122–129. doi: 10.1016/0090-8258(74)90003-1. [DOI] [PubMed] [Google Scholar]
- 100.Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20(1):54–60. doi: 10.1136/emj.20.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, Greene BT, DeMichele A. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115(16):3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayer-Davis EJ, D’Agostino R, Jr, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279(9):669–674. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 103.McGowan L, Norris HJ, Hartge P, Hoover R, Lesher L. Risk factors in ovarian cancer. Eur J Gynaecol Oncol. 1988;9(3):195–199. [PubMed] [Google Scholar]
- 104.McNeely ML, Courneya KS. Exercise programs for cancer-related fatigue: evidence and clinical guidelines. J Natl Compr Canc Netw. 2010;8(8):945–953. doi: 10.6004/jnccn.2010.0069. [DOI] [PubMed] [Google Scholar]
- 105.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 106.McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, Chubak J, Stanczyk FZ, Bowen D, Irwin ML, Rudolph RE, Potter JD, Schwartz RS. Effect of exercise on serum androgens in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1099–1105. [PubMed] [Google Scholar]
- 107.McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, Sorensen B, Rudolph RE, Bowen D, Stanczyk FZ, Potter JD, Schwartz RS. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64(8):2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 108.Menu E, van Valckenborgh E, van Camp B, Vanderkerken K. The role of the insulin-like growth factor 1 receptor axis in multiple myeloma. Arch Physiol Biochem. 2009;115(2):49–57. doi: 10.1080/13813450902736583. [DOI] [PubMed] [Google Scholar]
- 109.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol. 2010;144(1):3–15. doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 111.Muscaritoli M, Bossola M, Aversa Z, Bellantone R, Rossi Fanelli F. Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer. 2006;42(1):31–41. doi: 10.1016/j.ejca.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 112.Mustian KM, Palesh OG, Flecksteiner SA. Tai Chi Chuan for breast cancer survivors. Med Sport Sci. 2008;52:209–217. doi: 10.1159/000134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.National Cancer Institute: Division of Applied Research. Walking & Bicycling Related Items from Physical Activity Questionnaires. [Online]. http://appliedresearch.cancer.gov/tools/paq/reflist.html.
- 114.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18(1):11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 115.Nieman DC. Upper respiratory tract infections and exercise. Thorax. 1995;50(12):1229–1231. doi: 10.1136/thx.50.12.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nieman DC. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994;26(2):128–139. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 117.Nindl BC, Pierce JR. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med Sci Sports Exerc. 2010;42(1):39–49. doi: 10.1249/MSS.0b013e3181b07c4d. [DOI] [PubMed] [Google Scholar]
- 118.Olsen CM, Bain CJ, Jordan SJ, Nagle CM, Green AC, Whiteman DC, Webb PM Australian Ovarian Cancer Study Group. Recreational physical activity and epithelial ovarian cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2321–2330. doi: 10.1158/1055-9965.EPI-07-0566. [DOI] [PubMed] [Google Scholar]
- 119.O’Rorke MA, Cantwell MM, Cardwell CR, Mulholland HG, Murray LJ. Can physical activity modulate pancreatic cancer risk? a systematic review and meta-analysis. Int J Cancer. 2010;126(12):2957–2968. doi: 10.1002/ijc.24997. [DOI] [PubMed] [Google Scholar]
- 120.Oshima M, Taketo MM. COX selectivity and animal models for colon cancer. Curr Pharm Des. 2002;8(12):1021–1034. doi: 10.2174/1381612023394953. [DOI] [PubMed] [Google Scholar]
- 121.Pan SY, DesMeules M. Energy intake, physical activity, energy balance, and cancer: epidemiologic evidence. Methods Mol Biol. 2009;472:191–215. doi: 10.1007/978-1-60327-492-0_8. [DOI] [PubMed] [Google Scholar]
- 122.Pan SY, Morrison H. Physical activity and hematologic cancer prevention. Recent Results Cancer Res. 2011;186:135–158. doi: 10.1007/978-3-642-04231-7_6. [DOI] [PubMed] [Google Scholar]
- 123.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, Buchholz TA, Hortobagyi GN, Giordano SH. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23(30):7475–7482. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- 125.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50(2):167–178. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pienta KJ, Esper PS. Risk factors for prostate cancer. Ann Intern Med. 1993;118(10):793–803. doi: 10.7326/0003-4819-118-10-199305150-00007. [DOI] [PubMed] [Google Scholar]
- 127.Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, Lawton C, Machtay M, Grignon D. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50(5):1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 128.Pollock RE, Doroshow JH, Khayat D, Nakao A, O’Sullivan B, editors. UICC Manual of Clinical Oncology. Hoboken, NJ: Wiley-Liss; 2003. p. 917. [Google Scholar]
- 129.Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens. 2010;23(5):460–468. doi: 10.1038/ajh.2010.25. [DOI] [PubMed] [Google Scholar]
- 130.Rundle A. Molecular epidemiology of physical activity and cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(1):227–236. [PubMed] [Google Scholar]
- 131.Saarto T, Sievanen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, Kautiainen H, Jarvenpaa S, Penttinen HM, Utriainen M, Jaaskelainen AS, Elme A, Ruohola J, Palva T, Vertio H, Rautalahti M, Fogelholm M, Luoto R, Blomqvist C. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2011 doi: 10.1007/s00198-011-1761-4. [DOI] [PubMed] [Google Scholar]
- 132.Santen RJ. Clinical review: Effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96(2):308–319. doi: 10.1210/jc.2010-1679. [DOI] [PubMed] [Google Scholar]
- 133.Schatzkin A, Gail M. The promise and peril of surrogate end points in cancer research. Nat Rev Cancer. 2002;2(1):19–27. doi: 10.1038/nrc702. [DOI] [PubMed] [Google Scholar]
- 134.Schmitz K. Physical activity and breast cancer survivorship. Recent Results Cancer Res. 2011;186:189–215. doi: 10.1007/978-3-642-04231-7_8. [DOI] [PubMed] [Google Scholar]
- 135.Schmitz KH. Exercise for secondary prevention of breast cancer: moving from evidence to changing clinical practice. Cancer Prev Res (Phila) 2011;4(4):476–480. doi: 10.1158/1940-6207.CAPR-11-0097. [DOI] [PubMed] [Google Scholar]
- 136.Schmitz KH. Balancing lymphedema risk: exercise versus deconditioning for breast cancer survivors. Exerc Sport Sci Rev. 2010;38(1):17–24. doi: 10.1097/JES.0b013e3181c5cd5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmitz KH, Cappola AR, Stricker CT, Sweeney C, Norman SA. The intersection of cancer and aging: establishing the need for breast cancer rehabilitation. Cancer Epidemiol Biomarkers Prev. 2007;16(5):866–872. doi: 10.1158/1055-9965.EPI-06-0980. [DOI] [PubMed] [Google Scholar]
- 138.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, VON Gruenigen VE, Schwartz AL. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 139.Schmitz KH, Prosnitz RG, Schwartz AL, Carver JR. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer. 2012;118(S8):2270–2276. doi: 10.1002/cncr.27462. [DOI] [PubMed] [Google Scholar]
- 140.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer. 2007;110(4):918–925. doi: 10.1002/cncr.22862. [DOI] [PubMed] [Google Scholar]
- 141.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Exercise training manages cardiopulmonary function and fatigue during and following cancer treatment in male cancer survivors. Integr Cancer Ther. 2007;6(3):235–241. doi: 10.1177/1534735407305871. [DOI] [PubMed] [Google Scholar]
- 142.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91(13):1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 143.Schwartz AL. Physical activity after a cancer diagnosis: psychosocial outcomes. Cancer Invest. 2004;22(1):82–92. doi: 10.1081/cnv-120027582. [DOI] [PubMed] [Google Scholar]
- 144.Schwartz AL, Winters-Stone K. Effects of a 12-month randomized controlled trial of aerobic or resistance exercise during and following cancer treatment in women. Phys Sportsmed. 2009;37(3):62–67. doi: 10.3810/psm.2009.10.1730. [DOI] [PubMed] [Google Scholar]
- 145.Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation. 2011;124(5):642–650. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, Venner PM, Quinney HA, Jones LW, D’Angelo ME, Wells GA. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 147.Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, Malone SC, Wells GA, Scott CG, Slovinec D’Angelo ME. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. 2009;27(3):344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 148.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, Gerber LH. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 149.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344(26):1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 150.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 151.Shephard RJ, Shek PN. Effects of exercise and training on natural killer cell counts and cytolytic activity: a meta-analysis. Sports Med. 1999;28(3):177–195. doi: 10.2165/00007256-199928030-00003. [DOI] [PubMed] [Google Scholar]
- 152.Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, Schwartz AG, Brown LM, Greenberg RS, Schoenberg JB, Pottern LM, Hoover RN, Fraumeni JF., Jr Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80(11):1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 155.Sporn MB. The war on cancer. Lancet. 1996;347(9012):1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- 156.Stortecky S, Suter TM. Insights into cardiovascular side-effects of modern anticancer therapeutics. Curr Opin Oncol. 2010;22(4):312–317. doi: 10.1097/CCO.0b013e32833ab6f1. [DOI] [PubMed] [Google Scholar]
- 157.Stubblefield MD, O’Dell MW, editors. Cancer Rehabilitation: Principles and Practice. 386 Park Avenue South, Suite 301. New York, New York 10016: Demos Medical Publishing, LLC; 2009. p. 1093. [Google Scholar]
- 158.Stubblefield MD. Cancer rehabilitation. Semin Oncol. 2011;38(3):386–393. doi: 10.1053/j.seminoncol.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 159.Stubblefield MD. Radiation fibrosis syndrome: neuromuscular and musculoskeletal complications in cancer survivors. PMR. 2011;3(11):1041–1054. doi: 10.1016/j.pmrj.2011.08.535. [DOI] [PubMed] [Google Scholar]
- 160.Stubblefield MD, Custodio CM. Upper-extremity pain disorders in breast cancer. Arch Phys Med Rehabil. 2006;87(3 Suppl 1):S96–9. doi: 10.1016/j.apmr.2005.12.017. quiz S100–1. [DOI] [PubMed] [Google Scholar]
- 161.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 162.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23(27):6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 163.Tardon A, Lee WJ, Delgado-Rodriguez M, Dosemeci M, Albanes D, Hoover R, Blair A. Leisure-time physical activity and lung cancer: a meta-analysis. Cancer Causes Control. 2005;16(4):389–397. doi: 10.1007/s10552-004-5026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thompson WR, Gordon NF, Pescatello LS, editors. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010. [Google Scholar]
- 165.Thompson HJ, Jiang W, Zhu Z. Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009;61(9):895–901. doi: 10.1002/iub.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325(23):1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 167.Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33(6 Suppl):S530–50. doi: 10.1097/00005768-200106001-00025. discussion S609–10. [DOI] [PubMed] [Google Scholar]
- 168.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–1972. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 169.Twiss JJ, Waltman NL, Berg K, Ott CD, Gross GJ, Lindsey AM. An exercise intervention for breast cancer survivors with bone loss. J Nurs Scholarsh. 2009;41(1):20–27. doi: 10.1111/j.1547-5069.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 170.van’t Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: a meta-analytical review of 58 studies after 1980. Psychosom Med. 1997;59(3):280–293. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 171.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The Effect of Physical Exercise on Cancer-related Fatigue during Cancer Treatment: a Meta-analysis of Randomised Controlled Trials. Clin Oncol (R Coll Radiol) 2010;22(3):208–221. doi: 10.1016/j.clon.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 172.Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Voskuil DW, Monninkhof EM, Elias SG, Vlems FA, van Leeuwen FE Task Force Physical Activity and Cancer. Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiol Biomarkers Prev. 2007;16(4):639–648. doi: 10.1158/1055-9965.EPI-06-0742. [DOI] [PubMed] [Google Scholar]
- 174.Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr. 2010;92(3):471–490. doi: 10.3945/ajcn.2010.29005. [DOI] [PubMed] [Google Scholar]
- 175.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev. 2006;15(4):750–755. doi: 10.1158/1055-9965.EPI-05-0820. [DOI] [PubMed] [Google Scholar]
- 176.Weiderpass E. Lifestyle and cancer risk. J Prev Med Public Health. 2010;43(6):459–471. doi: 10.3961/jpmph.2010.43.6.459. [DOI] [PubMed] [Google Scholar]
- 177.Weiss EP, Holloszy JO. Improvements in body composition, glucose tolerance, and insulin action induced by increasing energy expenditure or decreasing energy intake. J Nutr. 2007;137(4):1087–1090. doi: 10.1093/jn/137.4.1087. [DOI] [PubMed] [Google Scholar]
- 178.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO Washington University School of Medicine CALERIE Group. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4(3):187–201. doi: 10.1007/s11764-010-0122-1. [DOI] [PubMed] [Google Scholar]
- 180.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- 181.Wolin KY, Tuchman H. Physical activity and gastrointestinal cancer prevention. Recent Results Cancer Res. 2011;186:73–100. doi: 10.1007/978-3-642-04231-7_4. [DOI] [PubMed] [Google Scholar]
- 182.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100(4):611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wonders KY, Reigle BS. Trastuzumab and doxorubicin-related cardiotoxicity and the cardioprotective role of exercise. Integr Cancer Ther. 2009;8(1):17–21. doi: 10.1177/1534735408330717. [DOI] [PubMed] [Google Scholar]
- 184.Yahalom J, Portlock CS. Long-term cardiac and pulmonary complications of cancer therapy. Hematol Oncol Clin North Am. 2008;22(2):305–18. vii. doi: 10.1016/j.hoc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 185.Yang EJ, Park WB, Seo KS, Kim SW, Heo CY, Lim JY. Longitudinal change of treatment-related upper limb dysfunction and its impact on late dysfunction in breast cancer survivors: a prospective cohort study. J Surg Oncol. 2010;101(1):84–91. doi: 10.1002/jso.21435. [DOI] [PubMed] [Google Scholar]
- 186.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 187.Zakikhani M, Blouin MJ, Piura E, Pollak MN. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat. 2010;123(1):271–279. doi: 10.1007/s10549-010-0763-9. [DOI] [PubMed] [Google Scholar]
- 188.Zhu Z, Jiang W, Sells JL, Neil ES, McGinley JN, Thompson HJ. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1920–1929. doi: 10.1158/1055-9965.EPI-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]