Abstract
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease leading to motor neuron dysfunction resulting in impairment of neuromuscular transmission. A2A adenosine receptors have already been considered as a potential therapeutical target for ALS but their neuromodulatory role at the neuromuscular junction in ALS remains to be clarified. In the present work, we evaluated the effects of A2A receptors on neuromuscular transmission of an animal model of ALS: SOD1(G93A) mice either in the pre-symptomatic (4–6 weeks old) or in the symptomatic (12–14 weeks old) stage. Electrophysiological experiments were performed obtaining intracellular recordings in Mg2+ paralyzed phrenic nerve-hemidiaphragm preparations. Endplate potentials (EPPs), quantal content (q. c.) of EPPs, miniature endplate potentials (MEPPs) and giant miniature endplate potential (GMEPPs) were recorded. In the pre-symptomatic phase of the disease (4–6 weeks old mice), the selective A2A receptor agonist, CGS 21680, significantly enhanced (p<0.05 Unpaired t-test) the mean amplitude and q.c. of EPPs, and the frequency of MEPPs and GMEPPs at SOD1(G93A) neuromuscular junctions, the effect being of higher magnitude (p<0.05, Unpaired t-test) than age-matched control littermates. On the contrary, in symptomatic mice (12–14 weeks old), CGS 21680 was devoid of effect on both the amplitude and q.c. of EPPs and the frequency of MEPPs and GMEPPs (p<0.05 Paired t-test). The results herein reported clearly document that at the neuromuscular junction of SOD1(G93A) mice there is an exacerbation of A2A receptor-mediated excitatory effects at the pre-symptomatic phase, whereas in the symptomatic phase A2A receptor activation is absent. The results thus suggest that A2A receptors function changes with ALS progression.
Introduction
Amyotrophic Lateral Sclerosis (ALS) is an adult-onset progressive neurodegenerative disease characterized by the selective loss of motor neuron function leading to muscle atrophy and weakness. After symptomatic onset disease progression lasts 4 to 5 years and patients ultimately die due to bulbar failure. Most of the diagnosed cases carry an unknown genetic link (sporadic ALS) and a few (5–10%) are related to known mutations in specific proteins (familial ALS). Both present similar pathological and clinical features [1], [2]. The first gene associated with the inherited form of the disease was the SOD1 gene encoding for the superoxide dismutase 1 enzyme which accounts for 20% of the familial forms of ALS [3]. This led to the design of the first animal model of ALS, the SOD1(G93A) mouse, which currently is the most used and well characterized rodent model for this disease [4]. Neuromuscular dysfunction at symptomatic SOD1(G93A) mice has been reported [5], [6]. We recently showed that the SOD1(G93A) mice neuromuscular transmission impairment starts long before symptomatic onset [7].
Adenosine is a key neuromodulator with implications in pathological conditions [8]. At the neuromuscular junction it can act on both A1 and A2A adenosine receptors, fine-tuning acetylcholine (ACh) release [9]. A2A receptors are known to have a neuroprotective role in some pathological conditions [8] and have been considered as a potential therapeutical target for ALS [10]–[12]. Some contradictory reports in the literature can however be found [10], [11] highlighting the need for an evaluation of the influence of A2A receptors in ALS models where disease progression and neuromuscular transmission impairment can be taken into account.
Given the unexplored role of A2A receptors at the neuromuscular junction in ALS, and considering that the neuromuscular transmission in the SOD1(G93A) mice starts to present alterations long before symptoms onset [7], we considered of interest to evaluate A2A receptor effects on neuromuscular transmission, in both pre-symptomatic (4–6 weeks old) and symptomatic (12–14 weeks old) SOD1(G93A) ALS mice. The results now reported show that the role of A2A receptors at the motor nerve terminals, changes upon ALS progression. In the pre-symptomatic phase the A2A receptor-mediated excitatory effects on neuromuscular transmission are exacerbated, probably acting as a compensatory mechanism towards delaying disease progression, whereas in the symptomatic phase the A2A receptor excitatory action disappears.
Methods
Ethics statement
This study was performed in accordance with the European Community guidelines (Directives 86/609/EU and 2010/63/EU, Recommendation 2007/526/CE, European Convention for the Protection of Vertebrate Animals used for Experimental or Other Scientific Purposes ETS 123/Appendix A) and Portuguese Laws on Animal Care (Decreto-Lei 129/92, Portaria 1005/92, Portaria466/95, Decreto-Lei 197/96, Portaria 1131/97). All the protocols carried in this study were under approval of the Portuguese National Authority (General Direction of Veterinary) and the Ethics Committee of the Instituto de Medicina Molecular of the Faculty of Medicine, University of Lisbon, Lisbon, Portugal.
Animals
Transgenic B6SJL-TgN (SOD1-G93A)1Gur/J males (Jackson Laboratory, No. 002726) overexpressing the human SOD1 gene carrying a glycine to alanine point mutation at residue 93 (G93A) [4] and wild-type B6SJLF1/J females were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and were breed at IMM rodent facilities where a colony was established. Mice were maintained on a background B6SJL by breeding SOD1(G93A) transgenic males with non-transgenic females in a rotational scheme. Males were crossed with non-transgenic females because transgenic females are infertile. F1 offspring was used in all experiments. Progeny was no longer used in breeding to avoid mSOD1 gene copy number loss and therefore deviation from ALS phenotype [4]. SOD1(G93A) mice were used to study pre-symptomatic (4–6 weeks old) and symptomatic (12–14 weeks old) phases of the disease. 4–6 and 12–14 weeks old wild type (WT) animals served as controls. Both male and female mice were used. The proportion of male and female mice was about the same in the WT (18 males and 19 females in total) and SOD1(G93A) mice (20 males and 17 females in total) groups. Furthermore, using the same mice strain (B6SJL-Tg(SOD1-G93A)1Gur/J) no gender influences over the intrinsic features of neuromuscular transmission have been detected [7], though gender differences could be detected in other mice strains (B6.Cg-Tg-(SOD1-G93A)1Gur/J) [6].
Littermates were identified by dermal ear punching and divided into cages by gender. The ear tissue was used to genotype the animals by polymerase chain reaction (PCR) [3]. Animals were housed 4–5 mice/cage, under a 12 h light/12 h dark cycle, and received food and water ad libitum.
Electrophysiological recordings
Animals were anaesthetised using halothane and rapidly decapitated. Both right and left phrenic-nerve attached to the hemidiaphragm muscle were isolated. One preparation was placed and stretched in a 3 mL Perspex chamber continuously perfused via a roller pump (3 mL.min−1) with a physiologic saline solution (Krebs and Henseleit solution, see Drugs section) under continuous oxygenation. The other phrenic-nerve hemidiaphragm preparation was kept in a beaker with an oxygenated saline solution before being set up the recording chamber. Since no functional differences were found between right and left phrenic nerve-hemidiaphragm muscles, different protocols were carried in each preparation.
Intracellular recordings were performed in the conventional way [13]–[15]. The phrenic-nerve was stimulated supramaximally by a suction electrode (Cu/Cu2+) connected to a S48 square pulse stimulator (Grass Tecnologies, West Warwick, RI, USA). Stimuli were applied in a low frequency of 0.5 Hz with a current duration of 20 µs. The reference electrode was an Ag-AgCl pellet placed in the bath. The recording electrode was a glass microelectrode filled with KCl (3 M) with resistance between 15–40 MΩ inserted into the motor endplate. A Digidata 1440A digitizer (Molecular Devices, Sunnyvale, CA, USA), designed to work with the Axoclamp 2B amplifier (Molecular Devices, Sunnyvale, CA, USA), performed data acquisition, allowing continuous monitoring and digital storage of evaluated parameters with adequate software (pCLAMP 10.3, Molecular Devices, Sunnyvale, CA, USA).
Endplates with a resting potential between –65 to –85 mV were chosen for experiment. Resting voltage was stable throughout all experiments with less than 5% variation of its initial value. Endplate Potentials (EPPs) amplitude was assessed as the average amplitude of 60 consecutive EPPs (with amplitudes ranging between 1 mV to 5 mV). To evaluate the percentage of the drug effect, the mean averaged EPP amplitudes in the last 10 minutes before adding any drug (control) was compared with the mean averaged EPP amplitudes from the last 10 minutes of drug perfusion (treatment). The quantal content (q. c.) of EPPs was calculated as the ratio between the mean EPP amplitude and the mean Miniature Endplate Potential (MEPP) amplitude acquired during the same period with the same resting membrane potential. MEPPs were recorded in gap-free intervals of 100 seconds before adding the drug and at the end of drug perfusion. MEPP detection threshold was set between 0.2 mV and 1 mV [7]. MEPP amplitude was defined as the mean of all spontaneous events and the frequency as the number of events registered during the 100 seconds. The minimum Giant Miniature Endplate Potential (GMEPP) threshold amplitude was set in 1 mV [7]. This indirect measure of spontaneous activity synchronism was analyzed as the frequency of giant events in the 100 seconds gap-free acquisition mode and the mean amplitude as the average of GMEPPs magnitude in the same interval. Only if GMEPP frequency was higher than 0.04 s−1 before adding the drug, the percentage of effect was considered for analysis. Evoked activity was analyzed with Clampfit software (Molecular Devices, Sunnyvale, CA, USA) and spontaneous events with Mini-Analysis software (Synaptosoft Inc., Decatur, GA, USA). Whenever perfusing two drugs, the % change was calculated by comparing acquired values with the ones obtained from the first drug perfused (considered then as control).
Drugs
The bathing solution was modified from Krebs and Henseleit [16] (NaCl 117 mM; KCl 5 mM; NaHCO3 25 mM; NaH2PO4 1.2 mM; glucose 11 mM; CaCl2 2.5 mM; MgCl2 1.2 mM; pH 7.4) continuously gassed with 95% O2 and 5% CO2 kept at room temperature (22–25°C). Muscle twitch was prevented by increasing [Mg2+] to 18.5–19.5 mM in 4–6 weeks old animals and 20.0–22.0 mM in 12–14 weeks old mice. This strategy reduces the q. c. of EPPs but preserves the main features of neuromuscular transmission [13].
Drugs used were: 2-p-(2-carboxyethyl) phenethylamino]-5′-N-ethylcarboxamido adenosinehydrochloride (CGS 21680) and 5-Amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo(4,3-e)-1,2,4-triazolo(1,5-c) pyrimidine (SCH 58261). Stock solutions (5 mM) were made in dimethyl sulfoxide. To avoid compound precipitation aliquots were kept frozen at –20°C until used. Dimethyl sulfoxide was devoid of effect in the performed experiments like previously reported [13].
Statistical analysis
Data are presented as mean ± standard error of the mean in each group, which n corresponds to the number of animals used (1 fiber per mouse).
Student’s t-test for independent samples (Unpaired t-test) was used to compare drug effect between two groups. One way analysis of variance (ANOVA) was applied whenever comparing more than 2 means. If p<0.05, Tukey’s pos-test was applied to compare drug-induced changes between different groups. Student’s t-test for paired samples (Paired t-test) was used to compare obtained measurement with the control parameter before adding the drug (e.g. mean EPP amplitude before drug perfusion). Values of p<0.05 were considered to represent statistically significant differences.
Results
In pre-symptomatic SOD1(G93A) mice the excitatory A2A receptor-mediated effects on neuromuscular transmission are exacerbated
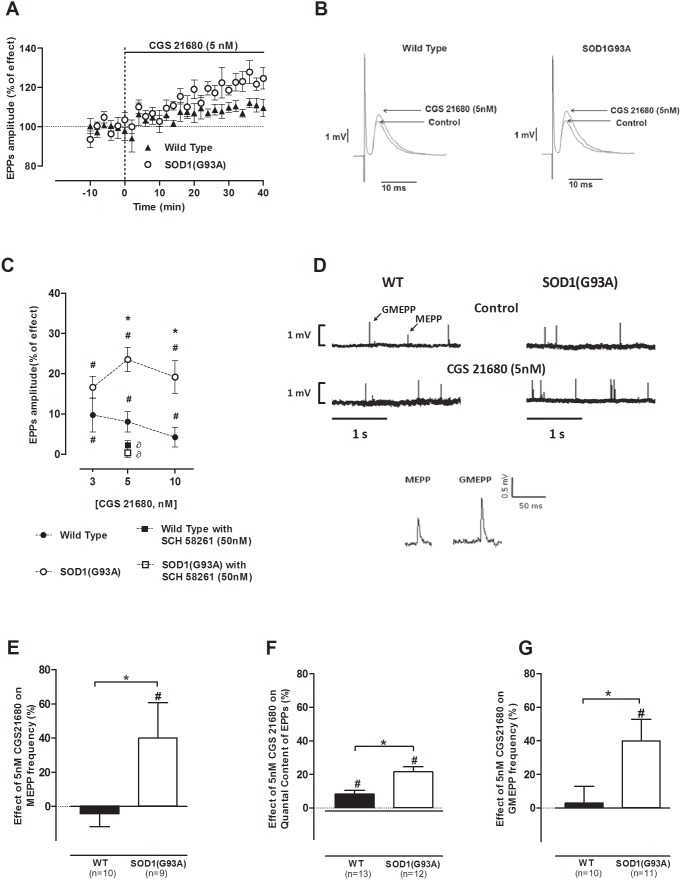
Though the selective A2A receptor agonist CGS 21680 [17] has been extensively used in research, namely at the neuromuscular junction [9], [13], [18], there are only few studies reporting its effects at the mouse neuromuscular junction, the existing being on K+-evoked ACh-release (e.g. [19]). We performed a dose-response study using 3, 5 to 10 nM of CGS 21680 in both pre-symptomatic SOD1(G93A) mice and their age-matched healthy controls. Figure 1A illustrates the time-course changes of mean EPP amplitude in the presence of CGS 21680 (5 nM). It clearly shows an exacerbation of the facilitatory effect of the A2A receptor agonist on EPP amplitude, in the pre-symptomatic SOD1(G93A) mice, a finding also illustrated in figure 1B. The difference between the two groups started to be evident 20 min after drug perfusion. As illustrated in Figure 1C, all the tested concentrations enhanced the mean amplitude of EPPs, when compared to the value measured before drug perfusion (p<0.05, Paired t-test). To evaluate the role of A2A receptors on neuromuscular transmission, while comparing the effect of CGS 21680 on the mean amplitude of EPPs recorded in both groups of animals, it could be concluded that at 3 nM there were no significant differences between groups (p>0.05, Unpaired t-test) while at 5 nM and 10 nM the facilitation caused by CGS 21680 on EPPs amplitude was significantly higher in pre-symptomatic SOD1(G93A) mice (5 nM: n = 13; 10 nM: n = 5; p<0.05, Unpaired t-test), when compared to the WT group (5 nM: n = 14; 10 nM: n = 7). Since the difference between groups was already pronounced at 5 nM, we decided to use this concentration in the remaining experiments. To exclude potential unspecific effects of CGS 21680, we evaluated the effect of this drug in the presence of the selective A2A receptor antagonist, SCH 58261 (50 nM) [20]. The blockade of A2A receptors did not change the mean amplitude of EPP in both groups of animals (data not shown, n = 5 for controls and n = 6 for SOD1G93A; p>0.05, Paired t-test) and effectively prevented the facilitatory effects of CGS 21680 (5 nM) (Figure 1C, n = 4 for controls and n = 6 for SOD1G93A; p<0.05, one-way ANOVA followed by Tukey’s pos-hoc). These results suggest that in the present experimental conditions A2A receptors are not tonically activated by endogenous adenosine and, also, that the effect of CGS 21680 (5 nM) results from specific A2A receptor action upon neuromuscular transmission.
Figure 1. CGS 21680 facilitation of evoked activity is exacerbated in pre-symptomatic mice; (A) representative time-course change of mean EPP amplitude throughout CGS 21680 (5 nM) perfusion and (B) representation of EPP amplitude increase in 4–6 weeks old WT (n = 5) and pre-symptomatic mice (n = 10) upon A2A receptor activation (CGS 21680 at 5 nM); (C) concentration-response changes in mean EPP amplitude in the presence of CGS 21680 (3 nM: n = 7, WT, n = 7, SOD1G93A; 5 nM: n = 14, WT, n = 13, SOD1G93A; 10 nM: n = 7, WT, n = 5, SOD1G93A) whose effect was blocked by SCH 58261 at 50 nM (n = 5, WT, n = 4, SOD1G93A); (D) raw recording of spontaneous release fluctuations from a 4–6 weeks old WT and pre-symptomatic SOD1G93A neuromuscular junction promoted by CGS 21680 (5 nM); effect of CGS 21680 (5 nM) perfusion regarding (E) MEPP frequency (n = 10, WT, n = 9, SOD1(G93A), (F) quantal Content of EPPs (n = 13, WT, n = 12, SOD1(G93A)) and (G) GMEPP frequency (n = 10, WT, n = 11, SOD1(G93A)) in pre-symptomatic SOD1(G93A) mice and respective healthy controls; *p<0.05 Unpaired t-test; ∂p<0.05 one-way ANOVA with Tukey’s pos-hoc; #p<0.05 Paired t-test (as compared with control value before drug perfusion); control corresponds to 100% in all cases.
To evaluate changes in the q. c. of EPPs, MEPPs and EPPs were recorded simultaneously. As illustrated in Figure 1D–E, when tested in WT animals, CGS 21680 (5 nM) was devoid of effect on both MEPPs amplitude and frequency (n = 10; p>0.05, Paired t-test). However, when applied to pre-symptomatic SOD1(G93A) neuromuscular junctions it caused a significant increase in the frequency of MEPPs, without changing its average amplitude (n = 9; p<0.05, Unpaired t-test). As it occurred for evoked changes in EPPs amplitude, the A2A receptor-mediated facilitatory effect on the mean frequency of MEPPs was more pronounced in pre-symptomatic SOD1(G93A) mice than in its age-matched healthy controls (Figure 1E). Also, SCH 58261 (50 nM), per se, did not significantly change MEPPs frequency in both studied animal groups (data not shown, n = 8 for controls and n = 4 for SOD1(G93A); p>0.05 Paired t-test), while preventing the facilitatory action of the A2A receptor agonist upon MEPP frequency in the pre-symptomatic SOD1(G93A) mice (n = 4, p<0.05, one-way ANOVA followed by Tukey’s pos-hoc). Regarding the q. c. of EPPs (Figure 1F) we observed that CGS 21680 (5 nM) caused a significantly higher facilitation in pre-symptomatic SOD1(G93A) mice (n = 12) than in its age-matched healthy mice (n = 13; p<0.05, Unpaired t-test); this effect was prevented by SCH 58261 (50 nM; n = 4 for controls and n = 6 for SOD1(G93A); p<0.05, one-way ANOVA followed by Tukey’s pos-hoc).
GMEPPs arise from intracellular Ca2+ disturbances resulting in a non-evoked “constitutive” secretion leading to abnormal spontaneous events at mammalian neuromuscular junctions [21], [22] and pre-symptomatic SOD1(G93A) mice present higher frequency of GMEPPs when compared to controls [7]. Considering the role of adenosine receptors in Ca2+ modulation [23], [24], the effect of A2A receptor activation, with its selective agonist, on the amplitude and frequency of giant spontaneous events was also evaluated. As illustrated in Figure 1D and 1G, CGS 21680 (5 nM) caused a significant increase in mean frequency of GMEPPs in pre-symptomatic SOD1(G93A) mice (n = 11; p<0.05, Unpaired t-test), whereas in the WT group it was devoid of effect (n = 10, p>0.05, Paired t-test). SCH 58261 (50 nM), per se, did not change the mean frequency of GMEPPs (data not shown, n = 7 for controls and n = 5 for SOD1(G93A)) and effectively prevented the facilitatory effect caused by 5 nM CGS 21680 in pre-symptomatic SOD1(G93A) mice (n = 5; p<0.05, one-way ANOVA). GMEPPs amplitude remained unchanged upon CGS 21680 (5 nM) perfusion and no statistical difference was found between the studied groups of animals.
Together, the results suggest that the A2A receptor-mediated facilitatory effects on neuromuscular transmission are exacerbated in the pre-symptomatic phase of the disease.
In symptomatic SOD1(G93A) mice the excitatory A2A receptor-mediated effects on neuromuscular transmission are absent
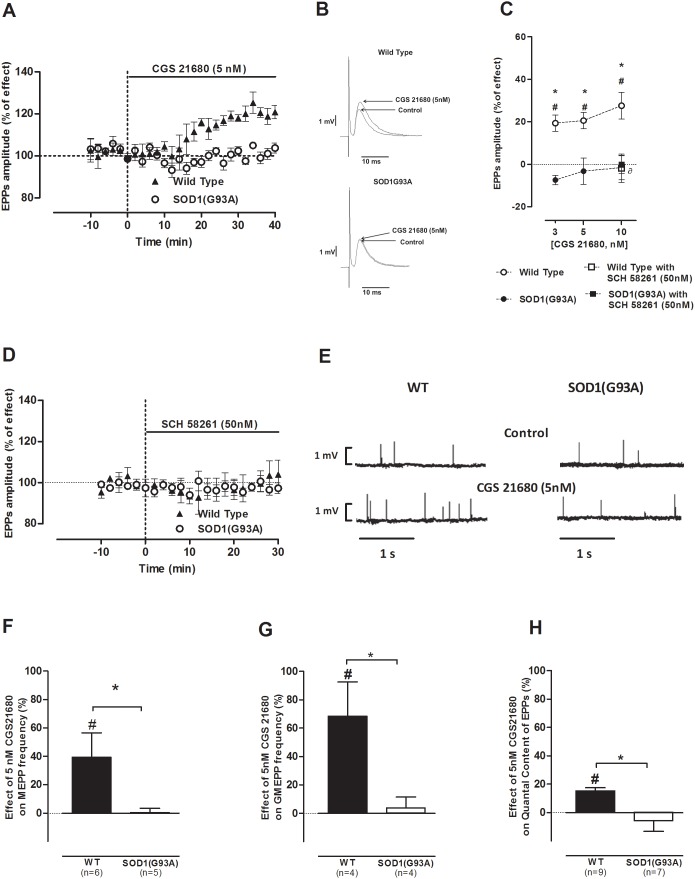
As in the pre-symptomatic phase of the disease, we performed a concentration-response study using the same concentrations (3, 5 and 10 nM) of CGS 21680 in both symptomatic SOD1(G93A) mice and their age-matched (12–14 weeks old) healthy controls. Figure 2A shows the time course of mean EPP amplitude changes in the symptomatic SOD1(G93A) mice throughout CGS 21680 (5 nM) perfusion. Figure 2B represents the profile of mean EPP amplitude changes in WT and symptomatic mice by 5 nM of CGS 21680. As expected, all the tested concentrations enhanced the mean averaged amplitude of EPPs in WT mice, when compared to the measured value before drug perfusion (Figure 2C; 3 nM: n = 8; 5 nM: n = 10; 10 nM: n = 11; p<0.05, Paired t-test). Remarkably, when applied to the symptomatic SOD1(G93A) neuromuscular junctions, none of the tested concentrations modified the amplitude of EPPs (Figure 2C; 3 nM: n = 10; 5 nM: n = 7; 10 nM: n = 7; p>0.05, Paired t-test). As illustrated in Figure 2D, SCH 58261 (50 nM) was devoid of effect in the mean EPP amplitude in both groups (WT: n = 4; SOD1(G93A): n = 7; p>0.05 Paired t-test), suggesting the absence of tonic activation of the A2A receptor agonist in symptomatic SOD1(G93A) mice as well in WT mice.
Figure 2. A2A receptor modulation is lost in symptomatic SOD1(G93A) mice endplates; (A) representative average time-course of mean EPP amplitude change during CGS 21680 (5 nM) bathing and (B) illustrative mean EPP profile facilitation in 12–14 weeks old control (n = 6) and symptomatic mice (n = 6); (C) dose-response alterations in mean EPP amplitude by CGS 21680 (3 nM: n = 8, WT, n = 10, SOD1G93A; 5 nM: n = 10, WT, n = 7, SOD1G93A; 10 nM: n = 11, WT, n = 7, SOD1G93A) were blocked by SCH 58261 at 50 nM in WT mice (n = 4, WT, n = 4, SOD1G93A); (D) SCH 58261 (50 nM) did not affect evoked activity throughout data acquisition (n = 4, WT, n = 7, SOD1G93A); (E) raw recording of spontaneous release variations from a 12–14 weeks old WT and symptomatic SOD1G93A endplate upon CGS 21680 (5 nM) perfusion; effect of A2A receptor activation by CGS 21680 (5 nM) on (F) MEPP frequency (n = 6, WT, n = 5, SOD1(G93A)) (G) GMEPP frequency (n = 4, WT, n = 4, SOD1(G93A)) and (H) quantal content of EPPs (n = 9, WT, n = 7, SOD1(G93A)); *p<0.05 Unpaired t-test; ∂p<0.05 one-way ANOVA with Tukey’s pos-hoc; #p<0.05 Paired t-test (as compared with control value before drug perfusion); control corresponds to 100% in all cases.
As illustrated in figures 2E, 2F and 2G, CGS 21680 (5 nM) caused an increase on MEPPs (n = 6) and GMEPPs frequency in WT mice (n = 4; p<0.05, Paired t-test) but when applied to symptomatic SOD1(G93A) neuromuscular junctions it was devoid of effect on MEPPs (n = 5) and GMEPP frequency (n = 4; p<0.05, Paired t-test). MEPPs amplitude remained unchanged in the presence of the A2A receptor agonist in both groups (p>0.05, Unpaired t-test). In relation to the q. c. of EPPs (Figure 2H), there was a significant increase in the q. c. of EPPs in 12–14 weeks old healthy mice, upon CGS 21680 (5 nM) perfusion (n = 9; p<0.05, Unpaired t-test), which was prevented by SCH 58261 (50 nM; n = 4; p<0.05, one-way ANOVA followed by Tukey’s pos-hoc). In contrast, the q. c. of EPPs was not modified by CGS 21680 (5 nM) perfusion in symptomatic SOD1(G93A) mice (n = 7; p>0.05 Paired t-test).
Comparison between the effect of A2A receptors activation at SOD1(G93A) neuromuscular junctions upon disease progression
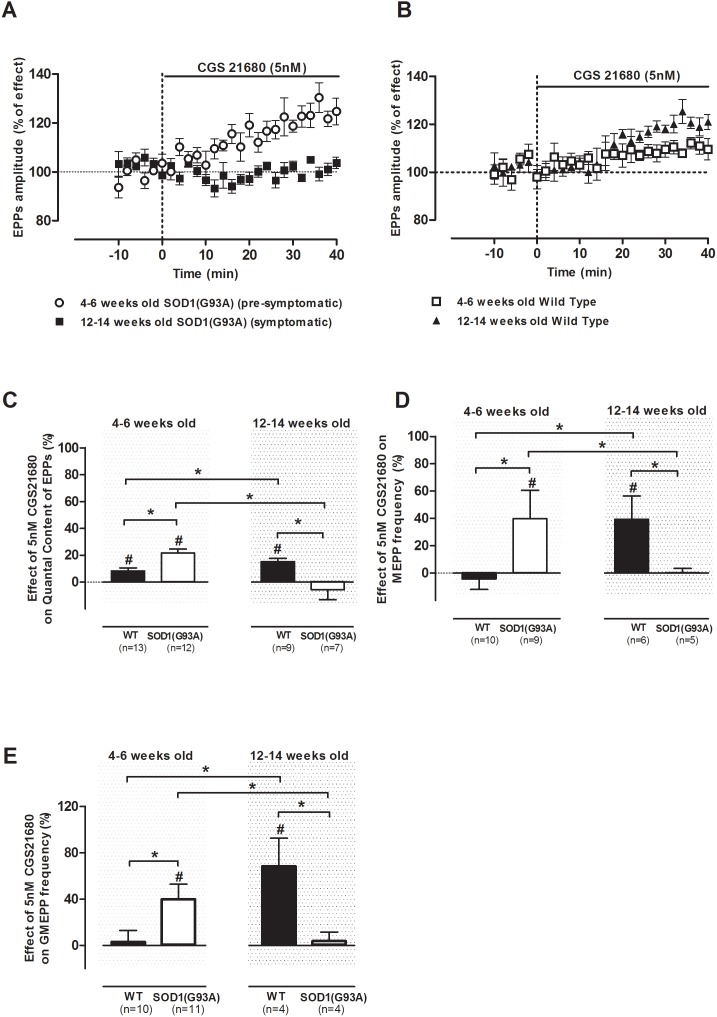
To allow the assessment of the role of A2A receptors throughout ALS progression, in Figure 3 are compared the effects of CGS 21680 (5 nM) in the pre-symptomatic and symptomatic SOD1(G93A) mice. Age-matched healthy controls were also subject of comparison to evaluate the maturation-associated alterations of neuromuscular transmission in physiological conditions. Figure 3A shows the superimposed time-course profiles of mean EPP amplitude change throughout CGS 21680 (5 nM) perfusion in pre-symptomatic and symptomatic SOD1(G93A) neuromuscular junctions. By this figure one can find that the role of A2A receptors dramatically changes with disease progression. It is concluded that the A2A receptor selective agonist induced a significantly higher enhancement of EPPs amplitude in 12–14 weeks old WT than in 4–6 weeks old control animals (p<0.05 Unpaired t-test) (Figure 3B), which was accompanied by a significant increase in the q. c. of EPPs (p<0.05, Unpaired t-test) (Figure 3C). Interestingly, the effect of CGS 21680 (5 nM) in pre-symptomatic SOD1(G93A) animals (4–6 weeks old) is similar to its effect in the 12–14 weeks old WT controls. This might be related to an ALS-associated early maturation process at the neuromuscular junction, as previously suggested [7].
Figure 3. Comparison of A2A receptor function upon disease progression in SOD1(G93A) mice and healthy controls; average time course of mean EPP amplitude facilitation by CGS 21680 (5 nM) in (A) pre-(n = 10) and symptomatic (n = 6) SOD1(G93A) rodents and (B) 4–6 weeks (n = 5) and 12–14 weeks (n = 6) old WT mice; effect of CGS 21680 perfusion at 5 nM on: (C) quantal content of EPPs (4–6 weeks old: n = 13, WT, n = 12, SOD1(G93A); 12–14 weeks old: n = 9, WT, n = 7, SOD1(G93A)); (D) MEPP frequency (4–6 weeks old: n = 10, WT, n = 9, SOD1(G93A); 12–14 weeks old: n = 6, WT, n = 5, SOD1(G93A)); and (E) GMEPP frequency (4–6 weeks old: n = 10, WT, n = 11, SOD1(G93A); 12–14 weeks old: n = 4, WT, n = 4, SOD1(G93A)) in both phases of the study from SOD1(G93A) mice and respective healthy controls; *p<0.05 Unpaired t-test; ∂p<0.05 one-way ANOVA with Tukey’s pos-hoc; #p<0.05 Paired t-test (as compared with control value before drug perfusion); control corresponds to 100% in all cases.
In relation to the effect of A2A receptor activation on MEPPs and GMEPPs frequency (figures 3D and 3E), there are some similarities between the pre-symptomatic SOD1(G93A) mice and the 12–14 weeks old wild-type controls. For example, the changes on the frequency of MEPPs and GMEPPs caused by A2A receptor activation, observed in pre-symptomatic SOD1(G93A) mice were not statistically different from the values recorded in the 12–14 weeks old wild type controls (p<0.05 Unpaired t-test).
Discussion
The main finding of the present work was that the role of adenosine A2A receptors at the neuromuscular junction of the ALS SOD1(G93A) mouse model changes with disease progression. In the pre-symptomatic phase, the magnitude of the excitatory effects on neuromuscular transmission, caused by A2A receptor, is enhanced compared to age-matched controls. In contrast, in the symptomatic SOD1(G93A) mice, the A2A receptor-mediated facilitation is absent.
The enhancement of neuromuscular transmission caused by the selective A2A receptor agonist, CGS 21680, results from an increase in the evoked release of ACh, since it increased the q. c. of EPPs without affecting the average amplitude of MEPPs recorded concomitantly. It is known that the activation of the adenosine A2A receptors induces an enhancement of neuromuscular transmission, which is hardly reversible [9], [13] and apparently more robust in 3–4 weeks old rats [13] than in 4–6 weeks old mice (present work). Interestingly, the A2A receptor signaling is apparently lost at the neuromuscular junction of aged (70–80 weeks old) rats as it is in the symptomatic SOD1(G93A) mice (present work), suggestive of an a disease induced early-ageing of A2A receptor influence upon neuromuscular transmission. The reason for the hardly reversible adenosine A2A receptor-mediated action might be the transducing system operated by the receptor, which binds to G-protein coupled receptors [25] involving cyclic AMP formation and Protein kinase A (PKA) activation [26] with subsequent protein phosphorylation, causing a long-lasting increase in synaptic strength. In addition, multiple interactions of A2A receptors with other proteins have been described both in peripheral and central nervous system [8]. At the neuromuscular junction, A2A receptors are known to interact with adenosine A1 receptors [13], [18], [27], presynaptic nicotinic autofacilitatory receptors [28], tyrosin receptor kinase B (TrkB) [29] or calcitonin gene-related peptide [30]. Furthermore, SOD1(G93A) mice pathogenesis is characterized by increased oxidative stress [2] and A2A receptors present redox-sensitive synchronizing action at the neuromuscular synapse [31]. Interestingly, in a recent work from our team where neuromuscular transmission of the SOD1(G93A) mouse was studied [7], it was show that ACh release at the neuromuscular junction is enhanced in the pre-symptomatic phase of the disease, since the average amplitude of EPPs recorded in the SOD(G93A) mice during the pre-symptomatic phase (4–6 weeks old) was similar to the values obtained in the healthy control group (12–14 weeks-old). Interestingly, the levels of brain-derived neurotrophic factor (BDNF) are strongly increased in post-mortem muscle samples of early phase of ALS patients [32]. It is known that A2A receptors, at motor nerve terminals, trigger the action of BDNF [29], which enhances transmitter release at developing neuromuscular junctions [33], improving neuromuscular transmission in the adult rat diaphragm [34] and facilitating synaptic efficacy by increasing presynaptic depolarization at the neuromuscular junction [35]. BDNF is also important for maintenance of ACh receptor clustering in the endplate [36], [37]. Whether the enhancement by A2A receptor in the pre-symptomatic phase of the disease, could account for the potentiation of endogenous BDNF actions that might occur at the neuromuscular junction, therefore, enhancing synaptic transmission and compensating an eventual early denervation needs to be investigated. Nevertheless, data herein reported suggests that activation of A2A receptors might be an important mechanism involved in the scenarios of pathology that leads to deficits in ACh release, like ALS.
Activation of A2A receptors with its selective agonist CGS 21680 markedly increased the frequency of spontaneous giant events in SOD1(G93A) mice, when compared to age matched controls. Interestingly, the magnitude of this effect in the pre-symptomatic SOD1(G93A) mice (4–6 weeks old) was not different from the one observed in the WT group with 12–14 weeks old, reinforcing the “early maturation” hypothesis [7]. Modulation of Ca2+ dynamics by A2A receptors could also be considered as an adenosine-related compensatory mechanism. In fact, it was shown that, at the mouse [19] and rat [23] neuromuscular junction, activation of A2A receptors can facilitate spontaneous and evoked ACh secretion by independent mechanisms: as result of (1) an increase in cytosolic nerve terminal Ca2+ concentration due to release of this ion from intracellular Ca2+ stores or (2) by increase of extracellular Ca2+ entry into the terminals via L-type voltage gated Ca2+ channels (VGCC). Both mechanisms lead to an intracellular Ca2+ rise that in turn increases ACh release. Furthermore, muscle strength depends on the firing frequency and motor unit recruitment [38] and presynaptic changes in Ca2+ homeostasis may induce adaptations to facilitate firing frequency, specifically during high-frequency stimulation [39]. Fuchs and colleagues [40], using visually guided patch-clamp recordings in combination with single cell Ca2+ imaging of motor neurons throughout the complete lifespan of the SOD1(G93A) ALS mouse, reported that the pre-symptomatic motor terminals (70 days ∼7 weeks) present hyperexcitability in association with remodeling of Ca2+ handling.
In symptomatic mice, A2A receptors modulation of both evoked and spontaneous activity was lost. Full occupancy of A2A receptors by high levels of endogenous adenosine cannot account for this lack of effect, because the selective antagonist was devoid of effect on neuromuscular transmission suggesting that, A2A receptors were not tonically activated by the endogenous ligand. Indeed, the experimental conditions used to evaluate changes in the quantal release of ACh (low frequency stimulation, low quantal content and muscle twitching prevented) favor reduced levels of extracellular adenosine at the endplate, since purines are released both from the nerve endings, in part together with ACh, and from the contracting muscle fibers [41], [42]. In addition, the extracellular levels of adenosine may be considerably decreased in ALS, as it occurs in other disorders of the motor endplate [42].
The loss of excitatory effect while directly activating the A2A receptors with the agonist in symptomatic SOD1(G93A) mice may result from a decrease in the number and/or a decrease in the affinity of the receptor to its ligand. A2A receptors expression was shown to be decreased in the spinal cord of symptomatic SOD1(G93A) animals [11]. Alterations in the transducing system operated by A2A receptors may also be altered in ALS. Thus, ALS patients have increased PKA expression (the intracellular target of A2A receptor activation) in the spinal cord [43], which could indicate a positive feedback response for a PKA saturation mechanism, where different proteins trigger the cAMP – PKA pathway, limiting A2A receptor effects. Also immunoglobulins from ALS patients sera increased spontaneous release [44] by rendering L-type VGCC sensitive to stimuli [45], the signaling target of A2A receptor activation. This could lead to abnormal interactions, resulting in impaired regulatory A2A receptor recruitment of L-type VGCCs. For example, in Myasthenia gravis, a deficient A2A modulation impairs recruitment of L-type VGCC rendering animals susceptible to tetanic depression [42].
Interestingly, we could observe some similarities between the symptomatic SOD1(G93A) mice (herein presented) and aged rats (70–80 weeks old; [18]) in what respects to the effect of the A2A receptor selective agonist, CGS21680. In both cases there is an absence of effect of A2A receptors. It remains to be clarified what are the consequences of the absence of A2A receptors actions for fine-tuning of motor control and whether this relates to the age-associated or ALS-related decline in neuromuscular control. So, the reported loss of A2A receptor-mediated excitatory effects in symptomatic SOD1(G93A) neuromuscular junctions could be an adaptive shift to slow motor neuron degeneration. Further studies designed to manipulate A2A receptors in vivo before or after symptoms appearance may help to clarify whether A2A receptors influence progression of the neuromuscular transmission deficits observed in ALS patients or if these A2A receptor changes are a consequence of the disease progression. The results herein reported also pave the way for further studies designed to assess whether A2A receptor changes occur in ALS patients and if so, whether they are restricted to those with SOD1 gene mutations or are present in all ALS forms.
Immuno-inflammatory processes are features present in ALS patients and in the SOD1(G93A) mouse model [46]. A2A receptors have a well described immunossupressive action on immune cells [47] and their activation has proven beneficial in neuromuscular inflammatory diseases such as experimental auto-immune myasthenia gravis [48]. Schwann cells participate in adenosinergic modulation at the level of the neuromuscular junction [49] and can also participate in the modulation of immune actions [50]. A2A receptors are also present on motor neurons, microglia and astrocytes helping to fine-tune motor neuron responses and participate in neuroinflammatory processes [8], [47]. A2A receptors are overexpressed in lymphocytes from ALS patients, resulting in increased levels of intracellular cAMP [51], which highlights a possible role for these receptors in immunosuppressive responses in ALS. Whether the now documented A2A receptor functional changes in the SOD1(G93A) also parallel with an immunological based response and relate with the previously reported A2A receptor-mediated delayed onset and reduced progression of motor neuron dysfunction in this ALS model [11], awaits further investigation.
In conclusion, the work herein reported clearly documents that at the neuromuscular junction of SOD1(G93A) mice there is an exacerbation of A2A receptor-mediated excitatory effects at the pre-symptomatic phase, whereas in the symptomatic phase A2A receptor activation is absent. The results thus suggest that A2A receptors function changes with ALS progression.
Acknowledgments
We thank Mr. João Baião for animals handling and the Rodent Facility from the Instituto de Medicina Molecular, Faculty of Medicine University of Lisbon.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Fundação do Ministério da Ciência e Tecnologia de Portugal [Grant PTDC/SAU-FAR/118787/2010] (http://www.fct.pt/index.phtml.pt). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, et al. (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12: 310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robberecht W, Philips T (2013) The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14: 248–64. [DOI] [PubMed] [Google Scholar]
- 3. Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364: 362. [DOI] [PubMed] [Google Scholar]
- 4. Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, et al. (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264: 1772–5. [DOI] [PubMed] [Google Scholar]
- 5. Kim YI, Joo C, Cheng CC, Davis CE, O’Shaughnessy TJ (1997) Neuromuscular transmission in a transgenic animal model of motor neuron disease. Proceedings of the 18th Annual International Conference of the Ieee Engineering in Medicine and Biology Society, Vol 18, Pts 1–5 18: 1773–1774. [Google Scholar]
- 6.Naumenko N, Pollari E, Kurronen A, Giniatullina R, Shakirzyanova A, et al. (2011) Gender-specific mechanism of synaptic impairment and its prevention by GCSF in a mouse model of ALS. Frontiers in Cellular Neuroscience 5. [DOI] [PMC free article] [PubMed]
- 7. Rocha MC, Pousinha PA, Correia AM, Sebastião AM, Ribeiro JA (2013) Early Changes of Neuromuscular Transmission in the SOD1(G93A) Mice Model of ALS Start Long before Motor Symptoms Onset. PLoS One 8: e73846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebastião AM, Ribeiro JA (2009) Adenosine receptors and the central nervous system. Handb Exp Pharmacol 471–534. [DOI] [PubMed]
- 9. Correia-de-Sá P, Sebastião AM, Ribeiro JA (1991) Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve ending of the rat. Br J Pharmacol 103: 1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beghi E, Pupillo E, Messina P, Giussani G, Chio A, et al. (2011) Coffee and amyotrophic lateral sclerosis: a possible preventive role. Am J Epidemiol 174: 1002–8. [DOI] [PubMed] [Google Scholar]
- 11. Potenza RL, Armida M, Ferrante A, Pezzola A, Matteucci A, et al. (2013) Effects of chronic caffeine intake in a mouse model of amyotrophic lateral sclerosis. J Neurosci Res 91: 585–92. [DOI] [PubMed] [Google Scholar]
- 12. Yanpallewar SU, Barrick CA, Buckley H, Becker J, Tessarollo L (2012) Deletion of the BDNF truncated receptor TrkB. T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS One 7: e39946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pousinha PA, Correia AM, Sebastião AM, Ribeiro JA (2010) Predominance of adenosine excitatory over inhibitory effects on transmission at the neuromuscular junction of infant rats. J Pharmacol Exp Ther 332: 153–63. [DOI] [PubMed] [Google Scholar]
- 14. Ribeiro JA, Sebastião AM (1987) On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol 384: 571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ribeiro JA, Walker J (1975) The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junctions. Br J Pharmacol 54: 213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krebs HA, Henseleit K (1932) Untersuchungen uber die Harnstoffbildung im Tierkoper. Hoppe-Seyler’s Z Physiol Chem 210: 33–37. [Google Scholar]
- 17. Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, et al. (1989) [H-3] Cgs-21680, a Selective A2 Adenosine Receptor Agonist Directly Labels A2-Receptors in Rat-Brain. Journal of Pharmacology and Experimental Therapeutics 251: 888–893. [PubMed] [Google Scholar]
- 18. Pousinha PA, Correia AM, Sebastião AM, Ribeiro JA (2012) Neuromuscular transmission modulation by adenosine upon aging. Neurobiol Aging 33: 2869–80. [DOI] [PubMed] [Google Scholar]
- 19. Palma AG, Muchnik S, Losavio AS (2011) Excitatory effect of the A2A adenosine receptor agonist CGS-21680 on spontaneous and K+-evoked acetylcholine release at the mouse neuromuscular junction. Neuroscience 172: 164–76. [DOI] [PubMed] [Google Scholar]
- 20. Fredholm BB, Lindstrom K, Dionisotti S, Ongini E (1998) [3H] SCH 58261, a selective adenosine A2A receptor antagonist, is a useful ligand in autoradiographic studies. J Neurochem 70: 1210–6. [DOI] [PubMed] [Google Scholar]
- 21. Sellin L, Molgo J, Tornquist K, Hansson B, Thesleff S (1996) On the possible origin of giant or slow-rising miniature end-plate potentials at the neuromuscular junction. Pflugers Arch 431: 325–34. [DOI] [PubMed] [Google Scholar]
- 22. Weinstein SP (1980) A comparative electrophysiological study of motor end-plate diseased skeletal muscle in the mouse. J Physiol 307: 453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Correia-de-Sá P, Timóteo MA, Ribeiro JA (2000) A(2A) adenosine receptor facilitation of neuromuscular transmission: influence of stimulus paradigm on calcium mobilization. J Neurochem 74: 2462–9. [DOI] [PubMed] [Google Scholar]
- 24. Oliveira L, Timóteo MA, Correia-de-Sá P (2004) Tetanic depression is overcome by tonic adenosine A(2A) receptor facilitation of L-type Ca(2+) influx into rat motor nerve terminals. J Physiol 560: 157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopes LV, Cunha RA, Ribeiro JA (1999) Increase in the number, G protein coupling, and efficiency of facilitatory adenosine A2A receptors in the limbic cortex, but not striatum, of aged rats. J Neurochem 73: 1733–8. [DOI] [PubMed] [Google Scholar]
- 26. Correia-de-Sá P, Ribeiro JA (1994) Evidence that the presynaptic A2a-adenosine receptor of the rat motor nerve endings is positively coupled to adenylate cyclase. Naunyn Schmiedebergs Arch Pharmacol 350: 514–22. [DOI] [PubMed] [Google Scholar]
- 27. Correia-de-Sá P, Timóteo MA, Ribeiro JA (1996) Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J Neurophysiol 76: 3910–9. [DOI] [PubMed] [Google Scholar]
- 28. Correia-de-Sá P, Ribeiro JA (1994) Tonic adenosine A2A receptor activation modulates nicotinic autoreceptor function at the rat neuromuscular junction. Eur J Pharmacol 271: 349–55. [DOI] [PubMed] [Google Scholar]
- 29. Pousinha PA, Diogenes MJ, Ribeiro JA, Sebastião AM (2006) Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neurosci Lett 404: 143–7. [DOI] [PubMed] [Google Scholar]
- 30. Correia-de-Sá P, Ribeiro JA (1994) Potentiation by tonic A2a-adenosine receptor activation of CGRP-facilitated [3H]-ACh release from rat motor nerve endings. Br J Pharmacol 111: 582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsentsevitsky A, Kovyazina I, Nikolsky E, Bukharaeva E, Giniatullin R (2013) Redox-sensitive synchronizing action of adenosine on transmitter release at the neuromuscular junction. Neuroscience 248: 699–707. [DOI] [PubMed] [Google Scholar]
- 32. Kust BM, Copray JC, Brouwer N, Troost D, Boddeke HW (2002) Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp Neurol 177: 419–27. [DOI] [PubMed] [Google Scholar]
- 33. Boulanger LM, Poo MM (1999) Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci 2: 346–51. [DOI] [PubMed] [Google Scholar]
- 34. Mantilla CB, Zhan WZ, Sieck GC (2004) Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve 29: 381–6. [DOI] [PubMed] [Google Scholar]
- 35. Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belluardo N, Westerblad H, Mudo G, Casabona A, Bruton J, et al. (2001) Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol Cell Neurosci 18: 56–67. [DOI] [PubMed] [Google Scholar]
- 37. Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, et al. (1999) Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron 24: 567–83. [DOI] [PubMed] [Google Scholar]
- 38. Norris FH Jr, Gasteiger EL (1955) Action potentials of single motor units in normal muscle. Electroencephalogr Clin Neurophysiol 7: 115–25. [DOI] [PubMed] [Google Scholar]
- 39. Catterall WA, Few AP (2008) Calcium channel regulation and presynaptic plasticity. Neuron 59: 882–901. [DOI] [PubMed] [Google Scholar]
- 40. Fuchs A, Kutterer S, Muhling T, Duda J, Schutz B, et al. (2013) Selective mitochondrial Ca2+ uptake deficit in disease endstage vulnerable motoneurons of the SOD1G93A mouse model of amyotrophic lateral sclerosis. J Physiol 591: 2723–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ribeiro JA, Cunha RA, Correia-de-Sá P, Sebastião AM (1996) Purinergic regulation of acetylcholine release. Prog Brain Res 109: 231–41. [DOI] [PubMed] [Google Scholar]
- 42. Noronha-Matos JB, Morais T, Trigo D, Timóteo MA, Magalhães-Cardoso MT, et al. (2011) Tetanic failure due to decreased endogenous adenosine A(2A) tonus operating neuronal Ca(v) 1 (L-type) influx in Myasthenia gravis. J Neurochem 117: 797–811. [DOI] [PubMed] [Google Scholar]
- 43. Hu JH, Zhang H, Wagey R, Krieger C, Pelech SL (2003) Protein kinase and protein phosphatase expression in amyotrophic lateral sclerosis spinal cord. J Neurochem 85: 432–42. [DOI] [PubMed] [Google Scholar]
- 44. Uchitel OD, Appel SH, Crawford F, Sczcupak L (1988) Immunoglobulins from amyotrophic lateral sclerosis patients enhance spontaneous transmitter release from motor-nerve terminals. Proc Natl Acad Sci U S A 85: 7371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fratantoni SA, Weisz G, Pardal AM, Reisin RC, Uchitel OD (2000) Amyotrophic lateral sclerosis IgG-treated neuromuscular junctions develop sensitivity to L-type calcium channel blocker. Muscle Nerve 23: 543–50. [DOI] [PubMed] [Google Scholar]
- 46. Phani S, Re DB, Przedborski S (2012) The Role of the Innate Immune System in ALS. Front Pharmacol 3: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hasko G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7: 759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li N, Mu L, Wang J, Zhang J, Xie X, et al. (2012) Activation of the adenosine A2A receptor attenuates experimental autoimmune myasthenia gravis severity. Eur J Immunol 42: 1140–51. [DOI] [PubMed] [Google Scholar]
- 49. Todd KJ, Robitaille R (2006) Purinergic modulation of synaptic signalling at the neuromuscular junction. Pflugers Arch 452: 608–14. [DOI] [PubMed] [Google Scholar]
- 50. Ydens E, Lornet G, Smits V, Goethals S, Timmerman V, et al. (2013) The neuroinflammatory role of Schwann cells in disease. Neurobiol Dis 55: 95–103. [DOI] [PubMed] [Google Scholar]
- 51. Vincenzi F, Corciulo C, Targa M, Casetta I, Gentile M, et al. (2013) A2A adenosine receptors are up-regulated in lymphocytes from amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener 14: 406–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.