Abstract
Objectives
Recent genome-wide association study found rs1801274, a functional single nucleotide polymorphism (SNP) in IgG receptor gene FCGR2A, was associated with increased risk of Kawasaki disease (KD). However, subsequent studies on the role of this SNP were limited and controversial.
Methods
A case-control study was conducted in a Chinese Han population including 428 KD patients and 493 controls to examine the association between rs1801274 and KD susceptibility. A meta-analysis was performed in combination with the relevant published studies to further clarify such an association.
Results
Our case-control study found that rs1801274 was significantly associated with increased risk of KD in the Chinese Han population, with an odds ratio (OR) of 1.58 (95% CI = 0.96–2.62) for the GA genotype and 1.93 (95% CI = 1.16–3.19) for the AA genotype compared with the GG genotype. The result of meta-analysis further demonstrated that the A allele of rs1801274 was significantly correlated with KD risk under the allelic model (OR = 1.35, 95% CI = 1.27–1.44) without heterogeneity by fixed-effects model analysis (Q = 17.30, p = 0.139). Moreover, sensitivity analysis supported the robustness of this meta-analysis.
Conclusion
These results further confirm that rs1801274 in the FCGR2A gene is significantly associated with increased risk of KD.
Introduction
Kawasaki disease (KD, OMIM 611775), first reported by a Japanese pediatric doctor Tomisaku Kawasaki [1], is an acute, multi-systemic vasculitis that specifically attacks children under 5 years old. Currently, KD has become one of the leading causes of acquired heart disease of children in developed countries [2]–[5]. Clinical manifestations of KD include fever at least 5 days, bilateral conjunctival congestion, polymorphous rashes, cervical lymphadenopathy, changes of extremities and mucous membranes of upper respiratory tract [6]. Diagnosis of KD is mostly based on clinical criteria due to absence of specific tests and pathogen markers [7]. About 50% of KD patients suffer from heart damages, and a quarter of untreated patients finally develop coronary artery lesions (CALs), including coronary artery dilatation, coronary fistula and even myocardial infarction [8]–[10]. So far, high dose intravenous immunoglobulin (IVIG) in combination with oral aspirin is the only evidence-based therapy for KD, which could decrease the occurrence of coronary artery complications to 5–16% [11], [12]. It is reported that the annual incidence of KD is 69/100 000 in Taiwan, the third highest worldwide, and 30.7/100000 in mainland China [3], which is on the increase in recent years.
So far, the etiology of KD remains unclear even clinical features indicates an infectious agent may be involved in the pathogenesis of KD. Evidence from epidemiological studies highly suggested that genetic factors play an important role in this process. First, twins and siblings of affected children have a risk of KD 10 times higher than general population [13]. Second, the risk of KD is doubled in the children when their parents had a history of KD [14]–[16]. At last, the incidence of KD is 10 to 20 times higher in North-East Asian populations including Japanese and Koreans than that reported in Caucasians [17], [18]. To date, genome-wide association study (GWAS) and linkage analysis of KD have identified 13 genetic loci, which are associated with KD or CALs in Japanese, Europeans, Koreans and Taiwanese [19]–[24], including IgG receptor gene FCGR2A (Fc gamma receptor IIa). The single nucleotide polymorphism (SNP) rs1801274 in FCGR2A gene encodes an H131R substitution, which may be related to the development of KD.
FCGR2A is commonly expressed on immune responsive cells like macrophages, neutrophils, monocytes and dendritic cells [25] to boost phagocytosis and production of inflammatory mediators [26]. The A allele of rs1801274 results in a point mutation from arginine to histidine, leading to increased immune responses. In a variety of human immune system diseases, such as systemic lupus erythematosus [27], arthritis [28] and ulcerative colitis [29], aberrant expression or allelic variants of FCGR2A have been identified.
Given its genetic function, FCGR2A gene was assumed to be a potential marker of KD risk. However, the results from previous studies were inconsistent [22], [23], [37]–[40]. Hence, we conducted a case-control study to examine the association between FCGR2A gene and KD risk in a Chinese Han population from Zhejiang Province in Southeast China. Additionally, a meta-analysis was performed by combining the data of this study with the results from other relevant, previously published case-control studies, and the data of transmission/disequilibrium test (TDT) to further confirm such an association.
Materials and Methods
Study population
In this present study, a total of 428 KD patients were recruited from April 2009 to September 2012 in Children's Hospital, Zhejiang University in China. All the patients were unrelated children of Chinese Han ethnic origin. The diagnosis of KD was based on the latest diagnostic criteria released by the Japan Kawasaki Disease Research Committee in 2002 [7]. The controls were 493 gender-matched unrelated healthy Chinese children of Han ethnic origin. They were randomly selected from the children taking physical examination in Children's Hospital, Zhejiang University for admission to primary school. All patients were subjected to two-dimensional echocardiography during febrile episode and after hospital discharge. And 414 patients received IVIG at daily dose of 1 g/kg for 2 days or a single infusion of 2 g/kg and oral aspirin at daily dose of 80 mg/kg. CAL was defined as coronary arteries with a diameter (inner border to inner border) ≥3 mm in children younger than 5 years old, >4 mm in children above 5 years, or the diameter was >1.5 times wider than that of the adjacent vessel [30], [31].
This study was approved by the Ethics Committee of Children's Hospital, Zhejiang University School of Medicine, and Huazhong University of Science and Technology (HUST). Written informed consent forms were obtained from parents/guardians of all the participants.
SNP Genotyping
Genomic DNA was extracted from 2 ml peripheral blood sample with the RelaxGene Blood DNA System DP319-02 (Tiangen, Beijing, China) following the manufacturer's instructions. TaqMan SNP Genotyping Assay was used to determine rs1801274 genotypes using a 7900 HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA).
Statistical analysis
The gender distribution and genotypic frequencies were estimated by Pearson χ2 test between patients and controls. Hardy-Weinberg equilibrium (HWE) was tested by a goodness-of-fit χ2 test for the distribution of genotypic frequencies among controls. After adjustment of gender, unconditional logistic regression analyses were performed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the effect of the SNP on KD and CALs in patients and controls, as well as the patients with and without CALs, respectively. The analysis was conducted under dominant, recessive and additive models respectively to avoid the bias of genetic models. All the statistical analyses were performed with SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). Power calculation was carried out using Power version 3.0 with the current sample size and genotype frequency. All p values were two-tailed. P<0.05 was considered statistically significant.
Meta-analysis for the association between rs1801274 and KD risk
Several databases including EMBASE, PubMed, and ISI Web of Science were searched up to August 28, 2013 to identify the relevant case-control and TDT studies on the association between rs1801274 and KD. The search terms rs1801274, Fc gamma receptor, polymorphism, and Kawasaki disease were used without language restriction. References cited in the retrieved articles, relevant studies or review articles on this topic were also included. The selected studies should meet the following criteria: (1) a case-control or family-based TDT study assessing the association between rs1801274 and KD; (2) containing integrated information about genotype or allele frequency for risk estimates, or original data; (3) genotypes in control groups were fit to Hardy-Weinberg equilibrium (p>0.05). Animal studies, commentaries and case reports were excluded. If subjects were overlapped in several studies, only the one with more complete design or larger sample size was selected.
The information extracted from each eligible article included the name of first author, year of publication, study group, ethnicity, method of genotyping, design type and diagnostic criteria for KD.
Cochran's χ2-based Q statistic test was used to estimate between-study heterogeneity. It was considered statistically significant when p<0.1. In this case, a random-effects model, using DerSimonian and Laird method, was further applied to calculate the pooled OR. Otherwise, a fixed-effects model, using Mantel-Haenszel method, was employed. In order to integrate the results from both case-control and TDT studies, the method described by Kazeem and Farrall [32] was referred. Estimate of combined OR and its standard error (SE) was calculated by a weighted analysis method [33]. All the evaluations were conducted using Catmap software, which could be downloaded from http://www.r-project.org [34]. In addition, sensitivity analysis was used to assess the influence of each study on the overall estimate after removal of this study [35]. Publication bias was assessed by funnel plot and regression test. All p values were from two-tailed test with a significant level at 0.05 except those for heterogeneity. The meta-analysis was carried out using Catmap software V1.6.
Results
Characteristics of study population
A total of 428 KD patients and 493 healthy controls were included in this case-control study. Characteristics of all the patients and controls were summarized in Table 1. Males accounted for 61.45% of te ptients and 61.46% of the controls (χ2 = 0.000, p = 0.997). Generally, CALs were detected in 29 patients. The statistical power was 0.846 for the current sample size (493/428), and 0.190 for CAL group (399/29).
Table 1. The characteristics of patients with Kawasaki disease and healthy controls in current study.
| Variables | Case (n = 428) | Control (n = 493) | P value |
| Gender, N (%) | 0.997a | ||
| Male | 263 (61.45) | 303 (61.46) | |
| Female | 165 (38.55) | 190 (38.54) |
P value was calculated by χ2 test.
Association analysis
The genotyping of SNP rs1801274 was successful in 99.35% of the study subjects (425/428 patients and 493/493 controls). The genotypic frequency distribution was in agreement with HWE (p = 0.486) in the controls. As shown in Table 2, rs1801274 was significantly associated with increased risk of KD in those carrying the A allele or AA genotype compared to those carrying the G allele or GG genotype (A versus G: OR = 1.29, 95% CI = 1.06–1.58; AA versus GG: OR = 1.93, 95% CI = 1.16–3.19). Similarly, such a significant association was also demonstrated in dominant model (OR = 1.75, 95% CI = 1.08–2.84), recessive model (OR = 1.31, 95% CI = 1.01–1.70), or additive model (OR = 1.31, 95% CI = 1.07–1.61).
Table 2. Polymorphism of rs1801274 of the FCGR2A gene in the KD patients and healthy controls in current study.
| Frequency | KD cases (%) | Controls (%) | OR | 95% CI | P a |
| Genotype | |||||
| GG | 27 (6.37) | 52 (10.59) | Reference | Reference | 1 |
| GA | 185 (43.63) | 226 (46.03) | 1.58 | 0.96–2.62 | 0.075 |
| AA | 212 (50.00) | 213 (43.38) | 1.93 | 1.16–3.19 | 0.011 b |
| Allelic model | — | — | 1.29 | 1.06–1.58 | 0.012 b |
| Dominant model | — | — | 1.75 | 1.08–2.84 | 0.024 b |
| Recessive model | — | — | 1.31 | 1.01–1.70 | 0.045 b |
| Additive model | — | — | 1.31 | 1.01–1.61 | 0.010 b |
Adjusted the effect of gender and age. KD, Kawasaki disease.
The values in bold indicate statistically significant (p<0.05).
The KD patients were then stratified into two groups based on development of CAL (Table S1 in File S1). Unexpectedly, no significant association was observed between the polymorphism and CAL formation in terms of genotype (A versus G: OR = 1.03, 95% CI = 0.57–1.87), dominant model (OR = 0.47 95% CI = 0.06–3.63), recessive model (OR = 1.08, 95% CI = 0.51–2.29), or additive model (OR = 1.04, 95% CI = 0.56–1.93).
Relevant studies included in meta-analysis
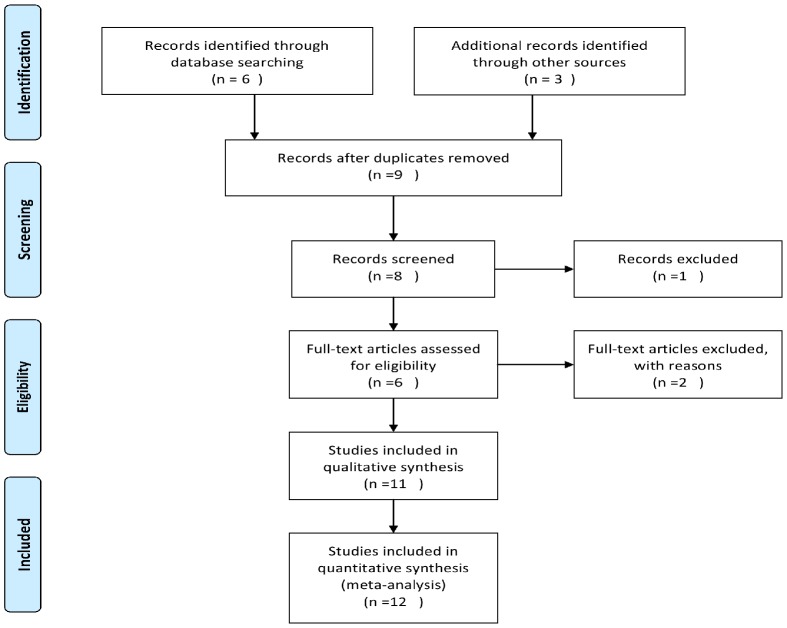
After comprehensive search, nine potentially relevant articles were retrieved, of which one review and two relevant studies were excluded due to ineligibility (Figure 1). As a result, the meta-analysis finally contained twelve (including this present study) individual case-control studies and one TDT study [22], [23], [36]–[39], involving 3673 cases, 14226 controls and 586 families (Table 3).
Figure 1. Flow chart of study selection.
Table 3. Characteristics of the studies on rs1801274 polymorphisms and risk of Kawasaki disease included in the meta-analysis.
| First Author | Year | Country | Ethnicity | Study Method | Study Design | Genotyping | T/NT | Case/Control |
| ShoichiroTaniuchi [37] | 2005 | Japan | Asian | CC | Replication | RFLP | 65/566 | |
| Maarten Biezeveld [38] | 2006 | Netherlands | Caucasian | CC | Replication | PCR-Direct sequencing | 176/239 | |
| ChieaChuenKhor [22] | 2011 | Singapore | European | CC | GWAS | IllumiaBeadChip | 405/6252 | |
| ChieaChuenKhor [22] | 2011 | Singapore | Asian | CC | Replication | SequenomMassARRAY | 438/446 | |
| ChieaChuenKhor [22] | 2011 | Singapore | Asian | CC | Replication | Taqman-PCR | 460/498 | |
| ChieaChuenKhor [22] | 2011 | Singapore | Asian | CC | Replication | Taqman-PCR | 130/568 | |
| ChieaChuenKhor [22] | 2011 | Singapore | Mixed | TDT | Replication | SequenomMassARRAY | 314/272 | |
| Yoshihiro Onouchi [23] | 2012 | Japan | Asian | CC | GWAS | IlluminaBeadChip | 428/3379 | |
| Yoshihiro Onouchi [23] | 2012 | Japan | Asian | CC | Replication | Invader Assay | 470/378 | |
| Yoshihiro Onouchi [23] | 2012 | Japan | Asian | CC | Replication | Invader Assay | 284/569 | |
| Yu Xiao Ji [39] | 2013 | China | Asian | CC | Replication | PCR-Direct sequencing | 35/25 | |
| Yuanlong Yan [36] | 2013 | China | Asian | CC | Replication | Taqman-PCR | 358/815 | |
| Current study | 2013 | China | Asian | CC | Replication | Taqman-PCR | 428/493 |
Abbreviations: CC, Case Control; TDT, transmission/disequilibrium test; T, transmission; NT, non-transmission.
Overall meta-analysis of the association between rs1801274 and KD risk
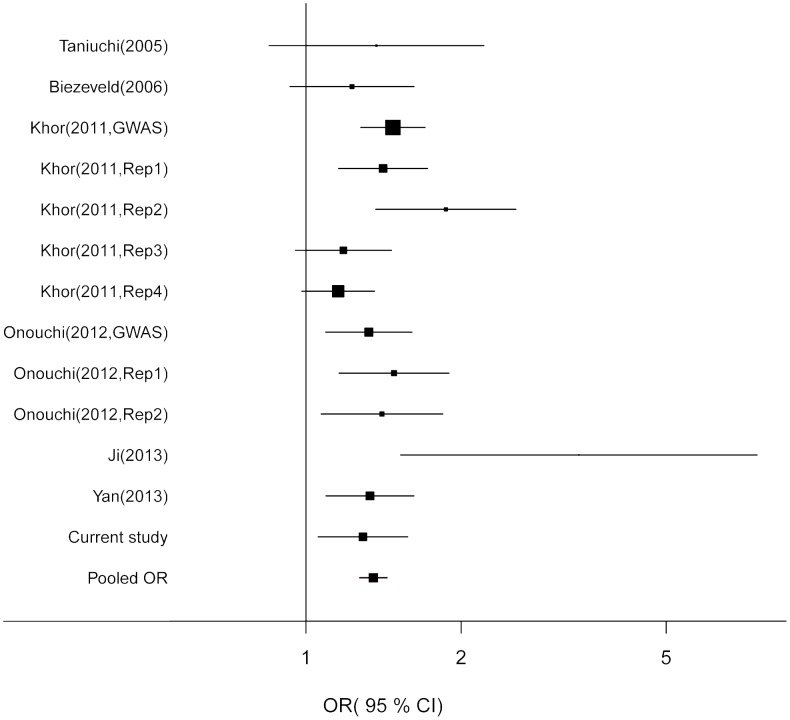
No evidence of significant heterogeneity was detected (Q = 17.30, p = 0.139). Therefore, a fixed-effects model was employed to calculate the pooled OR (Table 4). Compared with the G allele of rs1801274, the A allele showed a pooled OR of 1.35 (95% CI = 1.27–1.44). The forest plot was shown in Figure 2.
Table 4. FCGR2A rs1801274 allele A and the risk of Kawasaki disease in meta-analysis.
| Study ID | A/G in cases | A/G in controls | Transmitted A | Non-transmitted A | OR (95% CI) |
| Taniuchi (2005) [37] | 108/22 | 885/247 | — | — | 1.37 (0.85–2.21) |
| Biezeveld (2006) [38] | 202/150 | 250/228 | — | — | 1.23 (0.93–1.62) |
| Khor (2011) [22] | 616/260 | 559/333 | — | — | 1.41 (1.16–1.72) |
| Khor (2011) [22] | 200/60 | 728/408 | — | — | 1.87 (1.37–2.55) |
| Khor (2011) [22] | 726/194 | 757/239 | — | — | 1.18 (0.95–1.46) |
| Khor (2011) [22] | 459/351 | 5877/6627 | — | — | 1.47 (1.28–1.70) |
| Khor (2011) [22] | — | — | 314 | 272 | 1.15 (0.98–1.36) |
| Onouchi (2012) [23] | 720/136 | 5406/1352 | — | — | 1.32 (1.09–1.61) |
| Onouchi (2012) [23] | 790/150 | 590/166 | — | — | 1.48 (1.16–1.89) |
| Onouchi (2012) [23] | 482/86 | 910/228 | — | — | 1.40 (1.07–1.84) |
| Ji (2013) [39] | 55/15 | 26/24 | — | — | 3.38 (1.53–7.50) |
| Yan (2013) [36] | 528/188 | 1106/524 | — | — | 1.33 (1.09–1.62) |
| Current study (2013) | 609/239 | 652/330 | — | — | 1.29 (1.06–1.57) |
| Pooled OR | — | — | — | — | 1.35 (1.27–1.44)a |
Fixed-effects OR, Q = 17.30, P heterogeneity = 0.139, p<0.001.
Figure 2. The forest plot for the association between the A allelic variant and the risk of Kawasaki disease.
For each study, the estimate of OR and its 95% CI is plotted with a box and a horizontal line. Fixed-effects pooled OR = 1.35, 95% CI = 1.27–1.44, p<0.001; Q = 17.30, P heterogeneity = 0.139.
Subgroup analysis
The epidemiological characteristics of KD have shown that the risk of KD in Asian children is much higher than that in the children of other ethnic origin. So, Asian children were treated as a subgroup to further explore the susceptibility of KD in this single ethnicity. The pooled OR indicated that the A allele was still associated with a higher risk of KD in Asian children compared with the G allele (OR = 1.37, 95% CI = 1.27–1.48). No significant heterogeneity was detected under the fixed-effects model (Q = 11.61, p = 0.236, Table S2 in File S1).
Sensitivity analysis
To evaluate the effect of each single study on the pooled estimate, we performed a sensitivity analysis by removing one study each time in turn. It was found that all the results hardly changed after removal of each study, suggesting the robustness of our results (Table S3 in File S1).
Cumulative meta-analysis
Cumulative meta-analysis was also conducted in allelic model by gradually including each additional study according to chronological order. The 95% CI for pooled OR became progressively narrower after adding one more study (Figure S1 in File S1). It indicated that the precision of our estimation was gradually improved after adding more samples.
Publication bias evaluation
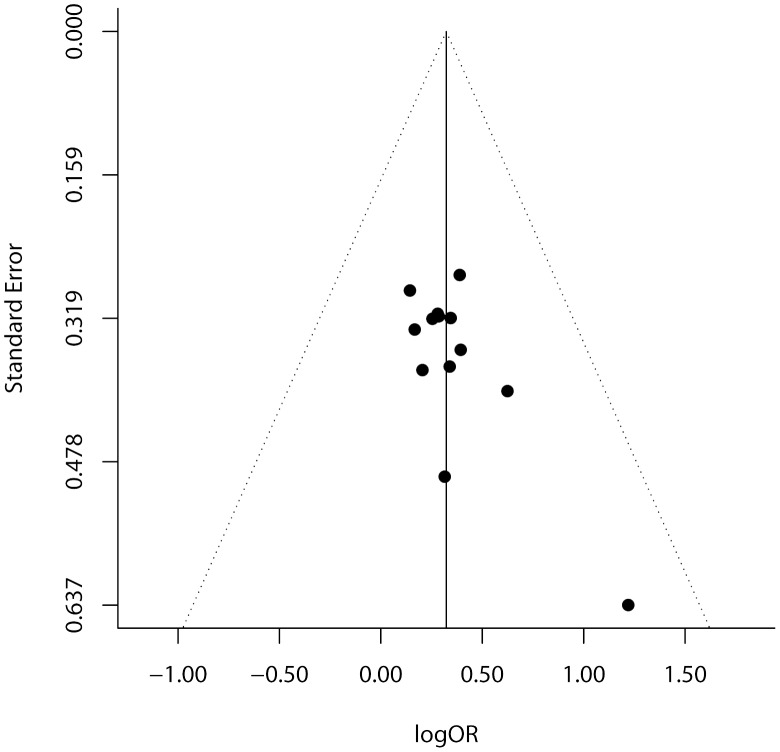
Publication bias was examined by funnel plot (Figure 3) and regression test. Dots in the funnel plot were mostly symmetrically distributed. The regression test further confirmed that there was no publication bias in this meta-analysis (p = 0.225).
Figure 3. The funnel plot of natural logarithm of OR against inverse standard error in each study.
Regression test for funnel plot asymmetry p = 0.224.
Discussion
In this present study, the individuals carrying the A allele or AA genotype of rs1801274 were at higher risk of developing KD but were not associated with CAL formation significantly. Subsequent meta-analysis further supported the association between this SNP with increased KD risk. Cumulative and sensitivity analyses confirmed the reliability and robustness of our results, and with little publication bias.
These findings are biologically plausible for the following reasons. First, the original GWAS on KD conducted by Khor et al [22] revealed this risk association in multi-ethnic populations and further tested this locus in four independent studies with different ethnic origins, including three case-control panels (Taiwanese, Korean and Chinese) and one TDT (non-European). Second, KD is an immune-mediated multi-systemic vasculitis and may have an unknown infectious trigger in addition to genetically susceptible factors. One function of FCGRs is to regulate the interaction between phagocytes and IgG antibodies. Functional assays demonstrated that this variant (G>A transition) enhanced FCGR2A's binding affinity to IgG indirectly, resulting in activation of FCGR2A signaling pathway and up-regulation of IgG2-opsonized phagocytosis [40], [41]. Excessive inflammatory responses can produce adverse effect on healthy tissues such as extremity edema, hyperalgesia and swelling tongue, and further leading to clinic manifestations of KD.
Moreover, as the most severe complication of KD, CAL is the leading cause of long-term morbidity and mortality of KD patients. A recent study on endothelial function indicated that the G allele of rs1801274 was associated with endothelium-dependent vasodilatation and nitric oxide activity when endothelial cells were stimulated [42]. Another relevant study suggested that FCGR2A on the cells with AA genotype had stronger binding affinity to C-reactive protein (CRP), which showed similar functional activities as IgG2 [43]–[45] and FCGRs [46], [47]. Plasma CRP level increases significantly during acute phase of KD, which is an independent predictor for acute coronary syndromes [48]–[51]. These results imply that FCGR2A may affect the pathophysiology of coronary artery indirectly. However, the present case-control study in Chinese Han ethnic children did not confirm whether rs1801274 was associated with CAL. This may be due to the lack of enough CAL cases in our study.
Several limitations should be kept in mind when interpreting the results of this study. First, the sample size was relatively small, which might affect the power to detect the association between this SNP and CAL. Second, KD is a complex disease associated with both genetic and environmental factors. Local environmental data were not included in this analysis which limited the interpretation of these results and evaluation of the gene-environment interaction.
In conclusion, this present study has provided more evidence to support the concept that the functional polymorphism rs1801274 in FCGR2A is significantly associated with the increased risk of KD.
Supporting Information
The forest plot for the cumulative meta-analysis. Fixed-effects pooled OR = 1.35, 95% CI = 1.27–1.44, P<0.001.
(TIF)
PRISMA Checklist.
(DOC)
Table S1, Polymorphism of rs1801274 of the FCGR2A gene in patients with CALs and non-CALs in current study. Table S2, FCGR2A rs1801274 risk allele A in Meta analysis (Asian subgroup).Table S3, Sensitivity analysis of the allelic model.
(DOCX)
Funding Statement
This work is supported, in part, by grants from The National Natural Science Foundation of China (81270177), Ministry of Health Research Foundation of China (201339378), The Health Bureau of Zhejiang Province (2009A124, 2009CA072), and population and family planning commission of Zhejiang province (JSW2013-A15). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kawasaki T (1967) [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi 16: 178–222. [PubMed] [Google Scholar]
- 2. Rowley AH, Baker SC, Orenstein JM, Shulman ST (2008) Searching for the cause of Kawasaki disease–cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol 6: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uehara R, Belay ED (2012) Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol 22: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K, et al. (2008) Increasing incidence of Kawasaki disease in Japan: nationwide survey. Pediatr Int 50: 287–290. [DOI] [PubMed] [Google Scholar]
- 5. Huang WC, Huang LM, Chang IS, Chang LY, Chiang BL, et al. (2009) Epidemiologic features of Kawasaki disease in Taiwan, 2003–2006. Pediatrics 123: e401–405. [DOI] [PubMed] [Google Scholar]
- 6. Fimbres AM, Shulman ST (2008) Kawasaki disease. Pediatr Rev 29: 308–316. [DOI] [PubMed] [Google Scholar]
- 7. Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, et al. (2005) Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int 47: 232–234. [DOI] [PubMed] [Google Scholar]
- 8. Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, et al. (1993) Diagnosis and therapy of Kawasaki disease in children. Circulation 87: 1776–1780. [DOI] [PubMed] [Google Scholar]
- 9. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, et al. (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110: 2747–2771. [DOI] [PubMed] [Google Scholar]
- 10. Burns JC, Glodé MP (2004) Kawasaki syndrome. The Lancet 364: 533–544. [DOI] [PubMed] [Google Scholar]
- 11. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, et al. (1991) A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 324: 1633–1639. [DOI] [PubMed] [Google Scholar]
- 12. Tse SML, Silverman ED, McCrindle BW, Yeung RSM (2002) Early treatment with intravenous immunoglobulin in patients with Kawasaki disease. J Pediatr 140: 450–455. [DOI] [PubMed] [Google Scholar]
- 13. Fujita Y, Nakamura Y, Sakata K, Hara N, Kobayashi M, et al. (1989) Kawasaki disease in families. Pediatrics 84: 666–669. [PubMed] [Google Scholar]
- 14. Holman RC, Curns AT, Belay ED, Steiner CA, Effler PV, et al. (2005) Kawasaki syndrome in Hawaii. Pediatr Infect Dis J 24: 429–433. [DOI] [PubMed] [Google Scholar]
- 15. Burns JC, Newburger JW (2012) Genetics insights into the pathogenesis of Kawasaki disease. Circ Cardiovasc Genet 5: 277–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uehara R, Yashiro M, Nakamura Y, Yanagawa H (2003) Kawasaki disease in parents and children. Acta Paediatr 92: 694–697. [DOI] [PubMed] [Google Scholar]
- 17. Park YW, Han JW, Hong YM, Ma JS, Cha SH, et al. (2011) Epidemiological features of Kawasaki disease in Korea, 2006–2008. Pediatr Int 53: 36–39. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, et al. (2012) Epidemiologic features of Kawasaki disease in Japan: results of the 2009–2010 nationwide survey. J Epidemiol 22: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JJ, Hong YM, Sohn S, Jang GY, Ha KS, et al. (2011) A genome-wide association analysis reveals 1p31 and 2p13.3 as susceptibility loci for Kawasaki disease. Hum Genet 129: 487–495. [DOI] [PubMed] [Google Scholar]
- 20. Burgner D, Davila S, Breunis WB, Ng SB, Li Y, et al. (2009) A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet 5: e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai FJ, Lee YC, Chang JS, Huang LM, Huang FY, et al. (2011) Identification of novel susceptibility loci for Kawasaki disease in a Han Chinese population by a genome-wide association study. PLoS One 6: e16853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khor CC, Davila S, Breunis WB, Lee YC, Shimizu C, et al. (2011) Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet 43: 1241–U1104. [DOI] [PubMed] [Google Scholar]
- 23. Onouchi Y, Ozaki K, Burns JC, Shimizu C, Terai M, et al. (2012) A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet 44: 517–521. [DOI] [PubMed] [Google Scholar]
- 24. Lee YC, Kuo HC, Chang JS, Chang LY, Huang LM, et al. (2012) Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet 44: 522–525. [DOI] [PubMed] [Google Scholar]
- 25. Pleass RJ, Woof JM (2001) Fc receptors and immunity to parasites. Trends Parasitol 17: 545–551. [DOI] [PubMed] [Google Scholar]
- 26. Gerber JS, Mosser DM (2001) Stimulatory and inhibitory signals originatingfrom the macrophage Fcγ receptors. Microbes Infect 3: 131–139. [DOI] [PubMed] [Google Scholar]
- 27. Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, et al. (2008) Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 40: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nimmerjahn F (2006) Activating and inhibitory FcγRs in autoimmune disorders; 2006. Springer. pp. 305–319. [DOI] [PubMed]
- 29. Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, et al. (2009) A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat Genet 41: 1325–1329. [DOI] [PubMed] [Google Scholar]
- 30. Akagi T, Rose V, Benson LN, Newman A, Freedom RM (1992) Outcome of coronary artery aneurysms after Kawasaki disease. J Pediatr 121: 689–694. [DOI] [PubMed] [Google Scholar]
- 31. Arjunan K, Daniels SR, Meyer RA, Schwartz DC, Barron H, et al. (1986) Coronary artery caliber in normal children and patients with Kawasaki disease but without aneurysms: an echocardiographic and angiographic study. J Am Coll Cardiol 8: 1119–1124. [DOI] [PubMed] [Google Scholar]
- 32. Kazeem GR, Farrall M (2005) Integrating case-control and TDT studies. Ann Hum Genet 69: 329–335. [DOI] [PubMed] [Google Scholar]
- 33. Peterman RM (1995) Statistical power of methods of meta-analysis. Trends Ecol Evol 10: 460. [DOI] [PubMed] [Google Scholar]
- 34. Nicodemus KK (2008) Catmap: case-control and TDT meta-analysis package. BMC Bioinformatics 9: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thakkinstian A, McElduff P, D'Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24: 1291–1306. [DOI] [PubMed] [Google Scholar]
- 36. Yan Y, Ma Y, Liu Y, Hu H, Shen Y, et al. (2013) Combined analysis of genome-wide-linked susceptibility loci to Kawasaki disease in Han Chinese. Hum Genet 1–12. [DOI] [PubMed] [Google Scholar]
- 37. Taniuchi S, Masuda M, Teraguchi M, Ikemoto Y, Komiyama Y, et al. (2005) Polymorphism of Fcγ RIIa may affect the efficacy of γ-globulin therapy in Kawasaki disease. J Clin Immunol 25: 309–313. [DOI] [PubMed] [Google Scholar]
- 38. Biezeveld M, Geissler J, Merkus M, Kuipers IM, Ottenkamp J, et al. (2007) The involvement of Fc gamma receptor gene polymorphisms in Kawasaki disease. Clin Exp Immunol 147: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ji YX, Zhang HY, Lin SX (2013) [Single nucleotide polymorphism of FCGR2A gene in Han Chinese children with Kawasaki disease]. Zhongguo Dang Dai Er Ke Za Zhi 15: 196–200. [PubMed] [Google Scholar]
- 40. Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ (1991) A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol 147: 1338–1343. [PubMed] [Google Scholar]
- 41. Shrestha S, Wiener H, Olson AK, Edberg JC, Bowles NE, et al. (2011) Functional FcγRIIB Gene Variants Influence Intravenous Immunoglobulin (IVIG) Response in Kawasaki Disease (KD) Patients. J Allergy Clin Immunol 128: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schneider MP, Leusen JH, Herrmann M, Garlichs CD, Amann K, et al. (2011) The Fcgamma receptor IIA R131H gene polymorphism is associated with endothelial function in patients with hypercholesterolaemia. Atherosclerosis 218: 411–415. [DOI] [PubMed] [Google Scholar]
- 43. Mortensen RF, Osmand AP, Lint TF, Gewurz H (1976) Interaction of C-reactive protein with lymphocytes and monocytes: complement-dependent adherence and phagocytosis. J Immunol 117: 774–781. [PubMed] [Google Scholar]
- 44. Kindmark CO (1971) Stimulating effect of C-reactive protein on phagocytosis of various species of pathogenic bacteria. Clin Exp Immunol 8: 941–948. [PMC free article] [PubMed] [Google Scholar]
- 45. Kilpatrick JM, Volanakis JE (1985) Opsonic properties of C-reactive protein. Stimulation by phorbol myristate acetate enables human neutrophils to phagocytize C-reactive protein-coated cells. J Immunol 134: 3364–3370. [PubMed] [Google Scholar]
- 46. Marnell LL, Mold C, Volzer MA, Burlingame RW, Du Clos TW (1995) C-reactive protein binds to Fc gamma RI in transfected COS cells. J Immunol 155: 2185–2193. [PubMed] [Google Scholar]
- 47. Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, et al. (2000) C-reactive protein binding to FcgammaRIIa on human monocytes and neutrophils is allele-specific. J Clin Invest 105: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zebrack JS, Muhlestein JB, Horne BD, Anderson JL (2002) C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol 39: 632–637. [DOI] [PubMed] [Google Scholar]
- 49. Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L (2000) Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med 343: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 50. Retterstol L, Eikvar L, Bohn M, Bakken A, Erikssen J, et al. (2002) C-reactive protein predicts death in patients with previous premature myocardial infarction–a 10 year follow-up study. Atherosclerosis 160: 433–440. [DOI] [PubMed] [Google Scholar]
- 51. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR (2002) Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347: 1557–1565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The forest plot for the cumulative meta-analysis. Fixed-effects pooled OR = 1.35, 95% CI = 1.27–1.44, P<0.001.
(TIF)
PRISMA Checklist.
(DOC)
Table S1, Polymorphism of rs1801274 of the FCGR2A gene in patients with CALs and non-CALs in current study. Table S2, FCGR2A rs1801274 risk allele A in Meta analysis (Asian subgroup).Table S3, Sensitivity analysis of the allelic model.
(DOCX)