The authors describe the incidence and impact of cytotoxic agents on the risk of acute iodinated contrast media-related adverse reactions (ARs) in a consecutive cohort of cancer patients. Results showed that such ARs are rare in cancer patients, with incidence similar to the general population. Treatment with taxanes and age <65 years seem to be risk factors.
Keywords: Iodinated contrast media, Hypersensitivity, Contrast-enhanced CT, Cancer patients, Risk of adverse event
Abstract
Background.
The impact of cytotoxic agents on the risk of acute allergy-like adverse reactions (ARs) to intravenous iodinated contrast media (ICM) injections is unknown.
Methods.
We retrospectively reviewed 13,565 computed tomography (CT) scans performed in a consecutive cohort of cancer patients from January 1, 2010 to December 31, 2012. Episodes of acute ICM-related ARs were reported to the pharmacovigilance officer. The following matched comparisons were made: tax code, gender, primary tumor, antineoplastic therapy, and date of last cycle. Concomitant antineoplastic treatment was classified into five groups: platinum, taxane, platinum plus taxane, other, and no treatment group (no therapy had been administered in the previous 24 months). Logistic regression was used to estimate odds ratio (OR) and 95% confidence interval (CI) to evaluate the risk of acute ICM-related ARs.
Results.
Of 10,472 contrast-enhanced CT scans, 97 (0.93%; 95% CI: 0.74–1.11) ICM-related ARs were reported, 11 of which (0.1%) were severe, including one fatality. The overall incidence was significantly higher in patients aged <65 years (p = .0062) and in the platinum plus taxane and taxane groups (p = .007), whereas no correlation was found with gender, number of previous CT scans, site of disease, or treatment setting. Multivariate analysis confirmed an increased risk for patients aged <65 years (OR: 1.73; 95% CI: 1.14–2.63) and for the taxane group (in comparison with the no treatment group; OR: 2.06; 95% CI: 1.02–4.16).
Conclusion.
Among cancer patients, concomitant treatment with taxanes and younger age would seem to be risk factors for ICM-related ARs.
Implications for Practice:
Cancer patients undergo routine computed tomography (CT) scans and iodinated contrast media (ICM) administration to assess the extent of disease and treatment response. Acute allergy-like adverse reactions (ARs) are a well-known complication of ICM injections. No data exist about the true incidence of ICM-related ARs in cancer patients. We describe the incidence and impact of cytotoxic agents on the risk of acute ICM-related ARs in a consecutive cohort of cancer patients. We showed that ICM-related ARs are rare in cancer patients, with incidence similar to the general population. Taxanes and age <65 years seem to be risk factors for ARs.
Introduction
Acute allergy-like adverse reactions (ARs) are a well-known complication of intravenous iodinated contrast media (ICM) injections and range from mild symptoms such as urticaria and itching to more severe reactions such as cardiopulmonary arrest and death. Acute ARs of high-osmolar contrast media have been reported among 12.7% of patients [1]. With the widespread use of low-osmolar contrast media, the incidence of likely acute ICM-related ARs has decreased to 0.2%–0.7% [2–4]. These are likely to occur independent of dose and concentration above a certain unknown threshold [5]. Serious acute reactions to ICM are rare, with a historical rate of approximately 0.04% [1] and a fatality rate of 0.9 per 100,000 ICM injections [6, 7].
Acute ARs to ICM manifest in a similar way to true allergic reactions seen with other drugs and allergens but, because an antigen-antibody response seldom can be identified, the pathogenesis of most allergy-like reactions remains unclear. It is believed that some ICM-related ARs may involve activation, deactivation, or inhibition of a variety of vasoactive substances or mediators (e.g., histamine, complement, and the kinin system) [5, 8, 9].
Cancer patients undergo routine computed tomography (CT) scans and, therefore, ICM administration to assess the extent of disease (staging) and response to treatment. Moreover, nearly all systemic agents used in cancer treatment today are associated with possible hypersensitivity reactions [10]. These reactions range in severity from mild flushing and itching to anaphylaxis and, in some rare cases, death. Although severe reactions are rare, mild to moderate hypersensitivity reactions occur frequently with many commonly used systemic cancer therapies, including platinum compounds [11] and taxanes [12]. One important difference among these agents is the time of onset of symptoms. Hypersensitivity to platinum compounds typically develops after multiple cycles of therapy, suggesting an acquired, anaphylactic reaction consistent with type 1 hypersensitivity [13]. In contrast, reactions to taxanes are immediate, often occurring within the first few minutes of the first infusion, suggesting that they may occur by alternative mechanisms [14].
To the best of our knowledge, no data exist about the true incidence of ICM-related ARs in cancer patients. This population could be at higher risk than the general population because of the number of examinations routinely performed due to treatment response assessment and follow-up, as stated in several international guidelines (e.g., National Comprehensive Cancer Network guidelines). In our study, we sought primarily to determine the frequency of this event in a consecutive cohort of cancer patients. In addition, because ICM-related ARs are frequently sporadic and unpredictable, and platinum compounds and taxanes used in cancer treatment are often associated with hypersensitivity reactions (albeit through different mechanisms), we aimed to evaluate whether cytotoxic agents may increase the risk of ICM-related ARs. This information would also be of interest in determining the risk-benefit ratio in relation to the time scheduled for the re-evaluation of the disease.
Patients and Methods
Patient Population
Using an institutional radiological database (Elefante, Agfa), we identified all consecutive CT scans with ICM performed at the Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS (Meldola, Italy), from January 1, 2010, to December 31, 2012. Only one CT examination per person per day was evaluated to avoid, for example, a CT scan of the thorax and abdomen being counted as two examinations rather than one. The hospital, which is an institute for cancer research and treatment, works only with adult cancer patients. The following data were extracted for each patient: tax code, date of the CT scan, ICM injection, and identification code of Elefante. From this database, we also obtained the number of previous CT scans in the 2 years preceding the date of the last examination.
This patient cohort was linked with an institutional medical records database (Log80) using the tax code as the common key. For each patient, we extracted the following data from Log80: tax code, birth date, gender, primary tumor, date of diagnosis, antineoplastic therapy, type and setting of antineoplastic therapy (adjuvant/neoadjuvant or advanced), start and end dates of the treatment, and date of the last cycle. All of the therapies administered in the 24 months before the date of the CT scan were analyzed. This study was approved by the medical scientific committee of IRST IRCCS.
CT Scanning and ICM Administration
All CT scans, most predominantly of the thorax and abdomen, were performed using a 256-slice CT scanner (Brilliance iCT; Philips Healthcare, Amsterdam, The Netherlands, http://www.philips.com). Written informed consent for the use of ICM was obtained from all patients. Patients fasted for at least 6 hours before the examination and were encouraged to drink abundantly in the 24 hours before examination, unless contraindicated.
In the preparation room, the anesthesiologist checked the signed informed consent form and the presence of any contraindication to the ICM injection. Thereafter, 80–150 mL ICM externally warmed to 37°C was infused into the cubital vein at 2–5 mL/second using a dual-barrel power injector via an i.v. catheter, followed by 20–30 mL of washing saline solution. Two main low-osmolar ICMs were used at our institution during the study period, predominantly (80%) iomeprol (Iomeron; Bracco Imaging Italia s.r.l., Milan, Italy, http://www.braccoimaging.com; 300–400 mg/mL) and less frequently (20%) iobitridol (Xenetix Guerbet S.p.A., Genoa, Italy, http://www.guerbet.com; 300–400 mgI/mL). All ICM injections were monitored by anesthesiologists, and all radiologists were certified in basic life support.
With regard to acute allergy-like ARs, our institution has existing protocols that suggest pretreatment of patients with any history of severe bronchospasm, severe laryngeal edema, angioedema, unresponsiveness, seizure activity, cardiac arrhythmias, or cardiopulmonary arrest following the administration of at least one drug or a history of allergies to more than one drug or reported AR to ICM. After a severe AR, patients were recommended to undergo another form of imaging, whenever possible. Premedication includes either prednisone (50 mg) by mouth every 6 hours starting 18 hours before the CT examination or methylprednisolone (32 mg) by mouth 12 hours before the CT examination plus ranitidine (150 mg) orally the evening before, followed by i.v. administration of dexamethasone (4 mg) plus chlorphenamine (10 mg) and ranitidine (100 mg), and hydration immediately before ICM administration.
Outcome Measures
The attending CT radiologist and anesthesiologist recorded any ARs that they observed or that patients described during or 1 hour after ICM injection. These ARs were then subsequently recorded by the hospital pharmacist (C.D.L.), who was responsible for pharmacovigilance. From these reports, each AR was entered into an electronic database with the tax code, the date of the CT scan, and a description of the AR.
We classified the severity of the ICM-related AR according to guidelines from the American College of Radiology Manual on Contrast Media, version 9.0 [15], as mild (nausea, vomiting, hitching), moderate (severe vomiting, diffuse rash, bronchospasm, facial or laryngeal edema, vasovagal reaction), or severe (shock, acute respiratory or heart failure, seizures). CT scans performed after an AR were excluded from the analysis.
Statistical Analysis
Time to CT evaluation was calculated as the time elapsed from the date of the CT scan and the date of the last chemotherapy administration and was classified into four groups (0–30 days, 31–90 days, 91–180 days, and ≥180 days). The antineoplastic treatment was classified into five groups: “platinum,” in the case of a platinum-based regimen (oxaliplatin, carboplatin, or cisplatin); “taxane,” in the case of a taxane-based regimen (either docetaxel or paclitaxel); “platinum plus taxane,” if the chemotherapy included both drugs; “other” (any chemotherapy without platinum or taxane); and “no treatment,” if no therapy was administered in the 24 months preceding the CT scan.
The distribution of patients and tumor characteristics were summarized by means of descriptive statistics. The incidence of AR between potential risk factors was evaluated using chi-square tests, whereas adverse reaction severity was evaluated on the basis of patient age using Fisher’s exact test. The multivariable logistic regression model was used to estimate the adjusted odds ratio (OR) and 95% confidence interval (CI) for each potential risk factor, including gender, age, tumor site, antineoplastic treatment, and number of previous CT scans. Logistic regression was fitted using the generalized estimating equations approach and the exchangeable correlation matrix to account for potential within-patient homogeneity of the outcome.
Time to CT evaluation and treatment setting were limited to evaluation in univariate analysis because they were calculated only for patients who had been treated in the 24 months before the CT scan. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
Total Patient Population
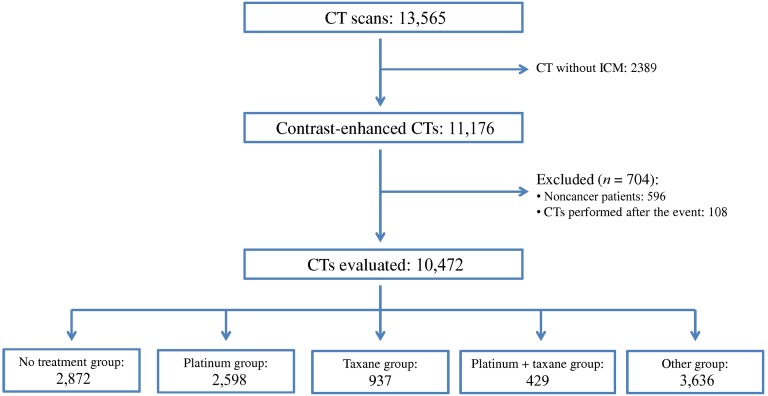
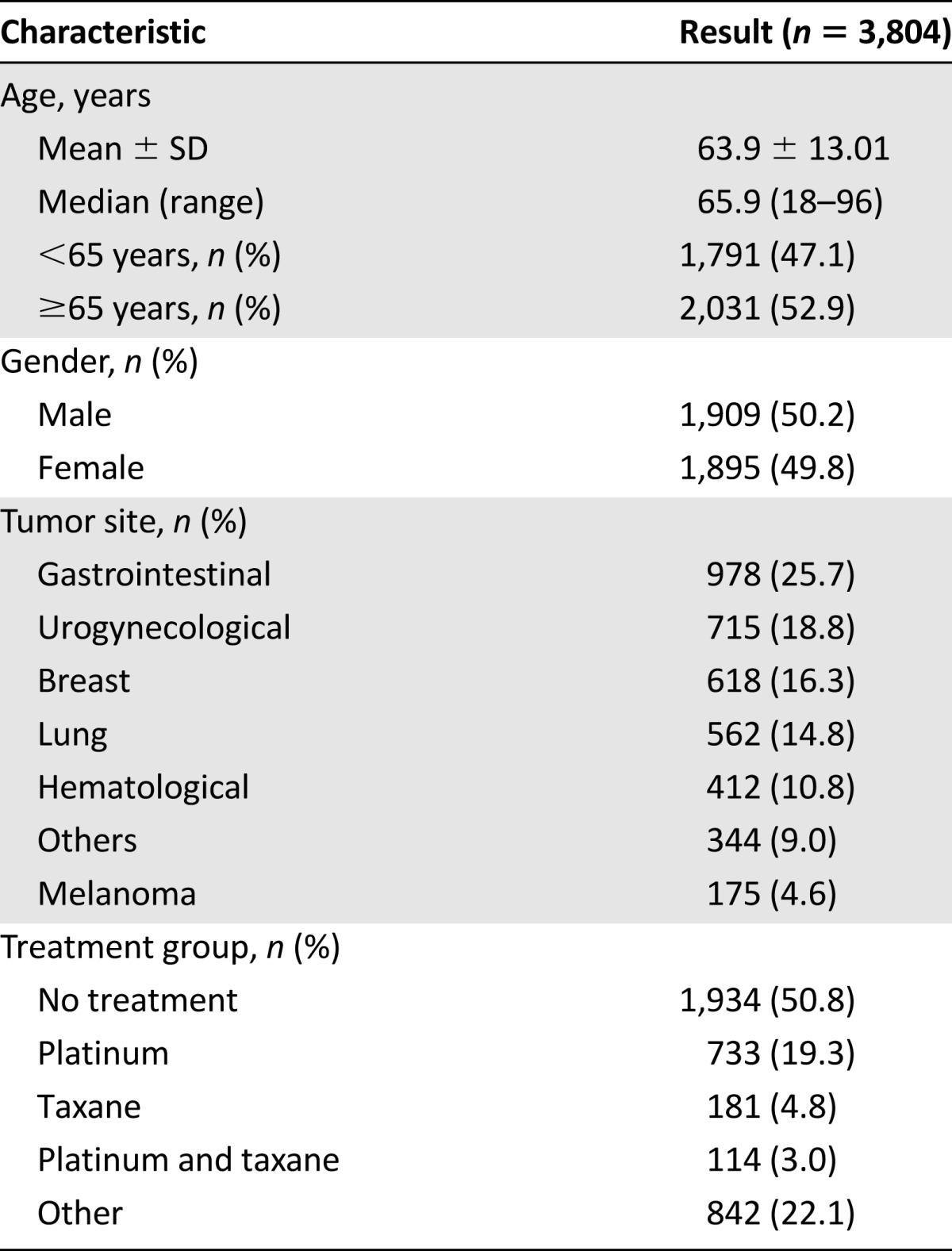
During the study time period, 13,565 CT scans were performed, of which 10,472 CT scans were eligible for further evaluation (Fig. 1). The contrast-enhanced CT scans were performed in 3,804 cancer patients with a median age of 65.9 years (range: 18–96 years). Patients’ principal baseline characteristics are listed in Table 1. The median number of CT scans for the sample of cancer patients was 2 (range: 1–24).
Figure 1.
Flow diagram of this study.
Abbreviations: CT, computed tomography; ICM, iodinated contrast media.
Table 1.
Baseline demographic and tumor characteristics of the patients
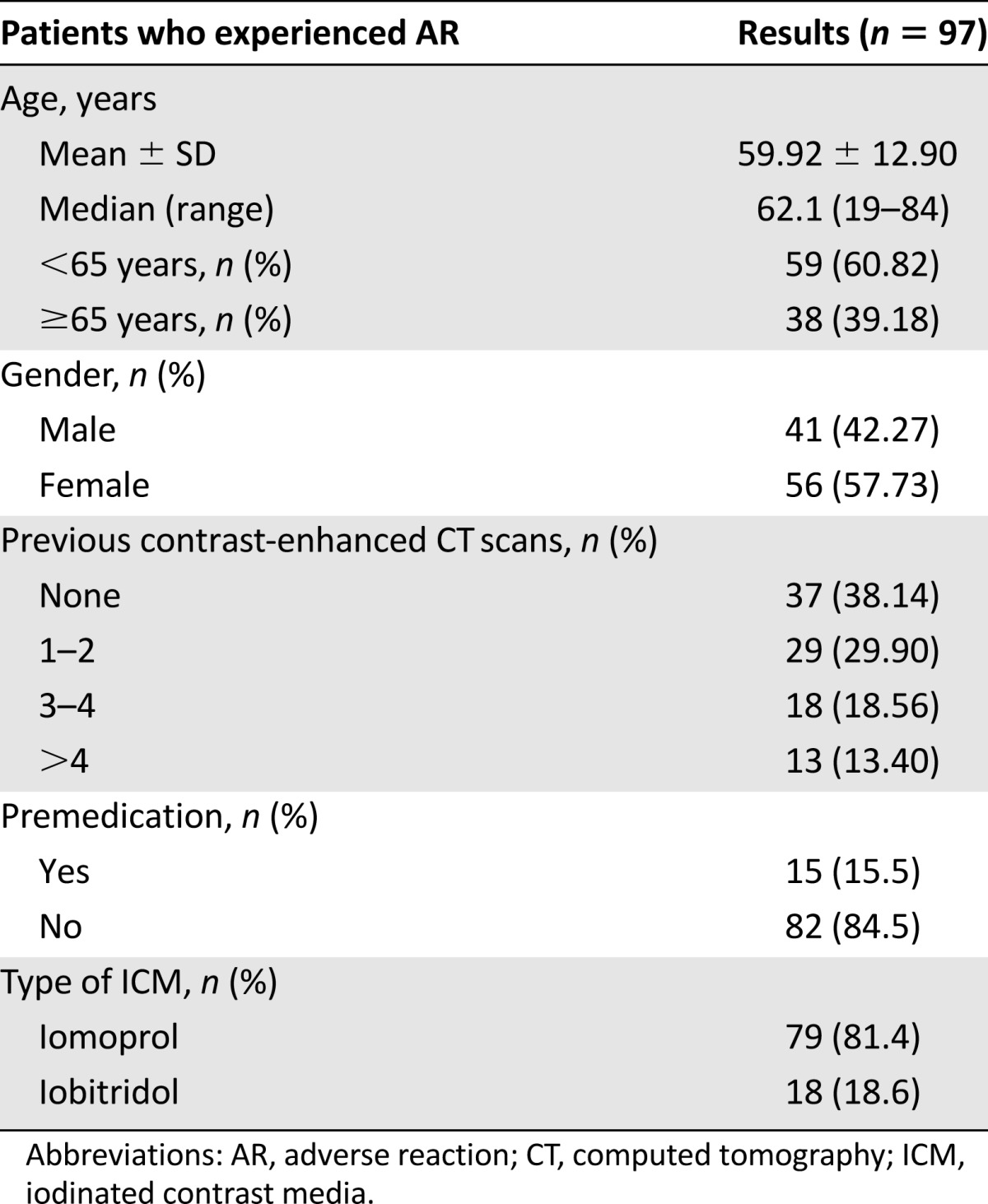
Ninety-seven acute ICM-related ARs (0.93%; 95% CI: 0.74–1.11), one per patient (Table 2), were reported. Twenty-two ARs (0.21%) were classified as mild, 64 (0.61%) were moderate, and 11 (0.11%) were severe. The latter had the following clinical patterns: shock in seven patients (0.067%), angioedema in three patients (0.029%), and death in one patient (0.010%). Each case was immediately handled by the attending anesthesiologist and treated with i.v. hydration, diphenhydramine, steroids, oxygen when indicated, and epinephrine in two cases. One case was transferred promptly to the emergency department.
Table 2.
Patient characteristics of the ICM-related ARs
The casualty concerned a female breast cancer outpatient in treatment with capecitabine who was scheduled for routine re-evaluation of the disease. Six contrast-enhanced CT scans were performed in the 2 years preceding the fatality, and the patient never presented symptoms of acute ARs. The final time, 7 minutes after ICM administration, she developed shortness of breath and cyanosis with bronchospasm and subsequent loss of consciousness. The anesthesiologist and cardiologist were promptly called and immediately administered i.v. hydration, steroids (1,000 mg of hydrocortisone) and 1 mg i.v. injections of epinephrine in refracted doses. Despite this, the patient developed a cardiopulmonary arrest. Cardiac massage and oxygen by face mask were performed. Electrocardiogram showed complete atrioventricular block with QRS complex enlargement. Intravenous epinephrine was administered again, unsuccessfully, so the patient was intubated; however, after another 35 minutes of advanced resuscitation, the patient died.
Risk Factors of ICM-Related ARs
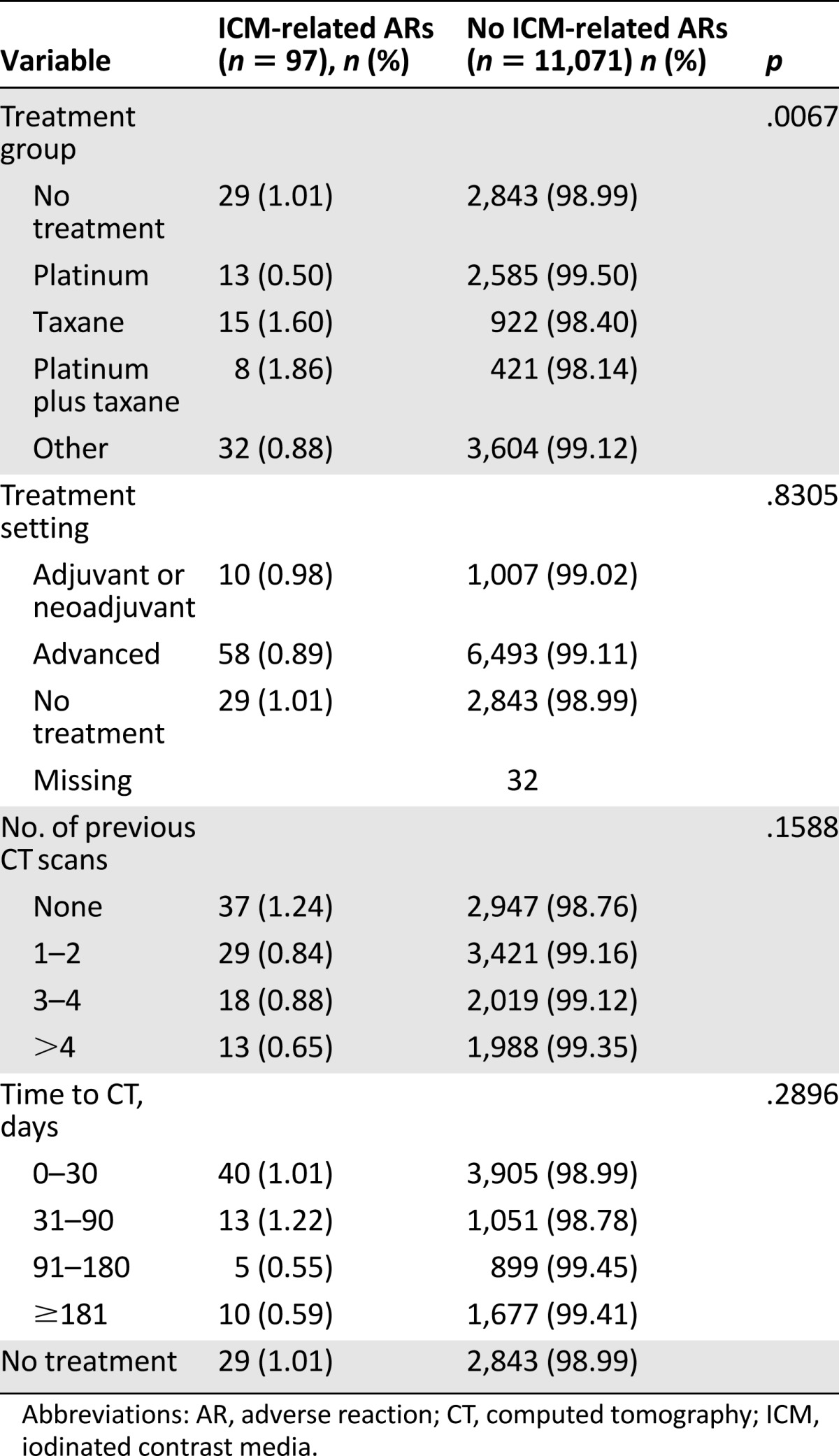
The overall incidence of acute ARs was significantly higher in those aged 64 or younger compared with those aged 65 or older (1.20% [95% CI: 0.89–1.50] vs. 0.69% [95% CI: 0.47–0.90]; p = .006). No significant differences were seen with regard to the severity of the ICM-related ARs and the age of patients: there were seven severe ARs (63.4%) in the younger group versus four (36.4%) in the older population and 52 mild to moderate ARs (60.5%) in those aged <65 years compared with 34 (39.5%) in those aged ≥65 years (p = 1.000). In comparison with the various treatment groups, the incidence of acute ARs was significantly higher (p = .007) in the platinum plus taxane and taxane groups than in the other groups. Conversely, no statistically significant relationship was found between the incidence of acute ICM-related ARs and gender, the number of previous contrast-enhanced CT scans, the time to CT evaluation, the site of disease, or the treatment setting (Table 3).
Table 3.
Univariate analysis of ICM-related ARs by possible risk factors
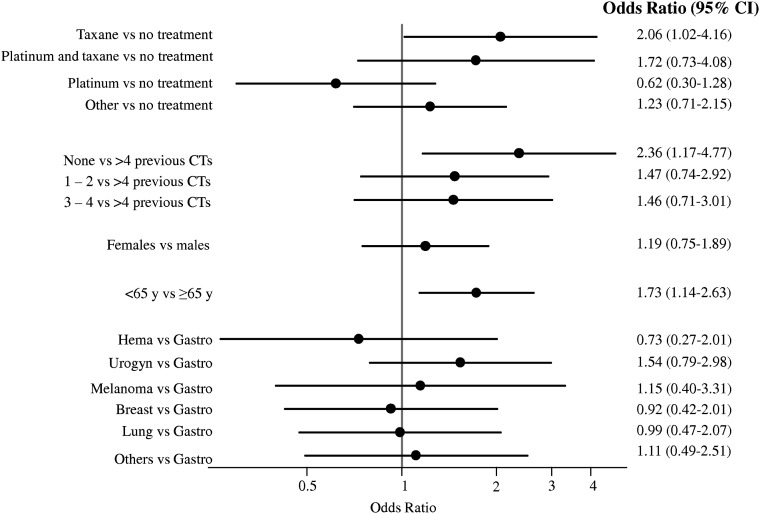
As shown in Figure 2, multivariate analysis confirmed a higher risk of ARs for patients aged <65 years (OR: 1.73; 95% CI: 1.14–2.63) and for patients in the taxane group compared with the no treatment group (OR: 2.06; 95% CI: 1.02–4.163). Moreover, an increased risk for patients at their first CT scan was seen compared with patients that had undergone six or more contrast-enhanced CT scans in the previous 2 years (OR: 2.36; 95% CI: 1.17–4.77).
Figure 2.
Forest plot of the risk of acute iodinated contrast media-related adverse reactions in cancer patients.
Abbreviations: CI, confidence interval; CT, computed tomography; Gastro, gastrointestinal tumors; Hema, hematological diseases; Urogyn, urogynecological cancer; y, years.
Discussion
A large number of radiological examinations, mainly contrast-enhanced CT scans, are conducted worldwide each year, especially in cancer patients. In fact, cancer mortality in Western countries has steadily declined since the late 1980s [16], resulting in the need for more prolonged patient follow-up. This situation, in association with the need for more appropriate drug prescriptions to decrease the costs of new drugs that do not always have a clear correlation with actual efficacy [17], has resulted in the need for more radiological examinations and, consequently, the risk of ICM-related ARs. In this study, acute ARs occurred in 0.93% of the examinations, with 0.1% considered severe, an incidence similar to that of the general population [2–4, 18]. It is not possible to compare the ICM-related mortality in our study (0.95 fatality per 10,000 ICM administrations) with that reported in the general population because an insufficient number of CT scans were analyzed.
Although it is clear that certain patients are at increased risk of experiencing ICM-related ARs, contrast reactions remain sporadic and unpredictable. In the general population, a prior allergy-like reaction to ICM is the most substantial risk factor for a recurrent AR [1, 3, 19–21]. In this context, our findings of the increased risk of acute AR in patients receiving ICM for the first time may be explained by the fact that patients subsequently tend to avoid further contrast-enhanced CT scans, as reported previously by others [18].
The pathogenesis of acute ICM-related ARs is unclear. Multiple mechanisms have been proposed, although allergic hypersensitivity is considered to be a frequently involved pathomechanism [8]. In fact, ICMs are known to directly cause histamine release from basophils and mast cells [22], possibly because they activate mast cells by bridging adjacent IgE molecules via attachment to their Fc segments. Other potentially involved mechanisms include activation of the complement and contact systems [23, 24].
Similar to ICM-related ARs, some chemotherapy agents, their metabolites, or vehicles may interact directly with mast cells and basophils, producing an anaphylactoid response that is indistinguishable from an IgE-mediated immune response [10]. Platinum salts (i.e., cisplatin, carboplatin, oxaliplatin) classically cause type 1 allergy [25]. Taxanes (e.g., paclitaxel and docetaxel) may induce hypersensitivity that is clinically similar to a type 1 allergy. Like ICM, taxanes may induce reactions at first administration [26]; therefore, it can be hypothesized that both drugs act via similar immune-mediated pathways. In this context, it would be interesting to understand whether the opposite is also true, namely, whether ICM increases the risk of taxane-induced hypersensitivity reactions. This is an area we intend to study in the future.
In our study, although ICM-related ARs were rare in cancer patients, the group treated with a taxane-based chemotherapy had a twofold risk of acute ICM-related ARs compared with the no treatment group. Patients treated with platinum plus taxanes, however, did not reach a significant OR in the multivariable regression model, possibly because of the low number of CT scans analyzed in this group. This result indicates that radiologists and anesthesiologists need to pay greater attention to a patient’s cancer history, especially with regard to current antineoplastic treatments, to adequately assess (in association with other known risk factors including age and prior allergic reactions) the basal risk of the patient developing an ICM-related AR.
We acknowledge some limitations of our study. In particular, the study was limited by the retrospective nature of the collected data. Although it is possible that we may have missed some minor reactions, which health care providers do not have a legal obligation to report, anesthesiologists at our institution were instructed to report any AR to the pharmacovigilance manager. Moreover, we analyzed only immediate reactions, namely, those that occur during the contrast agent injection or a maximum of 1 hour afterward. Although this definition is the most frequently used in the literature, we do not have data on delayed reactions because this was not the purpose of our study. In addition, information about the routine use of premedication was unavailable, but studies have thus far indicated that the main ICM-related ARs that benefit from premedication are minor ones, requiring no or minimal medical intervention [27]; these are the same that we failed to report. Finally, we used a system for classifying patients into treatment groups that has intrinsic biases. Despite these limitations, we believe that some type of classification is necessary to evaluate the effect of antineoplastic treatment and the risk of developing ICM-related ARs.
Conclusion
ICM-related ARs are rare among cancer patients, with an incidence similar to that found in the general population. The pathogenesis of these events appears to be secondary to the direct activation of mast cells. Taxanes would seem to be a risk factor for acute ICM-related ARs, possibly because they exert their antitumor effect via similar immune-mediated pathways. However, the mechanisms of action that elicit these reactions have yet to be fully understood.
Acknowledgment
We thank Ursula Elbling for editing the manuscript.
Author Contributions
Conception/Design: Alberto Farolfi, Giampaolo Gavelli
Provision of study material or patients: Corradina Della Luna, Elisa Carretta, Nicola Gentili, Carla Casadei, Michele Aquilina, Domenico Barone, Martina Minguzzi
Collection and/or assembly of data: Alberto Farolfi, Corradina Della Luna, Angela Ragazzini, Elisa Carretta, Nicola Gentili
Data analysis and interpretation: Alberto Farolfi, Elisa Carretta, Dino Amadori, Oriana Nanni, Giampaolo Gavelli
Manuscript writing: Alberto Farolfi, Corradina Della Luna, Elisa Carretta, Oriana Nanni, Giampaolo Gavelli
Final approval of manuscript: Giampaolo Gavelli
Disclosures
The authors indicated no financial relationships.
References
- 1.Katayama H. Adverse reactions to contrast media: What are the risk factors? Invest Radiol. 1990;25(suppl 1):16–17. doi: 10.1097/00004424-199009001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Cochran ST, Bomyea K, Sayre J. Trends in adverse events from iodinated contrast media. Acad Radiol. 2002;9(suppl 1):65–68. doi: 10.1016/s1076-6332(03)80399-9. [DOI] [PubMed] [Google Scholar]
- 3.Mortelé KJ, Oliva MR, Ondategui S, et al. Universal use of nonionic iodinated contrast medium for CT: Evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol. 2005;184:31–34. doi: 10.2214/ajr.184.1.01840031. [DOI] [PubMed] [Google Scholar]
- 4.Wang CL, Cohan RH, Ellis JH, et al. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008;191:409–415. doi: 10.2214/AJR.07.3421. [DOI] [PubMed] [Google Scholar]
- 5.Bush WH, Swanson DP. Acute reactions to intravascular contrast media: Types, risk factors, recognition, and specific treatment. AJR Am J Roentgenol. 1991;157:1153–1161. doi: 10.2214/ajr.157.6.1950858. [DOI] [PubMed] [Google Scholar]
- 6.Caro JJ, Trindade E, McGregor M. The risks of death and of severe nonfatal reactions with high- vs low-osmolality contrast media: A meta-analysis. AJR Am J Roentgenol. 1991;156:825–832. doi: 10.2214/ajr.156.4.1825900. [DOI] [PubMed] [Google Scholar]
- 7.Araoz PA, Haramati LB, Mayo JR, et al. Panel discussion: Pulmonary embolism imaging and outcomes. AJR Am J Roentgenol. 2012;198:1313–1319. doi: 10.2214/AJR.11.8461. [DOI] [PubMed] [Google Scholar]
- 8.Almen T. The etiology of contrast medium reactions. Invest Radiol. 1994;29(suppl 1):37–45. [PubMed] [Google Scholar]
- 9.Lasser EC. A coherent biochemical basis for increased reactivity to contrast material in allergic patients: A novel concept. AJR Am J Roentgenol. 1987;149:1281–1285. doi: 10.2214/ajr.149.6.1281. [DOI] [PubMed] [Google Scholar]
- 10.Zanotti KM, Markman M. Prevention and management of antineoplastic-induced hypersensitivity reactions. Drug Saf. 2001;24:767–779. doi: 10.2165/00002018-200124100-00005. [DOI] [PubMed] [Google Scholar]
- 11.Brandi G, Pantaleo MA, Galli C, et al. Hypersensitivity reactions related to oxaliplatin (OHP) Br J Cancer. 2003;89:477–481. doi: 10.1038/sj.bjc.6601155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadoyama K, Kuwahara A, Yamamori M, et al. Hypersensitivity reactions to anticancer agents: Data mining of the public version of the FDA adverse event reporting system, AERS. J Exp Clin Cancer Res. 2011;30:93. doi: 10.1186/1756-9966-30-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polyzos A, Tsavaris N, Kosmas C, et al. Hypersensitivity reactions to carboplatin administration are common but not always severe: A 10-year experience. Oncology. 2001;61:129–133. doi: 10.1159/000055363. [DOI] [PubMed] [Google Scholar]
- 14.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. The Oncologist. 2007;12:601–609. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 15.ACR manual on contrast media, version 9. Available at http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/2013_Contrast_Media.pdf Accessed November 15, 2013.
- 16.Bosetti C, Bertuccio P, Malvezzi M, et al. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol. 2013;24:2657–2671. doi: 10.1093/annonc/mdt301. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian HM, Fojo T, Mathisen M, et al. Cancer drugs in the United States: Justum pretium—the just price. J Clin Oncol. 2013;31:3600–3604. doi: 10.1200/JCO.2013.49.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomi T, Nagamoto M, Hasegawa M, et al. Are there any differences in acute adverse reactions among five low-osmolar non-ionic iodinated contrast media? Eur Radiol. 2010;20:1631–1635. doi: 10.1007/s00330-009-1698-6. [DOI] [PubMed] [Google Scholar]
- 19.Bettmann MA, Heeren T, Greenfield A, et al. Adverse events with radiographic contrast agents: Results of the SCVIR Contrast Agent Registry. Radiology. 1997;203:611–620. doi: 10.1148/radiology.203.3.9169677. [DOI] [PubMed] [Google Scholar]
- 20.Brockow K. Contrast media hypersensitivity—scope of the problem. Toxicology. 2005;209:189–192. doi: 10.1016/j.tox.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Kopp AF, Mortele KJ, Cho YD, et al. Prevalence of acute reactions to iopromide: Postmarketing surveillance study of 74,717 patients. Acta Radiol. 2008;49:902–911. doi: 10.1080/02841850802282811. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman PL, Seigle RL. Reactions to radiocontrast material. Anaphylactoid events in radiology. Clin Rev Allergy Immunol. 1999;17:469–496. doi: 10.1007/BF02737651. [DOI] [PubMed] [Google Scholar]
- 23.Lasser EC. Nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2009;192:W80–; author reply W81. doi: 10.2214/AJR.08.1692. [DOI] [PubMed] [Google Scholar]
- 24.Lasser EC. X-ray contrast media mechanisms in the release of mast cell contents: Understanding these leads to a treatment for allergies. J Allergy (Cairo) 2011:276258. doi: 10.1155/2011/276258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien ME, Souberbielle BE. Allergic reactions to cytotoxic drugs—an update. Ann Oncol. 1992;3:605–610. doi: 10.1093/oxfordjournals.annonc.a058285. [DOI] [PubMed] [Google Scholar]
- 26.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 27.Lasser EC, Berry CC, Mishkin MM, et al. Pretreatment with corticosteroids to prevent adverse reactions to nonionic contrast media. AJR Am J Roentgenol. 1994;162:523–526. doi: 10.2214/ajr.162.3.8109489. [DOI] [PubMed] [Google Scholar]