This manuscript presents the summary of the discussions and recommendations of an Advisory Board of European experts, which was convened to discuss current European treatment practices and the impact of new data, with the aim of providing recommendations for routine clinical practice that can be broadly applied.
Keywords: Multiple myeloma, Risk stratification, Consolidation, Maintenance, Autologous stem cell transplantation, Nontransplant setting, Elderly patients
Abstract
The treatment of multiple myeloma has undergone significant changes and has resulted in the achievement of molecular remissions, the prolongation of remission duration, and extended survival becoming realistic goals, with a cure being possible in a small but growing number of patients. In addition, nowadays it is possible to categorize patients more precisely into different risk groups, thus allowing the evaluation of therapies in different settings and enabling a better comparison of results across trials. Here, we review the evidence from clinical studies, which forms the basis for our recommendations for the management of patients with myeloma. Treatment approaches depend on “fitness,” with chronological age still being an important discriminator for selecting therapy. In younger, fit patients, a short three drug-based induction treatment followed by autologous stem cell transplantation (ASCT) remains the preferred option. Consolidation and maintenance therapy are attractive strategies not yet approved by the European Medicines Agency, and a decision regarding post-ASCT therapy should only be made after detailed discussion of the pros and cons with the individual patient. Two- and three-drug combinations are recommended for patients not eligible for transplantation. Treatment should be administered for at least nine cycles, although different durations of initial therapy have only rarely been compared so far. Comorbidity and frailty should be thoroughly assessed in elderly patients, and treatment must be adapted to individual needs, carefully selecting appropriate drugs and doses. A substantial number of new drugs and novel drug classes in early clinical development have shown promising activity. Their introduction into clinical practice will most likely further improve treatment results.
Implications for Practice:
Ongoing advances in the management of multiple myeloma have resulted in substantial improvements in outcomes for patients. It is necessary to critically evaluate trial results to assess how new findings should be incorporated into treatment strategies. The manuscript aims to provide recommendations for routine clinical practice that can be broadly applied based on the discussions among European experts and a review of evidence from clinical studies.
Introduction
Recent therapeutic advances have led to a multitude of treatment options for multiple myeloma (MM), which is in stark contrast to what was available until the year 2000. Although autologous stem cell transplantation (ASCT) was already introduced in the 1980s, it took several years and the availability of granulocyte colony-stimulating factor to improve the safety of the procedure. The next crucial advance came with the introduction of drugs with novel modes of action, such as immunomodulation and proteasome inhibition. Their widespread use has substantially impacted on treatment outcome with tangible improvements in survival seen across the patient spectrum [1–5]. Accordingly, these novel treatment options have been incorporated into guideline recommendations by several expert committees [6–8a].
The rapid progress is reflected by an increasing number of publications and presentations at congresses reporting on ever more treatment options, including new classes of agents, as well as novel combinations of existing options. One of the current challenges in the management of myeloma is not a lack of options, but rather the appropriate use of the available options. It is therefore important to review new data critically and to assess how novel approaches should be incorporated into treatment practices, taking into account not only the latest efficacy and tolerability data but also regulatory considerations and the diversity of health systems in different countries. An advisory board of European experts was convened to discuss current treatment practices and the impact of new data, with the aim of providing recommendations for routine clinical practice that can be broadly applied across Europe. This paper presents a summary of the discussions and recommendations, as well as a review of supporting data. Levels of evidence and grades of recommendation have been applied using the system shown in Tables 1 and 2 according to the Oxford Centre for Evidence-Based Medicine [8b]. Statements without grading were considered justified standard clinical practice by the experts.
Table 1.
Levels of evidence
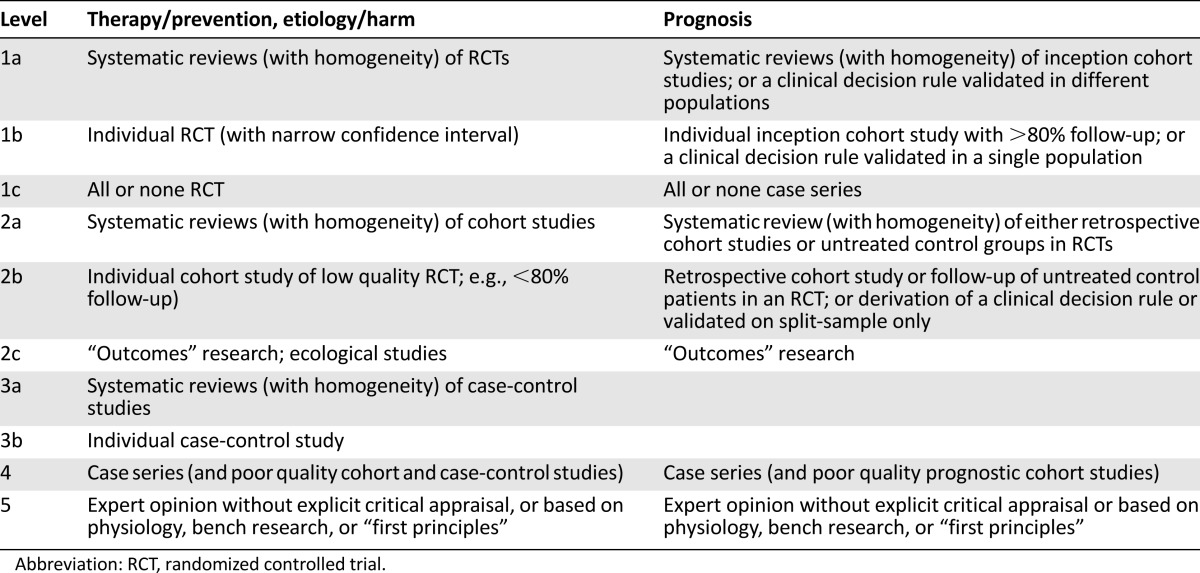
Table 2.
Grades of recommendation

Risk Stratification
The initial work-up of newly diagnosed patients includes a number of examinations designed to provide prognostic information. Foremost, the determination of the International Staging System (ISS) stage should routinely be carried out. In addition, levels of lactate dehydrogenase (LDH), the presence of extramedullary disease, plasma cell leukemia, and the proliferation rate provide valuable information regarding risk status. In addition, patient-specific factors, such as age, comorbidities, and complications (e.g., renal impairment, bone marrow function, anemia, and bone status), are taken into account when making treatment decisions.
It is well known that myeloma is associated with distinct cytogenetic abnormalities, some of which portend a poor prognosis. An analysis of cytogenetic aberrations using fluorescence in situ hybridization (FISH), in particular focusing on the translocation of chromosomes 4 and 14 (t(4;14)) or deletion in the short arm of chromosome 17 (del17p), is recommended. Analyzing risk status using routinely available methods (e.g., FISH analysis and ISS stage) can aid in the identification of a subset of patients with high-risk disease, who may benefit from innovative approaches, as shown in the study by Moreau et al. [9], who found that high LDH, ISS3, and the presence of cytogenetic abnormalities (either t (4; 14) or 17p deletion) was associated with an increased risk of death from progressive disease from the start of therapy. Using these three factors, they could develop a scoring system with which a subset of patients with a very poor prognosis could be clearly identified.
Providing a risk-adapted treatment strategy is a key goal in the ongoing research efforts aimed at providing treatment tailored to the individual genetic make-up, which would avoid unnecessary treatment and toxicity. Attempts at providing recommendations have been made in the so-called mSMART guidelines [10]; however, they are derived from clinical observations and experience rather than being based on results from randomized trials, and therefore prospective validation is desirable before widespread recommendation of these guidelines. The recent International Myeloma Working Group guidelines recommend the assessment of serum β2 microglobulin and serum albumin, as well as t(4;14), del17p, and gain in 1q by FISH, which will allow the stratification of patients into high-risk and standard-risk groups, thereby providing important prognostic information [11]. Furthermore, the authors conclude that there is currently no evidence base to suggest an adaptation of treatment based on risk groups, with the exception of administering prolonged proteasome inhibitor-based treatment to patients with t(4;14) and del17p and possibly using tandem transplantation in patients with these features who are eligible for ASCT [12]. Furthermore, pomalidomide plus dexamethasone has shown considerable activity in patients with relapsed/refractory disease with del17p [13, 14], but further studies are needed for a more extensive evaluation of its role in this patient population.
A number of new techniques to analyze genetic abnormalities are being investigated in clinical trials, such as gene expression profiling, analyses of single nucleotide polymorphisms, whole genome, exome or transcriptome sequencing, and methylation status [11, 15–18]. Although these techniques are providing ever more insights into the complexity of the genetic changes associated with myeloma, their use is currently only feasible in trials or specialist centers.
Conclusion: There is consensus that an analysis of specific factors to enable a better categorization of patients into different risk groups is valuable but subject to further improvement (level of evidence: 1b; grade of recommendation: A). Because prospective trials supporting a risk-adapted approach are not available, we recommend using the most effective treatment regardless of cytogenetic risk status and other risk factors (level of evidence: 2c; grade of recommendation: B). This will avoid the risk of undertreating those patients who will benefit from the most effective therapy but will result in overtreatment of a proportion of other patients.
Front-Line Transplant Setting
In Europe, high-dose therapy followed by ASCT represents the standard of care for patients without prohibitive comorbidities, with an age cutoff between 65 and 70 years, depending on the country. An impressive report on the use and outcome of ASCT as upfront therapy in more than 20,000 patients in the U.S. and Canada demonstrates that the use of ASCT has increased over time, as has the age of patients undergoing the procedure, as well as the number of patients with more advanced stages of disease and those having received any of the novel agents, thalidomide, bortezomib, or lenalidomide [19]. The analysis shows substantial improvements in survival over time, in part because of improvement in progression-free survival (PFS) following ASCT, but even more because of a better postrelapse survival, pointing to the value of incorporating novel agents into treatment strategies. These observations led the authors to conclude that ASCT and novel biological agents present complementary therapies that should be combined in the management of MM in suitable patients, affirming the role of ASCT in MM.
Induction Therapy
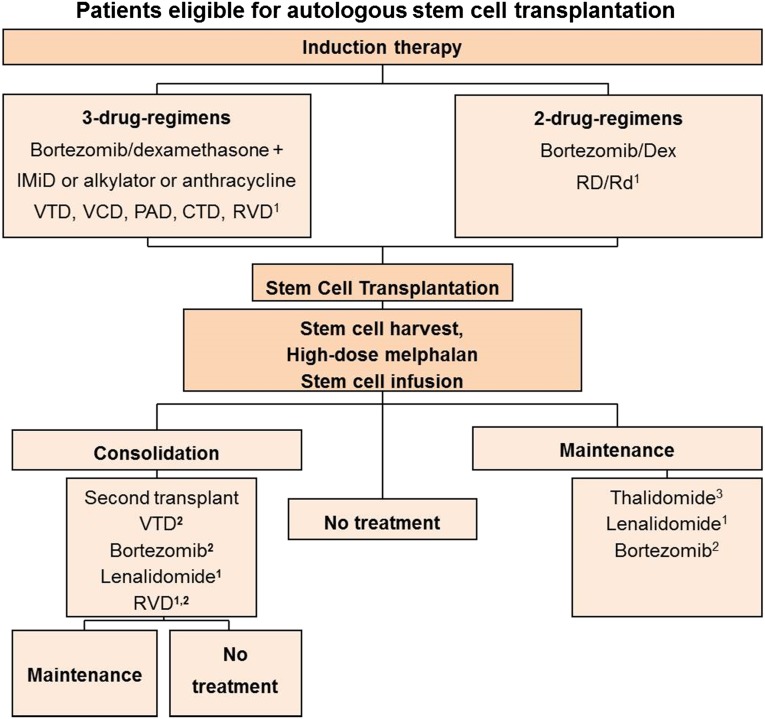
A survey during our consensus meeting revealed a homogeneous picture across Europe regarding typical induction regimens used outside clinical trials. Induction usually consists of a bortezomib-based regimen, with triple combinations finding increasing use. Based on available data [20–25], the combination of bortezomib and dexamethasone (VD) alone is seen as suboptimal for transplant-eligible patients, and the recommendation is to add an immunomodulatory agent, an alkylator or an anthracycline to VD, resulting in combinations such as bortezomib, thalidomide, and dexamethasone (VTD), bortezomib, cyclophosphamide, and dexamethasone (VCD), or bortezomib, doxorubicin, and dexamethasone (PAD) (Fig. 1). In addition, the combination of cyclophosphamide-thalidomide-dexamethasone is widely used in the United Kingdom. Of note, the combinations VD and VTD have been approved by the European Medicines Agency (EMA) for induction. The recommended dose of bortezomib is 1.3 mg/m2 twice weekly administered intravenously for four cycles (expert recommendation: in case of suboptimal response administer up to six cycles) with dexamethasone at 40 mg and thalidomide at 50 mg/day with possible increases to 100 or 200 mg/day. The use of the subcutaneous route of administration of bortezomib has resulted in improved tolerability at comparable efficacy to the intravenous route [26] and also presents a recommended option for induction.
Figure 1.
Treatment algorithm for patients eligible for autologous stem cell transplantation. 1Lenalidomide is currently not approved by the European Medicines Agency (EMA) for the treatment of newly diagnosed multiple myeloma or as consolidation or maintenance treatment. 2Bortezomib is currently not approved by the EMA as consolidation or maintenance treatment. 3Thalidomide is currently not approved by the EMA for the treatment of transplant-eligible patients or as consolidation or maintenance treatment.
Abbreviations: CTD, cyclophosphamide, thalidomide, and dexamethasone; Dex, dexamethasone; IMiD, immunomodulatory drug; PAD, bortezomib, doxorubicin, and dexamethaone; Rd, lenalidomide, low-dose dexamethasone; RD, lenalidomide, high-dose dexamethasone; RVD, lenalidomide, bortezomib, and dexamethasone; VCD, bortezomib, cyclophosphamide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.
The recommendation to use a bortezomib-based regimen is based on the results of randomized trials, which have recently been summarized in two meta-analyses and which confirmed the superiority of bortezomib-based regimens over conventional regimens [24, 25]. The analyses included data from four randomized controlled phase III trials: IFM 2005-01 (VD vs. vincristine-doxorubicin-dexamethasone [VAD] induction [21], HOVON-65/GMMG-HD4 (bortezomib-doxorubicin-dexamethasone vs. VAD [23]), PETHEMA GEM05MENOS65 (bortezomib-thalidomide-dexamethasone vs. thalidomide-dexamethasone [22]), and GIMEMA MM-BO2005 study (bortezomib-thalidomide-dexamethasone vs. thalidomide-dexamethasone [20]). The analysis of patient level data of these four trials by Sonneveld et al. [24] showed a significant superiority of bortezomib-based induction compared with non-bortezomib-based induction in terms of post-transplantation complete response (CR) + near CR rates (38% vs. 24%, p < .001), median PFS (35.9 months vs. 28.6 months, p < .001), and 3-year overall survival (OS) rates (79.7% vs. 74.7%, p = .0402). Bortezomib-based treatment resulted in a higher rate of peripheral neuropathy: 34% vs. 17% (grade ≥ 3, 6% v 1%), whereas the rate of death during induction was comparable between the two approaches (3% and 4% for bortezomib-based and non-bortezomib-based induction, respectively). Nooka et al. [25], following a meta-analysis of published results of the same trials, also concluded that bortezomib-containing regimens result in an improved depth of response, translating into improved post-transplant PFS and OS, albeit in the presence of a higher incidence of toxicity.
Conclusion: The consensus is that a bortezomib-based triple induction regimen should be given for four cycles before ASCT (level of evidence: 1a; grade of recommendation: A). The subcutaneous route of administration of bortezomib is recommended (level of evidence: 1b; grade of recommendation: A).
Conditioning
The standard conditioning regimen is 200 mg/m2 melphalan (MEL200); however, ongoing studies are assessing whether results can be improved by adding other agents. For example, intravenous busulfan is being combined with melphalan and in a matched comparison with melphalan alone, the combination was found to be effective and well tolerated [27]. A recent review summarizes additional results of studies incorporating busulfan during conditioning [28]. Because of synergistic effects between bortezomib and melphalan, this combination is also being investigated as a conditioning regimen. In a matched comparison with patients receiving melphalan alone, the combination showed a higher CR rate with comparable toxicity [29]. The combination has been investigated in other small studies and demonstrated beneficial effects both in the upfront, as well as the salvage transplant setting [30, 31]. Randomized studies are nevertheless needed to assess the role for the combination prospectively.
Conclusion: At the current time, MEL200 remains the standard conditioning regimen (level of evidence: 1b; grade of recommendation: A), but attempts to improve the efficacy by adding busulfan or bortezomib, among others, are ongoing.
Consolidation and Maintenance
Despite a lack of regulatory approval, the use of post-transplant therapy, in particular consolidation, defined as a short distinct course of treatment, is increasing across Europe in routine practice, with VTD being the predominant regimen used. In addition, maintenance therapy, the administration of prolonged treatment for 12 or 24 months or even until progression, consisting of thalidomide, lenalidomide, or bortezomib, is being used in some centers across Europe outside the clinical trial setting.
Consolidation with the aim of deepening the response after transplant is an attractive option with the evidence to support its use increasing. The most mature data come from a study by the GIMEMA group, who investigated two cycles of VTD vs. thalidomide and dexamethasone (TD) consolidation following VTD or TD induction and double ASCT [32, 33]. At the most recent data update, with a median follow-up of 59 months, the median PFS was 57 months for VTD versus 42 months for TD (p = .001) [33]. A landmark analysis from the start of consolidation treatment revealed a median PFS of 50 months in the VTD arm versus 38 months in the TD arm (p = .015). Of note, the PFS benefit of VTD over TD was retained across prespecified subgroups of patients irrespective of age, ISS stage, and the presence of t(4;14) and/or del17p. In addition, the duration of OS from progression was similar between the two groups (median 42 months for VTD versus 35 months for TD, p = .47), indicating the absence of more resistant relapses following VTD as compared with TD therapy [33]. Some experts recommend linking the number of cycles administered during consolidation to the number of cycles administered during induction, based on the hypothesis, which has so far not been investigated in trials, that the length of the entire treatment course is critical; for example, four cycles would be administered during induction plus two cycles during consolidation or three cycles of induction, followed by three cycles of consolidation. This proposal requires investigation in clinical trials. Of note, consolidation has been included in national treatment recommendations in some countries, such as in France.
Conclusion: There is consensus that consolidation seems to be beneficial, but at present it is not considered standard (level of evidence: 1b; grade of recommendation: A).
Maintenance therapy in the post-transplant setting has been investigated in a number of trials; however, they have so far not led to clear recommendations. Of note, none of the available agents is approved for use in maintenance.
A variety of agents is available, including interferon, which remains an option for some patients. With thalidomide, the treatment duration is limited by toxicity concerns, in particular peripheral neuropathy (PN); however, if tolerated, it can be considered. In patients with adverse risk cytogenetics, thalidomide maintenance was shown to result in shorter overall survival and should therefore not be used in the presence of these characteristics [34]. All six studies that have investigated the role of thalidomide maintenance therapy after ASCT showed a significant increase in PFS, and three of them also showed an increase in OS. The final analysis of the Australian trial with a median follow-up of 5.4 years revealed a significant benefit in both PFS and OS for the thalidomide/prednisone combination as compared with prednisolone alone (5-year PFS 27% versus 15%, p = .005; 5-year OS 66% versus 47%, p = .007, respectively) [35].
Lenalidomide maintenance versus no maintenance has been investigated in four randomized trials, three after ASCT [36–39] and one in transplant-ineligible patients [40], all of which demonstrated a remarkable PFS benefit with the agent, whereas OS was improved in two of the three post-transplant studies [36–39]. In the trial conducted by the IFM, PFS was 46 months in the lenalidomide arm and 24 months in the placebo arm (p < .001), whereas OS was comparable between the two arms (median OS 82 months vs. 81 months, respectively, p = .8) with a median follow up of 67 months. The time from the first to the second progression and the survival after the first progression were significantly shorter in the lenalidomide than in the placebo arm, whereas the cumulative incidence of second primary malignancies was significantly higher with lenalidomide [36]. In the trial of the CALGB, both median time to progression and OS were significantly superior with lenalidomide (time to progression: 50 months vs. 27 months, p < .001; OS: not reached vs. 73 months, p = .008), with a median follow-up of 48 months. The GIMEMA group used a 2 × 2 design and compared conventional melphalan, prednisone, and lenalidomide (MPR) chemotherapy (six cycles) to tandem ASCT, and, in the second part, lenalidomide maintenance to no therapy [38, 39]. All patients received four cycles of lenalidomide plus dexamethasone induction therapy. In this heterogeneously treated group of patients, lenalidomide maintenance led to a significantly improved PFS and OS compared with patients not receiving maintenance: the median PFS was 42.7 months for patients receiving lenalidomide maintenance versus 17.5 months for patients in the control arm (p < .0001) with a median follow-up of 48 months [38]. Importantly, there was also a significant survival benefit with lenalidomide maintenance: 4-year OS was 80% versus 62%, respectively (p = .02) [38].
In some studies investigating the long-term administration of lenalidomide, an increased risk of second primary malignancies (SPM) has been observed that has been carefully analyzed in different studies in the front-line and relapse settings [41]. It has been demonstrated that the increased risk of developing SPMs is related to melphalan exposure, being more pronounced with the oral administration of melphalan, as well as to advanced age [41]. The use of lenalidomide plus steroids does not appear to increase the risk of SPM. On the whole, the benefit in prolonged PFS and OS gained with lenalidomide maintenance outweighs by far the risk of developing an SPM.
One consideration regarding long-term treatment with any agent is whether its use until relapse will induce resistant relapses and will preclude the use of the agent in the future. So far there does not seem to be an indication that this occurs with long-term lenalidomide administration. This issue will impact the way we assess the benefit of new treatments and will lead to a new measure of outcome. The duration of the PFS of the second line therapy (second PFS) will be added to the length of the PFS of the first-line treatment (first PFS), creating a new measure of outcome consisting of the time from start of therapy to progression or death after first relapse treatment (PFS 2). Effective maintenance therapy should not be associated with a shorter second PFS or a shorter PFS 2 versus that of the control group. To really compare second PFS, a precise description of patients receiving this treatment is necessary.
Bortezomib has been investigated in the maintenance setting in two phase III studies, resulting in significant improvements in PFS in both and in OS in one study [42, 43]. In the study conducted by the Spanish myeloma group, the combination of bortezomib and thalidomide was associated with a significant PFS benefit compared with thalidomide or interferon, whereas OS was comparable between the three arms [42]. In the HOVON-65/GMMG-HD4 trial, with a median follow-up of 74 months, bortezomib-based treatment (during induction and maintenance) was found to improve both PFS (median 27 months vs. 36 months) and OS (median 84 months vs. not reached, p = .05) [43]. A landmark analysis from the start of the maintenance phase revealed a significant OS benefit with bortezomib maintenance treatment compared with thalidomide maintenance, whereas the PFS was comparable in the two arms.
In both of these maintenance trials, bortezomib was also used during induction therapy (bortezomib-based induction was compared with non-bortezomib-containing regimens) [42, 43]. Hence, it is as yet not clear which part of the bortezomib exposure contributed to the results.
Taken together, we appreciate the survival benefits obtained with lenalidomide maintenance in two of the three studies. Longer follow-up of the trials and additional studies about the optimal duration of lenalidomide maintenance therapy will provide further insights. Presently, patients’ preferences are integrated into clinical decision making in those countries where maintenance treatment is supported by the national health care system. A recent survey on the willingness of patients to accept toxicities in return for gain in outcome showed that the willingness among patients to receive maintenance was substantially influenced by the expected toxicity and survival gain [44]. In case of PFS but no OS benefit, only 23% of patients would choose maintenance if it was associated with moderate toxicity, but the proportion would rise to 92% in case of only mild side effects. Hence, decisions on the use of maintenance should be made after careful discussion of the pros and cons of maintenance therapy with the individual patient. Nevertheless, freedom from myeloma-related symptoms in real life may be less common than anticipated, as indicated by a recent European survey conducted in 11 U.K. and German centers. Twenty-five percent of patients reported severe myeloma-related symptoms, and 32% reported moderate symptoms from their disease at the time of the assessment [45].
Conclusion: There is consensus that lenalidomide significantly prolongs PFS. An increase in OS was seen in two of three post-transplant trials. Lenalidomide maintenance is as yet not approved in Europe (level of evidence: 2c; grade of recommendation: B). Other options are thalidomide (level of evidence: 2c; grade of recommendation: B) and bortezomib (level of evidence: 2c; grade of recommendation: B), both of which are also not approved. Maintenance therapy may be discussed with individual patients but presently cannot be considered standard.
Single versus Tandem ASCT
The use of single versus tandem ASCT varies among countries in Europe. A systematic Cochrane review of five randomized controlled studies involving 1,506 patients in total (two full-text publications, three conference presentations until 2011) reported a significant gain in event-free survival (EFS) in four and in OS in one of the studies but concluded that the inherent biases of the evaluated studies limited their value for deriving treatment decisions concerning the question of single versus double ASCT [46]. Furthermore, it was highlighted that none of the studies used novel agents, thalidomide, lenalidomide, or bortezomib, which are now considered standard in the treatment of MM.
A multivariate analysis of possible risk factors for mortality in four large prospective randomized European trials identified ISS stage 3, high-risk cytogenetics (t(4;14) and/or del17p), failure to achieve CR after induction, and assignment to receive a single ASCT as factors associated with mortality [12]. Patients assigned to receive a double ASCT had significantly longer PFS and OS compared with those undergoing a single ASCT (PFS: 50 vs. 38 months, p < .001, OS estimate at 5 years: 75% vs. 63%, p = .002). When patient level data of patients treated with bortezomib-based induction regimen only were analyzed, double ASCT was found to be significantly superior over single ASCT, particularly in patients with two adverse variables, with respect to both PFS and OS. Although these data require confirmation in prospective trials, they provide a strong rationale for patients with high-risk cytogenetics and those not achieving CR after bortezomib-based induction to undergo double ASCT.
Conclusion: There is consensus that double ASCT is likely to be beneficial in patients with high risk cytogenetics (t(4;14) and/or del17p) and those patients not achieving CR after induction (level of evidence: 4; grade of recommendation: C).
Can ASCT Be Delayed?
The availability of a number of very effective novel agents has resulted in the role of ASCT as upfront therapy being re-examined, and as a consequence, the question of whether the transplant step could be delayed to relapse is being investigated. In a retrospective analysis of patients who had received lenalidomide-dexamethasone as initial therapy, Kumar et al. [47] could show that OS was comparable in patients who underwent early stem cell transplantation (SCT) and in those for whom SCT was delayed to more than 12 months after diagnosis. A number of randomized trials are currently ongoing to address this question prospectively. The most advanced of these trials is the one conducted by the Italian Myeloma Network, in which 402 patients received induction with lenalidomide and low-dose dexamethasone and were then randomized to receive MPR or MEL200 plus ASCT, followed by another randomization to lenalidomide maintenance or observation. Patients who relapse after conventional therapy with or without lenalidomide maintenance will be subjected to ASCT. At a median follow-up of 48 months, the median PFS was significantly longer for patients undergoing initial ASCT (38.6 months vs. 24.2 months, respectively, p < .0001) [38]. Five-year OS was also superior in the transplant arm; however, the difference was not statistically significant (71% vs. 62%, p = .27). Longer follow-up is needed to assess the impact on OS. Two other trials, one conducted by the IFM in collaboration with the Dana Faber Cancer Institute and a second conducted by the European Myeloma Network, will help to shed light on the question of the role of ASCT upfront.
Conclusion: There is consensus that at the current time upfront ASCT is standard in transplant-eligible patients (level of evidence: 1b; grade of recommendation: A). Delaying ASCT until first relapse is feasible but is associated with a shorter PFS 1. Ongoing trials aimed at evaluating the efficacy of novel drug-based chemotherapy with upfront ASCT versus ASCT at first relapse will show whether early ASCT can be substituted by novel treatment regimens.
Role for ASCT at Relapse
Because of its potential for long-term disease control, ASCT is also an attractive option at relapse. A number of retrospective, single-center studies have suggested a benefit for salvage ASCT [48–53]. Recently, results of the first prospective study investigating ASCT in the relapse setting were published [54]. This trial included patients in first relapse after one previous transplant step, who had had a time to disease progression of ≥12 months following the first ASCT. Following reinduction with a PAD regimen, patients were randomized to salvage ASCT or weekly cyclophosphamide for 12 weeks. The investigators could demonstrate a significant benefit in PFS for the salvage procedure over cyclophosphamide treatment (19 months versus 11 months, respectively; p < .0001), whereas OS was comparable in the two arms with a median follow-up of 12 months [54].
Conclusion: There are robust data that indicate a benefit for salvage ASCT over conventional chemotherapy. Therefore, this modality should be considered after long PFS (≥18 months) following upfront ASCT (level of evidence: 1b; grade of recommendation: A).
Role for Allogeneic Stem Cell Transplantation
Although it is recognized that allogeneic stem cell transplantation (allo-SCT) may cure a minority of patients, its use outside clinical trials is not recommended because of a high treatment-related morbidity and mortality as stated by the International Myeloma Working Group Consensus Statement [55].
A number of trials have been conducted to evaluate the role of an autologous followed by allogeneic transplantation (auto-allo-SCT) in the front-line setting, with two of the studies supporting the auto-allo approach and four showing either no benefit or opposing this strategy [56–64]. A review and meta-analysis of four published randomized trials revealed that although autologous followed by allogeneic transplantation resulted in a higher CR rate than a tandem automatic approach (auto-auto-SCT), EFS and OS were comparable in the two arms, whereas nonrelapse mortality was significantly higher with auto-allo-SCT, leading the authors to conclude that the use of auto-allo-SCT for newly diagnosed MM should be restricted to clinical trials [65].
In patients with relapse after ASCT, a recently published comparison of outcomes of second ASCT versus nonmyeloablative or reduced-intensity conditioning allogeneic transplantation showed a higher treatment-related mortality and a lower survival with the allo-SCT procedure compared with the salvage autotransplant approach [66].
Further studies are needed to assess the value of allo-SCT, particularly in patients with t(4;14) and del17p, in whom a possible benefit with allo-SCT has been observed [67, 68]. In addition, the incorporation of novel agents into the allo-SCT procedure is showing promising results and requires further study [69, 70].
Conclusion: There is consensus that, in general, auto-allo-SCT should not be used outside clinical trials (levels of evidence: 1a; grade of recommendation: A). However, for patients with ultra-high-risk myeloma (i.e., de novo plasma cell leukemia), patients with both t(4;14) and del17p, and patients with high LDH, ISS3, and t(4;14) or del17p, the possibility of allo-SCT should also be discussed with patients outside the setting of clinical trials (level of evidence: 2c; grade of recommendation: B).
Minimal Residual Disease
Extensive efforts are currently focused on improving minimal residual disease (MRD) monitoring to evaluate the disease burden following treatment because many patients will have persistent levels of residual disease that are not detectable by morphological assessment of bone marrow alone. Therefore, more sensitive techniques are required to obtain information on response and prognosis, and importantly, such MRD assessments may be invaluable in guiding therapeutic decisions, such as the decision to administer prolonged treatment [71]. A number of techniques are being investigated in this setting, such as polymerase chain reaction-based methods, multiparameter flow cytometry, positron emission tomography-computed tomography, and, most recently, sequencing-based methods [72–80]. Both the Spanish and U.K. groups have shown that patients who achieve an MRD-negative status enjoy a significantly longer PFS and OS, and this was observed both in the transplant and nontransplant settings. Before incorporating such techniques in routine practice, standardization of methods is needed.
Conclusion: There is consensus that techniques for minimal residual disease assessment will be refined and standardized. Achievement of minimal disease negativity is considered to be an important treatment goal (level of evidence: 1b; grade of recommendation: A).
Front-Line Nontransplant Setting
Across Europe, treatment practices in the nontransplant setting outside clinical trials show a generally homogeneous picture, with melphalan, prednisone, thalidomide (MPT) and bortezomib, melphalan, prednisone (VMP) being the two most widely used regimens. When bortezomib is used as part of front-line therapy, administration is typically subcutaneous. On the whole, maintenance treatment is currently not widely used.
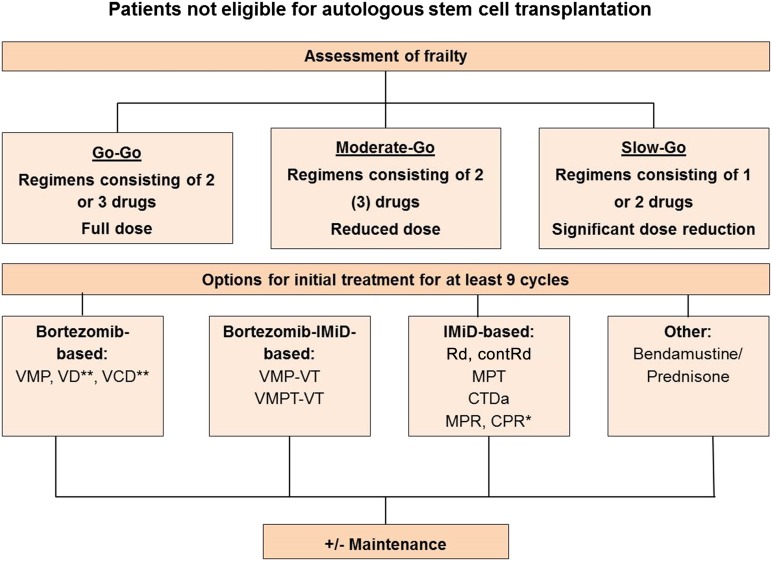
Novel agents play a crucial role in the management of patients not eligible for transplantation [81–84], and based on the results of randomized trials, which have previously been reviewed [85], the recommended treatments in the nontransplant setting include MPT and VMP (Fig. 2). Data from a trial comparing VMP to VD and VTD followed by weekly bortezomib maintenance for 25 weeks showed similar activity for the three regimens in patients ineligible for transplantation [86]. Similar results, showing no difference in major response parameters and other outcome measures, have been obtained by comparing a modified VMP, bortezomib, prednisone, and VCD regimen in elderly patients [87]. This indicates that melphalan can safely be omitted in elderly patients. Optimal duration of treatment seems crucial, and therapy should be administered for at least nine cycles; however, the optimal length of therapy has so far not been investigated prospectively. Regarding the dose and route of administration of bortezomib in the nontransplant setting, it is recommended to choose the subcutaneous route and weekly dosing. However, in case of renal impairment [88, 89] or extensive bone disease [90], twice weekly administration is recommended. MPR has recently been compared with lenalidomide, low-dose dexamethasone (Rd) and CPR, without showing significant differences in activity, but MPR and CPR proved to be more toxic [91]. In the most remarkable recent study involving 1,623 patients not eligible for transplantation, the combination of lenalidomide plus low-dose dexamethasone administered until disease progression or intolerance (contRd) was compared with MPT administered for 12 cycles (72 weeks) or Rd administered for 18 cycles (72 weeks) (Rd18) [92]. The trial revealed a significant benefit of contRd over Rd18 and MPT and a significant increase in OS over MPT. With a median follow-up of 37 months, the median PFS was 25.5 months for contRd compared with 20.7 months with Rd18 and 21.2 months for MPT (contRd versus MPT: p = .0006, contRd versus Rd18: p = .0001). Four-year OS was 59.4% for contRd, 55.7% for Rd18, and 51.4% for MPT (contRd versus MPT: p = .017, contRd versus Rd18: p = .307). Given the significant improvement in survival, the relatively acceptable toxicity, and the ease of administration of contRd, this combination will likely become a frequently used protocol in elderly patients.
Figure 2.
Treatment algorithm for patients not eligible for autologous stem cell transplantation. ∗, Rd and CPR showed similar activity in very elderly patients [90]; ∗∗, VD and VCD have activity similar to that of VMP [85, 86]. These treatments are not approved by the European Medicines Agency for treatment of transplant-ineligible patients.
Abbreviations: contRd, lenalidomide, low-dose dexamethasone until progression; CPR, cyclophosphamide, prednisone, and lenalidomide; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; MPR, melaphalan, prednisone, and lenalidomide; MPT, melphalan, prednisone, and thalidomide; Rd, lenalidomide, low-dose dexamethasone; VCD, bortezomib, cyclophosphamide, and dexamethasone; VD, bortezomib and dexamethasone; VMP, bortezomib, melphalan, and prednisone.
Conclusion: There is consensus that several options are available for the front-line treatment of patients not eligible for transplantation. MPT, VMP, and continuous treatment with Rd have proven to be superior over comparators in large prospective randomized trials (level of evidence: 1a; grade of recommendation: A). In addition, recent studies indicate that two- or three-drug combinations with either bortezomib or lenalidomide as backbone agents show similar activity to VMP or MPR in elderly patients (level of evidence: 1b; grade of recommendation: A). Several of the regimens, including continuous Rd, are not yet approved for first-line therapy in Europe. Front-line treatment should be administered for at least nine courses.
Maintenance Therapy
The role of maintenance in the nontransplant setting has been investigated in several trials with all the novel agents, thalidomide, bortezomib, and lenalidomide. Benefits in PFS have been seen with all of these [40, 85, 92–95]; however, an OS advantage was only seen in the GIMEMA study, which compared bortezomib, melphalan, prednisone, and thalidomide (VMPT) followed by bortezomib and thalidomide (VT) maintenance to VMP [95]. With a median follow up of 54 months, the median OS was not reached with VMPT-VT,5 and it was 60.0 months with VMP (5-year OS 61% vs. 51%, respectively). Because of the different regimens used during the initial phase of treatment, VMPT vs. VMP, it is not possible to attribute the survival effect exclusively to VT maintenance.
Lenalidomide maintenance following treatment with MPR (MPR-R) was shown to significantly prolong PFS over treatment with MPR or MP (MPR-R: 31 months versus MPR: 14 months [p < .001] versus MP 13 months [p < .001]), whereas for survival no difference was noted [40]. In addition, the continuous administration of lenalidomide plus low-dose dexamethasone (contRd) has been shown to result in significant improvements in PFS compared with Rd administration for 18 cycles and in significant increases in PFS and OS compared with 12 cycles of MPT as outlined above [92].
Although maintenance treatment cannot generally be recommended in transplant-ineligible patients, it may present an option for some patients, taking into account efficacy, tolerability, and also patient preference as discussed above.
Conclusion: There is consensus that maintenance treatment is an option for elderly patients not eligible for transplantation (level of evidence: 1b; grade of recommendation: A). A survival benefit has been observed in only one study; however, the results are difficult to interpret. Therefore, treatment should only be started after careful discussion of the limited benefits and relevant risks. Maintenance in the nontransplant setting is not approved in Europe and not standard.
Assessment of Elderly Patients
Patients not eligible for transplantation are a highly heterogeneous group. Aging is a complex, multifactorial process that is associated with substantial physiological, cognitive, and functional changes [96, 97]. The definition of elderly should not only include the biological age (versus chronological age), but also disability, comorbidity, and the degree of frailty/dependency on help. This complexity in the definition and diversity of characteristics necessitates a careful evaluation of the patient and a tailored approach to treatment.
Adapting treatment to individual risk factors is to some degree already incorporated into current management practices through the use of the ISS staging system, which appears to be particularly relevant for elderly patients, because it is suggested to represent a staging system for the age-related comorbidity burden [98]. Further assessments, such as a specific geriatric evaluation, are important to optimize the management of elderly patients. Older age alone should not be an argument for using less intensive therapy or not aiming for the deepest response possible because the achievement of CR in elderly patients has also been shown to be associated with improved PFS and OS [99]. Nevertheless, it is equally crucial to avoid overtreatment of patients risking severe toxicity, which will result in treatment interruption or early discontinuation, suboptimal treatment delivery, and inferior survival. This is supported by a recent publication that emphasized the substantial negative impact of severe toxicity (grade 3/4 hematological toxicity, cardiac toxicity, and infections) and treatment discontinuation on patient outcome [100]. Other factors significantly associated with decreased OS were age and increased creatinine levels. The recently reported Freiburg Comorbidity Index combines Karnofsky Performance Status, as well as lung and renal disease status, which allows the calculation of an index that appears to reliably reflect patient comorbidity and that, in combination with the ISS, was shown to contribute information that allowed the distinction of low-, intermediate-, and high-risk groups [101].
Palumbo et al. have developed a score based on age, the Charlson index, activities of daily living, and instrumental activities of daily living, which allows the classification of patients into fit, unfit, and frail groups [87, 102–104]. The investigators showed that frailty is a significant negative prognostic factor with higher prognostic relevance than unfavorable cytogenetics, strongly suggesting that frail patients require specifically adapted management approaches. Accordingly, a differentiation into “go-go,” “moderate-go,” and “slow-go” groups with resulting adaptations of dosing regimens is suggested, as indicated in Figure 3 [54, 102–104].
Figure 3.
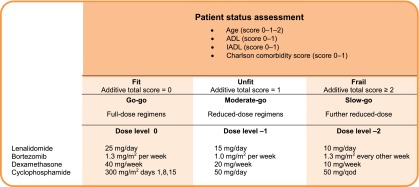
Treatment algorithm for elderly patients with myeloma (adapted from [104]).
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living; qod, every other day.
Although a prospective validation of the score suggested by Palumbo et al. is required, there is sufficient evidence to suggest the inclusion of geriatric assessment at diagnosis to enable the adaptation of treatment to ensure optimal patient management [103, 104].
Conclusion: There is consensus that frailty, disability, and comorbidity need to be assessed carefully in prospective trials and that the use of such assessments for treatment and dose selection is appropriate and valuable (level of evidence: 1b; grade of recommendation: A).
Smoldering Myeloma
Recent insights show that smoldering MM describes a heterogeneous state that is made up of entities with very different prognoses regarding the progression to symptomatic myeloma, and it has been proposed that a reclassification of smoldering MM may be needed [105, 106]. The new definition would classify high-risk smoldering MM as early myeloma, and it is thought that patients with high-risk smoldering myeloma may derive benefit from early treatment initiation; however, no general recommendation regarding this strategy exists so far. There are data from one phase III randomized trial, which demonstrate that patients with high-risk smoldering myeloma profit from early treatment initiation [107]. Patients receiving nine cycles of lenalidomide-dexamethasone followed by lenalidomide maintenance had a significantly longer time to active disease, as well as OS, compared with patients in the observation arm [107]. These results suggest a benefit for early initiation of treatment in a particular risk population, but further studies are needed to provide a general recommendation to do so. In particular, the definition of high-risk smoldering myeloma requires clarification [105, 108]. Patients with extensive marrow plasmacytosis (<60%) and a very high number of free light chains (FLCs) may be classified as ultra-high-risk and may very likely benefit from immediate treatment.
Conclusion: There is consensus that early initiation of therapy may be beneficial in high risk smoldering myeloma; however, further confirmatory results are needed before this approach can be recommended as standard (level of evidence: 1b; grade of recommendation: A).
Treatment at Relapse
Relapse situations are heterogeneous, and their management therefore requires an individual approach based on appropriate assessments to ensure that treatment is neither initiated too early nor too late [109]. First of all, a distinction between biochemical relapse, defined as a rise in M-protein without any of the typical myeloma-related complications, and clinical relapse, which is characterized by the presence of the typical myeloma symptoms, is crucial. Although a biochemical relapse in general does not require the immediate start of treatment but can be managed by observation, where the frequency of checkups depends on the kinetics of the M-protein increase, a symptomatic relapse requires the prompt start of therapy. Relapse requiring treatment is typically defined by the CRAB criteria (hypercalcemia, renal insufficiency, anemia, and bone lesions), which encompass direct indicators of increasing disease and/or end organ dysfunction; however, these criteria are seen as too conservative, and the indication to start treatment has been expanded to include significant paraprotein relapse even in patients without a clinical relapse [83]. Taken together, the following should constitute the indication to start treatment:
-
Clinical relapse
Development of new soft-tissue plasmacytomas or bone lesions
Definite increase in size of existing plasmacytomas or bone lesions
Hypercalcemia (>11.5 mg/dL; 2.65 mmol/L)
Decrease in hemoglobin of ≥2 g/dL (1.25 mmol/L), because of myeloma
Rise in serum creatinine by 2 mg/dL or more (177 mmol/L or more), because of myeloma
Hyperviscosity requiring therapeutic intervention
-
Significant biochemical relapse in patients without clinical relapse (IMW Paris 2011)
Doubling of the M-component in two consecutive measurements separated by < 2 months with the reference value of 5 g/L, or
-
In two consecutive measurements any of the following increases:
the absolute levels of serum M protein by ≥10 g/L, or
an increase of urine M protein by ≥500 mg per 24 hours, or
an increase of involved FLC level by ≥20 mg/dL (plus an abnormal FLC ratio) or 25% increase (whichever is greater)
Based on the careful assessment of the relapse situation, the following three groups can be identified: (a) patients in need of immediate treatment because of an aggressive relapse; (b) patients for whom close follow-up is required (e.g., monthly or bimonthly) to monitor paraprotein levels and to be in a position to initiate treatment at the first sign of disease; and (c) patients for whom only regular follow-up checks are needed.
In case of the presence of risk factors, such as aggressive clinical disease at diagnosis, a short treatment-free interval with a suboptimal response to the previous treatment line, imminent risk for organ dysfunction (patients with previous light chain-induced renal impairment), or unfavorable cytogenetics (t(4;14) or del17p), treatment should be initiated at the stage of biochemical relapse without waiting for symptoms.
Once the decision to initiate treatment has been made, a number of considerations will guide the choice of therapy, such as the components and efficacy of the initial line of treatment, as well as patient status and the type of relapse as mentioned above (Table 3).
Table 3.
General considerations when deciding on treatment in the relapse setting
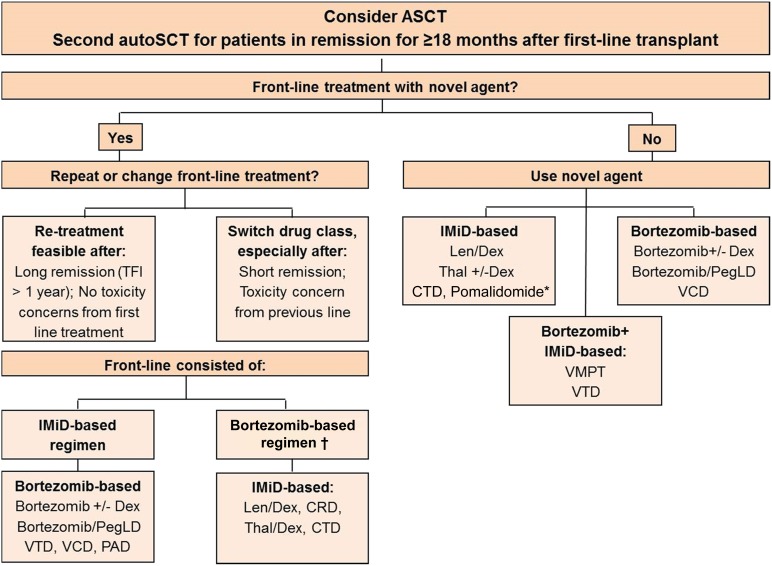
Treatment options at relapse are outlined in Figure 4. Retreatment with an agent used previously is considered feasible, provided the treatment produced a clinically meaningful response of adequate duration and acceptable toxicity. Studies with bortezomib and lenalidomide retreatment have shown that retreatment is feasible and effective and does not result in cumulative toxicity [110, 111]. If an effective alternative treatment, such as an agent from a different drug class, is available at relapse, switching drug class is preferable, and previously used agents should then be considered in later lines.
Figure 4.
Treatment algorithm for patients in first relapse. †, Retreatment with bortezomib after front-line bortezomib only if no peripheral neuropathy (PN) is present or if PN has recovered and there is no other therapeutic alternative. ∗, Pomalidomide is likely to be a valuable option, but presently the agent is approved for third or later line therapy only.
Abbreviations: autoSCT, autologous stem cell transplantation; CRD, cyclophosphamide, lenalidomide, and dexamethasone; CTD, cyclophosphamide, thalidomide, and dexamethasone; Dex, dexamethasone; IMiD, immunomodulatory drug; Len, lenalidomide; PAD, bortezomib, doxorubicin, and dexamethasone; PegLD: pegylated liposomal doxorubicin; TFI, treatment-free interval; Thal, thalidomide; VCD, bortezomib, cyclophosphamide, and dexamethasone; VTD, bortezomib, thalidomide, dexamethasone.
The novel agents bortezomib, thalidomide, and lenalidomide make up the mainstay of treatment at relapse [112–115]; however, other agents, such as bendamustine [116], also present effective options. Second generation proteasome inhibitors (carfilzomib, ixazomib, marizomib, and oprozomib) and third generation immunomodulatory agents (pomalidomide) have demonstrated efficacy [117–124]. The combination of pomalidomide with low-dose dexamethasone (Pom + ldDex) was investigated in a phase III study in patients with relapsed/refractory disease and was found to be superior to high-dose dexamethasone (hdDex) for both PFS and OS. The median PFS was 4.0 months for Pom + ldDex and 1.9 months for hdDex (p < .001), whereas median OS was 13.1 months and 8.1 months, respectively (p = .009) [13]. Of note, the presence of del17p did not impact the median PFS for patients receiving Pom + ldDex.
Carfilzomib has been investigated in the relapsed/refractory setting in phase II studies as single agent and as part of combination regimens. Single-agent carfilzomib resulted in an overall response rate (ORR) of 23.7%, a median duration of response of 7.8 months and a median OS of 15.6 months [123]. In combination with lenalidomide and low-dose dexamethasone, an ORR of 76.9% was observed, with a duration of response of 22.1 months and a median PFS of 15.4 months [122]. Phase III trials are currently ongoing. The oral proteasome inhibitor ixazomib has shown promising results in combination with lenalidomide and dexamethasone. In a phase I/II trial in patients with newly diagnosed MM, this combination resulted in an ORR of 93% with a 24% CR rate. Of note, grade 3 PN was observed in 5%, with no drug-related grade 4 adverse events noted [124].
Pomalidomide in combination with dexamethasone has been approved by the EMA for patients who have received at least two prior regimens and who have shown resistance to lenalidomide and bortezomib. Carfilzomib has received a positive opinion from the Committee for Medicinal Products for Human Use, and both pomalidomide and carfilzomib are approved by the U.S. Food and Drug Administration.
Conclusion: There is consensus that the initiation of treatment for relapsed/refractory disease depends on various factors, such as the rapidity and aggressiveness of relapse, as well as risk factors of the individual patient. Treatment selection should be based on results of the previous therapy and should take into account disease- and patient-related characteristics (level of evidence: 2c; grade of recommendation: B).
New Developments and Outlook
In the era of targeted treatments, a number of agents in the field of myeloma appear of particular interest, especially the monoclonal antibodies. Based on the success in other malignancies, it is hoped that these targeted agents will provide substantial benefits for patients. Monoclonal antibodies are thought to act through a range of mechanisms, including antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and the induction of apoptosis/growth arrest via the targeting of signaling pathways [125]. Elotuzumab, an anti-CS1 antibody, is the agent that has progressed furthest in development [126]. It has only modest single-agent activity; however, in combination with lenalidomide and dexamethasone, it showed high overall response rates and a PFS suggested to be superior to lenalidomide-dexamethasone alone in phase I and II studies [127, 128]. Based on these results, phase III trials in combination with lenalidomide in relapsed/refractory disease, as well as in front-line are ongoing (ELOQUENT-1 and ELOQUENT-2). The CD38 monoclonal antibody daratumumab appears to be a particularly promising agent because of its potent single-agent activity. In a phase I/II trial in patients with relapsed MM, substantial reductions in paraprotein in heavily pretreated patients were demonstrated [129]. In ongoing phase I/II and phase III trials, daratumumab is being investigated in combination with bortezomib and dexamethasone and with lenalidomide and dexamethasone in relapsed or refractory multiple myeloma. Other promising CD38 antibodies presently in early development are SAR650984 and MOR 202 [130, 131].
Histone deacetylases are known to be involved in the regulation of tumor suppressor proteins and oncogenes [132], making them attractive therapeutic targets. Single-agent studies with vorinostat and panobinostat showed only modest activity [133], and a randomized phase III trial comparing the combination of vorinostat and bortezomib to bortezomib alone showed an improvement in PFS of 28 days only [134]. The phase III PANORAMA 1 trial investigating panobinostat in combination with bortezomib and dexamethasone compared with bortezomib and dexamethasone alone, showed a gain of PFS by 4 months with panobinostat [135]. The role for HDAC inhibitors in myeloma requires further investigation, and the recent report by Minami et al. [136] of a new target for inhibition appears promising. Two new HDAC inhibitors, ACY-1215 and quisinostat, are currently undergoing investigation. A number of promising new agents, such as ARRY-520, a kinesin spindle protein inhibitor [137], and plitidepsin, a cyclodepsipeptide originally isolated from the Mediterranean tunicate Aplidium albicans [138], both of which show single-agent activity, are currently in clinical development, and additional results are awaited.
Conclusion
The substantial progress made over the recent years in developing effective treatments for myeloma and in the understanding of myeloma disease biology has resulted in substantially improved survival outcomes for patients across the age spectrum [3]. Such progress is finally questioning the long-held belief of myeloma being incurable in the vast majority of patients [139–141]. Continued efforts are needed in bringing together academic, clinical, regulatory, and pharmaceutical parties to develop treatment strategies that offer quality of life and survival prolongation, making cure a realistic goal for a larger proportion of patients. There is a strong expectation that novel drugs will increase the therapeutic spectrum, enabling a lymphoma-like approach to treatment, with immunotherapy being combined with chemotherapy and that such strategies are likely to improve outcome substantially.
Acknowledgments
The consensus meeting has been supported by an unrestricted grant by Janssen Pharmaceutical Companies of Johnson & Johnson in EMEA. This work was supported in part by the Austrian Forum Against Cancer. This is a position paper from the European Myeloma Network.
Footnotes
For Further Reading:Louis P. Garrison, Jr., Si-Tien Wang, Hui Huang et al. The Cost-Effectiveness of Initial Treatment of Multiple Myeloma in the U.S. With Bortezomib Plus Melphalan and Prednisone Versus Thalidomide Plus Melphalan and Prednisone or Lenalidomide Plus Melphalan and Prednisone With Continuous Lenalidomide Maintenance Treatment. The Oncologist 2013;18:27–36.
Implications for Practice:There is a growing number of treatment options available for previously untreated, transplant-ineligible multiple myeloma patients, based on the combination of melphalan and prednisone (MP) with bortezomib (VMP), thalidomide (MPT), or lenalidomide plus lenalidomide maintenance (MPR-R). These regimens confer substantial improvements in patient health outcomes compared with MP. However, they are also associated with higher costs than MP. With limited healthcare budgets, it is important to determine the most cost-effective treatment option. This paper presents the first published analysis of the potential cost-effectiveness of these regimens based on efficacy and safety data from randomized clinical trials. The findings show that VMP is projected to provide cost savings compared to MPT and MPR-R, and to be a cost-effective treatment option compared to MP, MPT, or MPR-R in previously untreated, transplant-ineligible multiple myeloma patients when managed within the U.S. healthcare system. These findings support the recommendation to use VMP for this patient population.
Author Contributions
Conception/Design: Heinz Ludwig, Pia Sondergeld
Data analysis and interpretation: Heinz Ludwig, Pia Sondergeld
Manuscript writing: Heinz Ludwig, Pieter Sonneveld, Faith Davies, Joan Bladé, Mario Boccadoro, Michele Cavo, Gareth Morgan, Javier de la Rubia, Michel Delforge, Meletios Dimopoulos, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Philippe Moreau, Hareth Nahi, Torben Plesner, Jesús San-Miguel, Roman Hajek, Pia Sondergeld, Antonio Palumbo
Final approval of manuscript: Heinz Ludwig, Pieter Sonneveld, Faith Davies, Joan Bladé, Mario Boccadoro, Michele Cavo, Gareth Morgan, Javier de la Rubia, Michel Delforge, Meletios Dimopoulos, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Philippe Moreau, Hareth Nahi, Torben Plesner, Jesús San-Miguel, Roman Hajek, Pia Sondergeld, Antonio Palumbo
Disclosures
Heinz Ludwig: Celgene, Mundipharma, Janssen-Cilag, Roche, Onyx, Millennium, Takeda (H); Pieter Sonneveld: Janssen, Celgene, Onyx, Millennium (C/A, RF); Faith Davies: Acetylon, Millennium, Janssen, Celgene (C/A); Janssen, Celgene (H); Joan Bladé: Celgene, Janssen (C/A, H); Mario Boccadoro: Celgene, Janssen (ET); Michele Cavo: Janssen, Celgene, Bristol-Myers Squibb, Millennium (C/A, H); Michel Delforge: Celgene, Janssen (C/A); Meletios Dimopoulos: Celgene, Ortho-Biotech, Onyx (H); Hermann Einsele: Celgene, Janssen, Onyx, Novartis (C/A, H, RF); Thierry Facon: Celgene, Millennium, Amgen, Bristol-MyersSquibb (C/A); Janssen, Celgene (H); Hartmut Goldschmidt: Janssen, Celgene, Novartis, Onyx, Millennium (C/A); Janssen, Celgene, Novartis, Chugai, Onyx, Millennium (H); Janssen, Celgene, Novartis, Chugai (RF); Philippe Moreau: Celgene, Janssen, Millennium (C/A); Torben Plesner: Genmab, Janssen, Celgene (C/A); Janssen (RF); Jesús San-Miguel: Millennium, Celgene, Novartis, Onyx, Janssen, Bristol-Myers Squibb, MSD Sharp & Dohme (C/A); Roman Hajek: Merck, Celgene (C/A); Merck, Celgene, Janssen (H); Antonio Palumbo: Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium, Onyx (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia. 2013;28 doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turesson I, Velez R, Kristinsson SY, et al. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: A population-based study. J Clin Oncol. 2010;28:830–834. doi: 10.1200/JCO.2009.25.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venner CP, Connors JM, Sutherland HJ, et al. Novel agents improve survival of transplant patients with multiple myeloma including those with high-risk disease defined by early relapse (<12 months) Leuk Lymphoma. 2011;52:34–41. doi: 10.3109/10428194.2010.531409. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KC, Alsina M, Bensinger W, et al. Multiple myeloma, version 1.2013. J Natl Compr Canc Netw. 2013;11:11–17. doi: 10.6004/jnccn.2013.0004. [DOI] [PubMed] [Google Scholar]
- 7.Moreau P, San Miguel J, Ludwig H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi133–vi137. doi: 10.1093/annonc/mdt297. [DOI] [PubMed] [Google Scholar]
- 8a.Engelhardt M, Terpos E, Kleber M, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99:232–242. doi: 10.3324/haematol.2013.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Oxford Centre for Evidence-Based Medicine. Levels of Evidence (March 2009). Available at http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 18, 2014. [Google Scholar]
- 9.Moreau P, Cavo M, Sonneveld P, et al. Combination of International Scoring System 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.0329. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88:360–376. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28:269–277. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 12.Cavo M, Salwender H, Rosinol L, et al. Double vs. single autologous stem cell transplantation after bortezomib-based induction regimens for multiple myeloma: An integrated analysis of patient-level data from phase European III studies. Blood. 2013;122:767a. [Google Scholar]
- 13.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–66. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 14.Leleu X, Karlin L, Macro M, et al. Pomalidomide plus low-dose dexamethasone in relapsed or refractory multiple myeloma (RRMM) with deletion (del)17p and/or translocation t(4;14) Blood. 2013;122:689a. [Google Scholar]
- 15.Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson SK, Heuck CJ, Albino AP, et al. The use of molecular-based risk stratification and pharmacogenomics for outcome prediction and personalized therapeutic management of multiple myeloma. Int J Hematol. 2011;94:321–333. doi: 10.1007/s12185-011-0948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan G, Johnsen HE, Goldschmidt H, et al. MyelomA Genetics International Consortium. Leuk Lymphoma. 2012;53:796–800. doi: 10.3109/10428194.2011.639881. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser MF, Johnson DC, Wu P, et al. Global methylation analysis identifies prognostically important epigenetically inactivated tumor suppressor genes in multiple myeloma. Blood. 2013;122:219–226. doi: 10.1182/blood-2013-03-487884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa LJ, Zhang MJ, Zhong X, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 21.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem cell transplantation in newly diagnosed multiple myeloma: Results of the IFM 2005-01 phase 3 trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 22.Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: A randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 23.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: Results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 24.Sonneveld P, Goldschmidt H, Rosiñol L, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: A meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- 25.Nooka AK, Kaufman JL, Behera M, et al. Bortezomib-containing induction regimens in transplant-eligible myeloma patients: A meta-analysis of phase 3 randomized clinical trials. Cancer. 2013;119:4119–4128. doi: 10.1002/cncr.28325. [DOI] [PubMed] [Google Scholar]
- 26.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 27.Blanes M, Lahuerta JJ, González JD, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: A matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 2013;19:69–74. doi: 10.1016/j.bbmt.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Reece D, Song K, LeBlanc R, et al. Efficacy and safety of busulfan-based conditioning regimens for multiple myeloma. The Oncologist. 2013;18:611–618. doi: 10.1634/theoncologist.2012-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roussel M, Moreau P, Huynh A, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: A phase 2 study of the Intergroupe Francophone du Myelome (IFM) Blood. 2010;115:32–37. doi: 10.1182/blood-2009-06-229658. [DOI] [PubMed] [Google Scholar]
- 30.Doo NW, Thompson PA, Prince HM, et al. Bortezomib with high dose melphalan conditioning for autologous transplant is safe and effective in patients with heavily pretreated and high risk multiple myeloma. Leuk Lymphoma. 2013;54:1465–1472. doi: 10.3109/10428194.2012.746682. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto T, Yoshimoto G, Kamimura T, et al. Combination of high-dose melphalan and bortezomib as conditioning regimen for autologous peripheral blood stem cell transplantation in multiple myeloma. Int J Hematol. 2013;98:337–345. doi: 10.1007/s12185-013-1402-0. [DOI] [PubMed] [Google Scholar]
- 32.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9–19. doi: 10.1182/blood-2012-02-408898. [DOI] [PubMed] [Google Scholar]
- 33.Cavo M, Galli M, Pezzi A, et al. Persistent improvement in clinical outcomes with bortezomib-thalidomide-dexamethasone vs. thalidomide-dexamethasone incorporated into double autologous transplantation for multiple myeloma: An updated analysis of Phase 3 Gimema-MMY-3006 study. Blood. 2013;122:2090a. [Google Scholar]
- 34.Morgan GJ, Davies FE, Gregory WM, et al. Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19:6030–6038. doi: 10.1158/1078-0432.CCR-12-3211. [DOI] [PubMed] [Google Scholar]
- 35.Kalff A, Kennedy N, Smiley A, et al. Thalidomide consolidation post autologous stem cell transplant (ASCT) for multiple myeloma (MM) is cost-effective with durable survival benefit at 5 years post randomisation: Final analysis of the ALLG MM6 study. Blood. 2013;122:537a. [Google Scholar]
- 36.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma: Follow-up analysis of the IFM 2005-02 trial. Blood. 2013;122:406a. [Google Scholar]
- 37.McCarthy P, Owzar K, Hofmeister C, et al. Analysis of overall survival (OS) in the context of cross-over from placebo to lenalidomide and the incidence of second primary malignancies (SPM) in the phase III study of lenalidomide versus placebo maintenance therapy following autologous stem cell transplant (ASCT) for multiple myeloma (MM) CALGB (ALLIANCE) ECOG BMTCTN 100104. Clin Lymphoma Myeloma Leuk. 2013;13(suppl 1):S15-5a. [Google Scholar]
- 38.Gay F, Cavallo F, Caravita T, et al. Maintenance therapy with lenalidomide significantly improved survival of young newly diagnosed multiple myeloma patients. Blood. 2013;122:2089a. [Google Scholar]
- 39.Palumbo A, Cavallo F, Hardan I, et al. Melphalan/prednisone/lenalidomide (MPR) versus high-dose melphalan and autologous transplantation (MEL200) in newly diagnosed multiple myeloma (MM) patients <65 years: Results of a randomized phase III study. Blood. 2011;118:3069a. [Google Scholar]
- 40.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 41.Palumbo A, Bringhen S, Rajkumar V, et al. Second primary malignancies (SPM) in newly diagnosed myeloma (MM) patients treated with lenalidomide (Len): Meta-analysis of 6,383 individual patient data (IPD) J Clin Oncol. 2013;31(suppl):8517a. [Google Scholar]
- 42.Rosinol L, Oriol A, Teruel AI, et al. Maintenance therapy after stem-cell transplantation for multiple myeloma with bortezomib/thalidomide vs. thalidomide vs. alfa2b-interferon: Final results of a phase III pethema/GEM randomized trial. Blood. 2012;120:334a. [Google Scholar]
- 43.Sonneveld P, Scheid C, van der Holt B, et al. Bortezomib induction and maintenance treatment iImproves survival in patients with newly diagnosed multiple myeloma: Extended follow-up of the HOVON-65/GMMG-HD4 trial. Blood. 2013;122:404a. [Google Scholar]
- 44.Burnette BL, Dispenzieri A, Kumar S, et al. Treatment trade-offs in myeloma: A survey of consecutive patients about contemporary maintenance strategies. Cancer. 2013;119:4308–4315. doi: 10.1002/cncr.28340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordan K, Proskorovsky I, Lewis P, et al. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: Results of a European, multicenter cohort study. Support Care Cancer. 2013 doi: 10.1007/s00520-013-1991-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naumann-Winter F, Greb A, Borchmann P, et al. First-line tandem high-dose chemotherapy and autologous stem cell transplantation versus single high-dose chemotherapy and autologous stem cell transplantation in multiple myeloma, a systematic review of controlled studies. Cochrane Database Syst Rev. 2012;10:CD004626. doi: 10.1002/14651858.CD004626.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar SK, Lacy MQ, Dispenzieri A, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–1592. doi: 10.1002/cncr.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook G, Liakopoulou E, Pearce R, et al. Factors influencing the outcome of a second autologous stem cell transplant (ASCT) in relapsed multiple myeloma: A study from the British Society of Blood and Marrow Transplantation Registry. Biol Blood Marrow Transplant. 2011;17:1638–1645. doi: 10.1016/j.bbmt.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Jimenez-Zepeda VH, Mikhael J, Winter A, et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: Impact on progression-free and overall survival. Biol Blood Marrow Transplant. 2012;18:773–779. doi: 10.1016/j.bbmt.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 50.Lemieux E, Hulin C, Caillot D, et al. Autologous stem cell transplantation: An effective salvage therapy in multiple myeloma. Biol Blood Marrow Transplant. 2013;19:445–449. doi: 10.1016/j.bbmt.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Michaelis LC, Saad A, Zhong X, et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant. 2013;19:760–766. doi: 10.1016/j.bbmt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonsalves WI, Gertz MA, Lacy MQ, et al. Second auto-SCT for treatment of relapsed multiple myeloma. Bone Marrow Transplant. 2013;48:568–573. doi: 10.1038/bmt.2012.183. [DOI] [PubMed] [Google Scholar]
- 53.Sellner L, Heiss C, Benner A, et al. Autologous retransplantation for patients with recurrent multiple myeloma: A single-center experience with 200 patients. Cancer. 2013;119:2438–2446. doi: 10.1002/cncr.28104. [DOI] [PubMed] [Google Scholar]
- 54.Cook G, Williams C, Brown JM, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014 doi: 10.1016/S1470-2045(14)70245-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Lokhorst H, Einsele H, Vesole D, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521–4530. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- 56.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 57.Giaccone L, Storer B, Patriarca F, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood. 2011;117:6721–6727. doi: 10.1182/blood-2011-03-339945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Björkstrand B, Iacobelli S, Hegenbart U, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: Long-term follow-up. J Clin Oncol. 2011;29:3016–3022. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 59.Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 60.Moreau P, Garban F, Attal M, et al. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood. 2008;112:3914–3915. doi: 10.1182/blood-2008-07-168823. [DOI] [PubMed] [Google Scholar]
- 61.Rosiñol L, Pérez-Simón JA, Sureda A, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3593. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): A phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lokhorst HM, van der Holt B, Cornelissen JJ, et al. Donor versus no-donor comparison of newly diagnosed myeloma patients included in the HOVON-50 multiple myeloma study. Blood. 2012;119:6219–6225; quiz 6399. doi: 10.1182/blood-2011-11-393801. [DOI] [PubMed] [Google Scholar]
- 64.Gahrton G, Iacobelli S, Björkstrand B, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs. autologous transplantation in multiple myeloma: Long-term results of the EBMT-NMAM2000 study. Blood. 2013;121:5055–5063. doi: 10.1182/blood-2012-11-469452. [DOI] [PubMed] [Google Scholar]
- 65.Kharfan-Dabaja MA, Hamadani M, Reljic T, et al. Comparative efficacy of tandem autologous versus autologous followed by allogeneic hematopoietic cell transplantation in patients with newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. J Hematol Oncol. 2013;6:2. doi: 10.1186/1756-8722-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freytes CO, Vesole DH, LeRademacher J, et al. Second transplants for multiple myeloma relapsing after a previous autotransplant-reduced-intensity allogeneic vs. autologous transplantation. Bone Marrow Transplant. 2014;49:416–421. doi: 10.1038/bmt.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Einsele H, Liebisch P, Hebart H, et al. Allogeneic stem cell transplantation for high-risk multiple myeloma. Haematologica. 2011;96(suppl 1):S11. [Google Scholar]
- 68.Kröger N, Badbaran A, Zabelina T, et al. Impact of high-risk cytogenetics and achievement of molecular remission on long-term freedom from disease after autologous-allogeneic tandem transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2013;19:398–404. doi: 10.1016/j.bbmt.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 69.El-Cheikh J, Crocchiolo R, Furst S, et al. Lenalidomide plus donor-lymphocytes infusion after allogeneic stem-cell transplantation with reduced-intensity conditioning in patients with high-risk multiple myeloma. Exp Hematol. 2012;40:521–527. doi: 10.1016/j.exphem.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Caballero-Velázquez T, López-Corral L, Encinas C, et al. Phase II clinical trial for the evaluation of bortezomib within the reduced intensity conditioning regimen (RIC) and post-allogeneic transplantation for high-risk myeloma patients. Br J Haematol. 2013;162:474–482. doi: 10.1111/bjh.12410. [DOI] [PubMed] [Google Scholar]
- 71.Munshi NC, Anderson KC. Minimal residual disease in multiple myeloma. J Clin Oncol. 2013;31:2523–2526. doi: 10.1200/JCO.2013.49.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korthals M, Sehnke N, Kronenwett R, et al. Molecular monitoring of minimal residual disease in the peripheral blood of patients with multiple myeloma. Biol Blood Marrow Transplant. 2013;19:1109–1115. doi: 10.1016/j.bbmt.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 73.Martinez-Lopez J, Fernández-Redondo E, García-Sánz R, et al. Clinical applicability and prognostic significance of molecular response assessed by fluorescent-PCR of immunoglobulin genes in multiple myeloma. Results from a GEM/PETHEMA study. Br J Haematol. 2013;163:581–589. doi: 10.1111/bjh.12576. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Lopez J, Garcia-Sanz R, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease (MRD) detection in multiple myeloma. J Clin Oncol. 2013;31(suppl):8511a. [Google Scholar]
- 75.Paiva B, Vidriales MB, Cerveró J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017–4023. doi: 10.1182/blood-2008-05-159624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- 77.Paiva B, Gutiérrez NC, Rosiñol L, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119:687–691. doi: 10.1182/blood-2011-07-370460. [DOI] [PubMed] [Google Scholar]
- 78.Puig N, Sarasquete ME, Balanzategui A, et al. Critical evaluation of ASO RQ-PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia. 2014;28:391–397. doi: 10.1038/leu.2013.217. [DOI] [PubMed] [Google Scholar]
- 79.Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: Impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–2547. doi: 10.1200/JCO.2012.46.2119. [DOI] [PubMed] [Google Scholar]
- 80.Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–5995. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 81.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN) Blood. 2011;118:4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- 82.Palumbo A, Mina R. Management of older adults with multiple myeloma. Blood Rev. 2013;27:133–142. doi: 10.1016/j.blre.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 83.Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600. doi: 10.1200/JCO.2013.48.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guglielmelli T, Palumbo A. Incorporating novel agents in the management of elderly myeloma patients. Curr Hematol Malig Rep. 2013;8:261–269. doi: 10.1007/s11899-013-0177-y. [DOI] [PubMed] [Google Scholar]
- 85.Ludwig H, Avet-Loiseau H, Bladé J, et al. European perspective on multiple myeloma treatment strategies: Update following recent congresses. The Oncologist. 2012;17:592–606. doi: 10.1634/theoncologist.2011-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niesvizky R, Flinn I, Rifkin RM, et al. Efficacy and safety of three bortezomib-based induction and maintenance regimens in previously untreated, transplant-ineligible multiple myeloma (MM) patients (Pts): Final results from the randomized, phase 3b, US community-based UPFRONT study ( NCT00507416) Blood. 2013;122:1966a. [Google Scholar]
- 87.Larocca A, Cavallo F, Magarotto V, et al. Reduced dose-intensity subcutaneous bortezomib plus prednisone (VP) or plus cyclophosfamide (VCP) or plus melphalan (VMP) for newly diagnosed multiple myeloma patients older than 75 years of age. Blood. 2013;122:539a. [Google Scholar]
- 88.Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: Results of a phase II study. J Clin Oncol. 2010;28:4635–4641. doi: 10.1200/JCO.2010.28.1238. [DOI] [PubMed] [Google Scholar]
- 89.Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: A subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99:148–154. doi: 10.3324/haematol.2013.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delforge M, Terpos E, Richardson PG, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol. 2011;86:372–384. doi: 10.1111/j.1600-0609.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 91.Palumbo A, Magarotto V, Bringhen S, et al. A randomized phase 3 trial of melphalan-lenalidomide-prednisone (MPR) or cyclophosphamide-prednisone-lenalidomide (CPR) vs. lenalidomide plus dexamethsone (Rd) in elderly newly diagnosed multiple myeloma patients. Blood. 2013;122:536a. [Google Scholar]
- 92. doi: 10.1056/NEJMoa1402551. Benboubker L, Dimopoulos MA, Dispenzieri A et al. Lenalidomide and dexamethasone in transplant-ineligible myeloma patients. NEJM (in press) [DOI] [PubMed] [Google Scholar]
- 93.Mateos MV, Oriol A, Martínez-López J, et al. Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. Blood. 2012;120:2581–2588. doi: 10.1182/blood-2012-05-427815. [DOI] [PubMed] [Google Scholar]
- 94.Morgan GJ, Gregory WM, Davies FE, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119:7–15. doi: 10.1182/blood-2011-06-357038. [DOI] [PubMed] [Google Scholar]
- 95.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: Updated follow-up and improved survival. J Clin Oncol. 2014;32:634–640. doi: 10.1200/JCO.2013.52.0023. [DOI] [PubMed] [Google Scholar]
- 96.Ludwig H, Bolejack V, Crowley J, et al. Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol. 2010;28:1599–1605. doi: 10.1200/JCO.2009.25.2114. [DOI] [PubMed] [Google Scholar]
- 97.Nigam Y, Knight J, Bhattacharya S, et al. Physiological changes associated with aging and immobility. J Aging Res. 2012;2012:468469. doi: 10.1155/2012/468469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bataille R, Annweiler C, Beauchet O. Multiple myeloma international staging system: “Staging” or simply “aging” system? Clin Lymphoma Myeloma Leuk. 2013;13:635–637. doi: 10.1016/j.clml.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Gay F, Larocca A, Wijermans P, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood. 2011;117:3025–3031. doi: 10.1182/blood-2010-09-307645. [DOI] [PubMed] [Google Scholar]
- 100.Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: Meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98:980–987. doi: 10.3324/haematol.2012.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kleber M, Ihorst G, Gross B, et al. Validation of the Freiburg Comorbidity Index in 466 multiple myeloma patients and combination with the international staging system are highly predictive for outcome. Clin Lymphoma Myeloma Leuk. 2013;13:541–551. doi: 10.1016/j.clml.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 103.Palumbo A. Tailoring multiple myeloma therapy for special populations. Clin Lymphoma Myeloma Leuk. 2013;13(suppl 1):S1-4a. [Google Scholar]
- 104.Larocca A, Bringhen S, Evangelista A, et al. A simple score, based on geriatric assessment, improves prediction of survival, and risk of serious adverse events in elderly newly diagnosed multiple myeloma patients. Blood. 2013;122:687a. [Google Scholar]
- 105.Dispenzieri A, Stewart AK, Chanan-Khan A, et al. Smoldering multiple myeloma requiring treatment: Time for a new definition? Blood. 2013;122:4172–4181. doi: 10.1182/blood-2013-08-520890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tageja N, Manasanch EE, Korde N, et al. Smoldering multiple myeloma: Present position and potential promises. Eur J Haematol. 2014;92:1–12. doi: 10.1111/ejh.12205. [DOI] [PubMed] [Google Scholar]
- 107.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369:438–447. doi: 10.1056/NEJMoa1300439. [DOI] [PubMed] [Google Scholar]
- 108.Dhodapkar MV, Sexton R, Waheed S, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120) Blood. 2014;123:78–85. doi: 10.1182/blood-2013-07-515239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fernández de Larrea C, Jiménez R, Rosiñol L, et al. Pattern of relapse and progression after autologous SCT as upfront treatment for multiple myeloma. Bone Marrow Transplant. 2014;49:223–227. doi: 10.1038/bmt.2013.150. [DOI] [PubMed] [Google Scholar]
- 110.Knopf KB, Duh MS, Lafeuille MH, et al. Meta-analysis of the efficacy and safety of bortezomib retreatment in patients with multiple myeloma. Clin Lymphoma Myeloma Leuk. 2013;13(suppl 1):P-228a. doi: 10.1016/j.clml.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Dimopoulos MA, Petrucci MT, Foa R, et al. Analysis of second-line lenalidomide following initial relapse in the MM-015 Trial. Blood. 2012;120:944a. [Google Scholar]
- 112.Laubach JP, Mitsiades CS, Mahindra A, et al. Management of relapsed and relapsed/refractory multiple myeloma. J Natl Compr Canc Netw. 2011;9:1209–1216. doi: 10.6004/jnccn.2011.0098. [DOI] [PubMed] [Google Scholar]
- 113.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17:1264–1277. doi: 10.1158/1078-0432.CCR-10-1805. [DOI] [PubMed] [Google Scholar]
- 114.Jakubowiak A. Management strategies for relapsed/refractory multiple myeloma: Current clinical perspectives. Semin Hematol. 2012;49(suppl 1):S16–S32. doi: 10.1053/j.seminhematol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 115.Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123:1461–1469. doi: 10.1182/blood-2013-07-517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ludwig H, Kasparu H, Leitgeb C, et al. Bendamustine-bortezomib-dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood. 2014;123:985–991. doi: 10.1182/blood-2013-08-521468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawasut P, Chauhan D, Laubach J, et al. New proteasome inhibitors in myeloma. Curr Hematol Malig Rep. 2012;7:258–266. doi: 10.1007/s11899-012-0141-2. [DOI] [PubMed] [Google Scholar]
- 119.Moreau P. The future of therapy for relapsed/refractory multiple myeloma: Emerging agents and novel treatment strategies. Semin Hematol. 2012;49(suppl 1):S33–S46. doi: 10.1053/j.seminhematol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Moreau P, Richardson PG, Cavo M, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–959. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 122.Wang M, Martin T, Bensinger W, et al. Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122:3122–3128. doi: 10.1182/blood-2013-07-511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Richardson PG, Hofmeister CC, Rosenbaum CA, et al. Twice-weekly oral MLN9708 (ixazomib citrate), an investigational proteasome inhibitor, in combination with lenalidomide (Len) and dexamethasone (Dex) in patients (Pts) with newly diagnosed multiple myeloma (MM): Final phase 1 results and phase 2 data. Blood. 2013;122:535a. [Google Scholar]
- 125.Tai YT, Anderson KC. Antibody-based therapies in multiple myeloma. Bone Marrow Res. 2011;2011:924058. doi: 10.1155/2011/924058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lonial S, Kaufman J, Laubach J, et al. Elotuzumab: A novel anti-CS1 monoclonal antibody for the treatment of multiple myeloma. Expert Opin Biol Ther. 2013;13:1731–1740. doi: 10.1517/14712598.2013.847919. [DOI] [PubMed] [Google Scholar]
- 127.Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012;30:1953–1959. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 128.Richardson PG, Jagannath S, Moreau P, et al. Phase 2 study of elotuzumab plus lenalidomide/dexamethasone (Len/Dex) in relapsed/refractory multiple myeloma (RR MM) Clin Lymphoma Myeloma Leuk. 2013;13(suppl 1):S145a. [Google Scholar]
- 129.Plesner T, Lokhorst H, Gimsing P, et al. Daratumumab, a CD38 monoclonal antibody in patients with multiple myeloma (MM): Data from a dose-escalation phase I/II study. Clin Lymphoma Myeloma Leuk. 2013;13(suppl 1):P-130a. [Google Scholar]
- 130.Martin TG, Strickland SA, Glenn M, et al. SAR650984, a CD38 monoclonal antibody in patients with selected CD38+ hematological malignancies- Data from a dose-escalation phase I study. Blood. 2013;122:284a. [Google Scholar]
- 131.Endell J, Boxhammer R, Wurzenberger C, et al. The activity of MOR202, a fully human anti-CD38 antibody, is complemented by ADCP and is synergistically enhanced by lenalidomide in vitro and in vivo. Blood. 2012;120:4018a. [Google Scholar]
- 132.Witt O, Deubzer HE, Milde T, et al. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 133.Richardson PG, Mitsiades CS, Laubach JP, et al. Preclinical data and early clinical experience supporting the use of histone deacetylase inhibitors in multiple myeloma. Leuk Res. 2013;37:829–837. doi: 10.1016/j.leukres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 134.Dimopoulos M, Siegel DS, Lonial S, et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): A multicentre, randomised, double-blind study. Lancet Oncol. 2013;14:1129–1140. doi: 10.1016/S1470-2045(13)70398-X. [DOI] [PubMed] [Google Scholar]
- 135.San Miguel J, Hungria VT, Yoon SS, et al. Randomized, double blind, placebo-controlled phase 3 study of panobinostat or placebo with bortezomi and dexamethasone in relapsed and relapsed and refractory multiple myeloma (Panorama I). EHA 2014:3347a. [Google Scholar]
- 136.Minami J, Suzuki R, Mazitschek R, et al. Histone deacetylase 3 as a novel therapeutic target in multiple myeloma. Leukemia. 2014;28:680–689. doi: 10.1038/leu.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lonial S, Shah JJ, Zonder J, et al. Prolonged survival and improved response rates with ARRY-520 in relapsed/refractory multiple myeloma (RRMM) patients with low α-1 acid glycoprotein (AAG) levels: Results from a phase 2 study. Blood. 2013;122:285a. [Google Scholar]
- 138.Mateos MV, Cibeira MT, Richardson PG, et al. Phase II clinical and pharmacokinetic study of plitidepsin 3-hour infusion every two weeks alone or with dexamethasone in relapsed and refractory multiple myeloma. Clin Cancer Res. 2010;16:3260–3269. doi: 10.1158/1078-0432.CCR-10-0469. [DOI] [PubMed] [Google Scholar]
- 139.Fassas A, Shaughnessy J, Barlogie B. Cure of myeloma: Hype or reality? Bone Marrow Transplant. 2005;35:215–224. doi: 10.1038/sj.bmt.1704757. [DOI] [PubMed] [Google Scholar]
- 140.Barlogie B, Shaughnessy JD., Jr Genomics and cure in multiple myeloma: Oral presentation at IMW 2011 during session on “High-risk entities of myeloma: From biology to treatment.”. Haematologica. 2011;96(suppl 1):S10. [Google Scholar]
- 141.San-Miguel JF, Mateos MV. Can multiple myeloma become a curable disease? Haematologica. 2011;96:1246–1248. doi: 10.3324/haematol.2011.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]