Despite the historically limited role of radiotherapy in the management of primary hepatic malignancies, modern advances in treatment design and delivery have renewed enthusiasm for radiation as a potentially curative treatment modality. This review aims to summarize modern advances in radiotherapy, particularly stereotactic body radiation therapy, in the treatment of primary hepatic malignancies.
Keywords: Hepatobiliary cancer, Stereotactic body radiation therapy, Radiation therapy, Cholangiocarcinoma, Hepatocellular carcinoma
Abstract
Despite the historically limited role of radiotherapy in the management of primary hepatic malignancies, modern advances in treatment design and delivery have renewed enthusiasm for radiation as a potentially curative treatment modality. Surgical resection and/or liver transplantation are traditionally regarded as the most effective forms of therapy, although the majority of patients with hepatocellular carcinoma and intrahepatic cholangiocarcinoma present with locally advanced or unresectable disease on the basis of local vascular invasion or inadequate baseline hepatobiliary function. In this context, many efforts have focused on nonoperative treatment approaches including novel systemic therapies, transarterial chemoembolization, ethanol ablation, radiofrequency ablation, and stereotactic body radiation therapy (SBRT). This review aims to summarize modern advances in radiotherapy, particularly SBRT, in the treatment of primary hepatic malignancies.
Abstract
摘要
尽管历史上放射疗法在原发性肝脏恶性肿瘤治疗中的作用有限,但其在现代治疗设计和实施上的进展重新激起了将放疗作为一种潜在根治性治疗方式的热情。手术切除和/或肝脏移植在传统上被视为最有效的治疗形式,然而根据局部血管浸润或基线肝胆功能不佳,大多数肝细胞癌和肝内胆管癌患者患有局部晚期或不可切除性疾病。在这种背景下,许多治疗努力偏重于非手术疗法,包括新型全身疗法、经动脉化疗栓塞、乙醇消融、射频消融和体部立体定向放射疗法 (SBRT)。本综述旨在总结现代放疗(特别是 SBRT)在原发性肝脏恶性肿瘤治疗方面的进展。 (The Oncologist) 2014;19:868–879
Implications for Practice:
Radiation therapy has historically served a limited role in the management of primary hepatic malignancies. Modern advances in treatment design and delivery have renewed enthusiasm for radiation as a potentially curative treatment modality. In particular, stereotactic body radiation therapy (SBRT) has emerged as a feasible and noninvasive treatment for patients with locally advanced or medically unresectable primary hepatic malignancies including hepatocellular carcinoma and intrahepatic cholangiocarcinoma. This review aims to summarize modern advances in radiotherapy, particularly SBRT, in the treatment of primary hepatic malignancies and to illustrate future areas of investigation.
Challenges of Liver Irradiation
The delivery of curative doses of radiation therapy (RT) for hepatic malignancies has traditionally been limited by the intrinsic tolerance of the liver and the occurrence of radiation-induced liver disease (RILD). RILD, or radiation hepatitis, is classically defined as a triad of anicteric hepatomegaly, ascites, and elevated alkaline phosphatase out of proportion to transaminitis or bilirubinemia and occurs between 2 weeks and 4 months after completion of RT. In a historic study of 12 patients who received liver RT to 3,000–5,900 cGy at Stanford University, pathologic hallmarks of RILD included veno-occlusive injury with fibrin deposition in central veins [1]. This process was occasionally fatal but appeared to resolve spontaneously among patients surviving more than 4 months after treatment. Modern studies have implicated transforming growth factor-β (TGF-β) in the stimulation of fibroblast migration and development of liver fibrosis [2, 3].
Historically, liver irradiation required large treatment fields, often encompassing the whole liver to account for early limitations in imaging and treatment delivery. In this context, in 1965 Ingold et al. published the first report of dose-limiting toxicity after whole-liver RT, demonstrating a 44% risk of RILD among patients treated with ≥35 Gy [4]. A subsequent dose-escalation study by the Radiation Therapy Oncology Group (RTOG), RTOG 84-05, evaluated whole-liver RT for patients with liver metastases and found rates of late liver injury among patients treated with 27–30 Gy and 33 Gy (1.5 Gy/fraction) of 0% and 10%, respectively, although the contribution from underlying cirrhotic progression and decompensation is unknown [5]. On the basis of these early studies, whole-liver RT was deemed unsafe at relatively low doses (i.e., 33 Gy in fractions of 1.5 Gy), far below doses needed for adequate tumor control. Consequently, early interest in whole-liver RT as a primary treatment modality for hepatic malignancies waned.
Technical Advances in Liver Irradiation
Three-Dimensional Conformal Radiation Therapy
Several modern advances in treatment planning have reinvigorated interest in liver-directed RT. The advent of three-dimensional conformal radiation therapy (3D-CRT), for example, introduced planning tools capable of selectively treating liver tumors while minimizing dose to uninvolved portions of the liver. Furthermore, 3D-CRT enabled a quantitative assessment of the relationships among dose, treatment volume, and risk of toxicity [6]. The ability to conform RT to prespecified targets led many to revisit the idea of delivering higher, tumoricidal doses of radiation while sparing normal hepatic tissue. In an effort to better quantify the relationship between dose and irradiated volumes and the probability of toxicity, the report by Emami et al. in 1991 outlined projected tolerance doses for partial liver irradiation [7]. Specifically, this report established TD 5/5 (the tolerated dose associated with a 5% complication risk at 5 years) to be 50 Gy, 35 Gy, and 30 Gy for irradiation of one third of the liver, two thirds of the liver, and the whole liver, respectively. TD 50/5 (the tolerated dose associated with a 50% complication rate at 5 years) was described as 55 Gy, 45 Gy, and 40Gy for the same volumes. A dose-escalation protocol at the University of Michigan with hyperfractionated RT and concurrent hepatic arterial fluorodeoxyuridine for patients with liver metastases tailored prescription doses to the percentage of normal liver treated. This protocol treated to 66 Gy when <33% of the normal liver received >50% of the prescribed dose and to 48 Gy when 33%–66% of the normal liver received >50% of the prescribed dose, with successive dose escalations in 10% increments. No patients who received partial-liver RT alone developed RILD, and outcomes included objective response rates of 50% and a median survival of 20 months [8].
Using this understanding, these investigators developed a normal tissue complication probability (NTCP) model designed to individualize prescription dose without exceeding a 10%–15% risk of RILD, as assessed by liver dosimetry on 3D planning [9–11]. A subsequent phase II study at the University of Michigan used this NTCP model to deliver conformal hyperfractionated RT with concurrent hepatic arterial fluorodeoxyuridine for 128 patients with liver tumors (37% with liver metastases, 28% with hepatocellular carcinoma, and 35% with cholangiocarcinoma) [6, 12]. This study reported median survival of 15.8 months and 3-year survival of 17%, both significantly superior to historical controls. Tumor dose was the only significant predictor for survival on multivariate analysis: doses ≥75 Gy were associated with higher overall survival (23.9 months vs. 14.9 months, p < .01). Patients with focal tumors had a 2-year actuarial freedom from hepatic progression of 72%. This study used separate models for patients with primary hepatobiliary versus metastatic tumors to account for the lower tolerance of the liver to radiation in primary hepatobiliary malignancies related to preexisting hepatic parenchymal disease. In addition, primary hepatobiliary tumors recurred primarily locally, illustrating the importance of local therapy for these tumors. These data demonstrated not only the feasibility of 3D-CRT but also the importance of intensified local therapy and dose response in liver-directed RT.
The feasibility of this approach in Child-Pugh (CP) class A and B cirrhotic patients was further explored through a multicenter prospective phase II RTF-1 trial in Lyon, France, evaluating 25 assessable patients with hepatocellular carcinoma (HCC) ≤5 cm for single nodules and ≤3 cm for 2 nodules treated with dose-escalated 3D-CRT to 66 Gy (2 Gy/fraction) [13]. This study reported high rates of complete response (80%), partial response (12%), and stable disease (8%) with sustained rates of local control (78%) at a median follow-up of 29 months. Grade 4 toxicities occurred only in CP class B patients (22%), all of whom had pre-existing grade 3 abnormalities prior to radiation. Among CP class A patients, 19% developed asymptomatic grade 3 toxicity, and none developed grade 4 toxicity.
Multiple retrospective series on liver-directed 3D-CRT have been published from Asia. The largest of these series is from South Korea and includes 398 patients with HCC treated at 10 centers, primarily with 3D-CRT (81.9%) to doses ≥45 Gy. This study reported 1-year overall survival of 45% without any observed grade 3 or higher toxicity [14]. Like the University of Michigan study, this study also identified radiation dose as a significant predictor for overall survival on multivariate analysis, with better outcomes seen for patients treated with a biological effective dose ≥53.1 Gy. However, this dose relationship may be confounded by the use of higher doses for smaller tumors, as would be expected by the NTCP model.
Taken together, these studies have demonstrated promising results with the use of 3D-CRT for liver-directed RT. By minimizing dose to uninvolved liver, this technique has allowed for safe and effective escalation of doses far beyond the conventional dose limitations of whole-liver irradiation. Although these studies were small and limited in design, these data greatly renewed interest in liver directed RT for localized hepatic malignancies.
By minimizing dose to uninvolved liver, [3D-CRT] has allowed for safe and effective escalation of doses far beyond the conventional dose limitations of whole-liver irradiation.
Stereotactic Body Radiation Therapy
More recently, the development of stereotactic body radiotherapy (SBRT) has generated further promise for liver-directed RT. SBRT is a technique that delivers high doses of RT in a single treatment or in a small number of fractions with high precision, thereby minimizing RT doses to adjacent normal tissues [15].
Standard fractionated radiotherapy is believed to rely on double-stranded DNA breakage to produce gradual cell death; in contrast, SBRT may use additional mechanisms such as vascular injury, resulting in a more ablative effect [16–19]. The relationship between radiation dose and cell death has historically been modeled using the linear-quadratic or α-β model, although these data suggest the potential to underestimate the degree of cell damage after SBRT using these models [20]. In light of these conflicting data, the radiobiological equivalence of SBRT remains highly controversial and requires further investigation. In practice, common doses of SBRT include 18–30 Gy in 1 fraction or 30–60 Gy in 3–5 fractions [21].
Despite longstanding experience with similar techniques such as stereotactic radiosurgery (SRS) for intracranial tumors with Gamma Knife technology, developed as early as 1951 [22], several technological advances were necessary before SBRT could be applied successfully to abdominal tumors. Specifically, such a precise system must account for the uncertainties of tumor motion, immobilize and localize the target consistently, and use a delivery system capable of creating highly conformal radiation.
With regard to tumor motion, the development of four-dimensional computed tomography (4D-CT) scanning illustrated the multitude of effects on abdominal tumors by respiratory motion and by adjacent viscous organs such as the stomach and bowels [23–25]. Several studies have characterized this range of liver motion using ultrasound, magnetic resonance imaging, breath-hold CT, or scintigraphy as ranging between 8 mm and 25 mm during normal breathing and between 37 mm and 55 mm on deep breathing [25–29]. 4D-CT, abdominal compression, and active breathing control (ABC) are some of the many techniques designed to account for this uncertainty. 4D-CT imaging involves the acquisition of multiple CT sets at various points throughout the respiratory cycle, which can be reconstructed to define the entire range of tumor motion during respiration [23]. This range of tumor motion can be used either to generate internal target volumes encompassing this range of motion or to dictate the subsequent need for additional motion-management strategies. Abdominal compression aims to reduce tumor motion altogether using devices that increase intra-abdominal pressure to limit diaphragmatic respiratory motion. Abdominal compression was initially described by the Karolinska Institute in Sweden to reduce organ motion to 5–10 mm [30], and subsequent studies including that of University of Texas Southwestern have illustrated 40% and 50% reductions in craniocaudal tumor motion with medium and high compression, respectively, possibly at the expense of left-right motion [31, 32]. Although this compression may theoretically deform the liver shape in unpredictable ways, investigators at Princess Margaret Hospital (PMH) in Toronto, Canada, have demonstrated that interfraction deformations tend to be small (<5 mm) and focal, based on registration of daily 4D cone-beam CTs [33, 34]. These data illustrate the reliability of a given treatment plan across multiple fractions using abdominal compression. ABC is a method that aims to deliver radiation only during predefined phases of a given patient’s respiratory cycle. ABC requires a degree of patient instruction to control respiration, as well as respiratory gating based on computerized video tracking to deliver radiation at only the appropriate times. Despite concerns about feasibility of coached breathing with patient comorbidity, early efforts showed excellent reproducibility (1- to 4-mm excursions) of ABC between successive fractions [35, 36]. Contemporary reports of SBRT with ABC showed image guidance and repositioning to reduce average absolute systemic errors from 4.1 mm to 1.1 mm craniocaudal, from 2.4 mm to 1.3 mm anterior-posterior, and from 3.1 mm to 1.6 mm medial-lateral, with an average intra- and interfraction craniocaudal offset of diaphragm position relative to vertebral bodies of 1.7 mm and 3.7 mm, respectively [37, 38].
SBRT further requires reproducible and accurate target localization, which has been accomplished through several systems in use today. Traditionally, SBRT included a body frame to effectively coordinate points in a patient with points in stereotactic space, as used in the Leksell Coordinate Frame G (Elekta, Stockholm, Sweden, http://www.elekta.com), Gamma Knife (Extend frame system; Elekta), and linear accelerator-based systems. A patient would be immobilized within this space using the frame and other devices such as vacuum cushions and thermal plastic restraints [39]. Alternatively, frameless SBRT systems as used in the BodyFIX 14 system (Elekta) and CyberKnife system (Accuray, Sunnyvale, CA, http://www.accuray.com) [40] have relied on surrogate structures such as spine, skull, or implanted fiducial markers for target localization [41, 42]. Respiratory motion of the liver unfortunately precludes reliable use of bony landmarks for image-guided radiation therapy (IGRT), and either inserted fiducial markers (including surgical clips or Lipiodol from prior transarterial chemoembolization [TACE] cavity) or soft tissue surrogates for the tumor (e.g., whole liver or liver dome contours on cone-beam CT) are needed instead. Complementary to IGRT is intrafraction monitoring during SBRT, which may be done with newer techniques including trackable electromagnetic transponders to ensure sustained accurate positioning and infrared emitters to record motion at the skin surface [43].
SBRT also requires a delivery system capable of creating highly conformal radiation. Photon-based RT accomplishes this end by using multiple variable-intensity, low-dose beamlets delivered from different beam angles or during rotation of the beams to create a summation of dosage at the tumor with lower doses surrounding the target. This process has been largely optimized by more efficient computing systems and the use of automated optimization as part of intensity-modulated radiation therapy (IMRT). Using these systems, a higher number of beamlets (or plan complexity) often affords higher target conformity of dose. Alternatively, charged particle-based RT such as proton-beam and carbon ion-beam therapy can allow for highly conformal planning with fewer beams and maximal normal tissue sparing, given the unique physical property of charged particles to deposit dose in tissues abruptly with a sharp peak known as a “Bragg peak” [44]. However, the more complex the plans (with either IMRT or ion therapy), the more sensitive plans are to motion and uncertainties, emphasizing the importance of breathing motion management and IGRT to ensure that the delivered doses are as close to the planned doses as possible.
These advances in accounting for tumor motion, target localization, and treatment delivery systems have allowed SBRT to create highly conformal, dose-escalated, and ablative treatment for primary hepatic malignancies. In this way, SBRT has offered a new and promising form of noninvasive and definitive treatment in this setting.
These advances in accounting for tumor motion, target localization, and treatment delivery systems have allowed SBRT to create highly conformal, dose-escalated, and ablative treatment for primary hepatic malignancies. In this way, SBRT has offered a new and promising form of noninvasive and definitive treatment in this setting.
Outcomes of SBRT in Primary Hepatic Malignancies
Liver-directed SBRT was first reported at the Karolinska Hospital by Blomgren et al. in 1995 as part of a pilot experience with extracranial SBRT [45]. This study included 9 patients with primary liver tumors, 8 with HCC, and 1 with intrahepatic cholangiocarcinoma (ICC), treated with 16–66 Gy in 1–3 fractions, with impressive objective response rates (70%). A contemporary phase I study at PMH by Tse et al. similarly demonstrated the efficacy of SBRT in patients with unresectable HCC (n = 31) and ICC (n = 10) [46], with RT doses individualized based on the NTCP model described above [9–11]. Doses were escalated in 3 predefined toxicity strata of 5%, 10%, and 20% to a median dose of 36 Gy (range: 24–54 Gy) in 6 fractions. Using this schema, investigators observed no incidence of RILD or treatment-related early grade ≥4 toxicity within 3 months after SRBT, and maximum tolerated dose was not achieved. Overall, 17% of patients demonstrated a decline in liver function from CP class A to class B within 3 months of SBRT, despite favorable baseline liver function (CP class A with >800 mL of uninvolved liver); however, the expected contribution from the progression of cirrhosis was not known. Median survival for all patients was 13.4 months, and rates of survival and local control at 1 year were 51% and 65%, respectively. These early experiences were highly encouraging for liver-directed SBRT and set the stage for subsequent prospective and retrospective investigations, as described below (Tables 1–3).
Table 1.
Stereotactic body radiotherapy outcomes for hepatocellular carcinoma

Table 3.
Liver-directed radiation outcomes for intrahepatic cholangiocarcinoma

SBRT for Hepatocellular Carcinoma
HCC is the third-leading cause of cancer-related death worldwide, resulting in approximately 700,000 deaths per year and endemic to portions of east and southeast Asia and middle and western Africa [47]. Chronic infection with hepatitis B virus accounts for the majority of HCC incidence, although rising rates of hepatitis C virus infection, obesity, and nonalcoholic fatty liver disease and alcohol-related cirrhosis may account for rising HCC incidence worldwide and in the U.S. [48, 49]. The majority of patients present with advanced disease, with median survival after diagnosis of 6–20 months and long-term survival limited to patients with early stage tumors [50]. Surgical resection with partial hepatectomy and orthotopic liver transplantation (OLT) remain the mainstay of curative therapy. OLT is primarily limited by donor liver availability, and most patients are ineligible for resection due to local tumor extent, vascular invasion, and/or underlying liver dysfunction. Consequently, several treatments have emerged as alternatives to surgery including radiofrequency ablation (RFA) and microwave ablation, percutaneous ethanol injection (PEI) or acetic acid ablation, TACE, radioembolization, cryoablation, systemic chemotherapy and molecularly targeted therapies, and SBRT.
Following the 2008 study by Tse et al., SBRT emerged as a promising alternative to surgery, and many centers have since reported on their SBRT experiences in HCC (Table 1). Perhaps the most robust is the recently updated phase I/II experience at PMH by Bujold et al., reporting on 102 patients with locally advanced HCC unsuitable for alternative locoregional treatment [51]. Similar to the earlier study by Tse et al., all patients had favorable baseline liver function (CP class A with >700 mL of uninvolved liver) and were treated to a mean dose of 36 Gy (range: 24-54 Gy) using the NTCP model, delivered in six fractions every other day over 2 weeks. This study included many patients with advanced disease, with tumor vascular thrombosis (TVT) present in 55% and tumor size ranging from 1.4 cm to 23.1 cm. Nonetheless, results were strikingly impressive, with 1-year local control and overall survival rates of 87% and 55%, respectively, and median survival of 17 months. In particular, complete radiographic response was observed in 11% and partial response was observed in 43%. Table 1 summarizes several recent SBRT studies for HCC, demonstrating 1-year local control rates ranging from 64% to 100% and 1-year survival rates from 48% to 100% [46, 51–69]. Differences between studies may be largely related to differences in patient characteristics such as CP class, tumor size or stage, and proportion of patients with TVT. For example, 2 centers in Japan have recently published impressive results with SBRT for early stage tumors (1–5 cm), with 1-year survival rates between 99% and 100% [56, 61]; these optimistic results have propelled ongoing prospective phase II studies at these centers. Other such studies have attempted to identify treatment characteristics such as SBRT dose to be prognostic for survival on multivariable analysis [58].
There are no prospective randomized studies to date comparing the relative efficacy of SBRT with other treatments for HCC such as surgery or TACE. In a retrospective series from Tianjin Medical University in China, 26 patients who underwent microscopic gross total resection (R0) were compared with 22 patients treated with CyberKnife SBRT for stage I HCC [62]. This series showed no significant difference in 3-year survival rates with surgery versus SBRT (69.2% vs. 57.1%, p = .49), although this study was nonrandomized and included a small number of patients. Another retrospective matched-pair analysis of patients with unresectable, recurrent HCC from Taiwan compared outcomes of 36 patients who were treated with CyberKnife SBRT with 138 matched patients who received other or no treatments (“no SBRT”) [63]. Two-year survival rates were superior for those undergoing SBRT versus no SBRT (72.6% vs. 42.1%, p = .013), and multivariable analysis showed treatment with SBRT, tumor size, stage at recurrence, and CP class as independent prognostic factors for survival.
Tumor Vascular Thrombosis
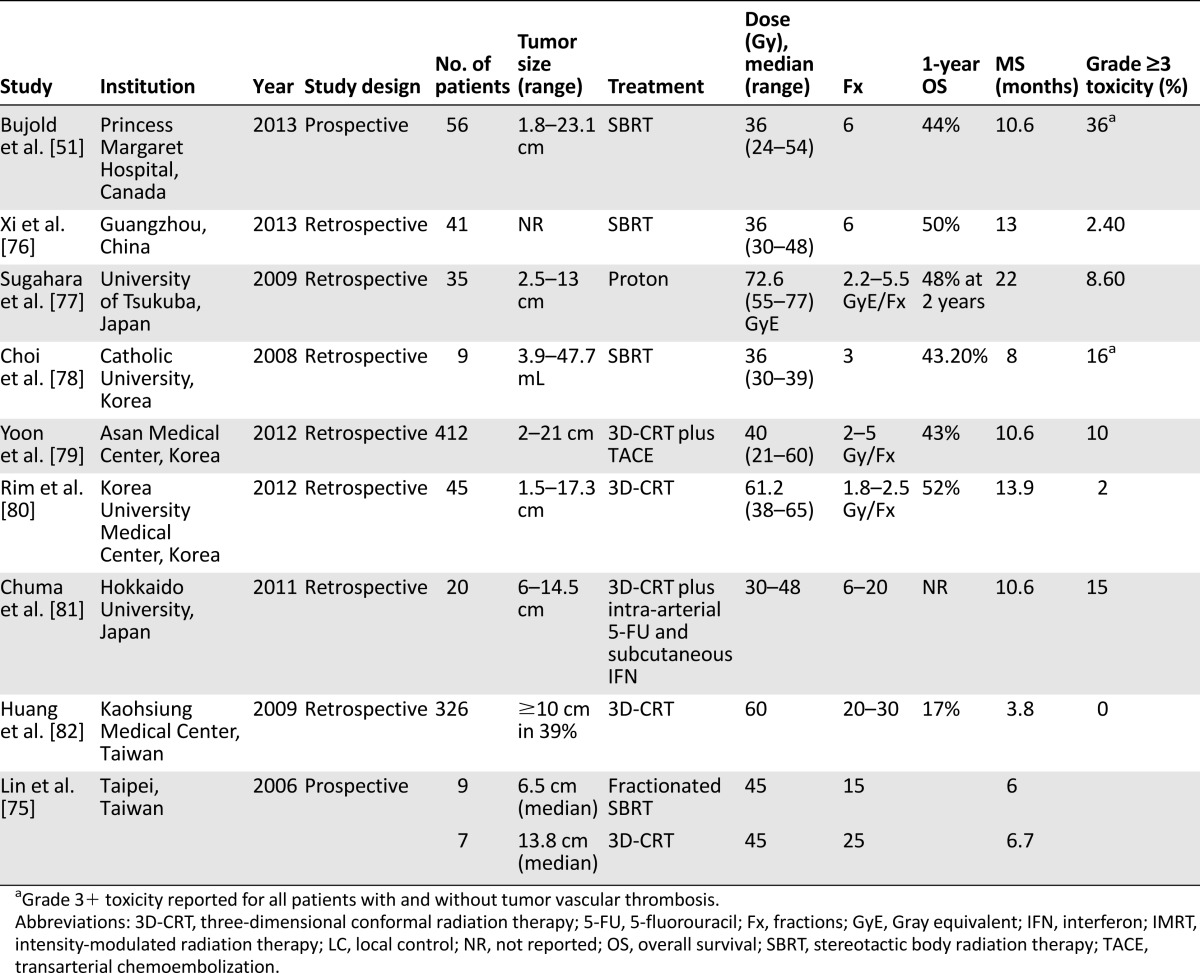
Novel uses of SBRT have also been investigated in certain subgroups of patients with HCC. For example, TVT, involving the portal or hepatic veins, represents a subset of patients with advanced HCC with a particularly poor prognosis. Unfortunately, patients with TVT are ineligible for many standard treatments such as surgery, PEI, and RFA, particularly when the thrombus affects hilar or major portal veins; untreated patients with TVT have median survival of only 2–4 months [70]. TACE has some efficacy in this setting, but the efficacy has never been definitively demonstrated in randomized prospective studies. Furthermore, it is often limited by treatment-related ischemic injury and risk of liver failure [71–73]. Consequently, investigators explored liver-directed radiation with or without TACE as a means of portal venous recanalization. Radiation to portal venous tumor thrombus was first reported by Takagi et al. in 1989 and achieved a histologic and/or angiographic response in 2 of 7 patients (29%) [74]. A subsequent prospective study from Taiwan similarly reported a 71% rate of partial venous recanalization as seen on CT with contrast following fractionated stereotactic RT or 3D-CRT among 16 evaluable patients [75]. Other series have since emerged describing outcomes of liver-directed RT for TVT, with reported median survival times ranging between 3.8 months and 22 months (Table 2) [51, 75–82]. The most favorable outcomes were seen in a series by the University of Tsukuba, Japan, that described median survival of 22 months and median local progression-free survival of 21 months after fractionated proton radiation to a median dose of 72.6 Gy equivalent (GyE) in 2.2- to 5.5-GyE fractions [77]. Response rates after SBRT to a median dose of 36 Gy (range: 30-48) in 6 fractions targeting the tumor thrombus have been reported by Xi et al. to produce rates of complete response, partial response, stable disease, and progressive disease of 36%, 39%, 17%, and 7%, respectively [76]. The largest series of SBRT for TVT draws from the prospective study by Bujold et al. described above, in which 56 patients with TVT were treated to a median dose of 36 Gy (range: 24–54 Gy) in six fractions [51]. These patients experienced 1-year overall survival of 44% and median survival of 10.6 months, with TVT emerging on multivariable analysis as the strongest adverse prognostic factor for survival (hazard ratio: 2.47 [95% confidence interval: 1.25–4.88], p = .01). Despite limited numbers, these data have presented SBRT as a potential therapy with or without TACE for patients with advanced HCC and TVT.
Table 2.
Liver-directed radiation outcomes for hepatocellular carcinoma with tumor vascular thrombosis
SBRT Bridge to Liver Transplantation
OLT is a potentially curative treatment option for some patients with advanced HCC, particularly those within the Milan criteria [83]. Given the limited availability of donor organs, up to 20% of patients with advanced HCC experience progressive disease while on a waitlist for OLT; therefore, local therapy may be used to “bridge” patients until a donor organ is available. Although RFA and TACE have traditionally served as the primary bridging treatment modalities, liver-directed RT has emerged as a new option in this setting. A series from PMH has recently reported on 10 patients with disease refractory to or ineligible for other therapies who were treated with 3D-CRT as a bridge to OLT [84]. Patients received a median dose of 33 Gy (range: 8.5–54 Gy) over 1–6 fractions, resulting in 100% local control and tumor volume regression of 10%–50%. Among the 5 patients who underwent OLT, tumors showed treatment effect with 40%–90% necrosis and fibrosis, and all transplanted patients were without recurrence at a median follow-up of 14 months. Other studies have described outcomes after SBRT as a bridging therapy. A series from Mount Sinai University of 27 patients treated with SBRT (26–36 Gy in 2–4 fractions) demonstrated complete response in 14%, partial response in 23%, and stable disease in 63% [85]. O’Connor et al. published a similar series from Baylor Medical Center with a 27% rate of pathologic complete response in explanted tumors [86]. In this context, 3D-CRT and SBRT may be safe and effective to bridge some patients with advanced HCC to OLT.
Charged Particle Therapy
The role of charged particle-based RT in the treatment of HCC is also an area of active investigation. As described above, charged particle therapy, such as proton-beam and carbon ion-beam therapy, may offer distinct dosimetric and physical advantages including the ability to minimize radiation exposure of normal liver tissue. Regarding proton radiotherapy, the largest series has been published by the University of Tsukuba including 318 patients with HCC and primarily CP class A treated with fractionated doses adjusted for anatomic considerations [87]. RT prescriptions included 77.0 GyE in 35 fractions for tumors within 2 cm of digestive organs, 72.6 GyE in 22 fractions for tumors within 2 cm of the porta hepatis, and 66 GyE in 10 fractions for peripheral tumors >2 cm away from digestive organs and the porta hepatis. The 5-year overall survival rate was 44.6%, with only 5 patients developing grade 3 toxicities (1 gastrointestinal, 4 skin). Nearly 20% of patients received some form of additional proton RT for either synchronous tumors or salvage, with 5-year survival of 51%. A more recent phase II prospective study reported on 76 patients treated with proton RT for HCC with cirrhosis, with 63 GyE in 15 fractions over 3 weeks [88]. This study included a significant number of patients with advanced stage and decompensated liver disease, with 54% outside of the Milan criteria, 24% with CP class C, and 16% with Model For End-Stage Liver Disease score >15. Despite otherwise poor prognosis for this cohort of patients, Bush et al. reported median progression-free survival of 3 years and a local failure rate of 20% occurring at an average of 18 months (range: 2–60 months). Furthermore, this treatment was very well tolerated, with no reported grade ≥3 toxicities.
Data with carbon ion therapy for HCC are more limited, although a prospective study by Kato et al. in Japan has shown promising results [89]. This dose escalation protocol treated 24 patients with doses between 49.5 GyE and 79.5 GyE in 15 fractions, resulting in 5-year local control and survival rates of 81% and 25% with no grade >3 toxicities. Based on these results, the authors recommended a dose of 72 GyE in 15 fractions as the minimum dose required for the greatest local control rate. A more recent phase I study from the University of Heidelberg treated 6 patients with HCC with carbon ion therapy (40 GyE in 4 fractions) with 100% local control at median follow-up of 11 months (4 lesions with partial response and 3 lesions with stable disease) [90]. One retrospective analysis of 343 consecutive patients treated with either proton radiotherapy (n = 242) or carbon ion therapy (n = 101) in Kobe, Japan, showed nearly equivalent outcomes after proton versus carbon ion therapy, including 5-year overall survival (90.2% vs. 93%) and local control (38% vs. 36.3%). This study showed only seven grade 3 toxicities, including panniculitis, biloma, upper gastrointestinal ulcer, and transaminitis.
These limited data have suggested a potential role for charged particle therapy in the treatment of primary hepatic malignancies. Nonetheless, the relative efficacy of these therapies versus conventional photon SBRT is unknown, and future prospective clinical studies and cost-effective analyses will be necessary before widespread use of these treatment modalities.
Cholangiocarcinoma
ICC is a primary hepatic malignancy with a particularly poor prognosis, with 5-year survival rates of 5%–10%. As in HCC, surgical resection is the only known curative treatment option for ICC, although up to 50%–90% of patients present with unresectable disease due to regional nodal metastasis, vascular invasion, or involvement of adjacent organs [91]. Radiation therapy can theoretically improve local control and survival for unresectable patients, although the role of RT in this setting is less well characterized. Regarding ICC, a phase II study of conformal hyperfractionated radiation therapy with concurrent hepatic arterial fluorodeoxyuridine included 46 patients with ICC. Median survival among these patients was 13.3 months and significantly improved compared with historical controls [6, 12]. Table 3 summarizes several other recent studies of RT for ICC [12, 45, 46, 55, 69, 92–94]. In a notable series from the Mayo Clinic, Barney et al. reported on 10 patients with unresectable or recurrent ICC receiving a median dose of 55 Gy (range: 45–60 Gy) in 3–5 fractions [93]. These patients demonstrated impressive rates of 1-year local control and survival of 100% and 73%, respectively, albeit with 2 significant late toxicities, including one patient with grade 3 biliary stenosis and another with grade 5 liver failure. In this context, among carefully selected patients, SBRT may prove increasingly important in the treatment of unresectable ICC, although additional prospective studies are needed.
Response Assessment
Assessment of tumor response after SBRT can be extremely challenging and a major limitation of many of the cited studies, particularly with regard to HCC. The Response Evaluation Criteria in Solid Tumors (RECIST) on axial imaging has been used by most prospective studies to evaluate tumor response [95]; meanwhile, the European Association for the Study of the Liver (EASL) issued criteria for treatment effect based on tumor nonenhancement by spiral CT as a surrogate for tumor necrosis [96]. Price et al. from Indiana University recently evaluated these competing response criteria in 26 patients with HCC treated with SBRT in a phase I or II study [97]. Eligible patients had solitary tumors ≤6 cm or up to 3 lesions with the sum of the diameters ≤6 cm and well-compensated cirrhosis. Patients received SBRT to a median dose of 42 Gy (range: 24–48 Gy) with follow-up imaging every 3–6 months after treatment and were subject to evaluation by RECIST or EASL criteria. At a median follow-up of 13 months, mean tumor shrinkage at 3 months, 6 months, 9 months, and 12 months was, respectively, 35%, 37%, 48%, and 55% by RECIST criteria, whereas mean percentage of necrosis during the same intervals was 59%, 69%, 81%, and 92% by EASL criteria. Given the higher percent of necrosis over tumor shrinkage at each time point, the authors concluded that nonenhancement served as a more useful indicator of treatment response after SBRT. The kinetics of this response are still incompletely characterized, with a recent study from Hiroshima University in Japan demonstrating different patterns of early and late enhancement among patients treated with TACE and SBRT [98]. Among 36 patients with residual arterial enhancement at an early follow-up of 3 months, only 2 patients did not demonstrate remission >6 months following SBRT, emphasizing that the time to maximal response or tumor ablation may be long and stable tumor at 3 months does not correlate with ultimately progressive disease. Future studies will be needed to better characterize tumor response after SBRT and other liver-directed therapies.
Limitations of SBRT
Despite these encouraging data, there are several potential limitations of liver-directed SBRT. In the absence of long-term follow-up and multi-institutional prospective data, the curative potential and the durability of this treatment remain unknown. Second, because SBRT uses dose-fractionation schemes based on normal liver tolerance, an inverse relationship exists between tumor size and allowable dose. This inherent dose-volume relationship may theoretically limit the efficacy of this treatment for larger tumors. Furthermore, the impact of SBRT on future treatment and dosing strategies for regional recurrences, new primary tumors, and locally recurrent tumors remains largely unstudied and investigational.
Radioembolization
Transarterial radioembolization (TARE), also known as selective internal RT, is an alternative strategy for delivering local ablative RT to liver tumors [99]. TARE selectively delivers radioactive particles to liver tumors through hepatic artery embolization. Unlike TACE, TARE involves only minimal embolization and vascular occlusion (microembolization) with the intention of preserving native blood flow to enhance radiation-induced free radical cell death [100].
TARE has been described with either of 2 radioactive isotopes: Lipiodol labeled with iodine 131 (131I) and microspheres tagged with yttrium 90 (90Y). The primary decay products of 131I are electrons with mean energy of 190 keV and 364 keV γ rays, and tissue penetration of these electrons is between 0.6 mm and 2 mm [101]. There are few reports on the use of 131I Lipiodol for unresectable HCC or ICC. A French randomized trial of 27 patients with HCC and TVT compared 5 treatments with 131I Lipiodol with control and demonstrated a significant survival benefit with TARE at 6 months (48% vs. 0%) and 9 months (7% vs. 0%) [102]; however, a similar study of 129 patients comparing 131I Lipiodol and TACE with cisplatin showed no significant differences in survival or response rates but significantly improved tolerability among patients treated with TARE [103]. Finally, a retrospective series from Sydney, Australia, illustrated encouraging results for 71 patients with unresectable HCC treated with 131I Lipiodol with median survival of 14 months and 3-year survival of 20%, with response to treatment as the sole independent predictor of survival on multivariate analysis [104]. Nonetheless, 131I Lipiodol is not readily available in the U.S. and thus is not commonly used in the management of locally advanced HCC.
In contrast, 90Y-tagged microspheres are readily available in North America in either glass-based systems (TheraSphere; MDS Nordion, Ottawa, Canada, http://www.therasphere.com) or resin-based systems (SIR-Spheres; SIRTeX Medical, Lake Forest, IL, http://www.sirtex.com). 90Y emits high-energy β decay (mean: 0.93 MeV) with mean tissue penetration of 2.5 mm. A large prospective series from Northwestern University of 291 patients with HCC treated with 90Y demonstrated response rates between 42% and 57%, depending on assessment criteria, and median time to progression of 7.9 months, with least favorable outcomes seen for CP class B patients with TVT [105]. In this series, toxicities included fatigue (57%), pain (23%), and nausea or vomiting (20%), and up to 19% demonstrated grade 3–4 bilirubinemia. There are no randomized comparisons of TARE with 90Y versus TACE, although several retrospective series suggest comparable outcomes with reduced rates of complications like abdominal pain after TARE [106, 107]. Currently there are no clear guidelines for the use of TARE over TACE, although presence of minor branch or lobar portal vein thrombosis may be one such scenario. Additional studies will be necessary to elucidate the role of this treatment in relation to other nonsurgical modalities.
Future Perspectives
Many questions remain regarding the treatment of primary hepatic malignancies. The cited studies highlight the heterogeneous practice of liver-directed SBRT, and the ideal dose and the fractionation schema are unclear. In light of the many options for locoregional therapy available today, the ideal selection criteria for SBRT is equally important, including considerations such as tumor size or location, extent of vascular invasion, and baseline liver function. Meanwhile, the majority of studies to date have excluded patients with decompensated liver function (i.e., CP classes B and C), and the role of RT in this population remains unknown. Furthermore, advances in metabolic and functional imaging may further enable SBRT to overcome tumor resistance while sparing normal liver.
Retrospective and small prospective studies dominate the literature in this area, and randomized comparisons of SBRT with other therapies such as surgery, TACE, and RFA will be critical to defining the role of liver-directed RT. In the absence of level 1 evidence, RT has not been widely incorporated into international treatment consensus guidelines for primary liver tumors. Given the abundance of early, encouraging data on liver-directed RT, large, prospective, multi-institutional, phase III studies will be needed to establish radiation therapy as an accepted paradigm of treatment.
The optimal integration of SBRT with other treatments such as systemic therapy or TACE is under active investigation. For example, sorafenib is a small molecule multikinase inhibitor that has emerged as a standard systemic therapy for HCC. Following the results of the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial, which demonstrated a 2.8-month median survival benefit with sorafenib compared with placebo [108], active investigation has been under way to integrate SBRT with sorafenib therapy. This agent has been shown to act as an in vitro radiosensitizer in HCC [109], although a recent abstract by Dawson et al. raised concerns about increased toxicity with higher doses of sorafenib (400 mg daily) combined with six-fraction SBRT to a higher effective liver volume (30%–60%) [110]. Based on these data, RTOG launched a phase III protocol, RTOG 1112, to definitively evaluate the role of liver-directed RT in HCC in the context of active systemic therapy. This trial aims to randomize patients with unresectable HCC and CP class A cirrhosis to either sorafenib monotherapy or sequential SBRT (27.5–50 Gy in 5 fractions) followed by sorafenib (delivered at half dose for the first month). This study is currently open to accrual, and the investigators hope to directly study the role of SBRT in a randomized, controlled fashion. In parallel, a clinical trial by the NRG (cancer clinical cooperative group of the National Surgical Adjuvant Breast and Bowel Project [NSABP], the Radiation Therapy Oncology Group [RTOG], and the Gynecologic Oncology Group [GOG]) is currently in development for unresectable, liver-confined ICC, with plans to randomize patients to gemcitabine/cisplatin chemotherapy with or without SBRT. This trial has been approved by the Cancer Therapy Evaluation Program.
Similarly, effort has been devoted to exploring the combination of SBRT with TACE, particularly for patients with TVT, in whom TACE monotherapy may be precluded by limited vascular supply. As discussed above, Yoon et al. described favorable outcomes with TVT-directed SBRT following TACE [79], and another series by Zhang et al. showed improved 1-year survival (32% vs. 6.9%, p < .01) associated with the addition of RT to percutaneous transhepatic portal vein stenting with TACE [111]. An ongoing randomized phase III study at Loma Linda University Medical Center aims to compare TACE with proton-beam radiotherapy for patients with HCC within the Milan criteria and has accrued 65 of a planned sample of 220 patients with the primary end point of overall survival (ClinicalTrials.gov identifier NCT00857805). Future, prospective study will be necessary to further elucidate the effects and risks of such therapies for HCC and other liver tumors.
In the absence of consensus guidelines for the use of SBRT, several centers have adopted unofficial guidelines to guide treatment. At our institutions, all hepatobiliary cases are discussed at a weekly multidisciplinary tumor board including members from hepatobiliary and transplant surgery, medical oncology, radiation oncology, and interventional radiology. SBRT is considered only for patients with intrahepatic-confined disease (either HCC or ICC) not amenable to surgical resection or OLT, which remain the treatments of choice for resectable patients. Specifically, SBRT is recommended for patients with HCC >3 cm or with TVT or for patients with tumors in anatomically challenging locations such as the liver dome that are not amenable to other nonsurgical options. TACE or TARE is recommended for HCC patients with multifocal or extensive regional disease. RFA is generally recommended for lesions measuring ≤3 cm and not in close proximity to major vessels. For metastatic lesions, our institution also offers liver SBRT in context of a prospective clinical trial.
Still, many patients present with locally advanced or end-stage disease and remain ineligible for high-dose ablative RT such as SBRT based on tumor size or baseline liver function. Liver-directed RT has recently emerged as a powerful palliative therapy for patients with symptomatic HCC or liver metastases. A recent phase II study at PMH has demonstrated substantial improvements in patient-reported quality of life after single-fraction RT to 8 Gy to at least 75% of the liver, with 48% of patients in this study reporting improvement in symptoms on average at 1 month follow-up [112]. On the basis of these results, the National Cancer Institute of Canada Clinical Trials Group is preparing for a phase III study to further investigate palliative RT in this setting, illustrating the expanding role of RT both in the definitive and palliative settings.
These future directions illustrate the relatively nascent stage of liver-directed RT at present and will hopefully shed great insight into the role of SBRT for primary hepatic malignancies.
Conclusion
In summary, these data illustrate the evolution of liver-directed radiation and its re-emergence as an effective, feasible, and noninvasive treatment for patients with locally advanced or medically unresectable primary hepatic malignancies including HCC and cholangiocarcinoma. Treatment with 3D-CRT allows for escalation of conventional radiation doses and understanding of dose, irradiated liver volume, and the risk of RILD.
SBRT delivers high doses of RT in one or a few treatment fractions and has emerged as an effective ablative therapy for liver tumors. SBRT relies on precise target definition, understanding of tumor and organ motion, target immobilization, and precise radiation delivery systems. Several retrospective and few prospective studies have illustrated promising results with SBRT for HCC and cholangiocarcinoma. Most favorable outcomes have been shown for patients with early stage (T1–T2) tumors, although other studies have demonstrated favorable outcomes for patients who are otherwise inoperable and ineligible for other therapies. SBRT has been effectively combined with TACE for the treatment of HCC patients with TVT and may be used to bridge patients with HCC to liver transplantation. Liver-directed SBRT may be delivered with photon therapy or with charged particle therapy, which remains investigational.
Randomized comparison of liver-directed SBRT to other treatment modalities is lacking, and active investigation is under way to assess the role of SBRT in conjunction with systemic therapies like sorafenib for HCC.
Author Contributions
Conception/Design: Shyam K. Tanguturi, Andrew X. Zhu, Laura A. Dawson, Theodore S. Hong
Collection and/or assembly of data: Shyam K. Tanguturi, Theodore S. Hong
Data analysis and interpretation: Shyam K. Tanguturi, Theodore S. Hong
Manuscript writing: Shyam K. Tanguturi, Jennifer Y. Wo, Andrew X. Zhu, Laura A. Dawson, Theodore S. Hong
Final approval of manuscript: Shyam K. Tanguturi, Jennifer Y. Wo, Andrew X. Zhu, Laura A. Dawson, Theodore S. Hong
Disclosures
Theodore S. Hong: Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Reed GB, Jr, Cox AJ., Jr The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol. 1966;48:597–611. [PMC free article] [PubMed] [Google Scholar]
- 2.Du SS, Qiang M, Zeng ZC, et al. Radiation-induced liver fibrosis is mitigated by gene therapy inhibiting transforming growth factor-β signaling in the rat. Int J Radiat Oncol Biol Phys. 2010;78:1513–1523. doi: 10.1016/j.ijrobp.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Sakata R, Ueno T, et al. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247–255. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- 4.Ingold JA, Reed GB, Kaplan HS, et al. Radiation Hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 5.Russell AH, Clyde C, Wasserman TH, et al. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: Results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27:117–123. doi: 10.1016/0360-3016(93)90428-x. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Josef E, Lawrence TS. Radiotherapy for unresectable hepatic malignancies. Semin Radiat Oncol. 2005;15:273–278. doi: 10.1016/j.semradonc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 8.Robertson JM, Lawrence TS, Dworzanin LM, et al. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol. 1993;11:1286–1293. doi: 10.1200/JCO.1993.11.7.1286. [DOI] [PubMed] [Google Scholar]
- 9.Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 10.Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45:856–864. doi: 10.1080/02841860600936369. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence TS, Tesser RJ, ten Haken RK. An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. Int J Radiat Oncol Biol Phys. 1990;19:1041–1047. doi: 10.1016/0360-3016(90)90031-e. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 13.Mornex F, Girard N, Beziat C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies—mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152–1158. doi: 10.1016/j.ijrobp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Seong J, Lee IJ, Shim SJ, et al. A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int. 2009;29:147–152. doi: 10.1111/j.1478-3231.2008.01873.x. [DOI] [PubMed] [Google Scholar]
- 15.Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:326–332. doi: 10.1016/j.ijrobp.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Clement JJ, Song CW, Levitt SH. Changes in functional vascularity and cell number following x-irradiation of a murine carcinoma. Int J Radiat Oncol Biol Phys. 1976;1:671–678. doi: 10.1016/0360-3016(76)90149-8. [DOI] [PubMed] [Google Scholar]
- 17.Clement JJ, Tanaka N, Song CW. Tumor reoxygenation and postirradiation vascular changes. Radiology. 1978;127:799–803. doi: 10.1148/127.3.799. [DOI] [PubMed] [Google Scholar]
- 18.Song CW, Cho LC, Yuan J, et al. Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. Int J Radiat Oncol Biol Phys. 2013;87:18–19. doi: 10.1016/j.ijrobp.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Kocher M, Treuer H, Voges J, et al. Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiother Oncol. 2000;54:149–156. doi: 10.1016/s0167-8140(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 22.Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316–319. [PubMed] [Google Scholar]
- 23.Pan T, Lee TY, Rietzel E, et al. 4D-CT imaging of a volume influenced by respiratory motion on multi-slice CT. Med Phys. 2004;31:333–340. doi: 10.1118/1.1639993. [DOI] [PubMed] [Google Scholar]
- 24.Rietzel E, Chen GT, Choi NC, et al. Four-dimensional image-based treatment planning: Target volume segmentation and dose calculation in the presence of respiratory motion. Int J Radiat Oncol Biol Phys. 2005;61:1535–1550. doi: 10.1016/j.ijrobp.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 25.Langen KM, Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50:265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 26.Harauz G, Bronskill MJ. Comparison of the liver’s respiratory motion in the supine and upright positions: Concise communication. J Nucl Med. 1979;20:733–735. [PubMed] [Google Scholar]
- 27.Suramo I, Päivänsalo M, Myllylä V. Cranio-caudal movements of the liver, pancreas and kidneys in respiration. Acta Radiol Diagn (Stockh) 1984;25:129–131. doi: 10.1177/028418518402500208. [DOI] [PubMed] [Google Scholar]
- 28.Davies SC, Hill AL, Holmes RB, et al. Ultrasound quantitation of respiratory organ motion in the upper abdomen. Br J Radiol. 1994;67:1096–1102. doi: 10.1259/0007-1285-67-803-1096. [DOI] [PubMed] [Google Scholar]
- 29.Balter JM, Ten Haken RK, Lawrence TS, et al. Uncertainties in CT-based radiation therapy treatment planning associated with patient breathing. Int J Radiat Oncol Biol Phys. 1996;36:167–174. doi: 10.1016/s0360-3016(96)00275-1. [DOI] [PubMed] [Google Scholar]
- 30.Lax I, Blomgren H, Näslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33:677–683. doi: 10.3109/02841869409121782. [DOI] [PubMed] [Google Scholar]
- 31.Heinzerling JH, Anderson JF, Papiez L, et al. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys. 2008;70:1571–1578. doi: 10.1016/j.ijrobp.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Wunderink W, Méndez Romero A, de Kruijf W, et al. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fiducial markers implanted in liver. Int J Radiat Oncol Biol Phys. 2008;71:907–915. doi: 10.1016/j.ijrobp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Eccles CL, Dawson LA, Moseley JL, et al. Interfraction liver shape variability and impact on GTV position during liver stereotactic radiotherapy using abdominal compression. Int J Radiat Oncol Biol Phys. 2011;80:938–946. doi: 10.1016/j.ijrobp.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Case RB, Sonke JJ, Moseley DJ, et al. Inter- and intrafraction variability in liver position in non-breath-hold stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:302–308. doi: 10.1016/j.ijrobp.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 35.Dawson LA, Ten Haken RK, Lawrence TS. Partial irradiation of the liver. Semin Radiat Oncol. 2001;11:240–246. doi: 10.1053/srao.2001.23485. [DOI] [PubMed] [Google Scholar]
- 36.Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44:911–919. doi: 10.1016/s0360-3016(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 37.Dawson LA, Eccles C, Bissonnette JP, et al. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62:1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 38.Eccles C, Brock KK, Bissonnette JP, et al. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:751–759. doi: 10.1016/j.ijrobp.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 39.Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother Oncol. 2000;57:225–236. doi: 10.1016/s0167-8140(00)00226-7. [DOI] [PubMed] [Google Scholar]
- 40.Adler JR, Jr, Chang SD, Murphy MJ, et al. The Cyberknife: A frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124–128. doi: 10.1159/000099863. [DOI] [PubMed] [Google Scholar]
- 41.Shirato H, Harada T, Harabayashi T, et al. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:240–247. doi: 10.1016/s0360-3016(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 42.Sharp GC, Jiang SB, Shimizu S, et al. Prediction of respiratory tumour motion for real-time image-guided radiotherapy. Phys Med Biol. 2004;49:425–440. doi: 10.1088/0031-9155/49/3/006. [DOI] [PubMed] [Google Scholar]
- 43.Schweikard A, Shiomi H, Adler J. Respiration tracking in radiosurgery. Med Phys. 2004;31:2738–2741. doi: 10.1118/1.1774132. [DOI] [PubMed] [Google Scholar]
- 44.Delaney TF, Kooy HM. Proton and Charged Particle Radiotherapy. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 45.Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 46.Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 47.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 48.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) Hepatocellular carcinoma - United States, 2001-2006. MMWR Morb Mortal Wkly Rep. 2010;59:517–520. [PubMed] [Google Scholar]
- 50.A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 51.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 52.Méndez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase I-II study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 53.Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 54.Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 55.Ibarra RA, Rojas D, Snyder L, et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol. 2012;51:575–583. doi: 10.3109/0284186X.2011.652736. [DOI] [PubMed] [Google Scholar]
- 56.Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: A retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 57.Takeda A, Sanuki N, Eriguchi T, et al. Stereotactic ablative body radiotherapy for previously untreated solitary hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:372–379. doi: 10.1111/jgh.12350. [DOI] [PubMed] [Google Scholar]
- 58.Jang WI, Kim MS, Bae SH, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. doi: 10.1186/1748-717X-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon SM, Lim YS, Park MJ, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8:e79854. doi: 10.1371/journal.pone.0079854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bibault JE, Dewas S, Vautravers-Dewas C, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: Prognostic factors of local control, overall survival, and toxicity. PLoS One. 2013;8:e77472. doi: 10.1371/journal.pone.0077472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honda Y, Kimura T, Aikata H, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530–536. doi: 10.1111/jgh.12087. [DOI] [PubMed] [Google Scholar]
- 62.Yuan Z, Tian L, Wang P, et al. Comparative research on the efficacy of CyberKnife® and surgical excision for stage I hepatocellular carcinoma. Onco Targets Ther. 2013;6:1527–1532. doi: 10.2147/OTT.S51452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 64.Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Son SH, Choi BO, Ryu MR, et al. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: Dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys. 2010;78:1073–1080. doi: 10.1016/j.ijrobp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102:209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 68.Louis C, Dewas S, Mirabel X, et al. Stereotactic radiotherapy of hepatocellular carcinoma: Preliminary results. Technol Cancer Res Treat. 2010;9:479–487. doi: 10.1177/153303461000900506. [DOI] [PubMed] [Google Scholar]
- 69.Goyal K, Einstein D, Yao M, et al. Cyberknife stereotactic body radiation therapy for nonresectable tumors of the liver: Preliminary results. HPB Surg. 2010;2010 doi: 10.1155/2010/309780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 71.Georgiades CS, Hong K, D’Angelo M, et al. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–1659. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 72.Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: A prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 73.Pentecost MJ, Daniels JR, Teitelbaum GP, et al. Hepatic chemoembolization: Safety with portal vein thrombosis. J Vasc Interv Radiol. 1993;4:347–351. doi: 10.1016/s1051-0443(93)71873-4. [DOI] [PubMed] [Google Scholar]
- 74.Takagi H, Takayama H, Yamada S, et al. Radiation therapy of hepatocellular carcinoma [in Japanese] Nippon Shokakibyo Gakkai Zasshi. 1989;86:237–245. [PubMed] [Google Scholar]
- 75.Lin CS, Jen YM, Chiu SY, et al. Treatment of portal vein tumor thrombosis of hepatoma patients with either stereotactic radiotherapy or three-dimensional conformal radiotherapy. Jpn J Clin Oncol. 2006;36:212–217. doi: 10.1093/jjco/hyl006. [DOI] [PubMed] [Google Scholar]
- 76.Xi M, Zhang L, Zhao L, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8:e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugahara S, Nakayama H, Fukuda K, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–788. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 78.Choi BO, Choi IB, Jang HS, et al. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: Preliminary analysis. BMC Cancer. 2008;8:351. doi: 10.1186/1471-2407-8-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: Long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 80.Rim CH, Yang DS, Park YJ, et al. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol. 2012;42:721–729. doi: 10.1093/jjco/hys082. [DOI] [PubMed] [Google Scholar]
- 81.Chuma M, Taguchi H, Yamamoto Y, et al. Efficacy of therapy for advanced hepatocellular carcinoma: Intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol. 2011;26:1123–1132. doi: 10.1111/j.1440-1746.2011.06745.x. [DOI] [PubMed] [Google Scholar]
- 82.Huang YJ, Hsu HC, Wang CY, et al. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–1163. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 83.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 84.Sandroussi C, Dawson LA, Lee M, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010;23:299–306. doi: 10.1111/j.1432-2277.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 85.Facciuto ME, Singh MK, Rochon C, et al. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: Evaluation of radiological and pathological response. J Surg Oncol. 2012;105:692–698. doi: 10.1002/jso.22104. [DOI] [PubMed] [Google Scholar]
- 86.O’Connor JK, Trotter J, Davis GL, et al. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18:949–954. doi: 10.1002/lt.23439. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: The University of Tsukuba experience. Cancer. 2009;115:5499–5506. doi: 10.1002/cncr.24619. [DOI] [PubMed] [Google Scholar]
- 88.Bush DA, Kayali Z, Grove R, et al. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: A phase 2 prospective trial. Cancer. 2011;117:3053–3059. doi: 10.1002/cncr.25809. [DOI] [PubMed] [Google Scholar]
- 89.Kato H, Tsujii H, Miyamoto T, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59:1468–1476. doi: 10.1016/j.ijrobp.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 90.Habermehl D, Debus J, Ganten T, et al. Hypofractionated carbon ion therapy delivered with scanned ion beams for patients with hepatocellular carcinoma - feasibility and clinical response. Radiat Oncol. 2013;8:59. doi: 10.1186/1748-717X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109–114. doi: 10.1055/s-2007-1007302. [DOI] [PubMed] [Google Scholar]
- 92.Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 93.Barney BM, Olivier KR, Miller RC, et al. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol. 2012;7:67. doi: 10.1186/1748-717X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dewas S, Bibault JE, Mirabel X, et al. Prognostic factors affecting local control of hepatic tumors treated by stereotactic body radiation therapy. Radiat Oncol. 2012;7:166. doi: 10.1186/1748-717X-7-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: Are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 96.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 97.Price TR, Perkins SM, Sandrasegaran K, et al. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer. 2012;118:3191–3198. doi: 10.1002/cncr.26404. [DOI] [PubMed] [Google Scholar]
- 98.Kimura T, Takahashi S, Kenjo M, et al. Dynamic computed tomography appearance of tumor response after stereotactic body radiation therapy for hepatocellular carcinoma: How should we evaluate treatment effects? Hepatol Res. 2013;43:717–727. doi: 10.1111/hepr.12007. [DOI] [PubMed] [Google Scholar]
- 99.Lau WY, Sangro B, Chen PJ, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: The emerging role for radioembolization using yttrium-90. Oncology. 2013;84:311–318. doi: 10.1159/000348325. [DOI] [PubMed] [Google Scholar]
- 100.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 101.Skugor M, Wilder JB. Thyroid Disorders: A Cleveland Clinic Guide. Cleveland, OH: Cleveland Clinic Press; 2006. [Google Scholar]
- 102.Raoul JL, Guyader D, Bretagne JF, et al. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: Intra-arterial iodine-131-iodized oil versus medical support. J Nucl Med. 1994;35:1782–1787. [PubMed] [Google Scholar]
- 103.Raoul JL, Guyader D, Bretagne JF, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 104.Chua TC, Chu F, Butler SP, et al. Intra-arterial iodine-131-lipiodol for unresectable hepatocellular carcinoma. Cancer. 2010;116:4069–4077. doi: 10.1002/cncr.25283. [DOI] [PubMed] [Google Scholar]
- 105.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 106.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 107.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497.e2–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 109.Huang CY, Lin CS, Tai WT, et al. Sorafenib enhances radiation-induced apoptosis in hepatocellular carcinoma by inhibiting STAT3. Int J Radiat Oncol Biol Phys. 2013;86:456–462. doi: 10.1016/j.ijrobp.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 110.Dawson LA, Brade A, Cho C, et al. Phase I study of sorafenib and SBRT for advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:S10–S11. [Google Scholar]
- 111.Zhang XB, Wang JH, Yan ZP, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: Treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245–1252. doi: 10.1002/cncr.24139. [DOI] [PubMed] [Google Scholar]
- 112.Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol. 2013;31:3980–3986. doi: 10.1200/JCO.2013.49.9202. [DOI] [PubMed] [Google Scholar]


