Abstract
Objective
The major histocompatibility complex (MHC) is the primary genetic contributor to multiple sclerosis (MS) and experimental allergic encephalomyelitis (EAE), but multiple additional interacting loci are required for genetic susceptibility. The identity of most of these non-MHC genes is unknown. In this report, we identify genes within evolutionarily conserved genetic pathways leading to multiple sclerosis (MS) and experimental allergic encephalomyelitis (EAE).
Methods
To identify non-MHC binary and quantitative trait loci (BTL/QTL) important in the pathogenesis of EAE, we generated phenotype-selected congenic mice using EAE-resistant B10.S and EAE-susceptible SJL mice. We hypothesized that genes linked to EAE BTL/QTL and MS-GWAS can be identified if they belong to common evolutionarily conserved pathways, which can be identified with a bioinformatic approach using Ingenuity software.
Results
Many known BTL/QTL were retained and linked to susceptibility during phenotype selection, the most significant being a region on chromosome 17 distal to H2 (Eae5). We show in pathway analysis that T helper (TH)-cell differentiation genes are critical for both diseases. Bioinformatic analyses predicted that Eae5 is important in CD4 T-effector and/or Foxp3+ T-regulatory cells (Tregs), and we found that B10.S-Eae5SJL congenic mice have significantly greater numbers of lymph node CD4 and Tregs than B10.S mice.
Interpretation
These results support the polygenic model of MS/EAE, whereby MHC and multiple minor loci are required for full susceptibility, and confirm a critical genetic dependence on CD4 TH-cell differentiation and function in the pathogenesis of both diseases.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) with axonal damage leading to progressive paralysis in affected individuals.1 The heritability of MS is estimated to be ~35%, largely associated with the inheritance of susceptible HLA haplotypes that can be present in up to 70% of these MS cases.2 However, polymorphisms in many other genes have also been associated with MS in genome-wide association studies (GWAS). In GWAS analyses, the contribution of each susceptibility gene is small, and thus both the importance of different genes in pathogenesis and the mechanisms by which they act are difficult to test individually.
A suitable inbred rodent model is useful for discerning the effect of each of these human disease-modifying genes. Experimental allergic encephalomyelitis (EAE), the autoimmune model for MS, results in a progressive paralysis due to the activation of myelin-specific CD4+ T cells that initiate disease pathogenesis.3, 4 Like MS, EAE is genetically complex, and susceptibility in H2S-matched progeny of EAE-resistant B10.S and EAE-susceptible SJL mice is modified by at least 30 different binary and quantitative trait loci (BTL/QTL).5-12
Identifying the gene underlying each BTL or QTL can be accomplished by positional cloning using traditional isolation of the QTL interval in congenic mice, facilitated by marker-assisted selection (MAS).13 However, complications arise for congenic production based solely on MAS because EAE is highly influenced by intrinsic genetic and environmental factors such as age, sex, and season of induction.11, 14 We attempted to circumvent these issues by creating phenotype-selected congenic mice, in which the trait is stable by definition. Such analyses have been used in the identification of genetic elements underlying simple and complex traits such as autoimmune and infectious diseases.15
In the present study, we selected congenic lines based on their EAE susceptibility as the first step toward the identification of positional candidate genes. Our results demonstrate, however, that MAS of advanced generations of originally phenotype-selected congenic lines did not identify regions amenable to positional candidate gene cloning. This finding is likely due to the polygenic nature of the disease and the dilution of multiple epistatic loci in advanced generations.
We nevertheless were able to use results from congenic production to identify the combinations of genes linked to EAE and MS that belong to common evolutionary conserved pathways. We undertook a pathway analysis to find non-MHC genes that underlie full expressivity of EAE. Our results underscore the importance of genetic epistasis in EAE and MS and identify potentially interacting evolutionarily conserved gene pathways that influence susceptibility to CNS autoimmune disease. Furthermore, our results provide evidence that the genetic dysregulation of TH-cell development, differentiation, and/or activation underlies the disease mechanism of both MS and EAE.
Materials and Methods
Animals
SJL/J (SJL) and B10.S/SgMcdJ (B10.S) were purchased from The Jackson Laboratory (Bar Harbor, ME). For phenotype selection, we selected for EAE resistance or susceptibility among (B10.S × SJL)F1 × SJL and (B10.S × SJL)F1 × B10.S progeny, respectively. Resistant families from SJL backcrosses were kept for breeding the next generation. Susceptible families from B10.S backcrosses were bred before testing the mothers for susceptibility due to the difficulty in breeding affected animals. Progeny derived from susceptible mothers were used to breed the next generation. We obtained phenotype and genome-wide genotype data from the backcross mice and followed the segregation of donor marker loci for 4 subsequent generations. After this phenotype selection stage, multiply heterozygous progeny of B10.S-SJL-EaeS and SJL-B10.S-EaeR N5 founder pairs were (1) crossed to their respective congenic partners to continue the lines as multi-BTL lines and (2) intercrossed to generate homozygous and heterozygous F2 progeny to test linkage of the retained segments to EAE incidence and severity. Two B10.S-SJL-Eae5S lines were selected for derivation into traditional congenic lines by MAS. The experimental procedures performed in this study were approved by the animal care and use committees of the University of Vermont and Drexel University College of Medicine.
EAE induction protocol
For the phenotype selection phase of the study, EAE was induced using SJL mouse spinal cord homogenate (MSCH) in complete Freund’s adjuvant without pertussis toxin as previously published.7, 16 For the cross-intercross stage of the phenotype-selection study, PLP139-151 was used according to established protocols.17
Genotyping and linkage analysis
Samples of tissue were harvested for DNA, and genotyping was performed as described in our previous publications.8 During the phenotype selection stage, the multigenerational groups were analyzed for linkage as follows: a χ2 goodness of fit test was performed for each marker locus to look for deviations from the expected segregation pattern of heterozygous and homozygous individuals for both backcross lines. Significant deviation in favor of the heterozygous genotypes for a particular marker indicates an effect of this locus on susceptibility. For the cross-intercross stage, traditional F2 linkage analysis was performed with χ2 and logistic regression testing of the linkage to EAE incidence, and ANOVA was used to determine significant EAE-BTL.8, 16 Fully congenic lines were assessed for the strength of their retained QTL by the same statistical methods. A p value of 0.01 was considered significant, and p = 0.05 was considered suggestively significant.
In silico analysis
Mouse homologues of 40 genes listed as MS-GWAS gene candidates (msgene.org) were identified using Mouse Genome Informatics (http://www.informatics.jax.org/) and Ingenuity programs, and their interacting genes were determined using the network tool in Ingenuity Pathway Analysis Software (IPA; Ingenuity® Systems, www.ingenuity.com). This list (~1600 genes, Supplemental Table 5) was used to create either “known EAE-BTL” (Supplemental Table 6, from previous linkage testing) or significantly retained “phenotype-selected EAE-BTL” (Supplemental Table 7) from the logistic regression described above. The two lists were filtered to contain only genes that were likely to be polymorphic between B10.S (using C57BL/10J as a reference because it is mostly sequenced and is the background strain for B10.S) and SJL using the Mouse Phenome Database (http://phenome.jax.org/) and selecting as polymorphic any allelic pair for which there was more than one nucleotide difference between SJL and C57BL/10J. The genes for which SNP information was missing for SJL were evaluated and included in the lists by employing the imputation data available in the Mouse Phenome Database.
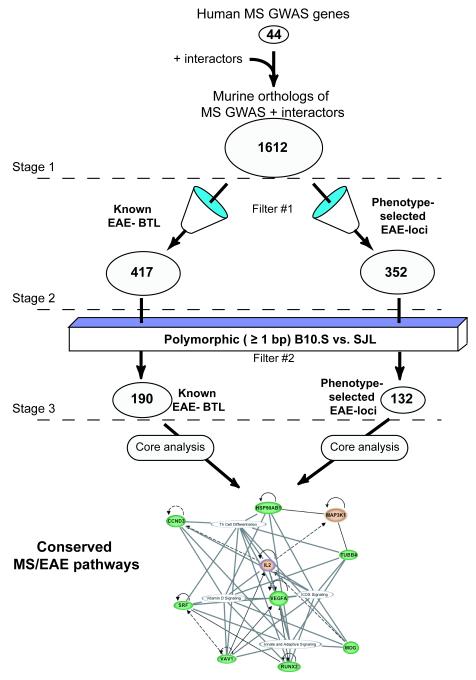
We then subjected the three lists to pathway analysis using the core analysis tool in IPA, and used the comparison analysis function to determine which pathways were enriched in all three lists. The P-values associated with each pathway were adjusted for multiple hypotheses testing within IPA.18 After enriched pathways were identified, epistasis modeling was conducted by first identifying the Eae5 candidates that were still present in both lists by stage three (Figure 2). Then, connect tool in IPA was used to test for interactions between those Eae5 candidates and the genes involved in each of the significantly enriched pathways.
Figure 2.
In silico analysis to determine epistatic interactions in EAE and MS. Starting with known 44 MS-GWAS genes and their interactors (defined as protein-protein interactions or gene regulation relationships), a total of 1612 murine orthologues formed our initial analysis point in Stage 1. For Stage 2, we filtered these genes against positions of known EAE-BTL or against regions retained during phenotype selection. In Stage 3 we queried these genes against the mouse phenome database to determine polymorphisms (≥ 1 bp) in the coding and noncoding regions of these genes as well as 20MB up- and downstream of the boundaries. These refined lists were then combined and analyzed by Core pathway analysis in Ingenuity. A highly significant (p<0.001 based on permutation test) interaction map for Conserved EAE/MS Pathways was then generated to elucidate epistatic interactions important in EAE and MS.
Antibodies and flow cytometric analysis
Lymph nodes (inguinal, braquial, axillary) were excised and dissociated into single cell suspensions. For the identification and phenotypic analysis of Treg cells (CD4+ CD8− TCRβ+ Foxp3+), the following surface anti-mouse mAb were used: anti-CD4 (MCD0417, Caltag); anti-CD8, (53-6.7, BD Pharmingen); anti-TCRβ and anti-Foxp3 (H57-5987, FJK-16s; eBioscience). Intracellular Foxp3 was stained with the mouse/rat Foxp3 staining set (eBioscience), according to the manufacturer’s instructions. Viable cells were selected for flow cytometric analysis (LSR II, BD) based on forward and side scatter light properties and analysis was performed using FlowJo software (TreeStar Software, Inc).
Results
EAE phenotype-selected lines
We generated bi-directional, phenotype-selected congenic lines using EAE-resistant B10.S and EAE-susceptible SJL parental strains (creating the reciprocal lines B10.S-SJL-EaeS and SJL-B10.S-EaeR). By the 5th backcross generation (N5), we observed 30.6% susceptibility in the B10.S-SJL-EaeS lines and 64.7% susceptibility in the SJL-B10.S-EaeR lines (Supplemental Table 1). We used microsatellite marker genotypes at each generation to track donor alleles that were retained during trait selection (Supplemental Tables 2, 3). A χ2 goodness of fit test was performed for each marker (Table 1), where significant deviations in favor of heterozygous genotypes indirectly indicate an effect of this locus on EAE susceptibility. Selection for phenotype for five backcross generations resulted in the retention of donor intervals on chromosomes (chr) 2, 3, 7, 12, 13, 16, and 17 (Table 1, Supplemental Table 4). These seven retained intervals harbor known EAE QTLs; thus, these results confirm previous linkages.
Table 1.
Significantly retained and linked BTL in phenotype-selected generations (N2-N5).
| Retained intervals Known EAE BTL |
Retention in B10.S-SJL- EaeS (p) a |
Linkage to EAE (p) b |
OR, rangec | Retention in SJL-B10.S- EaeR (p) a |
Linkage to EAE (p) b | OR, rangec |
|---|---|---|---|---|---|---|
| Chr 2: 18-122 Mb d Eae8,21, 33 |
0.03 | 0.001 | 0.6 - 3.1 | 6.1 × 10−22 | 0.03 | 1.4 - 4.2 |
| Chr 3: 29-141 Mb d Eae3, 10, 20a, b |
0.05 | 0.03 | 1.2 - 4.2 | 0.004 | NS | 0.7 – 1.8 |
| Chr 7: 63-132 Mb Eae4 |
NS | 0.02 | 2.1 – 4.7 | 3.0 × 10−15 | 0.05 | 1.6 - 2.4 |
| Chr 12: 12–120 Mb d Eae16 |
1.2 × 10-06 | NS | 1.1 – 2.2 | 2.0 × 10-09 | 0.02 | 0.4 - 0.5 |
| Chr 13: 3–117 Mb d | 0.02 | NS | 0.7 – 1.4 | 4.4 × 10−05 | NS | 0.3 - 2.7 |
| Chr 16: 4–57 Mb Eae11 |
0.0001 | NS | 1.9 – 2.1 | 0.001 | 0 .001 | 3.8 - 15.2 |
| Chr 17: 37-63 Mb b Eae5 |
1.6 × 10−25 | 0.005 | 2.7 – 3.9 | NS | NS | 1.2 – 4.0 |
Intervals that were retained during phenotype selection are shown. All but those on chr 7 and chr 17 are bi-directional and were retained in both B10.S-SJL-EaeS congenics as well as SJL-B10.S-EaeR congenics, often with significant direct linkage to EAE, as assessed by logistic regression. The regions in total cover ~18% of the mouse genome.
p value for most significantly retained marker in interval.
p value for most significant linkage to EAE in interval.
Odds ratios (OR) were calculated by logistic regression, corrected for the sex of the mice, and those greater than 1 indicate a ratio consistent with the expected effect of a retained allele (B10.S alleles that convey greater resistance, or SJL-derived alleles that convey greater susceptibility). The “range” of OR for individual markers in each interval is listed. Bold font indicates intervals where linkage to EAE was consistently seen with OR>1.
Intervals on chr 2, 3, 12, and 13, together with Eae5 on chr 17, were also retained in the N6 progenitor of the B10.S-SJL-Eae5S lines. The chr 2, 3, 12, and 13 regions were selected for the analysis of genes that are candidates for epistatic interactions with eae5 on chr 17.
NS, not significant
Markers were also analyzed for significant linkage to either resistance or susceptibility to EAE, and an odds ratio (OR) was calculated for each linkage (Table 1). Eae4 was significantly retained in the SJL-B10.S-EaeR congenics and significantly determined EAE incidence in both crosses, as did intervals located on chr 2 (comprising Eae8, Eae21, and Eae33). Eae5 was significantly retained in the B10.S-SJL-EaeS lines and nearly significantly retained in the SJL-B10.S-EaeR congenics (p = 0.08); this locus showed ORs consistently >1 across the narrow Eae5 interval on chr 17 in both crosses.
Derivation of single-interval EAE BTL congenic lines from phenotype-selected lines
The generation of SJL-B10.S-EaeR single-interval lines on the SJL background was unsuccessful due to the poor breeding performance of SJL mice. Eae5 was the only locus in B10.S-SJL-EaeS mice that showed strong significance in the N5 generation by both retention and linkage (Table 1). Therefore, we generated single-interval congenic lines for Eae5 by MAS (B10.S-SJL-Eae5S). Two separate congenic B10.S-SJL-Eae5S lines were created. In addition to Eae5, early generations of these congenic lines carried intervals bearing Eae3, Eae8, Eae16, Eae20, Eae21, and a region on chr 13, all of which were serially eliminated by MAS.
We observed that EAE susceptibility no longer segregated with Eae5 in later MAS backcross generations (Fig 1). There was no CNS inflammation in B10.S-SJL-Eae5S congenic mice in the absence of clinical signs in any generation (data not shown). Attempts were made to selectively promote EAE susceptibility in B10.S-SJL-Eae5S congenic mice, using MSCH and pertussis toxin in the induction, which resulted in a modest increase in susceptibility, but at the expense of increased susceptibility in B10.S control animals19 making positional candidate gene identification in B10.S-SJL-Eae5SJL nearly impossible. Thus, although the phenotype selection confirmed the requirement for known EAE BTL, we could not use this approach for physical positional candidate gene identification.
Figure 1.

The risk of EAE susceptibility in B10.S-SJL-Eae5S cross-intercross progeny bearing Eae5S/S or Eae5S/b is compared to progeny bearing Eae5b/b after immunization with 2×PLP+CFA. The relative odds ratio in B10.S-SJL-Eae5S mice that carried susceptible alleles at Eae5 over those that carried resistant Eae5b/b alleles, classified by the year of the study. The year 2003 corresponds primarily to N6 mice, 2004 to N7 and N8 mice, etc. The Eae5S/S-dependent susceptibility was lost after several generations of backcrossing to B10.S. One attempt to rescue the phenotype of these Eae5- bearing mice is shown for 2008, when they were inoculated with 1×MSCH+PTX. s/s, homozygous SJL at Eae5; s/b, heterozygous SJL/B10.S at Eae5; b/b, homozygous B10.S at Eae5; *, P<0.05; **, P<0.01. Percentage of affected mice (Eae5 s/− vs. Eae5b/b): 2008 with PTX 97% vs.77%; 2008, 10% vs.15%; 2007, 32% vs.42%; 2006, 38% vs.55%; 2005, 49% vs.67%; 2004, 51% vs.34%; 2003, 39% vs.10%
In silico candidate gene identification
Because gene identification by traditional positional cloning alone was not feasible for Eae5, we used a bioinformatic approach to identify genes that are important in both EAE and MS. Such genes have been identified using traditional genetic linkage studies and by genetic association studies including GWAS. For this analysis, we selected murine orthologues for the top 44 MS-GWAS genes (http://www.msgene.org). We used the network tool in IPA to identify interacting genes whose functions directly impact the action of each MS-GWAS homolog (Stage 1 of Fig 2). For example, IL2 was included in the model, because of its interaction with IL2RA, a gene identified in a previous MS-GWAS study.20 We did not include H2 because B10.S and SJL have identical H2 class I and class II alleles.8 From this list of ~1600 mouse genes (Supplemental Table 5), consisting of GWAS murine orthologues and interacting genes, we generated two additional lists: “known EAE-BTL,” containing BTL known to distinguish B10.S from SJL mice,6, 8, 11 and “phenotype-selected EAE-loci”, containing mouse genes that reside in intervals that were significantly retained and/or linked to EAE during the generation of the B10.S-SJL-Eae5S congenic lines (Stage 2 of Fig 2; Table 1). These two lists were filtered to include only polymorphic loci, using genomic SNP data from the Mouse Phenome Database (see Methods). The result was a list of 190 “known EAE-BTL” and a list of 132 “phenotype-selected EAE-loci” (Stage 3 of Fig 2) (Supplemental Tables 6-9). To determine physiological and functional networks of importance to autoimmune demyelination, we then subjected the three lists to pathway analysis in IPA.
The results of pathway analysis showed a significant enrichment of 10 canonical pathways within the MS-GWAS list, 21 in the known EAE-BTL list, and 22 in the phenotype-selected EAE-loci list (p < 0.01). The top 10 MS-GWAS-associated pathways were shared between mouse and human; they were conserved with high significance in all three models (Table 2; all pathways with p < 0.05 are in Supplemental Table 10). More than half of the shared pathways function in CD4 T helper (TH)-cell differentiation or activation.
Table 2.
Shared MS/EAE pathways show the close correspondence of human MS-GWAS pathways and both H2s mouse EAE BTL pathways.
| Ingenuity Canonical Pathways | List Name | p value | Molecules |
|---|---|---|---|
|
| |||
| 1. T Helper Cell Differentiation | MS-GWAS | 1.0E-09 | IL12A, CD40, TGFB1, TNFRSF1A, IL21R, HLA-DRB1, IL2RA, STAT3 |
| Known EAE-QTL | 3.0E-09 | IL21, IFNG, IL4R, IL5, CD40, IL2, IL12RB1, TNFRSF1A, NGFR, IL21R, RORC, IL4 |
|
| Phenotype-selected | 1.7E-04 | IL21, IL6ST, IL4R, IL2, IL21R, RORC, TRG (TCR gamma family) | |
|
| |||
| 2. Role of JAK1 and JAK3 in γc Cytokine Signaling |
MS-GWAS | 4.0E-05 | IL7R, IL21R, IL2RA, STAT3, IL7 |
| Known EAE-QTL | 7.0E-05 | IL21, IL4R, FES, IL2, IL21R, JAK3, IL7, IL4 | |
| Phenotype-selected | 5.1E-03 | IL21, IL4R, FES, IL2, IL21R | |
|
| |||
| 3. Altered T Cell & B Cell Signaling in Rheumatoid Arthritis |
MS-GWAS | 9.0E-05 | IL12A, CD40, TGFB1, HLA-DRB1, CHUK |
| Known EAE-QTL | 6.0E-03 | IL21, IFNG, CD40, IL2, CSF2, IL4 | |
| Phenotype-selected | 2.5E-02 | TLR2, IL21, IL2, TRG(TCR gamma family) | |
|
| |||
| 4. ICOS-ICOSL Signaling in T Helper Cells |
MS-GWAS | 2.0E-04 | CD40, HLA-DRB1, IL2RA, VAV1, CHUK |
| Known EAE-QTL | 8.0E-04 | GAB2, LCK, CD40, IL2, NFATC2, NFATC1, VAV1, ITK | |
| Phenotype-selected | 1.4E-02 | GAB2, AKT1, IL2, VAV1, TRG(TCR gamma family) | |
|
| |||
| 5. Communication between Innate & Adaptive Immune Cells |
MS-GWAS | 2.0E-03 | CXCL10, IL12A, CD40, HLA-DRB1 |
| Known EAE-QTL | 8.0E-03 | IFNG, IL5, CD40, IL2, CSF2, IL4 | |
| Phenotype-selected | 2.8E-02 | TLR2, B2M, IL2, TRG@ | |
|
| |||
| 6. IL-12 Signaling & Production in Macrophages |
MS-GWAS | 3.0E-03 | IL12A, CD40, TGFB1, CHUK |
| Known EAE-QTL | 4.5E-02 | IFNG, CD40, IL12RB1, IL4, PRKCB | |
| Phenotype-selected | 4.1E-02 | TLR2, TRAF6, AKT1, PRKCB | |
|
| |||
| 7. Colorectal Cancer Metastasis Signaling |
MS-GWAS | 5.0E-03 | TGFB1, TNFRSF1A, TYK2, STAT3, PTGER4 |
| Known EAE-QTL | 3.5E-02 | VEGFA, IFNG, RHOG, ARRB1, TNFRSF1A, LEF1, JAK3, WNT5B | |
| Phenotype-selected | 9.0E-03 | VEGFA, TLR2, IL6ST, AKT1, RHOG, ARRB1, EGF, LEF1 | |
|
| |||
| 8. Role of Macrophages, Fibroblasts & Endothelial Cells in Rheumatoid Arthritis |
MS-GWAS | 9.3E-03 | TGFB1, TNFRSF1A, STAT3, CHUK, IL7 |
| Known EAE-QTL | 3.0E-04 | TNFRSF1A, NFATC1, FCGR1A, IL7, IL16, VEGFA, NLK, CCL2, NGFR, NFATC2, LEF1, CSF2, WNT5B, PRKCB |
|
| Phenotype-selected | 2.0E-04 | IL6ST, VCAM1, PDIA3, DAAM1, FCGR1A, VEGFA, TLR2, TRAF6, IL16, AKT1, RIPK1, LEF1, PRKCB |
|
|
| |||
| 9. VDR/RXR Activation | MS-GWAS | 9.0E-03 | CXCL10, IL12A, CYP27B1 |
| Known EAE-QTL | 4.0E-03 | DEFB4A/DEFB4B, IFNG, IL2, RUNX2, CSF2, PRKCB | |
| Phenotype-selected | 7.0E-03 | WT1, YY1, IL2, RUNX2, PRKCB | |
|
| |||
| 10. Regulation of IL-2 Expression in Activated & Anergic T Lymphocytes |
MS-GWAS | 9.5E-03 | TGFB1, VAV1, CHUK |
| Known EAE-QTL | 1.5E-02 | IL2, VAV3, NFATC2, NFATC1, VAV1 | |
| Phenotype-selected | 2.1E-02 | IL2, VAV3, MAP3K1, VAV1 | |
Results are listed in order of significance for human GWAS pathways. P values for each pathway’s significance in the three lists, as determined by Ingenuity, are listed in center column. Significance of each was calculated after correction for multiple testing in IPA.
Genes were chosen from the Eae5 interval (Chr 17, between 37 and 57Mb) that likely play a functional role in these shared, conserved pathways. Ten polymorphic genes within the Eae5 interval were found, each of which function in at least one of the “shared MS/EAE pathways”: Ccnd3, C6orf130 homolog AI314976, Hsp90ab1, Mog, Runx2, Srf, Tubb4, Trip10, Vav1, and Vegfa (Table 2, and Supplemental Table 8). Seven of these Eae5 candidate genes are expressed by CD4 T cells, and all ten are expressed in Foxp3+ T regulatory cells (Tregs) 21, 22 as determined using the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/sites/geo).
We then set out to identify epistatic genes (on chr 2 [Eae21], chr 3 [Eae3, Eae20], chr 12 [Eae16], or chr 13) whose interactions with Eae5 are critical for EAE but that were lost in the B10.S-SJL-Eae5S congenic lines during MAS (Supplemental Table 9). We therefore located genes from these intervals that (1) are known to interact with an Eae5 candidate and (2) do so in the context of one of the top “shared MS/EAE pathways.” For each of the CD4 T-cell functional pathways, there was a “core” of molecules present, with distinct interactions (Fig 3). The core consists of four Eae5 candidates on chr 17 (Runx2, Srf, Vegfa, and Vav1) and IL2 (on chr 3 [Eae3/20]), all of which biologically unify five of the top canonical pathways involving CD4 T cells (Fig 3, Table 2). Again, the identification of evolutionarily conserved pathways that lead to demyelination in human and mouse species underscores the importance of TH cells in both MS and EAE. Epistasis modeling also shows there is clearly the potential for interaction of the Eae5 candidates with other genes in each of the significant pathways and that these interactions may play a critical role in susceptibility to disease.
Figure 3.
IPA analysis of polymorphic genes retained in B10.S-SJL-Eae5S phenotype selected congenic mice. Eae5 candidate genes on chr 17 are indicated in green, and the polymorphic genes retained in the B10.S-SJL-Eae5S congenic mice that interact with the eae5 candidates are in blue (chr 2), purple (chr 3), yellow (chr 12), and brown (chr 13). The Eae5 candidate genes are present in pathways that function within CD4 T cells, and these 5 pathways are unified by IL2, VEGFA, VAV1, SRF, and RUNX2. The loss of any one or more of the connected non-chr 17 genes could lead to the loss of the EAE-phenotype associated with Eae5.
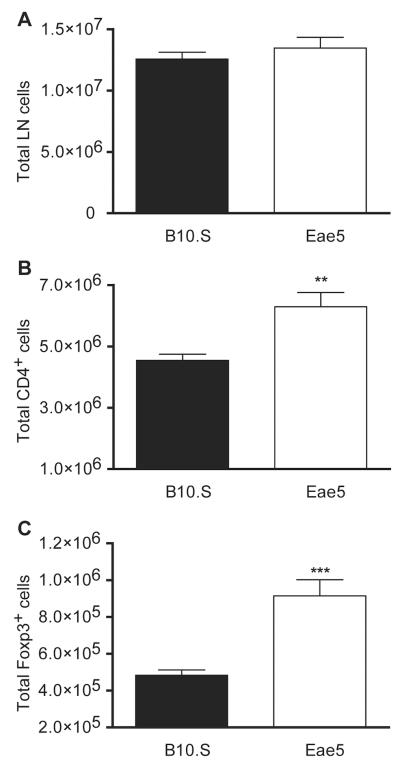
A test of this hypothesis is to show that Eae5 functions in T cells. The expression of Eae5 candidates in TH-cell pathways suggested that either CD4 TH cells or Tregs might be affected as a result of the inheritance of SJL alleles at Eae5. The frequency of natural Tregs (nTregs) in the lymph node is under the control of a gene within or tightly linked to H2.23 The Eae5 locus lies just distal to H2 (Fig 4). We hypothesized that the Eae5 candidate genes identified by in silico analysis would regulate the frequency or number of nTregs and susceptibility to EAE. We therefore quantified the total number of CD4+TCRβ+ T cells and the number of CD4+TCRβ+FoxP3+ nTregs in the lymph nodes of B10.S and B10.S-Eae5SJL congenic mice. Surprisingly, the absolute numbers of both TH cells and nTregs were significantly greater in B10.S-Eae5SJL mice compared to B10.S mice (Fig 5). These data establish that Eae5 plays a significant role in controlling the number of TH and nTregs and confirm the in silico approaches used.
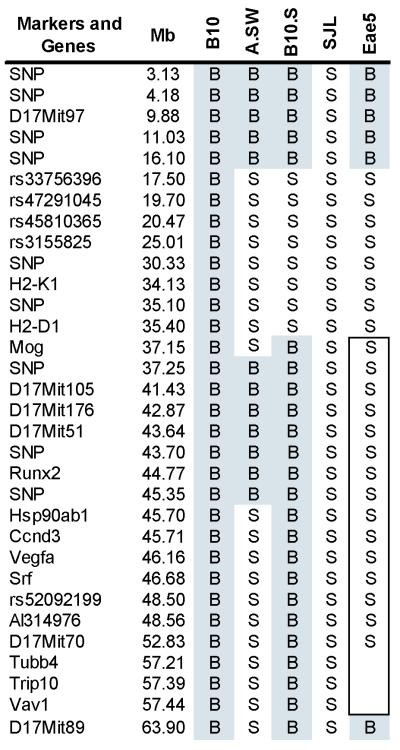
Figure 4.
The Eae5 haplotype and location of candidate genes. Traditional microsatellite mapping and SNP genotyping by phototyping44 were used to identify breakpoints in chr 17 for key strains. SNPs used are labeled by their position in megabases (Mb) and were developed from genomic databases at MPD. C57Bl/10J and A.SW were the parental strains used to create the B10.S H2 congenic and B10.S and SJL were the parental strains used to create the B10.S-Eae5SJL congenic strain. The distal border of Eae5 in the B10.S-Eae5SJL congenics is indeterminate.
Figure 5.
B10.S-Eae5SJL mice have an increased number of lymph node CD4+ T cells and Foxp3+ Treg cells. Total number of cells (A), CD4+ T cells (B), and CD4+Foxp3+ Treg cells (C) in the lymph nodes of naïve B10.S (■, n = 10) and B10.S-Eae5SJL (□, n = 6) mice. Statistical significance was determined using the Mann-Whitney test (**p<0.01; ***p<0.001). Data are representative of two independent experiments.
Discussion
The overpowering impact of HLA on MS susceptibility impinges on the identification of other genetic contributors necessary for disease. Therefore, we used the EAE model to identify non-MHC loci essential for disease by generating H2s-matched phenotype-selected congenic lines. Over the period of this selection, the incidence of EAE did not vary substantially in either of the crosses of as a consequence of trait selection (Supplemental Table 1). The phenotype selection approach was useful, however, for accumulating progeny mice that were significantly more likely to carry selected alleles of known EAE BTL and to transmit the selected EAE phenotype to their progeny.
We were able to selectively retain donor intervals that significantly conveyed resistance or susceptibility in the host strain. However, selection of mice at each generation that had the right phenotype and, presumably, the right EAE BTL alleles, did not lead to a high proportion of mice bearing the selected trait after five generations. This result indicates that numerous BTL must be retained in individual mice to govern EAE incidence. Therefore, in this strain combination, the generation of phenotype-selected and MAS congenic lines cannot be used for positional cloning of EAE candidate genes due to the polygenic nature of the disease. However, this finding does not apply to all animal models of complex genetic diseases, as witnessed in the non-obese diabetic (NOD) model.24 It is widely accepted that the selection of phenotypes for gene discovery is more successful when a susceptible genetic background is converted to resistance, as seen in NOD.B6 congenic mice. Unfortunately, we were unable to maintain SJL-B10.S-EaeR lines because of the poor breeding performance and aggressive nature inherent in the SJL strain.
To circumvent the difficulties pertaining to positional cloning and subsequent gene identification in the B10.S-SJL-Eae5S congenic lines, we developed an in silico method to ascertain polymorphic positional candidate genes within Eae5. This approach identified a core of Eae5 candidates—Runx2, Srf, Vegfa, and Vav1—that interacted with genes within the top ten canonical pathways. Of these genes, Vav1 has been shown to influence both EAE and MS susceptibility by controlling T-cell expansion and proinflammatory cytokine production,25, 26 and Vav1 has itself been suggested as a candidate gene.25 Additionally, TUBB4 (6.49 Mb), TRIP10 (6.74 Mb), and VAV1 (6.77 Mb), syntenic with human chr 19, are in linkage disequilibrium with TNFSF14 (LIGHT) (6.61 Mb), which has been confirmed as an MS-GWAS candidate in a forthcoming study from The International Multiple Sclerosis Genetics Consortium and the Wellcome Trust Case Control Consortium.27 Dysregulation of TNFSF14/LIGHT activity results in the disturbance of T-cell homeostasis, including an enlarged T-cell compartment, and the breakdown of peripheral tolerance.28
The most highly significant set of pathways emerging from our analysis governs TH-cell differentiation. Cellular and molecular techniques have defined a clear role for CD4 T cells in the pathogenesis of MS and EAE. Our results genetically support these data and suggest that the dysregulation of TH-cell differentiation may be the primary mechanism leading to MS and EAE susceptibility. Furthermore, pathways involving the communication between innate and adaptive immune systems; regulation of IL-2 expression; vitamin D receptor and retinoic acid signaling; and ICOS signaling are all unified by the core of Eae5 candidates through their function within CD4 T cells (Figure 2). Therefore, the altered regulation of one or more Eae5 candidate genes may impact CD4 T-cell differentiation and function and contribute to MS and EAE susceptibility, perhaps due to their role in the differentiation and/or function of Tregs. In the B10.S × SJL intercrosses and in the phenotype selection cohort, homozygous or heterozygous expression of the SJL-derived allele of Eae5 was associated with higher incidence of EAE, indicating that either the increased number of lymph node CD4 T cells or nTregs seen in the B10.S-Eae5S congenic mice leads to an increased susceptibility to disease. Studies to determine which of these alternative explanations is true are underway.
Our in silico approach also identified genes in other retained loci that epistatically interact with the Eae5 candidates within the context of CD4 T cells. Interestingly, Il2, which resides within Eae3/20 on chr 3, is central to all five top canonical pathways that signal in CD4 T cells. In human subjects, functional polymorphisms in the IL2 and IL2Rα genes have been identified in genetic studies29, 30 and in GWAS for other autoimmune disorders (http://t1dbase.org/page/Regions). IL-2 signaling plays a key role in immunomodulation through control of regulatory T-cell homeostasis and function. Indeed, small perturbations in IL-2 levels or in Treg frequency have been associated with differential susceptibility to EAE.23, 31 In addition, the environmentally derived factor VitD is physiologically immunoregulatory due, in part, to positive effects on Tregs.32, 33 In humans, low VitD levels are an environmental factor that is strongly associated with increased MS34 and relapse severity35 and exogenous VitD confers protection from EAE in mice.23, 36-38
Importantly, the EAE results accurately reflect the polygenic and multifactorial nature of MS in humans. In line with the transient nature of interval retention during phenotype selection, each GWAS-determined SNP associated with MS is relatively weak and contributes only a small fraction to the variance seen in patients. Our data are not incompatible with the hypothesis that stronger MS risk alleles are rare and would probably not be included in any GWAS that limited the minor allele frequency to > 0.05.39 Searching for the rare alleles of MS risk genes has already yielded some interesting results.40 A rare allele of TYK2 (present in the homozygous state in only 0.164% of individuals studied [http://www.msgene.org]) is associated with MS resistance. In this regard, the autoimmune-resistant Tyk2E775K allele is also rare (it is present only in B10.D1-H2q/SgJ mice and not found to be segregating among >100 different classical laboratory and wild-derived inbred strains [unpublished data]), and it controls full susceptibility and resistance to EAE.41
In summary, by using an evolutionarily conserved network-based pathway analysis, we have established a critical role for CD4 T-cell differentiation and function in the pathogenesis of EAE and MS. Importantly, our results provide a functional framework in which animal models can provide insight into understanding the evolutionary overlap between the non-MHC genetic basis for susceptibility to different autoimmune diseases, i.e., the shared autoimmune disease susceptibility gene hypothesis, that first arose from animal studies looking at the genetic control of susceptibility to EAE and experimental autoimmune orchitis.42, 43 Moreover, such studies may identify pathways that may be optimally suited to therapeutic intervention for the prevention or treatment of autoimmune disease.
Supplementary Material
Acknowledgements
We are grateful for the assistance of numerous technicians who helped by genotyping the mice over the course of this study. This work was supported by the NIH grants NS061014, NS069628, and NS36526 to CT and NMSS grant RG3575 to EPB.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat.Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt H, Williamson D, Ashley-Koch A. HLA-DR15 haplotype and multiple sclerosis: a HuGE review. American journal of epidemiology. 2007;165:1097–1109. doi: 10.1093/aje/kwk118. [DOI] [PubMed] [Google Scholar]
- 3.Fazekas G, Tabira T. What transgenic and knockout mouse models teach us about experimental autoimmune encephalomyelitis. Rev.Immunogenet. 2000;2:115–132. [PubMed] [Google Scholar]
- 4.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 5.Blankenhorn EP, Butterfield RJ, Rigby R, et al. Genetic analysis of the influence of pertussis toxin on experimental allergic encephalomyelitis susceptibility: an environmental agent can override genetic checkpoints. J Immunol. 2000;164:3420–3425. doi: 10.4049/jimmunol.164.6.3420. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield RJ, Blankenhorn EP, Roper RJ, et al. Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunogenetically distinct. J Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- 7.Butterfield RJ, Blankenhorn EP, Roper RJ, et al. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am J Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterfield RJ, Sudweeks JD, Blankenhorn EP, et al. New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 9.Encinas JA, Lees MB, Sobel RA, et al. Genetic analysis of susceptibility to experimental autoimmune encephalomyelitis in a cross between SJL/J and B10.S mice. J.Immunol. 1996;157:2186–2192. [PubMed] [Google Scholar]
- 10.Encinas JA, Lees MB, Sobel RA, et al. Identification of genetic loci associated with paralysis, inflammation and weight loss in mouse experimental autoimmune encephalomyelitis. Int.Immunol. 2001;13:257–264. doi: 10.1093/intimm/13.3.257. [DOI] [PubMed] [Google Scholar]
- 11.Teuscher C, Bunn JY, Fillmore PD, et al. Gender, age, and season at immunization uniquely influence the genetic control of susceptibility to histopathological lesions and clinical signs of experimental allergic encephalomyelitis: implications for the genetics of multiple sclerosis. Am J Pathol. 2004;165:1593–1602. doi: 10.1016/S0002-9440(10)63416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teuscher C, Butterfield RJ, Ma RZ, et al. Sequence polymorphisms in the chemokines Scya1 (TCA-3), Scya2 (monocyte chemoattractant protein (MCP)-1), and Scya12 (MCP-5) are candidates for eae7, a locus controlling susceptibility to monophasic remitting/nonrelapsing experimental allergic encephalomyelitis. J Immunol. 1999;163:2262–2266. [PubMed] [Google Scholar]
- 13.Abiola O, Angel JM, Avner P, et al. The nature and identification of quantitative trait loci: a community’s view. Nature Reviews Genetics. 2003;4:911–916. doi: 10.1038/nrg1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teuscher C, Doerge RW, Fillmore PD, et al. eae36, a locus on mouse chromosome 4, controls susceptibility to experimental allergic encephalomyelitis in older mice and mice immunized in the winter. Genetics. 2006;172:1147–1153. doi: 10.1534/genetics.105.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai YD, Marrero IG, Gros P, et al. Slc11a1 enhances the autoimmune diabetogenic T-cell response by altering processing and presentation of pancreatic islet antigens. Diabetes. 2009;58:156–164. doi: 10.2337/db07-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillmore PD, Blankenhorn EP, Zachary JF, et al. Adult gonadal hormones selectively regulate sexually dimorphic quantitative traits observed in experimental allergic encephalomyelitis. Am.J.Pathol. 2004;164:167–175. doi: 10.1016/S0002-9440(10)63107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fillmore PD, Brace M, Troutman SA, et al. Genetic analysis of the influence of neuroantigen-complete Freund’s adjuvant emulsion structures on the sexual dimorphism and susceptibility to experimental allergic encephalomyelitis. Am J Pathol. 2003;163:1623–1632. doi: 10.1016/S0002-9440(10)63519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behavioural brain research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 19.Falk K, Rotzschke O, Santambrogio L, et al. Induction and suppression of an autoimmune disease by oligomerized T cell epitopes: enhanced in vivo potency of encephalitogenic peptides. The Journal of experimental medicine. 2000;191:717–730. doi: 10.1084/jem.191.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matesanz F, Caro-Maldonado A, Fedetz M, et al. IL2RA/CD25 polymorphisms contribute to multiple sclerosis susceptibility. Journal of neurology. 2007;254:682–684. doi: 10.1007/s00415-006-0416-4. [DOI] [PubMed] [Google Scholar]
- 21.Vinuesa CG, Cook MC, Angelucci C, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 22.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature immunology. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 23.del Rio R, Sun Y, Alard P, et al. H2 control of natural T regulatory cell frequency in the lymph node correlates with susceptibility to day 3 thymectomy-induced autoimmune disease. Journal of Immunology. 2011;186:382–389. doi: 10.4049/jimmunol.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 25.Jagodic M, Colacios C, Nohra R, et al. A role for VAV1 in experimental autoimmune encephalomyelitis and multiple sclerosis. Science translational medicine. 2009;1:10ra21. doi: 10.1126/scitranslmed.3000278. [DOI] [PubMed] [Google Scholar]
- 26.Korn T, Fischer KD, Girkontaite I, et al. Vav1-deficient mice are resistant to MOG-induced experimental autoimmune encephalomyelitis due to impaired antigen priming. Journal of Neuroimmunology. 2003;139:17–26. doi: 10.1016/s0165-5728(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 27.The International Multiple Sclerosis Genetics Consortium. Wellcome Trust Case Control Consortium Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011 doi: 10.1038/nature10251. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Zhu M, Miller M, et al. Immunoregulation by tumor necrosis factor superfamily member LIGHT. Immunological reviews. 2009;229:232–243. doi: 10.1111/j.1600-065X.2009.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matesanz F, Fedetz M, Collado-Romero M, et al. Allelic expression and interleukin-2 polymorphisms in multiple sclerosis. Journal of Neuroimmunology. 2001;119:101–105. doi: 10.1016/s0165-5728(01)00354-x. [DOI] [PubMed] [Google Scholar]
- 30.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl.J.Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 31.del Rio R, Noubade R, Subramanian M, et al. SNPs upstream of the minimal promoter control IL-2 expression and are candidates for the autoimmune disease-susceptibility locus Aod2/Idd3/Eae3. Genes Immun. 2008;9:115–121. doi: 10.1038/sj.gene.6364455. [DOI] [PubMed] [Google Scholar]
- 32.Matejuk A, Bakke AC, Hopke C, et al. Estrogen treatment induces a novel population of regulatory cells, which suppresses experimental autoimmune encephalomyelitis. J Neurosci Res. 2004;77:119–126. doi: 10.1002/jnr.20145. [DOI] [PubMed] [Google Scholar]
- 33.Polanczyk MJ, Carson BD, Subramanian S, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 34.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 35.Wingerchuk DM, Lesaux J, Rice GP, et al. A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1294–1296. doi: 10.1136/jnnp.2004.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175:4119–4126. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 37.Joshi S, Pantalena LC, Liu XK, et al. 1,25-Dihydroxyvitamin D3 Ameliorates Th17 Autoimmunity via Transcriptional Modulation of Interleukin-17A. Molecular and cellular biology. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayne CG, Spanier JA, Relland LM, et al. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:822–832. doi: 10.1002/eji.201040632. [DOI] [PubMed] [Google Scholar]
- 39.Pritchard JK. Are rare variants responsible for susceptibility to complex diseases? Am J Hum Genetics. 2001;69:124–137. doi: 10.1086/321272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakkula E, Leppa V, Sulonen AM, et al. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. American Journal of Human Genetics. 2010;86:285–291. doi: 10.1016/j.ajhg.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spach KM, Noubade R, McElvany B, et al. A single nucleotide polymorphism in Tyk2 controls susceptibility to experimental allergic encephalomyelitis. J Immunol. 2009;182:7776–7783. doi: 10.4049/jimmunol.0900142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teuscher C. Experimental allergic orchitis in mice. II. Association of disease susceptibility with the locus controlling Bordetella pertussis-induced sensitivity to histamine. Immunogenetics. 1985;22:417–425. doi: 10.1007/BF00418088. [DOI] [PubMed] [Google Scholar]
- 43.Teuscher C, Blankenhorn EP, Hickey WF. Differential susceptibility to actively induced experimental allergic encephalomyelitis and experimental allergic orchitis among BALB/c substrains. Cellular immunology. 1987;110:294–304. doi: 10.1016/0008-8749(87)90124-9. [DOI] [PubMed] [Google Scholar]
- 44.Bunce M, O’Neil CM, Barnardo MC, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.