Abstract
Aims
To evaluate the efficacy and safety of the PROMETA™ Protocol for treating methamphetamine dependence.
Design
A double-blind, placebo-controlled 108-day study with random assignment to one of two study conditions: active medication with flumazenil (2 mg infusions on days 1, 2, 3, 22, 23), gabapentin (1200 mg to day 40) and hydroxazine (50 mg to day 10) versus placebo medication (with active hydroxazine only).
Setting
Three substance abuse treatment clinics: two in-patient, one out-patient.
Participants
Treatment-seeking, methamphetamine-dependent adults (n = 120).
Measurements
Primary outcome was percentage of urine samples testing negative for methamphetamine during the trial.
Findings
No statistically significant between-group differences were detected in urine drug test results, craving, treatment retention or adverse events.
Conclusions
The PROMETA protocol, consisting of flumazenil, gabapentin and hydroxyzine, appears to be no more effective than placebo in reducing methamphetamine use, retaining patients in treatment or reducing methamphetamine craving.
Keywords: Methamphetamine, pharmacotherapy, PROMETA protocol, substance abuse treatment
INTRODUCTION
Methamphetamine is the most abused illicit drug worldwide after cannabis, with about 15–16 million regular users [1]. Epidemiological findings show that methamphetamine use decreased between 2002 and 2006 in the United States [2,3], although the number of Americans who met criteria for methamphetamine abuse or dependence doubled between 2002 and 2004. In 2008, 529 000 Americans over age 12 used methamphetamine in the past month, 1.2 million in the past year [3], with more than one-half residing in the Western United States and substantial proportions of users in the Midwest and the South [2,3].
There are no approved medications for the treatment of methamphetamine dependence. Efficacious treatments include behavioral and psychosocial approaches of contingency management, cognitive–behavioral therapy, motivational enhancement strategies [4] and 12-Step programs [5].
Ideally, a pharmacotherapy for methamphetamine dependence should address one or more aspects of the neurobiological processes involved in supporting chronic use of methamphetamine and/or facilitating its discontinuation. Dopaminergic processes are central to the reinforcing and behavioral properties involved with methamphetamine use [6], yet, with the exception of a single report showing direct dopamine agonist effect using methylphenidate [7], trials of dopamine agonist and antagonist compounds [8] have been disappointing. Some positive findings have emerged from efforts to evaluate compounds that have effects that project onto the dopaminergic system, including naltrexone, acting on the opioid system, and bupropion, affecting a combination of amine systems +/− nicotinic acetylcholine receptor (nACHr) inhibition (bupropion) [9,10]. Limited success has been shown using the irreversible gamma amino butyric acid (GABA) transaminase inhibitor vigabatrin for cocaine and methamphetamine dependence in human research [11].
The proprietary system of treatment for methamphetamine dependence, the PROMETA™ protocol, combines medications purported to normalize altered dopamine, glutamate and GABA neurotransmitter function produced by chronic psychostimulant use, along with psychosocial treatment designed to minimize withdrawal symptoms, help prevent relapse and reduce drug cravings. The multiple components are thought to provide an integrated neurological, physiological and psychosocial approach for methamphetamine dependence, to redress specific effects of chronic psychostimulant exposure and the corresponding symptoms of psychostimulant withdrawal, and to restore balance in inhibitory neurological circuits.
The three medications included in the proprietary protocol provide theoretical underpinning for the approach. The principal element in the approach is flumazenil, a specific benzodiazepine antagonist used in the operating room to facilitate recovery from deep sedation, and in the emergency room as an antidote for benzodiazepine overdose [12]. Flumazenil reverses the central nervous system (CNS) depressant effects of a number of nonbenzodiazepine drugs [12,13] and may act both as partial agonist and inverse agonist at GABA receptors [14]. Although the purported therapeutic effects of the PROMETA protocol is conceptualized to be from the combined effects of the component medications, the key element of the treatment protocol is presumed to be the flumazenil infusion from its activity on GABAergic thresholds at the benzodiazepine receptors.
Gabapentin, an anti-convulsant acting as a GABA agonist, is a second medication in the proprietary protocol that is also thought to decrease brain glutamate concentrations [15]. Gabapentin has been shown in animal models to have anxiolytic-like effects and has been used as an analgesic [16]. Gabapentin has been reported to reduce craving [17,18] and other subjective effects of cocaine administration [19].
Hydroxyzine hydrochloride, an H1 histamine receptor antagonist used for the treatment of generalized anxiety disorders, has been widely used in the management of withdrawal from substance dependence during the initial phase of detoxification and throughout treatment. It has also been shown to be an anti-emetic, a sedative and a skeletal muscle relaxant [20].
The purpose of this study was to evaluate the efficacy and safety of a combination medication protocol of flumazenil, gabapentin and hydroxyzine (the PROMETA™ protocol) for treating methamphetamine dependence compared to placebo in participants treated at one of three Los Angeles area study sites. Study recruitment occurred during a local advertising campaign conducted by Hythiam, Inc., that featured radio, website and newspaper advertisements. Individuals residing in the Los Angeles area, who contacted the PROMETA Call Center, operated by the owner of the proprietary procedure, were often referred to the study team for telephone screening of eligibility.
METHODS
Participants
Eligibility criteria included: age 18 years and older; methamphetamine abuse or dependence per DSM-IV-TR criteria [21,22]; treatment seeking; reporting methamphetamine use on at least 4 of the last 30 days; and if female, neither pregnant nor lactating and willing to use birth control. Participants were excluded for current dependence on any psychoactive substance other than methamphetamine, alcohol, nicotine or marijuana; needing medical detoxification from alcohol; psychiatric or neurological disorders involving seizures, loss of consciousness or dementia; suicidal ideation; uncontrolled hypertension, significant heart disease or other disorders that could significantly alter metabolism of study agents; clinically significant abnormal laboratory values; benzodiazepine use within 15 days of treatment; acquired immune deficiency syndrome (AIDS) or active tuberculosis; an allergy or sensitivity to any of the study medications; or treatment with PROMETA in the prior 12 months.
Procedures
Research procedures were overseen by the UCLA Human Subjects Protection Committee, the Mission Hospital Ethics Committee, and the UCLA Addiction Medicine Data and Safety Monitoring Board. The trial was registered on Clinicaltrials.com.
Study staff were trained on the treatment protocol by members of Hythiam, Inc., the owner of the proprietory protocol, in order to use treatment procedures identical to those being used in private medical settings. Guidance documents were also utilized to assist the study team in developing the study protocol and operations manual.
Individuals meeting preliminary screening criteria completed a full screening appointment at one of three study sites (two in-patient infusion sites; one out-patient infusion site). Screening included the consent process and assessments to determine eligibility. Eligible participants were scheduled for the first infusion series and randomized to treatment group by the UCLA Data Management Center (DMC) using an urn randomization computer program [23] to ensure multivariate balance across gender (male, female) and severity of reported methamphetamine use in the 30 days prior to baseline (≤10 days; 11+ days). Randomization assignment was faxed to the university pharmacy for preparation of study medications. Participants were scheduled for 14 weekly clinic assessment visits across the study duration. Incentives, designed to compensate for time, travel costs, parking, and childcare, included $50 for screening, $25 for each of 12 clinic visits, $75 for each of 5 infusion days and $50 for the final visit.
Pharmacotherapy
Blinded study drugs were prepared to appear identical: flumazenil and saline were both clear liquids in transparent vials; gabapentin and placebo were over-encapsulated using opaque capsules. Prior to each 2 mg infusion on days 1, 2, 3, 22 and 23, participants were administered a 50-mg dose of oral hydroxyzine, with 50 mg take-home hydroxyzine to day 10. As hydroxyzine is not considered the key element of the PROMETA protocol, all participants received active hydroxyzine in order to reduce the anxiety that might be experienced during the medical procedures, and to assist with sleep. On day 1, participants began gabapentin or placebo, increasing by one capsule (300 mg) per day to reach the maximum dose of 1200 mg on study day 4. Down-titration began on study day 38 with the final gabapentin or placebo dose on day 40.
Cognitive behavioral therapy
A standardized treatment manual, adapted from the Carroll [24] manual for treatment of stimulant dependence and used in multiple medication studies for treating stimulant dependence, provided 14 weekly cognitive–behavioral therapy (CBT) sessions by a trained and supervised master's level counselor over the course of the study. Topics included: internal and external triggers to drug use, phases of recovery from stimulant abuse, scheduling, teaching problem-solving skills and increasing self-efficacy.
Measures
Assessments
Measures included: (i) the Addiction Severity Index (ASI) [25]; (ii) the SCID-IV-TR or the DSM-IV-TR checklist; (iii) medical history, physical examination, laboratory tests including an infectious disease panel and urinalysis, 12-lead electrocardiogram, vital signs, and pregnancy test, administered at screening; (iv) urine drug tests at every clinic contact to test for metabolites of methamphetamine, benzodiazepines, cocaine, opioids and cannabis using radioimmune assay (Phamatech, Inc., San Diego, CA, USA); (v) the Brief Symptom Craving Scale (BSCS) [26], collected weekly to record self-reported craving for methamphetamines; (vi) retention, measured by the number of days between the first infusion and the last clinic visit; and (vii) adverse events, documented at each clinic visit.
Statistical analyses
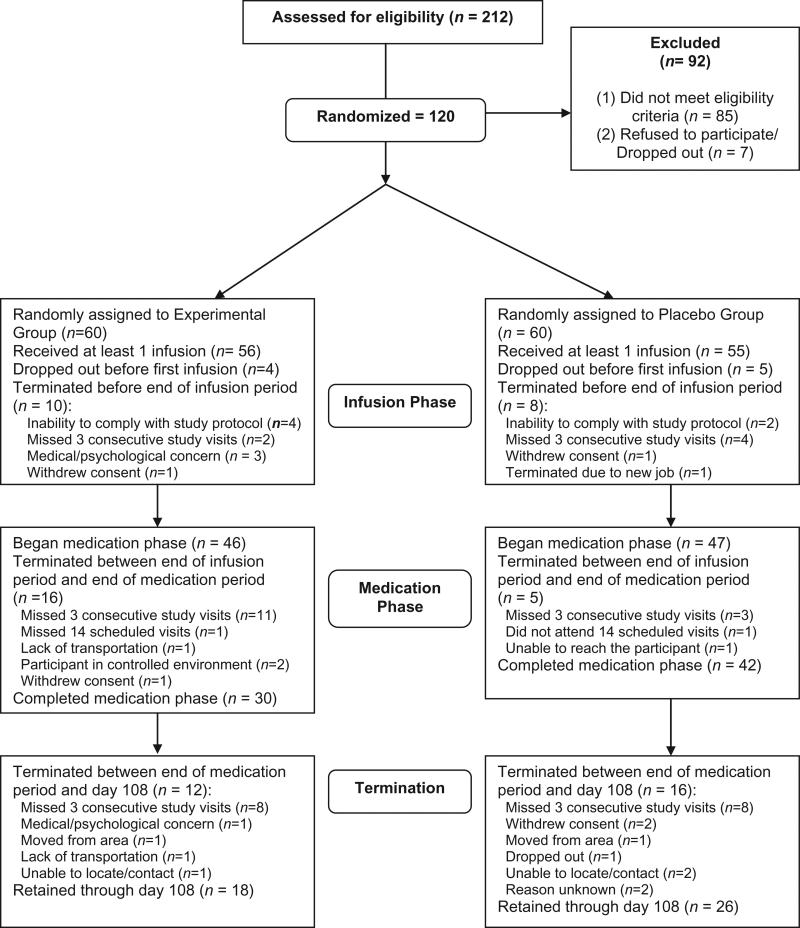
Analyses use a modified version of intent-to-treat, as some participants were randomized but never received a first infusion and were lost to follow-up. These participants did not contribute treatment-related data and were excluded from analyses. As seen in Fig. 1, they were balanced across the groups.
Figure 1.
Consort diagram
Safety and efficacy were measured using urine drug tests for methamphetamine, self-reported drug use, addiction behaviors, craving, adverse events, and the number of days in treatment. Analyses of the primary outcome, methamphetamine use, used urine drug test results to compute a proportion based on the number of methamphetamine-negative urine tests over the number of tests possible through each study time-point (day 23, end of infusion phase; day 40, end of medication phase; day 108, end of trial). Analyses use urine samples collected at each weekly clinic visit and the first day of the second infusion series at all sites, and were also collected on second and third infusion days at the out-patient site, for a total of 18 at the out-patient infusion site and 15 at the in-patient infusion sites.
Univariate analyses were conducted between groups on baseline variables using χ2 tests for categorical data and t-tests or Wilcoxon Mann–Whitney tests for numerical data. Variables on which the treatment groups differed significantly at baseline were used as covariates in the main outcome analyses. Where possible, baseline scores showing differences between groups were covaried when testing for condition effects. Statistical tests were two-sided, with alpha set at 0.05. No probability adjustment was applied for analysis of multiple outcomes. All analyses were carried out using SPSS (version 16.0; SPSS, Inc.) and SAS (version 9.1.3; SAS Institute, Inc.).
A sample of 90 was planned to detect a medium effect between groups. After the study began, the sample size was increased to 120 to adjust for participant dropout over the course of the study. Power was set at 0.80 with a medium effect size expected for the experimental condition over placebo with d = 0.3.
RESULTS
Participant characteristics
Comparisons of baseline demographic and drug use characteristics between the experimental and placebo groups in Table 1 shows that the groups differed only by age, with the mean age of the experimental group older than the placebo group.
Table 1.
Baseline characteristics of the experimental and placebo medication groups.
| Medication groups | ||||
|---|---|---|---|---|
| Characteristic | Experimental (n = 56) | Placebo (n = 55) | P-value | |
| Mean age (years) (SD) | 40.77 (7.46) | 35.98 (8.75) | 0.0033 | |
| Gender (%) | Male | 80 | 75 | NS |
| Ethnicity: (%) | White | 65.4 | 55.4 | NS |
| Hispanic (all) | 21.8 | 37.4 | ||
| Black | 9.1 | 5.4 | ||
| Marital status (%) | Married | 20.0 | 21.4 | NS |
| Widowed/divorced/separated | 16.4 | 17.9 | ||
| Never married | 63.6 | 60.7 | ||
| Employment (%) | Full time | 58.2 | 53.6 | NS |
| Part-time | 18.2 | 32.1 | ||
| Unemployed | 20.0 | 10.7 | ||
| Other | 3.6 | 3.6 | ||
| Criminal justice status | On probation/parole (%) | 89.1 | 82.1 | NS |
| Has criminal history (%) | 43.6 | 33.9 | NS | |
| Abuse history | Physically abused (%) | 60.0 | 57.1 | NS |
| Sexually abused (%) | 69.1 | 75.0 | NS | |
| Drug treatment/drug use/craving | Mean no. of treatments | 1.69 (2.76) | 2.02 (5.40) | NS |
| Mean no. of days amphetamine/methamphetamine | ||||
| Use in past 30 days | 17.82 (9.90) | 17.13 (9.45) | NS | |
| Mean years of use | 10.84 (8.18) | 9.66 (6.60) | NS | |
| Mean craving (BSCS) | 6.62 (2.91) | 6.59 (2.93) | NS | |
BSCS: The Brief Symptom Craving Scale; SD: standard deviation; NS: not significant. [Correction added after online publication 7 December 2011; amphetamine/methadone has been changed to amphetamine/methamphetamine].
Methamphetamine use
Table 2 provides comparisons of methamphetamine use by group across study phases. After controlling for age, the mean proportion of methamphetamine-negative urine drug tests did not differ significantly between the groups from screening to the end of the infusion phase (F(2108) = 0.60; P = 0.55), medication phase (F(2108) = 0.87; P = 0.42) or study end (F(2108) = 1.28; P = 0.28). The percentage of missing urine tests was computed for both the completer and non-completer groups, with the completer group (n = 41) missing a mean of 16.6% of urine tests and the non-completer group (n = 70) missing a mean of 68.5% of urine tests. (Table 2 shows that 45 participants were retained to the study end; however, four participants missed three or more consecutive visits so did not meet ‘completer’ criteria.)
Table 2.
Study outcomes by study day and treatment groups.
| Condition |
||
|---|---|---|
| Outcome variable: study time-point | Experimental (n = 56) | Placebo (n = 55) |
| Mean proportion methadone-negative urine testsa (SD) | ||
| Day 23: end of infusion phase | 0.5 (0.4) | 0.5 (0.4) |
| Day 40: end of medication phase | 0.4 (0.4) | 0.5 (0.4) |
| Day 108: end of study | 0.3 (0.3) | 0.4 (0.3) |
| Craving (mean, SD)b | ||
| Day 23: end of infusion phase | 2.8 (2.9) | 2.9 (3.0) |
| Day 40: end of medication phase | 3.1 (3.4) | 2.9 (3.2) |
| Day 108: end of study | 2.2 (2.7) | 2.4 (3.4) |
| % 3 consecutive negative UA tests (n) | ||
| Day 40: end of medication phase | 35.7 (20) | 45.5 (25) |
| Day 108: end of study | 39.3 (22) | 50.9 (28) |
| % retained in study (n) | ||
| Day 23: end of infusion phase | 82.1 (46) | 85.5 (47) |
| Day 40: end of medication phase | 53.6 (30) | 76.4 (42) |
| Day 108: end of study | 32.1 (18) | 47.3 (26) |
Total of urine tests possible: three for in-patient sites and six for out-patient site to end of infusion phase, five for in-patient sites and eight for out-patient site to end of medication phase, and 15 for in-patient sites and 18 for out-patient sites to end of study.
The craving score was computed from three items with Likert scale responses from 0–4, with a possible total score ranging from 0 to 12.
SD: standard deviation; UA: urinalysis.
The percentage of participants who provided 3 consecutive weeks of methamphetamine metabolite-free urine samples did not differ significantly by study group when controlling for age to the end of the medication phase (χ2(2) = 2.19; P = 0.34) or to study end (χ2(2) = 1.89; P = 0.39).
Although there were no significant between-group differences in urine test results, post-hoc analyses of the full sample showed a significant decrease in methamphetamine use between the baseline urine sample (positive, n = 73; negative, n = 38) and the urine sample results at day 23 (χ21 = 31.91, P < 0.0001) at day 40 (χ21 = 25.75, P < 0.0001) and at day 108 (χ21 = 17.03, P < 0.0001).
Reduction in self-reported days of methamphetamine use
There was a three- to fourfold reduction in the number of days of self-reported methamphetamine use in the past 30 days from baseline [mean = 17.29; standard deviation (SD) = 9.89] to the end of treatment for all participants (mean = 4.45; SD = 8.03; t(50) = 8.70; P < 0.001). No statistical differences were observed in days of meth-amphetamine use between groups at screening or study end. The experimental group reported using methamphetamine a mean of 17.12 days (SD = 9.45) at screening, compared to a mean of 17.82 days (SD = 9.90) for the placebo group (t(109) = 0.38; P = 0.71). At study end, mean days of methamphetamine use for the experimental group (n = 23) was 5.04 days (SD = 9.09) and was 3.96 days (SD = 7.17) for the placebo group (n = 28) (t(49) = –0.47; P = 0.64).
Retention
Table 2 includes retention numbers by study phase for participants who received at least one infusion. In addition to numbers of participants who completed each study phase, mean number of days retained was also computed. The experimental group was retained in the study for a mean of 57.21 days (SD = 39.68) and the placebo group was retained for a mean of 74.04 days (SD = 39.10). Cox regression analysis shows that these differences were not statistically significant when covarying for age (χ2(2) = 2.44; P = 0.30); however, analyses not controlling for age indicate a significant group difference (SD = 39.10; t(109) = –2.25, P = 0.03).
Craving
There were no statistically significant differences between groups in craving scores at any assessment (Table 2). Combining groups, craving ratings decreased significantly from baseline to end of the infusion phase (F(1291) = 70.89; P < 0.001), end of the medication phase (F(1291) = 55.46; P < 0.001) and to study end (F(1291) = 68.89; P < 0.001).
Adverse events
A total of 157 adverse events (AEs) were documented over the study duration, including 75 from the experimental group and 82 from the placebo group, a non-significant difference. AEs were reported by 62 participants with a mean of 2.5 events each. Combining groups, most AEs (65%) were deemed mild, with 32% moderate and 3% severe. No life-threatening events occurred. By treatment group, AE severity was statistically different, with the experimental group experiencing a higher percentage of mild AEs and the placebo group experiencing a higher percentage of moderate AEs: 73% of the experimental group and 59% of the placebo group reported mild AEs; 23% of the experimental group and 40% of the placebo group reported moderate AEs; and 4% of the experimental group and 1% of the placebo group reported severe AEs (χ2(2) = 6.08, P = 0.05). No AE was deemed definitely related to the study drug, and only one was judged to be probably related. Four serious adverse events occurred, with three deemed not related to study medication. One participant in the experimental group neglected to report a history of seizures, and was terminated after experiencing a generalized tonic clonic seizure during the first infusion. Table 3 presents the most often-occurring AEs by treatment group.
Table 3.
Most often occurring adverse events (incidencea) by medication group.
| Event | Experimental | Placebo | Total |
|---|---|---|---|
| Headache | 19 | 15 | 34 |
| Cold/flu/fever/cough | 3 | 12 | 15 |
| Back pain | 7 | 4 | 11 |
| Sleep problems/insomnia | 8 | 1 | 9 |
| Tingling/numbness | 5 | 3 | 8 |
| Depression | 2 | 4 | 6 |
| Hot flashes | 0 | 6 | 6 |
| Dizziness/lightheaded | 1 | 4 | 5 |
| Nausea/vomiting | 2 | 3 | 5 |
| Anxiety | 1 | 3 | 4 |
| Sinus/allergy | 3 | 1 | 4 |
| Toothache | 1 | 3 | 4 |
| Leg/arm pain | 2 | 1 | 3 |
| Pain | 2 | 1 | 3 |
| Panic attacks | 2 | 1 | 3 |
Incidence is defined as the reporting of the symptom at any time during the trial; 62 participants reported adverse events (AEs).
Treatment compliance
A total of 58.9% of the experimental group received all five infusions compared to 69.1% of the placebo group. Not all those receiving all five infusions were retained for the entire study. For fewer than the maximum number of infusions, of the experimental group 12.5%, 23.2%, 3.6% and 1.8%, respectively, received four, three, two and one infusions; of the placebo group: 9.1%, 16.4%, 1.8% and 3.6%, respectively, received four, three, two and one infusions.
Comparing self-reported take-home medication use by group (verified by pill counts), no differences were found between groups for gabapentin (experimental mean = 19.31 days SD = 11.65; placebo mean = 20.88 days, SD = 12.90; t(104) = –0.66; P = 0.51) or for hydroxyzine (experimental mean = 5.45 days, SD = 3.08; placebo mean = 6.78 days, SD = 4.20; t(104) = –1.87, P = 0.07). Results show no significant difference between the experimental and placebo groups in CBT session attendance: the experimental group attended a mean of 2.95 sessions (SD = 2.83) and the placebo group attended a mean of 2.86 sessions (SD = 2.59) (t(104) = 0.16, P = 0.88).
DISCUSSION
This study was conducted when the proprietary treatment protocol was heavily publicized and there was a great deal of debate and controversy within the drug abuse research and treatment community, as well as the investment community and the news media. Proponents were buoyed by anecdotal reports and uncontrolled studies, whereas opponents cited the lack of data from placebo-controlled trials. Although Hythiam, the owner of the proprietary treatment protocol, provided funding for the study, the conduct of the study and interpretation of the results were entirely in the hands of the investigative team and were carried out with approval and oversight of the UCLA IRB. The results were negative and clear: the active medication and placebo groups showed no statistically significant difference in drug use as measured by urine drug testing, self-reported drug use, or self-reported craving. Both groups substantially reduced their reported methamphetamine use from the 30-day period prior to baseline compared to all three designated treatment time-points. The placebo group remained in the study for approximately 17 days longer than the medication group, which was not statistically significant when controlling for age using multivariate analyses. No differences were observed between groups for the incidence of adverse events, and although there was a statistically significant difference between groups for adverse event severity, there were no clinically relevant differences observed in the pattern or severity of adverse events that would imply a greater risk in either group. The one participant who suffered a seizure after the first infusion turned out to have a history of seizure disorder that he failed to disclose at intake.
These results differ from the findings by Urschel [27], who compared the same medication combination and placebo in 135 study participants over a 30-day period. Study assessments were completed at days 6, 13, 20 and 30 with an additional urine drug screen at day 4. Over the entire study duration, participants in the active drug group had significantly fewer methamphetamine-positive urine drug test results compared to the placebo group (P = 0.02). When addressing each assessment day, however, the only significant difference in urine test results between the treatment groups was found at day 6, the visit immediately following the last of the first three infusions.
In trying to determine possible explanations for the differences in results between Urschel's study and ours, we looked at the differences in methods of analyses between the two studies. Because we had not examined urine results separately for each assessment day, we conducted post-hoc analyses to determine whether our groups differed in urine test results for day 6, as this was the day that appeared to drive the differences between Urschel's groups. We found no differences in day 6 urine test results between our two treatment groups.
One possible explanation for the difference in outcomes between the Urschel study and our study could be the influence of the marketing campaign that occurred during our trial, which may have elevated the placebo effect. Furthermore, participants’ perception of the complex medical procedures, including in-patient hospitalization for infusions at two sites, may have also contributed to a strong placebo effect. When surveying participants at one site at the end of treatment, twice as many participants believed that they received active medication as those who believed they received placebo. Post-hoc analyses suggests that these participants’ beliefs about their assigned treatment condition may have influenced treatment outcome. In this study, those who believed they were given the active medications were twice as likely to be abstinent at the end of the study compared to those who believed that they received placebo, regardless of what they actually received.
Our findings cannot tell us whether our participants are different from individuals presenting for this treatment in private medical offices. No information about private patients receiving this treatment is accessible. We did, however, adhere to the procedural guidelines provided by Hythiam, Inc. Possible differences between the procedures used in this trial and those that may be used in private practice settings include that we provided counseling by a certified counselor, whereas PROMETA-licensed physicians may have provided their own counseling to their patients. We also scheduled weekly clinic visits for counseling, assessments and collection of urine samples over the entire 108-day study duration, whereas private physicians may not offer an extended treatment period. Finally, our participants may have seen multiple physicians and other study staff during their tenure on this study, whereas patients in a private practice setting may have interacted only with a treating physician and nurse.
CONCLUSIONS
We conclude that under the conditions of this study, treatment with the combination medication protocol was no more effective than placebo in reducing participants’ methamphetamine use, keeping them in treatment, or reducing their methamphetamine craving.
Acknowledgements
This study was made possible by unrestricted funding received from Hythiam, Inc. for an investigator-initiated study. The funding agency played no role in study design or procedures except to provide specific information requested regarding the PROMETA protocol. The Principal Investigator and lead author, independent of any commercial funder, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
No author reports any conflict of interest.
Clinical trial registration
Trial registration was initiated and maintained on clinicaltrials.com (NCT00260481).
Declarations of interest
None.
References
- 1.United Nations Office on Drugs and Crime . World Drug Report, 2008. United Nations; Vienna, Austria: 2008. [Google Scholar]
- 2.Office of Applied Statistics Substance Abuse and Mental Health Services Administration (SAMHSA). The NSDUH Report. Methamphetamine Use, Abuse, and Dependence: 2002, 2003, and 2004. 2005 Available at: http://www.drugabusestatistics.samhsa.gov/2k5/meth/meth.htm (accessed 20 September 2011; archived by Webcite at http://www.webcitation.org/61qQK6dQD)
- 3.Office of Applied Statistics Abuse and Mental Health Services Administration (SAMHSA). National Survey on Drug Use and Health: National Findings. 2007 Available at: http://www.oas.samhsa.gov/nsduh/2k7nsduh/2k7results.pdf (accessed 20 September 2011; archived by Webcite at http://www.webcitation.org/61qQ38n6g)
- 4.Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–17. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinelli-Casey P, Hillhouse MP, Rawson R. 12-Step participation of methamphetamine-dependent adults previously participating in the Methamphetamine Treatment Project (MTP).. Presented at the annual meeting of the College on Problems of Drug Dependence; Scottsdale, AZ.. June 2006. [Google Scholar]
- 6.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–2. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 8.Vocci FJ, Elkashef A, Appel NM. Pharmacological treatment of methamphetamine addiction. In: Roll JM, Rawson RA, Ling L, Shoptaw S, editors. Methamphetamine Addiction: From Basic Science to Treatment. Guilford Press; New York: 2009. pp. 202–29. [Google Scholar]
- 9.Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 10.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;1:222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodie JD, Figueroa E, Laska EM, Dewey SL. Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2005;55:122–5. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- 12.Roberge RJ, Lin E, Krenzelok EP. Flumazenil reversal of carisoprodol (Soma) intoxication. J Emerg Med. 2000;18:61–4. doi: 10.1016/s0736-4679(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 13.Rubio F, Quintero S, Hernandez A, Fernandez S, Cozar L, Lobato IM, et al. Flumazenil for coma reversal in children after cannabis. Lancet. 1993;341:1028–9. doi: 10.1016/0140-6736(93)91120-b. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Ohmori T, Abekawa T, Koyama T. The role of benzodiazepine receptors in the acquisition and behavioral expression of sensitization to methamphetamine. Pharmacol Biochem Behav. 2000;65:705–10. doi: 10.1016/s0091-3057(99)00263-4. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty JA, Rhoney DH. Gabapentin: a unique anti-epileptic agent. Neurol Res. 2001;23:821–9. doi: 10.1179/016164101101199414. [DOI] [PubMed] [Google Scholar]
- 16.Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;20:742–50. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myrick H, Henderson S, Brady KT, Malcolm R, Measom M. Divalproex loading in the treatment of cocaine dependence. J Psychoact Drugs. 2001;33:283–7. doi: 10.1080/02791072.2001.10400575. [DOI] [PubMed] [Google Scholar]
- 18.Raby WN. Gabapentin therapy for cocaine cravings. Am J Psychiatry. 2000;157:2058–9. doi: 10.1176/appi.ajp.157.12.2058-a. [DOI] [PubMed] [Google Scholar]
- 19.Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine related subjective effects, but not self-administration by human. Drug Alcohol Depend. 2004;73:279–87. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for the DSM-IV (SCID) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 22.American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Author; Washington, DC: 1994. [Google Scholar]
- 23.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 24.Carroll KM. A Cognitive–Behavioral Approach: Treating Cocaine Addiction. NIDA Therapy Manuals for Drug Addiction. Department of Health and Human Services; Bethesda, MD: 1998. [Google Scholar]
- 25.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 26.Mezinskis J, Dryenforth S, Goldsmith R, Cohen R, Somoza E. Craving and withdrawal symptoms for various drugs of abuse. Psychiatr Ann. 1998;28:577–83. [Google Scholar]
- 27.Urschel HC, III, Hanselka LL, Baron M. A controlled trial of flumazenil and gabapentin for initial treatment of methylamphetamine dependence. J Psychopharmacol. 2011;25:254–62. doi: 10.1177/0269881109349837. [DOI] [PubMed] [Google Scholar]