Abstract
Aim
Invasive urothelial carcinoma of the bladder (UCB) is characterized by alterations in cell-cycle regulatory pathways. Defects in the expression of cyclin D1, a key cell-cycle regulator, have been implicated in progression of various types of cancer. In the present study, we investigated whether cyclin D1 expression is associated with clinicopathological parameters and whether it has any potential prognostic value in determining risk of UCB recurrence.
Patients and Methods
Tissue microarrays containing bladder cancer specimens (n=212) and adjacent normal bladder tissues (n=131) were immunostained using an antibody against cyclin D1. The association between cyclin D1 and clinicopathological parameters including stage, lymph node metastasis, and disease-free survival, were evaluated. Cyclin D1 mRNA expression data from human normal bladder (n=14) and cancer specimens (n=28) were extracted from the public Oncomine database.
Results
Cyclin D1 mRNA and protein expression were significantly higher in UCB compared to adjacent non-malignant bladder tissue (for mRNA p=0.003, for protein p=0.001). Cyclin D1 protein expression was significantly higher in non-invasive tumors than in muscle-invasive UCB (p=0.016). Among patients with muscle-invasive UCB, increased cyclin D1 expression in tumor cells significantly correlated with lymph node metastasis (p<0.001), and there was a trend of cyclin D1 together with lymph node positivity to be associated with disease recurrence (p=0.678). Loss of nuclear cyclin D1 expression in tumor cells was likewise significantly associated with the presence of lymph node metastasis (p<0.001).
Conclusion
Altered expression of cyclin D1 is associated with lymph node metastasis and risk of UCB recurrence. Cyclin D1 expression may therefore have clinical value as a prognostic marker and potential therapeutic target.
Keywords: Biomarker, bladder cancer, cyclin D1, muscle-invasive
In the United States, bladder cancer is the fourth most common cancer (1), and it is estimated that bladder cancer will account for 72,570 new cases and 15,210 deaths in the US alone in 2013 (2). Urothelial carcinoma of the bladder (UCB) is the most frequent histological subtype of bladder cancer and accounts for more than 90% of the cases in USA and Europe (3). UCB is classified into two major groups for treatment and prognostic purposes, namely: i) non-muscle invasive bladder cancer NMIBC (Ta/Tis/T1) and muscle-invasive bladder cancer (MIBC) (T2-T4). A majority of patients with UCB present with NMIBC that although clinically heterogeneous is typically associated with a favorable prognosis and a relatively low risk for recurrence following cystectomy. However, NMIBC is associated with a high risk (60-75%) of recurrence in patients with intact bladders (4). In contrast, MIBC accounts for only 25-30% of cases, yet the five-year survival rate, even following radical cystectomy, is greatly reduced as compared with NMIBC, particularly when patients have lymph node metastases (5-8). In both NMIBC and MIBC, available histopathological parameters, including tumor stage and grade do not always predict the course of disease in individual patients, including those who have an increased risk of post-cystectomy recurrence. There is therefore an urgent need to identify novel biomarkers that indicate UCB cases with increased risk for recurrence and metastases.
Multiple cellular pathways are altered in UCB (9-12). It is well-known that cell-cycle regulatory proteins and pathways are particularly important in controling normal growth and differentiation. Cyclins are critical cell-cycle proteins that control cell-cycle progression by activating cyclin-dependent kinases (CDK). Overexpression of cyclins is implicated in numerous human malignancies and contributes to cell-cycle dysfunction in various human cancer types. Indeed overexpression of cyclin D1 is detected in breast, prostate, uterine, ovarian, and colorectal cancer, as well as squamous carcinoma of the head and neck (13, 14). Overexpression of cytoplasmic cyclin D1 independently predicted poor outcome, such as overall survival and event-free survival, in patients with prostate cancer metastases (15). Overexpression of cyclin D1 has been also shown to be associated with significantly shorter event-free survival in ovarian cancer (16), suggesting the clinical importance of cyclin D1 expression in predicting cancer progression.
Interestingly, overexpression (17) and polymorphic variability of cyclin D1 gene also has recently been implicated in human bladder cancer (18), and the cyclin D1b variant has been shown to promote invasiveness in an experimental bladder cancer model (19). However, clinical evidence implicating cyclin D1 in UCB is conflicting. Whereas a recent report showed that nuclear cyclin D1 expression was associated with NMIBC (9), an earlier report found no association of cyclin D1 expression with pathological parameters or clinical outcome (20). Given the role of cyclin D1 in the cell cycle and in cancer progression, we therefore aimed to examine the expression of this factor in patient bladder cancer tissue specimens, and to evaluate the clinical importance of cyclin D1 expression in predicting clinical outcomes in NMIBC and MIBC.
Materials and Methods
Tissue samples and tissue microarrays
Local Institutional Review Board (Fox Chase Cancer Center, Philadelphia, PA, USA) approval was granted (Approval number: IRB 09-815) to obtain bladder cancer specimens from patients treated with radical cystectomy. The cohort represents the available subset of patients reported in Mahklin et al. 2011 (21). The study was approved by the local Ethical Committees of Fox Chase Cancer Center and Lund University (Approval number 2010/110), and the Helsinki Declaration of Human Rights was strictly observed. Tissue microarrays (TMAs) were constructed from 1 mm cores (two per sample) which contained both malignant and adjacent non-malignant urothelium, where possible. The TMAs included 212 bladder cancer cases; the clinical information was available for 204 cases, comprising of NMIBC (N=34) and MIBC (N=170). In addition, non-malignant specimens (N=131) of tissue adjacent to bladder cancer were included in the TMAs. Since some cores did not show clear staining, 188 out of 212 cases were available for evaluation (Table I). Patient demographics are summarized in Table I. Out of the 170 patients with MIBC, 68 had lymph node metastases (40%). The age of these patients ranged from 37 to 90 years and information about gender and other clinical characteristics are included in Table I. Tumors were staged according to the TNM classification and graded using the 1998 WHO classification (22). The patients were followed-up from 0 to 120 months from the time of surgery, with a mean of 80.77 months. Clinical information, such as surgical margin status, stage, grade, multifocality, number of previous bladder tumors, and disease-free survival, was obtained.
Table I. Clinicopathological characteristics of the cohort.
| Characteristics | No. of patients (%) |
|---|---|
| Total | 188 |
| Age (years) (n=156) | |
| ≤65 | 53 (33.9) |
| >65 | 103 (66.1) |
| Median | 71 |
| Range | 37-90 |
| p-Value | 0.981 |
| Gender (n=154) | |
| Male | 114 (74.0) |
| Female | 40 (25.9) |
| p-Value | 0.584 |
| Race (n=153) | |
| White | 130 (84.9) |
| Black | 17 (11.1) |
| Asians | 4 (2.6) |
| Unknown | 2 (1.3) |
| p-Value | 0.734 |
| Primary tumor stage (n=184) | |
| pTis | 7 (3.8) |
| pTa | 10 (5.4) |
| pT1 | 11 (5.9) |
| pT2 | 28 (15.2) |
| pT3 | 79 (42.9) |
| pT4 | 49 (26.3) |
| p-Value | 0.014 |
| Histology (n=171) | |
| Urothelial carcinoma (pure) | 140 (81.9) |
| Urothelial carcinoma with mixed histology | 31 (18.1) |
| p-Value | 0.054 |
| Lymph node status (n=169) | |
| pN0 | 106 (62.7) |
| pN+ | 63 (37.3) |
| p-value | 0.560 |
Source of antibodies and immunohistochemistry (IHC)
The following antibodies and dilutions were used for IHC and western blotting: mouse monoclonal against cyclin D1, 1:50 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for immunohistochemistry; rabbit polyclonal against cyclin D1, 1:500 (Cell Signaling, Beverly, MA, USA); mouse monoclonal against actin, 1:15000 (MP Biomedicals, Solon, OH, USA) for immunoblot analysis. Formalin-fixed, paraffin-embedded tissue samples were de-paraffinized in xylene for 10 min, followed by washing in a decreasing concentration of ethanol (95, 75, 50%) for 2 min each. After de-paraffinization, antigen retrieval was performed by boiling the slides in 0.01 M citrate buffer, pH 6.0, in a microwave for 10 min. The slides were then applied to a semi-automatic IHC diagnostic system (Ventana ES, Ventana Inc., Tucson, AZ) and IHC staining was performed using the antigen-specific antibodies as indicated above. The scoring of all the tumor cores was performed and later reanalyzed independently by a specialist urological pathologist (BDR). An intensity score was assigned and represented the average intensity of positively stained cells (0=no staining, 1=weak, 2=intermediate and 3=strong staining). The fraction of cells stained with antibodies to cyclin D1 were counted as a percentage and scored (0%=0, <15%=1, 15-50%=2, >50%=3).
Analysis of cyclin D1 mRNA expression from gene expression profiles
We analyzed the DNA microarray gene expression profiles (23) available in the public Oncomine database (24). The raw signal intensities of the arrays were preprocessed by setting the intensity to the minimum value if the intensity was below a minimum value of 10, and normalized by subtracting the median log ratio of an array by all the log ratios on that array. A p-value of less than 0.05 was considered as significant.
Cell lines and immunoblot analysis
Three human UCB lines [HTB1 (J82), HTB5 (TCCSUP), HT1376] and one bladder squamous cell carcinoma cell line [HTB3 (ScaBER)] were a generous gift from Dr. Douglas Scherr, Weill Cornell Medical College, and cultured as previously described (25). The cells were harvested and lysed in ice-cold RIPA buffer (120 mM NaCl, 50 mM Tris-HCL pH 7.6, 50 mM NaF, 0.1mM Na3VO4, 1% NP40, 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma, St. Louis, MO, USA) and 15% Complete Mini protease inhibitor cocktail (Roche, Basel, Switzerland). Protein lysates (18 μg) were loaded on 10% sodium dodecyl sulfate Polyacrylamide gel electrophoresis (SDS-PAGE) gels and bands were separated and were transferred onto nitrocellulose membranes (Trans-Blot Transfer Medium, Bio-Rad, Solna, Sweden). Signals were visualized using the Enhanced ChemiLuminescence detection system (Millipore Corp. Sweden, Solna, Sweden) and documented with an Alpha Imager CCD system (Bio-Rad).
Statistical analysis
Statistical analysis was performed using SPSS Version 18 & 20 (SPSS Inc., Chicago, IL, USA). Non-parametric analysis using Mann–Whitney test was performed for different variables for comparison between non-malignant bladder and malignant bladder tumors and cancer sub groups. Statistics with Pearson chi-square analysis was performed to determine associations for age, gender, race, TNM staging and lymph node status. Box plot analysis was performed for comparing the expression level of cyclin D1 between non-tumor and tumor bladder samples, as well as between NMIBC and MIBC specimens. Kaplan–Meier survival analysis was performed for assessment of cancer recurrence and disease-free survival functions, and log-rank tests were used to compare the differences of survival or recurrence in subgroups.
Results
Expression of cyclin D1 mRNA is significantly higher in bladder cancer specimens than that in normal bladder tissues
We evaluated mRNA expression of cyclin D1 in normal bladder tissues (n=14) and NMIBC specimens (n=28) using the existing database of gene expression profiles from Oncomine (23). Bladder cancer specimens had significantly higher levels of cyclin D1 expression, with a 3-fold increase compared to the normal bladder or mucosa tissues (p=0.003) (Figure 1). This suggests that level of cyclin D1 mRNA is elevated in bladder cancer specimens in this patient cohort.
Figure 1.
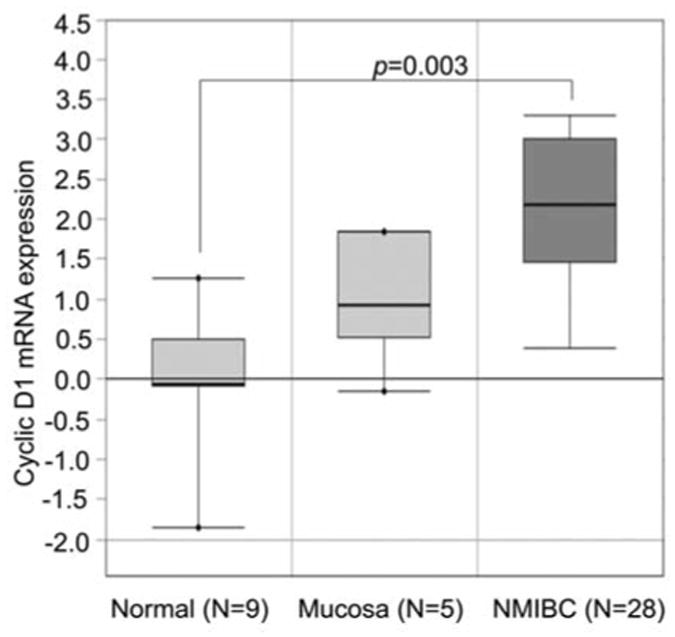
Expression of cyclin D1 mRNA in normal bladder tissues and specimens of non-muscle invasive bladder cancer (NMIBC). Box-plot analysis shows differences in cyclin D1 mRNA among three groups Normal bladder cancer tissues are indicated as ‘Normal’ and ‘Mucosa’. The bladder cancer specimens are the non-muscle invasive subtypes as indicated as “NMIBC”. The data was extracted from public Oncomine data-bases.
Expression of cyclin D1 is higher in bladder cancer specimens than that in adjacent non-malignant bladder tissues
Next, we immunostained TMAs containing bladder cancer specimens (n=212) and adjacent non-malignant bladder tissues (n=131) using an antibody against cyclin D1. Expression of cyclin D1 was evaluable in 188 bladder carcinoma specimens and 107 samples of adjacent non-malignant urothelium. Cyclin D1 expression was significantly elevated in bladder cancer specimens compared with non-malignant bladder tissues (p<0.001) (Figure 2A). In non-malignant urothelium, cyclin D1 was predominantly present in the nucleus (Figure 2B). In bladder cancer specimens, cyclin D1 was present in the nucleus and cytoplasm of the tumor cells (Figure 2C). These data are consistent with the above observation that cyclin D1 mRNA level is higher in bladder cancer than in non-malignant bladder tissues.
Figure 2.
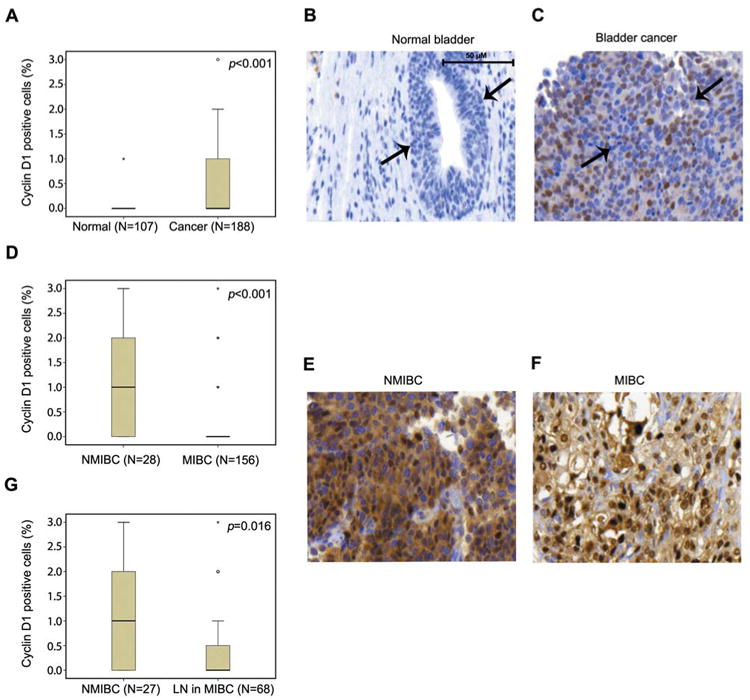
Immunohistochemical analysis of cyclin D1 protein expression in normal bladder tissues and bladder cancer specimens. A: Box-plot graph shows that cyclin D1 expression is significantly higher in bladder cancer tissues than in normal bladder tissues. Bars shows the range of cyclin D1 expression. The difference in cyclin D1 expression between normal bladder tissues and cancer specimens was assessed using the Mann–Whitney test. Representative microphotographs of normal (B) and bladder cancer (C) specimens stained with antibody against cyclin D1. The normal mucosa cells and cancer cells are indicated by arrows. D: Cyclin D1 expression in bladder cancer subgroups of NMIBC and MIBC is presented using box-blot graph. Representative microphotographs of cyclin D1 protein expression in tumor cells from NMIBC (E) and MIBC (F) are shown. G: Box-plot of cyclin D1 expression in NMIBC and in the subgroup with lymph node metastasis (×40 magnification).
Association between cyclin D1 expression and clinical characteristics
Cyclin D1 expression was evaluable in 188/212 tumor cases in this patient cohort. Clinical information is presented in Table I. The majority of patients were Caucasians (84.9%), compared with 11.1% African-American and 4% Asians. However, there was no significant difference between patient, age, gender and ethnicity. There was a significantly higher number of cases (84.7%) of MIBC (≥pT2) compared to NMIBC (pTis/pTa/pT1) cases (15.3%) (p<0.014) (Table I). We next compared cyclin D1 expression patterns between the NMIBC and MIBC groups. The percentage of cyclin D1-positive tumors cells was higher in the NMIBC group compared to the one in the MIBC group (p<0.001) (Figure 2D). The staining pattern of cyclin D1 in tumor cells from NMIBC and MIBC groups are shown in Figure 2E and F. Similarly, cyclin D1 expression was higher in the NMIBC group compared to those with lymph node metastasis (p=0.016) (Figure 2G). To assess whether cyclin D1 expression is associated with tumor invasiveness, we evaluated cyclin D1 expression in the aggressive MIBC group in which patients were divided into the two groups based on the presence or absence of lymph node metastasis. We observed that cyclin D1 expression was significantly higher in patients with lymph node metastasis compared to those without (p<0.001) (Figure 3).
Figure 3.
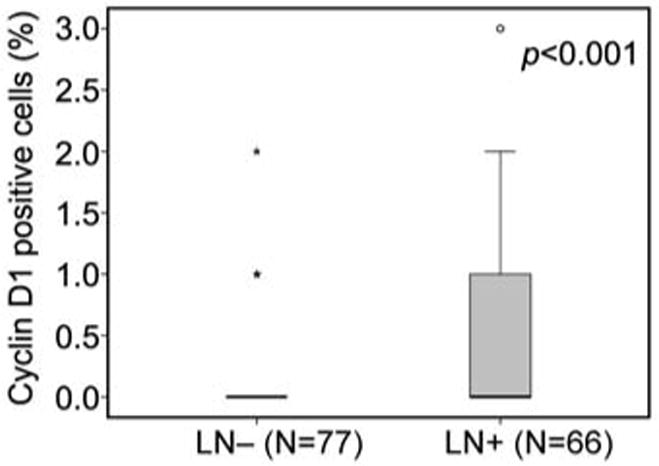
Evaluation cyclin D1 expression in lymph node-positive and –negative subgroups of patients with muscle-invasive bladder cancer (MIBC). Box-plot of cyclin D1 expression in patients with lymph node metastasis and those without lymph node metastasis. Difference in cyclin D1 expression between the two subgroups was assessed using Mann-Whitney test.
Correlation between cyclin D1 and disease recurrence in the MIBC group
We next analyzed whether cyclin D1 expression together with tumor metastasis may be used to predict disease recurrence within the aggressive MIBC-group. We stratified patients into two groups based on cyclin D1 expression and lymph node status, that is: high-cyclin D1 and lymph node metastasis vs. low-cyclin D1 and no lymph node metastasis. There was a clear trend that patients with high cyclin D1 expression and lymph node metastasis had worse disease-free survival compared to those with lower level of cyclin D1 and no lymph node metastasis (Figure 4).
Figure 4.
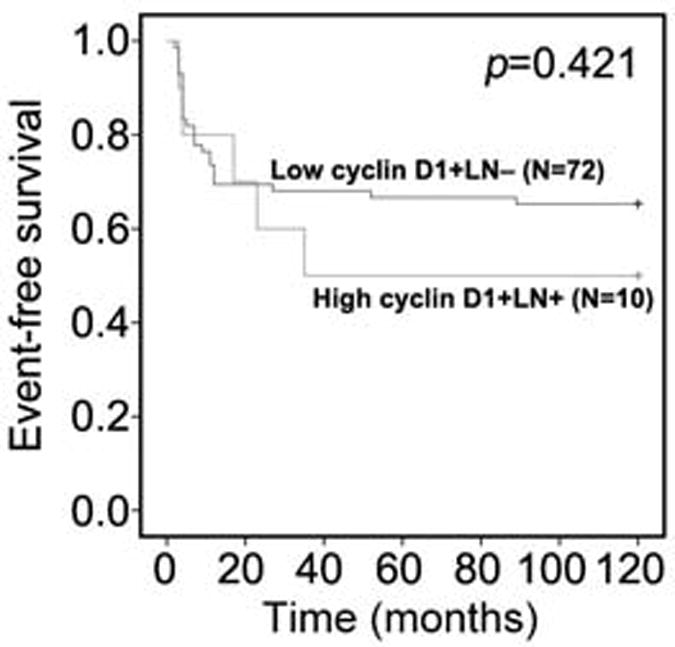
Analysis of the predictive value of cyclin D1 in patients with MIBC. Disease-free survival of patients with high cyclin D1 expression and lymph node metastasis is compared to that of patients with low cyclin D1 expression and had no lymph node metastasis. Patients were followed-up for 120 months. p=0.421.
Absence of nuclear cyclin D1 expression is associated with the invasive UCB
We next assessed the cellular expression of cyclin D1 in each evaluable (177/188) tumor specimen (Figure 5A). Strikingly, nuclear cyclin D1 expression was absent from the majority of invasive tumors and from lymph node metastasis within MIBC group (p<0.001) (Figure 5B and C).
Figure 5.
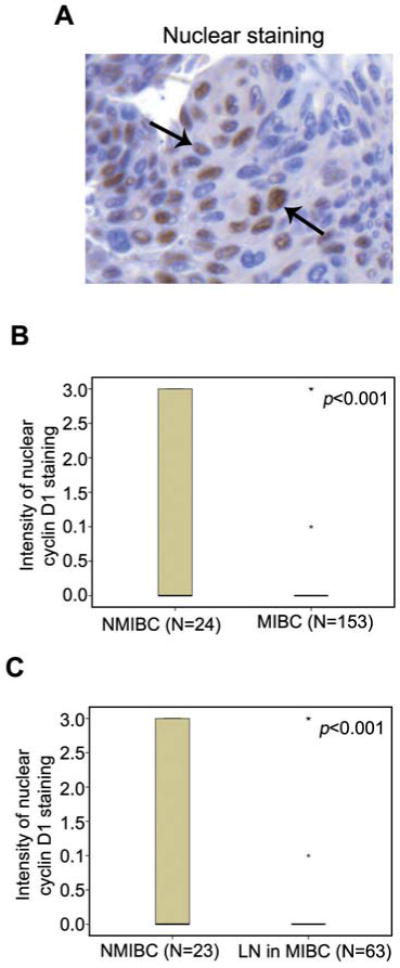
Correlation between nuclear cyclin D1 staining and bladder cancer metastasis. A: Representative image from immunohistochemical analysis shows that cyclin D1 expression is present in the nucleus of tumor cells in bladder cancer specimens. B: Difference in nuclear cyclin D1 expression between NMIBC and MIBC shown in a box-plot. C: Difference in the nuclear cyclin D1 expression between NMIBC and MIBC with lymph node metastasis (LN+) is shown in a box-plot.
Patients with elevated cyclin D1 expression tended to have a shorter time to cancer recurrence within the group of patients with lymph node metastasis
We divided patients into two groups based on their lymph node status and we observed that patients with lymph node metastasis tended to have poorer recurrence-free survival, although statistical significance was not achieved (p=0.275) (Figure 6A). We next evaluated whether combined cyclin D1 expression and lymph node status had any prognostic value in predicting cancer recurrence. We divided patients into four groups: (i) patients without both tumor cyclin D1 expression and lymph node metastasis; (ii) patients with cyclin D1 expression in tumor cells and without lymph node metastasis; (iii) patients without cyclin D1 expression in tumor cells but with lymph node metastasis; (iv) patients with both cyclin D1 expression in tumor cells and lymph node metastasis. Recurrence-free survival analysis showed that patients who had cyclin D1-positive tumor cells and lymph node metastasis tended to have a worse recurrence-free survival compared to the other three groups (p=0.678) (Figure 6B). This suggests that among patients with lymph node metastasis, those who had an elevated level of cyclin D1 expression tended to have a shorter time-to-cancer recurrence, although statistical significance was not achieved.
Figure 6.
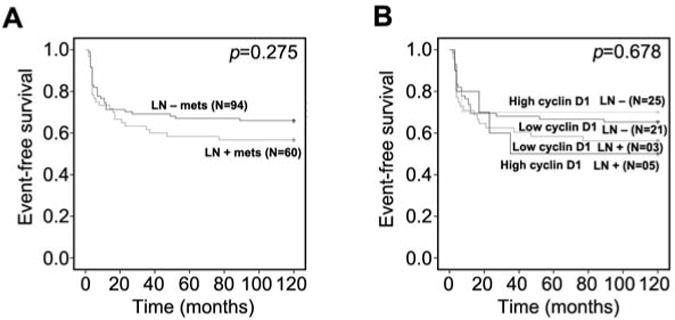
Cyclin D1 expression in combination with lymph node status is associated with disease recurrence. A: Disease-free survival of patients with lymph node metastasis (LN+) is compared to that of patients without lymph node metastasis (LN−). B: Disease-free survival of four subgroups of patients. Patients were stratified into four different groups based on the level of cyclin D1 expression and lymph node metastasis: Patients with high cyclin D1 expression but no lymph node metastasis; patients with low cyclin D1 expression and no lymph node metastasis; patients with low cyclin D1 but with lymph node metastasis; patients with high cyclin D1 expression and lymph node metastasis. Log-rank test was performed.
Cyclin D1 expression in bladder cancer cell lines
Next, we examined the cyclin D1 protein expression in a series of bladder cancer cell lines including: HTB1, J82 (transitional cell carcinoma); HTB3, Scaber (squamous cell carcinoma); HTB5, grade IV female transitional cell carcinoma; HT1376, grade III transitional cell carcinoma cells. We found that cyclin D1 was expressed in HTB1 and HT1376 cells, but was undetectable in HTB5 cells, derived from high-grade invasive bladder cancer. Cyclin D1 was also absent in HTB3 cells and highest levels of cyclin D1 were observed in HTB1 and HT1376 cells (Figure 7).
Figure 7.
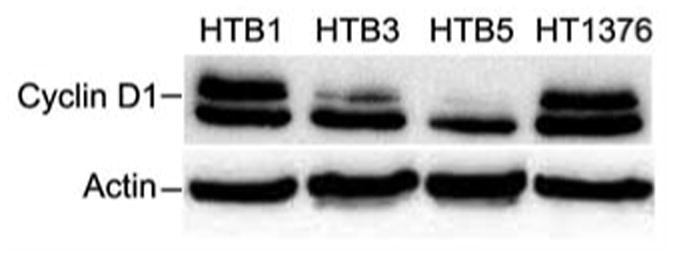
Western blot analysis using an antibody against cyclin D1 in bladder cancer cell lines: (HTB1, J82 [transitional cell carcinoma (TCC)]; HTB3, Scaber (squamous cell carcinoma); HTB5, TCCSUP (grade IV female TCC); HT1376, Grade III TCC).
Discussion
Cyclin D1 is a key cell-cycle regulator that controls the G0/G1 progression and contributes to cell proliferation. In the field of bladder cancer, conflicting data on the importance of this target have been reported: Cyclin D1 protein expression has been correlated with both poor prognosis (26) and good prognosis (27). However, the majority of the studies on cyclin D1 conducted in bladder cancer included patients with NMIBC. In the present study, we examined primarily patients with MIBC and found increased expression of cyclin D1 mRNA in UCB specimens. We further showed that cyclin D1 protein expression is higher in UCB versus the adjacent non-malignant bladder tissues. Moreover, stage-specific expression of cyclin D1 was noted. MIBC cases and cases with lymph node metastasis displayed lower cyclin D1 expression in the tumor cells compared with that of NMIBC. A recent report by Fristrup and colleagues showed that high cyclin D1 expression is predominantly nuclear in tumor cells from non-muscle invasive (Ta/T1) UCB, and that this nuclear cyclin D1 expression is associated with poor patient outcome (9). In our study, the patient cohort mainly consisted of MIBC cases (84.7%), and only a small number (15.3%) of NMIBC. Thus, our findings in NMIBC are in agreement with the reported study. We further showed that the absence of nuclear cyclin D1 expression in tumor cells was significantly associated with MIBC. Thus, cyclin D1 may have some value as a predictive marker for a sub-group of patients with UCB. It will be important to examine cyclin D1 expression and its clinical relevance in larger cohorts of UCB.
It is known that high-grade bladder tumors are more likely to recur than low-grade ones (28). In the present study, expression of cyclin D1 was linked to the degree of invasiveness and cancer recurrence. Lack of or low-cyclin D1 expression in tumors was found to be associated with cancer recurrence. Because cyclin D1 is a nuclear protein in normal cells, altered subcellular localization of cyclin D1 may result in alterations in tumor cell behavior, such as of proliferation, survival and invasion (29, 30). Our study suggests that cyclin D1 may be used as a potential marker for stratifying subtypes of bladder cancer for individual treatment.
The present study has some limitations. The majority of patients included in the current cohort had MIBC, and this was limited to patients who had undergone radical cystectomy. There is also a limitation with respect to the number of events, which affected the statistical power. Future studies are therefore warranted to extend this study to larger and more diverse UCB cohorts. Xenograft and in vitro functional studies are also required to validate the role of cyclin D1 in UCB.
Acknowledgments
This work was supported by a grant from the Swedish Cancer Foundation, the Swedish National Research Council, a Government Health Grant, a Lund University Medical Faculty Grant, the Malmö Cancer Foundation, the Skåne University Hospital Foundation to JLP, and the University of Nottingham (NPM). We thank Elise Nilsson for technical support.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Moreira JM, Ohlsson G, Gromov P, Simon R, Sauter G, Celis JE, Gromova I. Bladder cancer-associated protein, a potential prognostic biomarker in human bladder cancer. Mol Cell Proteomics. 2010;9:161–177. doi: 10.1074/mcp.M900294-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nargund VH, Tanabalan CK, Kabir MN. Management of non-muscle-invasive (superficial) bladder cancer. Semin Oncol. 2012;39:559–572. doi: 10.1053/j.seminoncol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Proctor I, Stoeber K, Williams GH. Biomarkers in bladder cancer. Histopathology. 2010;57:1–13. doi: 10.1111/j.1365-2559.2010.03592.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: How far have we come? CA Cancer J Clin. 2010;60:244–272. doi: 10.3322/caac.20077. [DOI] [PubMed] [Google Scholar]
- 7.Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ, Sagalowsky AI, Schoenberg MP, Lerner SP. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: A contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006;176:2414–2422. doi: 10.1016/j.juro.2006.08.004. discussion 2422. [DOI] [PubMed] [Google Scholar]
- 8.Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T, Sternberg CN, Sonpavde G. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–533. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Fristrup N, Birkenkamp-Demtroder K, Reinert T, Sanchez-Carbayo M, Segersten U, Malmstrom PU, Palou J, Alvarez-Mugica M, Pan CC, Ulhoi BP, Borre M, Orntoft TF, Dyrskjot L. Multicenter validation of cyclin D1, MCM7, TRIM29, and UBE2C as prognostic protein markers in non-muscle invasive bladder cancer. Am J Pathol. 2012 doi: 10.1016/j.ajpath.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Dyrskjot L, Zieger K, Real FX, Malats N, Carrato A, Hurst C, Kotwal S, Knowles M, Malmstrom PU, de la Torre M, Wester K, Allory Y, Vordos D, Caillault A, Radvanyi F, Hein AM, Jensen JL, Jensen KM, Marcussen N, Orntoft TF. Gene expression signatures predict outcome in non-muscle-invasive bladder carcinoma: A multicenter validation study. Clin Cancer Res. 2007;13:3545–3551. doi: 10.1158/1078-0432.CCR-06-2940. [DOI] [PubMed] [Google Scholar]
- 11.Dyrskjot L, Zieger K, Kruhoffer M, Thykjaer T, Jensen JL, Primdahl H, Aziz N, Marcussen N, Moller K, Orntoft TF. A molecular signature in superficial bladder carcinoma predicts clinical outcome. Clin Cancer Res. 2005;11:4029–4036. doi: 10.1158/1078-0432.CCR-04-2095. [DOI] [PubMed] [Google Scholar]
- 12.Mitra AP, Cote RJ. Molecular pathogenesis and diagnostics of bladder cancer. Annu Rev Pathol. 2009;4:251–285. doi: 10.1146/annurev.pathol.4.110807.092230. [DOI] [PubMed] [Google Scholar]
- 13.Johansson M, Persson JL. Cancer therapy: Targeting cell-cycle regulators. Anticancer Agents Med Chem. 2008;8:723–731. doi: 10.2174/187152008785914833. [DOI] [PubMed] [Google Scholar]
- 14.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 15.Fleischmann A, Rocha C, Saxer-Sekulic N, Zlobec I, Sauter G, Thalmann GN. High-level cytoplasmic cyclin D1 expression in lymph node metastases from prostate cancer independently predicts early biochemical failure and death in surgically treated patients. Histopathology. 2011;58:781–789. doi: 10.1111/j.1365-2559.2011.03800.x. [DOI] [PubMed] [Google Scholar]
- 16.Bali A, O'Brien PM, Edwards LS, Sutherland RL, Hacker NF, Henshall SM. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res. 2004;10:5168–5177. doi: 10.1158/1078-0432.CCR-03-0751. [DOI] [PubMed] [Google Scholar]
- 17.Lee CC, Yamamoto S, Morimura K, Wanibuchi H, Nishisaka N, Ikemoto S, Nakatani T, Wada S, Kishimoto T, Fukushima S. Significance of cyclin D1 overexpression in transitional cell carcinomas of the urinary bladder and its correlation with histopathologic features. Cancer. 1997;79:780–789. doi: 10.1002/(sici)1097-0142(19970215)79:4<780::aid-cncr15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Yuan L, Gu X, Shao J, Wang M, Zhu Q, Zhang Z. Cyclin D1 G870A polymorphism is associated with risk and clinicopathologic characteristics of bladder cancer. DNA Cell Biol. 2010;29:611–617. doi: 10.1089/dna.2010.1018. [DOI] [PubMed] [Google Scholar]
- 19.Kim CJ, Nishi K, Isono T, Okuyama Y, Tambe Y, Okada Y, Inoue H. Cyclin D1b variant promotes cell invasiveness independent of binding to CDK4 in human bladder cancer cells. Mol Carcinog. 2009;48:953–964. doi: 10.1002/mc.20547. [DOI] [PubMed] [Google Scholar]
- 20.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Association of cyclin D1 and E1 expression with disease progression and biomarkers in patients with nonmuscle-invasive urothelial cell carcinoma of the bladder. Urol Oncol. 2007;25:468–475. doi: 10.1016/j.urolonc.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Makhlin I, Zhang J, Long CJ, Devarajan K, Zhou Y, Klein-Szanto AJ, Huang M, Chernoff J, Boorjian SA. The mTOR pathway affects proliferation and chemosensitivity of urothelial carcinoma cells and is up regulated in a subset of human bladder cancers. BJU Int. 2011;108:E84–90. doi: 10.1111/j.1464-410X.2010.09844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Dyrskjot L, Kruhoffer M, Thykjaer T, Marcussen N, Jensen JL, Moller K, Orntoft TF. Gene expression in the urinary bladder: A common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauffman EC, Robinson BD, Downes MJ, Powell LG, Lee MM, Scherr DS, Gudas LJ, Mongan NP. Role of androgen receptor and associated lysine-demethylase co-regulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol Carcinog. 2011;50:931–944. doi: 10.1002/mc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, Quintero A, Merlo F, Carrasco JC, Requena MJ, Montironi R. Prognostic factors in stage T1 grade 3 bladder cancer survival: The role of G1-S modulators (p53, p21Waf1, p27kip1, Cyclin D1, and Cyclin D3) and proliferation index (ki67-MIB1) Eur Urol. 2004;45:606–612. doi: 10.1016/j.eururo.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, Quintero A, Merlo F, Requena MJ, Montironi R. Prognostic factors in survival of patients with stage Ta and T1 bladder urothelial tumors: The role of G1-S modulators (p53, p21Waf1, p27Kip1, cyclin D1, and cyclin D3), proliferation index, and clinicopathologic parameters. Am J Clin Pathol. 2004;122:444–452. doi: 10.1309/LTFU-3UUM-BY09-5HUM. [DOI] [PubMed] [Google Scholar]
- 28.Raman JD, Ng CK, Boorjian SA, Vaughan ED, Jr, Sosa RE, Scherr DS. Bladder cancer after managing upper urinary tract transitional cell carcinoma: Predictive factors and pathology. BJU Int. 2005;96:1031–1035. doi: 10.1111/j.1464-410X.2005.05804.x. [DOI] [PubMed] [Google Scholar]
- 29.Dhar KK, Branigan K, Parkes J, Howells RE, Hand P, Musgrove C, Strange RC, Fryer AA, Redman CW, Hoban PR. Expression and subcellular localization of cyclin D1 protein in epithelial ovarian tumour cells. Br J Cancer. 1999;81:1174–1181. doi: 10.1038/sj.bjc.6690826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]


