Abstract
Background
When kidney transplants fail, transplant medications are discontinued to reduce immunosuppression-related risks. However, retransplant candidates are at risk for allosensitization which prolonging immunosuppression may minimize. We hypothesized that for these patients, a prolonged immunosuppression withdrawal after graft failure preserves nonsensitization status (PRA 0%) better than early immunosuppression withdrawal.
Methods
We retrospectively examined subjects transplanted at a single center between 7/1/1999-12/1/2009 with a non-death related graft loss. Subjects were stratified by timing of immunosuppression withdrawal after graft loss: early (≤3 months) or prolonged (>3 months). Retransplant candidates were eligible for the main study where the primary outcome was nonsensitization at retransplant evaluation. Non-retransplant candidates were included in the safety analysis only.
Results
We found 102 subjects with non-death related graft loss of which 49 were eligible for the main study. Nonsensitization rates at retransplant evaluation were 30% and 66% for the early and prolonged immunosuppression withdrawal groups respectively (p=0.01). After adjusting for cofactors such as blood transfusion and allograft nephrectomy, prolonged immunosuppression withdrawal remained significantly associated with nonsensitization (adjusted odds ratio=5.78, 95% C.I. [1.37-24.44]). No adverse safety signals were seen in the prolonged immunosuppression withdrawal group compared to the early immunosuppression withdrawal group.
Conclusions
These results suggest that prolonged immunosuppression may be a safe strategy to minimize sensitization in retransplant candidates and provide the basis for larger or prospective studies for further verification.
Keywords: kidney, transplantation, sensitization, immunosuppression withdrawal
INTRODUCTION
Kidney transplantation is life-sparing for patients with end stage renal disease (ESRD) who are on the waitlist (1-3). Projected patient lifetime almost doubles after deceased donor kidney transplantation compared with remaining on the waitlist (4). However when a kidney transplant fails, the survival benefit is lost (5,6). Additionally, more sepsis and infection-related deaths have been reported after transplant failure compared to patients who retain transplant function (5,7). Yet, patients who are retransplanted benefit from a reduction in mortality when compared with their wait-listed counterparts with prior transplant failure (8).
A growing number of patients with a failed kidney transplant are relisted for a subsequent transplant. United States registry data show that approximately 20% of all ESRD patients on the kidney transplant waiting list have a prior failed transplant (9). However, retransplanting these patients is challenging because prior transplantation is a risk factor for human leukocyte antigen (HLA) sensitization which may limit compatible organs and prolong waitlist times (10,11). Furthermore, kidney transplant failure itself carries a high risk of sensitization which is probably related to several factors like transplant nephrectomy, blood transfusion, and sudden immunosuppression cessation (12-15).
When kidney transplants fail, transplant medications are commonly stopped to reduce the risks associated with immunosuppression, namely infection and premature death (16,17). However, when to withdraw immunosuppression remains an unanswered question. Potential benefits of prolonged immunosuppression withdrawal include a reduction in graft rejection and preservation of residual renal function (18,19), but another more intriguing benefit may be minimizing the risk of sensitization after transplant failure (15). By minimizing sensitization risk, patients may have a greater opportunity to receive a subsequent life-sparing kidney transplant. We hypothesized that in patients referred for retransplantation, prolonged immunosuppression withdrawal after kidney transplant failure reduces the risk of sensitization and better preserves their nonsensitization status compared to early immunosuppression withdrawal. In addition, we evaluated whether prolonged immunosuppression withdrawal was associated with additional safety risks compared to early withdrawal.
RESULTS
Out of 1448 subjects who received a kidney transplant between 7/1/1999-12/1/2009, 102 subjects were identified with a non-death related graft failure. Forty-nine subjects were evaluated for kidney retransplantation (main study group). Of the main study participants, 20 and 29 subjects had their immunosuppression withdrawn at ≤3 months (early) and >3 months (prolonged) respectively (Figure 1). The main study group baseline characteristics were similar in both withdrawal groups (Table 1). The median durations for immunosuppression after graft failure were 24 days [25%,75% Quartiles: 16, 41 days] and 357 days [25%,75% Quartiles: 210, 595 days] for the early and prolonged withdrawal groups respectively. Prolonged immunosuppression after graft failure was seen in 5 subjects with a functioning non-renal solid organ transplant. Rates of antibody mediated rejection were similar and no subject received a prolonged treatment lasting ≥7 days. To salvage graft function, no differences were seen in the use of depleting antibody (4 v. 5 subjects), IVIG (2 v. 0 subjects), or plasmapheresis (1 v. 0 subjects) between the early and prolonged withdrawal groups respectively. A third of grafts failed within a year of transplantation—largely due to primary nonfunction, thrombosis, or acute rejection—which resulted in a low median graft survival.
Figure 1. Subject Selection.
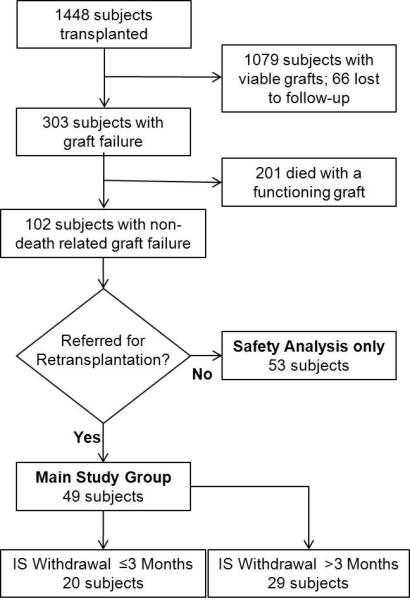
IS = immunosuppression.
Table 1.
Baseline Subject Characteristics
| Baseline Characteristics | Main Study Group N=49 | All Subjects N=102 | ||||
|---|---|---|---|---|---|---|
| IS Withdrawal ≤ 3 months N=20 | IS Withdrawal > 3 months N=29 | P Value | IS Withdrawal ≤ 3 months N=52 | IS Withdrawal > 3 months N=50 | P Value | |
| Age at Graft Failure, Years, Mean ± SD | 43 ± 12 | 43 ± 15 | 0.9262 | 49 ± 14 | 46 ± 14 | 0.2818 |
| Female Gender, N (%) | 11 (55) | 9 (31) | 0.0934 | 21 (41) | 18 (38) | 0.7083 |
| Prior Pregnancy, N (%)a | 6 (30) | 6 (21) | 0.4563 | N/A | N/A | N/A |
| Black Race, N (%) | 6 (30) | 9 (31) | 0.9384 | 20 (39) | 15 (31) | 0.1618 |
| ESRD due to Diabetes, N (%) | 4 (25) | 2 (10) | 0.2058 | 8 (15) | 5 (10) | 0.4149 |
| Deceased Donor, N (%) | 17 (85) | 24 (83) | >0.999 | 42 (82) | 41 (85) | 0.6790 |
| Nonsensitized Prior to 1° Graft, N (%) | 12 (60) | 23 (79) | 0.1414 | 34 (65) | 39 (78) | 0.1580 |
| ≥ 3 HLA Mismatches, N (%) | 15 (79) | 18 (75) | 0.7609 | 40 (80) | 26 (68) | 0.2140 |
| Induction LDA, N (%) | 6 (30) | 6 (21) | 0.4563 | 16 (31) | 10 (21) | 0.2578 |
| Acute Rejection, N (%)b | 5 (25) | 9 (31) | 0.6458 | 18 (35) | 16 (32) | 0.7794 |
| Immunologic Graft Failure, N (%) | 5 (25) | 5 (17) | 0.5078 | N/A | N/A | N/A |
| Immunologic Graft Failure <1 year from Transplant, N (%) | 3 (38) | 2 (22) | 0.4902 | N/A | N/A | N/A |
| 1° Graft Survival, months, Median [25%, 75% Quartile] | 20 [6, 48] | 31 [9, 60] | 0.3089 | 23 [7, 49] | 14 [3, 49] | 0.6348 |
| Nephrectomy after Graft Failure, N (%) | 7 (44) | 11 (52) | 0.6028 | 21 (47) | 24 (48) | 0.4387 |
| Evaluated for Retransplantation, N (%) | 20 (100) | 29 (100) | N/A | 20 (38) | 29 (58) | 0.0483 |
| Time from Graft Failure to Re-Evaluation PRA, days, Median [25%, 75% Quartile] | 141 [64, 367] | 147 [66, 228] | 0.1072 | N/A | N/A | N/A |
| Blood Transfusion, N (%) | 9 (45) | 8 (28) | 0.2082 | N/A | N/A | N/A |
Table 1 Footnotes
No female subjects became pregnant after transplantation or transplant failure.
All acute rejections were biopsy confirmed Banff 1A or higher or biopsy confirmed antibody mediated rejection.
IS = immunosuppression. ESRD = end stage renal disease. HLA = human leukocyte antigen. LDA = lymphocyte depleting agent. PRA = panel reactive antibody. N/A = not available or not applicable.
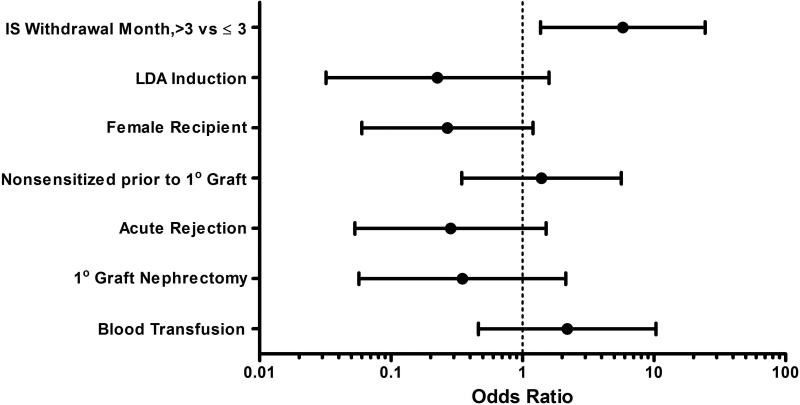
Prior to initial kidney transplantation, similar rates of nonsensitized subjects were observed in the two immunosuppression withdrawal groups. However after transplant failure, a notable reduction in nonsensitized subjects was seen in the early withdrawal group, while only a mild reduction was seen in the prolonged withdrawal group. Consequently, a significant separation in nonsensitization rates (30% v. 66%, p=0.01) developed after transplant failure favoring the prolonged withdrawal group (Figure 2). In subjects who converted from nonsensitized prior to primary graft placement to sensitized, 20% had testing for HLA antibodies at the time of graft failure and none had detectable antibodies at that time. After adjusting for covariates including nonsensitization prior to kidney transplantation, the multivariate logistic regression model showed that the prolonged withdrawal group demonstrated better preservation of nonsensitization status (aOR =5.78, 95% C.I. [1.37-24.44]) compared to the early withdrawal group (Figure 3). None of the other model covariates were significantly associated with nonsensitization after transplant failure.
Figure 2. Nonsensitization Stratified by Immunosuppression Withdrawal Duration after Graft Failure.
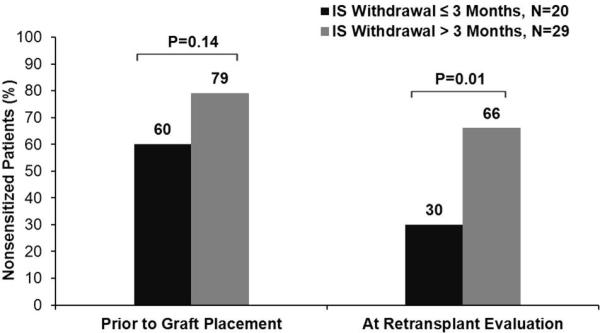
IS = immunosuppression.
Figure 3. Multivariate Analysis of Nonsensitization after Graft Failure, N=49.
IS = immunosuppression. LDA = lymphocyte depleting antibody.
A secondary analysis of PRA levels (0-100) was performed in 38 subjects of whom 16 and 22 came from the early and prolonged withdrawal groups respectively. Eleven subjects were excluded due to discordant PRA assays over time. Prior to primary kidney transplant, mean PRA levels were similar in both immunosuppression withdrawal groups. However at retransplant evaluation, the mean PRA level was significantly higher, 40.6±9.6 versus 12.5±5.1 (p=0.016), in the early versus prolonged withdrawal group respectively (Figure 4).
Figure 4. Mean Panel Reactive Antibody Stratified by Immunosuppression Withdrawal Duration after Graft Failure.
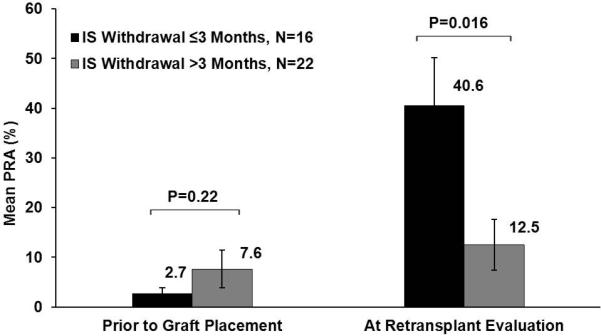
Eleven subjects were excluded from this analysis due to discordant PRA detection techniques prior to graft placement and after graft failure. IS = immunosuppression. PRA = panel reactive antibody.
Subjects relisted within 3 years of graft failure in the early and prolonged withdrawal groups were 11 (55%) and 22 (76%) respectively (p=0.13). Among relisted subjects, 3 (27%) and 14 (64%) subjects were retransplanted within 3 years of graft failure in the early and prolonged withdrawal groups respectively (p=0.049). All three subjects in the early withdrawal group were not on immunosuppression at retransplantation. By contrast in the prolonged withdrawal group, only one of 14 subjects was no longer on immunosuppression at retransplantation. Retransplantation with a living donor kidney in the early and prolonged withdrawal groups occurred in 1 (33%) and 4 (29%) subjects respectively.
In the 49 subject main study group, the 3-year mortality rate was 14% (7 subjects). Deaths in the early and prolonged withdrawal groups were 5 and 2 respectively. In the early withdrawal group, 3 subjects died from cardiovascular events, one from sepsis, and one from chronic myelogenous leukemia. In the prolonged withdrawal group, one subject died from an abdominal bleed and the other from untreated renal failure, but no deaths were attributable to infection or malignancy. Due to the low number of death events, no statistical conclusion was derived from the main study group.
A combined safety analysis was performed for all 102 subjects (main study group + safety analysis only group). Baseline characteristics were generally well matched between the early (52 subjects) and prolonged (50 subjects) withdrawal groups (Table 1). The 3-year mortality showed that 44% and 22% of subjects died the early and prolonged withdrawal groups respectively. Also, 3-year infection related mortality showed that 23% and 4% of subjects died of an infection in the early and prolonged withdrawal groups respectively. In the early withdrawal group, 48% (11/23) of deaths and 67% (8/12) of infection related deaths occurred within 3 months of graft failure. Per study design, no deaths could occur within 3 months of graft failure in the prolonged withdrawal group. To control for this bias, time-dependent multivariate Cox models were employed which showed that mortality and infection related mortality were no worse in the prolonged withdrawal group compared to the early withdrawal group (SDC, Table 1). Excluding non-melanoma skin cancers, four subjects in the early withdrawal group developed cancers—chronic myelogenous leukemia, renal cell carcinoma, melanoma, and pancreatic cancer—and one subject died from cancer. No subjects developed cancer in the prolonged withdrawal group.
The study was not powered to show differences in maintenance immunosuppression regimens but some general comparisons were feasible. At the time of graft failure, the most common regimen was a calcineurin inhibitor (CNI) + mycophenolic acid (MPA) + corticosteroid (73%), followed by a CNI + corticosteroid regimen (10%). Both regimens were employed similarly across both withdrawal groups (SDC Table 2). Among CNI based regimens, the most common CNI was tacrolimus (71%) which was similarly utilized in both withdrawal groups. In subjects weaned off combination CNI + antimetabolite therapy, half of the subjects had the CNI weaned first while the other half had the antimetabolite weaned first. In the prolonged withdrawal group three months after graft failure, 62% of subjects remained on CNI + MPA + corticosteroid and 21% were on CNI + corticosteroid (SDC Table 2).
DISCUSSION
Since nonsensitized patients are more likely to be transplanted (10,20), preserving nonsensitization status is crucial for patients who hope to be retransplanted. We found that prolonged immunosuppression withdrawal after transplant failure was associated with better preservation of a subject's nonsensitized status and a lower mean PRA level. Our results are consistent with recent single center studies that suggest that immunosuppression withdrawal after transplant failure may be a risk factor for HLA sensitization (15,21). In a prior study of transplant failure patients from our center, 11 patients continued immunosuppression and remained nonsensitized despite 7 who underwent transplant nephrectomy or transfusion (15). In single center study by Augustine and colleagues (21), 24 subjects who continued immunosuppression after graft failure were compared with subjects who had immunosuppression weaned. They observed that weaning immunosuppression was associated with sensitization independent of transplant nephrectomy, but their study was limited by the absence of blood transfusion data—a significant risk factor for sensitization (21).
After transplant failure, infection is a common cause of death (22) and the risks of sepsis and infection related death are higher compared to subjects with a functioning transplant (5,7). Prior authors have suggested that immunosuppression should be tapered off as quickly as feasible (16,23). Others have advocated a gradual withdrawal of immunosuppression after noting few infection related complications (18). In other words, there is no consensus on the optimal duration of immunosuppression withdrawal after graft failure (17,24).
An important component of this study was to monitor for safety outcomes associated with prolonged immunosuppression withdrawal after transplant failure. In the main study group, no definitive conclusions could be drawn due to few event numbers. However in all study patients (main study group + safety analysis only group), a higher mortality rate was seen with early withdrawal and almost half of these deaths occurred within 3 months of graft failure. This suggests that immunosuppression may have been tapered more rapidly in higher risk subjects. In contrast, no deaths occurred within 3 months of graft failure in the prolonged withdrawal group since subjects had to survive at least 3 months to be categorized as such. Therefore, a time bias favored the prolonged withdrawal group because survival was guaranteed throughout the first 3 months after graft failure. To account for this time bias, we used time-dependent Cox models for our survival analyses. The Cox models found no associations between prolonged immunosuppression withdrawal and excessive mortality or infection related death, but our study was not originally powered to show differences in mortality.
Mortality rates in our study appear to be in-line with other publications. Mortality in the main study group (14%) and all study patients (33%) were comparable to those seen in a United States registry study of patients with failed kidney transplants (8). Also in subjects not referred for retransplantation their three-year mortality rate (51%) was almost identical to that seen in incident United States dialysis patients (25). Differences in mortality between the main study and safety analysis only groups are important because potential retransplant candidates are probably healthier and may be better suited to handle the risks of prolonged immunosuppression.
In the design of our study, there was no uniform immunosuppression withdrawal protocol. But if there were no life-threatening complications, then our program considered prolonged immunosuppression after graft failure in subjects with either an anticipated short waitlist time or significant residual renal function. In retransplant candidates, therapeutic FK506 levels were maintained until a subsequent transplant was obtained barring no immunosuppressive complications. If immunotherapy was not weaned immediately, then the general strategy for the two most common drugs—tacrolimus and mycophenolate mofetil—was to maintain FK506 trough levels between 3-6 ng/mL and reduce the dose of mycophenolate mofetil to 250-500 mg twice a day. MPA levels were not routinely measured after transplant failure.
Study Strengths
Our study has several strengths which also address some shortcomings from prior studies. First, we believe our study has the largest cohort of patients to date on prolonged (>3 months) immunosuppression after transplant failure available for HLA antibody and safety analysis. Second, unlike prior studies, we address the optimal timing for immunosuppression withdrawal by comparing efficacy and safety outcomes across different durations of immunosuppression withdrawal. Third, important potential confounders for HLA sensitization such as acute rejection (26), induction medication (27), HLA matching (10,28), graft nephrectomy (12,29), and importantly blood transfusions (30-34) are controlled for in our multivariate models. Finally, we provide a more detailed safety analysis of prolonged immunosuppression in comparison to prior studies.
Study Limitations
As in prior studies, our study's main limitation is sample size which may cause insufficient power and an inconclusive death analysis. However, significant differences in nonsensitization among both immunosuppression withdrawal groups were identified out of the small cohort, which drives the results more conservative. Residual confounders may exist from unrecorded events such as blood transfusions and infections that may have occurred in the local community and may not have been captured by the study database. Finally, our retrospective study design does not predict cause and effect, but rather reports associations seen in the duration of immunosuppression withdrawal and the detection of HLA antibodies. Validation will require prospective randomized control trials. However at the time of our study's inception, we felt a prospective study of this nature would not have been ethical because we knew that prolonged immunosuppression can be harmful and there was scant evidence for benefit. Now, with these and other data, a prospective trial may be more realistic in the future.
CONCLUSION
In this study, the strategy of prolonged immunosuppression withdrawal after kidney transplant failure seemed well tolerated and appeared to preserve nonsensitization status in subjects referred for retransplantation. In addition, we observed no adverse safety signals in the prolonged withdrawal group compared to the early withdrawal group, but this study was not originally powered for safety outcomes so further investigation with a larger sample size is required. Hopefully, these results will serve as a basis for future prospective studies. In summary, prolonged immunosuppression withdrawal after kidney transplant failure may be a safe strategy to minimize sensitization in retransplantation candidates especially those who anticipate a short stay on the transplant waitlist.
MATERIALS AND METHODS
This retrospective cohort study utilized data from the transplant database and electronic medical record system at the Shands Transplant Center at the University of Florida. The study protocol was approved by the University of Florida Institutional Review Board. Study participants consisted of adult kidney transplant recipients transplanted between 7/1/1999-12/1/2009 who experienced non-death related graft failure with follow-up through 8/31/2011. Subjects subsequently evaluated for kidney retransplantation were eligible for the main study group and subjects not evaluated for subsequent retransplantation were included in the safety analysis. Subjects were analyzed by duration of immunosuppression withdrawal after transplant failure: ≤3 months (early) and >3 months (prolonged). The 3 months withdrawal cutoff was based on a prior examination of practices at our center and was defined prior to this analysis. The duration of immunosuppression therapy after graft failure was determined when one of the following occurred: withdrawal of all noncorticosteroid immunosuppressant drugs, death, or retransplantation, whichever came first. Death and retransplantation are natural immunosuppression withdrawal endpoints since debate over immunosuppression ceases after these events. Since our program typically continued 5 mg of prednisone in all subjects who retained a failed transplant, low-dose corticosteroids were not analyzed as maintenance immunosuppression for the purposes of this study.
Study Outcomes
The primary outcome of the main study group was the rate of nonsensitization among subjects evaluated for retransplantation. Nonsensitization was defined as a panel reactive antibody (PRA) level of 0%. We chose nonsensitization because it was a simple endpoint that conferred the best retransplantation potential. Prior to the primary transplant, the peak PRA was used to determine nonsensitization status. After graft failure, the PRA at the time of retransplant evaluation was used. Secondary outcomes from the main study group were PRA level at retransplant evaluation and 3-year relisting and retransplantation rate. All subjects—whether evaluated or not for retransplantation—were studied for 3-year mortality, infection related mortality, and malignancy (excluding non-melanoma skin cancer). The date of graft failure served as the starting point for all 3-year outcomes. Main study subjects were seen in clinic at least annually and queried about prior infections, blood transfusions, and malignancies. All deaths were confirmed by death certificate and/or Centers for Medicare & Medicaid Services (CMS) Death Notification (form CMS-2746).
Antibody Detection
Throughout the entire study, HLA antibodies were screened (antibody positive or negative) by solid phase assay (ELISA or flow bead techniques) for the primary outcome in all 49 main study subjects. ELISA is slightly less sensitive but comparable to flow bead techniques when detecting HLA antibodies (35,36) and both solid phase assays are considered adequate to confirm nonsensitization status (37). In the secondary analysis, a quantitative PRA level (0-100) was determined by cytotoxicity (years 1999-2006) or by calculated PRA using single antigen beads (years 2006-present). However, consistent PRA levels across these two different techniques have not been proven so any subject with PRA levels from discordant techniques was excluded from this secondary analysis. All solid-phase assays used commercial reagents for enzyme immunoassay and flow cytometry. The tests used to measure HLA antibodies were enzyme immunoassay (GTI, Waukesha, WI), Luminex screen, Flow PRA and single antigen beads (One Lambda, Canoga Park, CA). For the latter test, raw median fluorescence intensity values > 1,000 were considered positive and values of 1,000 to 3,000 were considered positive but acceptable for potential donors carrying the target antigen.
Statistical Analysis
Continuous variables were compared using the Student's t-test and categorical variables were analyzed using the chi-squared or Fisher's exact test. Multivariate logistic regression was employed to identify risk factors for nonsensitization at retransplant evaluation. Since immunosuppression exposure after transplant failure was categorized by time, subjects in the prolonged withdrawal group were “immortal” for the first 3 months after transplant failure since they had to survive at least 3 months to be categorized into the prolonged withdrawal group (38). Therefore, a time-dependent, multivariate Cox model was utilized to adjust for this time bias since it has been reported as an effective tool for similar survival analyses (39,40). All statistical analyses were conducted with SAS 9.2 (Cary, NC). Statistical significance level was defined as P<0.05.
Supplemental Digital Content
ACKNOWLEDGEMENTS
Special thanks to Dr. Mark Segal and Dr. David Nelson for their assistance in preparing this manuscript. This work supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064.
Abbreviations
- aHR
Adjusted Hazard Ratio
- aOR
Adjusted Odds Ratio
- CI
Confidence Interval
- CNI
Calcineurin Inhibitor
- ELISA
Enzyme-linked Immunosorbent Assay
- ESRD
End Stage Renal Disease
- HLA
Human Leukocyte Antigen
- IS
Immunosuppression
- LDA
Lymphocyte Depleting Agent
- MPA
Mycophenolic Acid
- PRA
Panel Reactive Antibody
- SD
Standard Deviation
- SDC
Supplemental Digital Content
Contributor Information
Michael J. Casey, Department of Medicine, University of Florida, Gainesville, FL, United States.
Xuerong Wen, Department of Medicine, University of Florida, Gainesville, FL, United States.
Liise K. Kayler, Department of Surgery, Montefiore Medical Center, Bronx, NY, United States.
Ravi Aiyer, Department of Medicine, University of Washington Medical Center, Seattle, WA, United States.
Juan C. Scornik, Department of Pathology, University of Florida, Gainesville, FL, United States.
Herwig-Ulf Meier-Kriesche, Bristol-Meyers Squibb, Princeton, NJ, United States..
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589. doi: 10.1681/ASN.V123589. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant. 2004;4:1662–1668. doi: 10.1111/j.1600-6143.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 4.Danovitch GM, editor. Handbook of Kidney Transplantation. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2005. pp. 19–20. [Google Scholar]
- 5.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2:970–974. doi: 10.1034/j.1600-6143.2002.21015.x. [DOI] [PubMed] [Google Scholar]
- 6.Knoll G, Muirhead N, Trpeski L, Zhu N, Badovinac K. Patient survival following renal transplant failure in Canada. Am J Transplant. 2005;5(7):1719–1724. doi: 10.1111/j.1600-6143.2005.00921.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnston O, Zalunardo N, Rose C, Gill JS. Prevention of Sepsis during the Transition to Dialysis May Improve the Survival of Transplant Failure Patients. J Am Soc Nephrol. 2007;18:1331–1337. doi: 10.1681/ASN.2006091017. [DOI] [PubMed] [Google Scholar]
- 8.Ojo A, Wolfe RA, Agodoa LY, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 1998;66(12):1651–9. doi: 10.1097/00007890-199812270-00014. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Renal Data System . USRDS 2011 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. Table E.3. [Google Scholar]
- 10.Meier-Kriesche HU, Scornik JC, Susskind B, Rehman S, Schold JD. A lifetime versus a graft life approach redefines the importance of HLA matching in kidney transplant patients. Transplantation. 2009;88(1):23–9. doi: 10.1097/TP.0b013e3181a9ec89. [DOI] [PubMed] [Google Scholar]
- 11.Mizutani K. HLA mismatches and PRA in kidney retransplants. Clin Transpl. 2007:19–27. [PubMed] [Google Scholar]
- 12.Adeyi OA, Girnita AL, Howe J, et al. Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol. 2005;14(1):53–62. doi: 10.1016/j.trim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Sumrani N, Delaney V, Hong JH, Daskalakis P, Sommer BG. The influence of nephrectomy of the primary allograft on retransplant graft outcome in the cyclosporine era. Transplantation. 1992;53(1):52–5. doi: 10.1097/00007890-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Khakhar AK, Shahinian VB, House AA, et al. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc. 2003;35(2):862–3. doi: 10.1016/s0041-1345(02)04031-9. [DOI] [PubMed] [Google Scholar]
- 15.Scornik JC, Meier-Kriesche H-U. Human leukocyte antigen sensitization after transplant loss: timing of antibody detection and implications for prevention. Human Immunology. 2011;72:398–401. doi: 10.1016/j.humimm.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Smak Gregoor PJH, Zietse R, van Saase JLCM, et al. Immunosuppression should be stopped in patients with renal allograft failure. Clin Transplant. 2001;15:397–401. doi: 10.1034/j.1399-0012.2001.150606.x. [DOI] [PubMed] [Google Scholar]
- 17.Kendrick EA, Davis CL. Managing the failing allograft. Semin Dial. 2005 Nov-Dec;18(6):529–39. doi: 10.1111/j.1525-139X.2005.00100.x. [DOI] [PubMed] [Google Scholar]
- 18.Morales A, Gavela E, Kanter J, et al. Treatment of Renal Transplant Failure. Transplantation Proceedings. 2008;40:2909–2911. doi: 10.1016/j.transproceed.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Jassal SV, Lok CE, Walele A, Bargman JM. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: results of a decision analysis. Am J Kidney Dis. 2002;40(1):178–83. doi: 10.1053/ajkd.2002.33927. [DOI] [PubMed] [Google Scholar]
- 20.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN / SRTR 2010 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2011. Table 5.2. [Google Scholar]
- 21.Augustine JJ, Woodside KJ, Padiyar A, Sanchez EQ, Hricik DE, Schulak JA. Independent of nephrectomy, weaning of immunosuppression leads to late sensitization after kidney transplant failure. Transplantation. 2012 Oct 15;94(7):738–43. doi: 10.1097/TP.0b013e3182612921. [DOI] [PubMed] [Google Scholar]
- 22.Gill JS, Abichandani R, Kausz AT, Pereira BJ. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002 Nov;62(5):1875–83. doi: 10.1046/j.1523-1755.2002.00640.x. [DOI] [PubMed] [Google Scholar]
- 23.Vanrenterghem Y, Khamis S. The management of the failed renal allograft. Nephrol Dial Transplant. 1996;11(6):955–7. [PubMed] [Google Scholar]
- 24.Bennett WM. The failed renal transplant: in or out? Semin Dial. 2005 May-Jun;18(3):188–9. doi: 10.1111/j.1525-139X.2005.18306.x. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Renal Data System, USRDS 2011 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Vol. 2. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. Table 5a. [Google Scholar]
- 26.Katznelson S, Bhaduri S, Cecka JM. Clinical aspects of sensitization. Clin Transpl. 1997:285–96. [PubMed] [Google Scholar]
- 27.Dawson KL, Patel SJ, Xu J, Knight RJ, Gaber AO. Effect of immunosuppression for first kidney or kidney/pancreas transplant on sensitization at the time of second transplant. Transplantation. 2011 Apr 15;91(7):751–6. doi: 10.1097/TP.0b013e31820cfd5b. [DOI] [PubMed] [Google Scholar]
- 28.Sanfilippo F, Goeken N, Niblack G, Scornik J, Vaughn WK. The effect of first cadaver renal transplant HLA-A, B match on sensitization levels and re-transplant rates following graft failure. Transplantation. 1987;43:240–4. doi: 10.1097/00007890-198702000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Billen EVA, Christians MHL, Lee J, van den Berg-Loonen EM. Donor-directed HLA antibodies before and after transplantectomy detected by the Luminex single antigen assay. Transplantation. 2009;87:563–9. doi: 10.1097/TP.0b013e3181949e37. [DOI] [PubMed] [Google Scholar]
- 30.Fuller TC, Delmonico FL, Cosimi B, Huggins CE, King M, Russell PS. Impact of blood transfusion on renal transplantation. Ann Surg. 1978 Feb;187(2):211–8. doi: 10.1097/00000658-197802000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scornik JC, Ireland J, Howard RJ, Pfaff WW, Fennell RS., 3rd Sensitization by blood transfusions in previously transplanted patients. Transplantation. 1983;35:505–6. doi: 10.1097/00007890-198305000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Hyun J, Park KD, Yoo Y, et al. Effects of different sensitization events on HLA alloimmunization in solid organ transplantation patients. Transplant Proc. 2012 Jan;44(1):222–5. doi: 10.1016/j.transproceed.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Scornik JC, Schold JD, Bucci M, Meier-Kriesche HU. Effects of blood transfusions given after renal transplantation. Transplantation. 2009;87:1381–6. doi: 10.1097/TP.0b013e3181a24b96. [DOI] [PubMed] [Google Scholar]
- 34.Scornik JC, Brunson ME, Howard RJ, Pfaff WW. Alloimmunization, memory, and the interpretation of crossmatch results for renal transplantation. Transplantation. 1992;54:389–94. doi: 10.1097/00007890-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Gebel HM, Bray RA. Sensitization and sensitivity: defining the unsensitized patient. Transplantation. 2000 Apr 15;69(7):1370–4. doi: 10.1097/00007890-200004150-00027. [DOI] [PubMed] [Google Scholar]
- 36.Lucas DP, Paparounis ML, Myers L, et al. Detection of HLA class I-specific antibodies by the QuikScreen enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1997 May;4(3):252–7. doi: 10.1128/cdli.4.3.252-257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebel HM, Bray RA, Nickerson P. Pre-Transplant Assessment of Donor-Reactive, HLA-Specific Antibodies in Renal Transplantation: Contraindication vs. Risk. Am J Transplant. 2003 Dec;3(12):1488–500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 38.Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 39.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 40.Cassuto JR, Reese PP, Sonnad S, et al. Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients. Am J Transplant. 2010 Nov;10(11):2502–11. doi: 10.1111/j.1600-6143.2010.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.