Abstract
The endoplasmic reticulum (ER) is the primary intracellular organelle responsible for protein and lipid biosynthesis, protein folding and trafficking, calcium homeostasis, and several other vital processes in cell physiology. Disturbance in ER function results in ER stress and subsequent activation of the unfolded protein response (UPR). The UPR up-regulates ER chaperones, reduces protein translation, and promotes clearance of cytotoxic misfolded proteins to restore ER homeostasis. If this vital process fails, the cell will be signaled to enter apoptosis, resulting in cell death. Sustained ER stress also can trigger an inflammatory response and exacerbate oxidative stress, both of which contribute synergistically to tissue damage. Studies performed over the past decade have implicated ER stress in a broad range of human diseases, including neurodegenerative diseases, cancer, diabetes, and vascular disorders. Several of these diseases also entail retinal dysfunction and degeneration caused by injury to retinal neurons and/or to the blood vessels that supply retinal cells with nutrients, trophic and homeostatic factors, oxygen, and other essential molecules, as well as serving as a conduit for removal of waste products and potentially toxic substances from the retina. Collectively, such injuries represent the leading cause of blindness world-wide in all age groups. Herein, we summarize recent progress on the study of ER stress and UPR signaling in retinal biology and discuss the molecular mechanisms and the potential clinical applications of targeting ER stress as a new therapeutic approach to prevent and treat neuronal degeneration in the retina.
Keywords: endoplasmic reticulum stress, unfolded protein response, retinal degeneration, cell death, apoptosis, inflammation
1. Introduction
The endoplasmic reticulum (ER) has long been recognized as the cell’s protein factory, engaging in the biosynthesis, post-translational modification, folding, and trafficking of proteins (Alberts et al., 2002). Only properly folded proteins with native configurations can be released from the ER and transported successfully to the Golgi apparatus. Similar to its function in protein production, the ER serves as the primary site for the de novo synthesis of phospholipids and sterols, which constitute the major lipid components of the plasma membrane and the membranes of subcellular organelles. In addition, the ER is the central reservoir for storage of intracellular calcium and actively modulates calcium homeostasis (Timmins et al., 2009). Activation of the calcium channels on the ER membrane leads to calcium release from the ER into cytoplasm, which in turn activates calcium-dependent kinases and phosphatases, resulting in a diverse variety of cellular responses as well as detrimental events such as apoptosis.
Apart from its traditional roles in protein, lipid and calcium homeostasis, emerging evidence demonstrates that the ER is centrally involved in sensing of subtle metabolic changes within the cell and transmittal of the signal to the nucleus for gene regulation (Ron and Walter, 2007; Todd et al., 2008). This novel role of the ER is mediated by three major signal transducers: PKR-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). These proteins are activated in response to increased concentrations of misfolded or unfolded proteins in the ER lumen, a condition known as ER stress. In turn, IRE1, PERK and ATF6 initiate their downstream signaling pathways, collectively comprising the unfolded protein response (UPR), to combat ER stress through three complementary strategies: 1) up-regulating chaperones and folding enzymes to facilitate recovery of the damaged protein’s original three dimensional structure; 2) attenuating global protein translation to reduce the influx of client proteins to the ER; and 3) enhancing ER-associated degradation (ERAD) to facilitate clearance of misfolded proteins from the ER (Schroder and Kaufman, 2005) (Fig. 1). However, if the duration and intensity of ER stress overwhelms the capacity of the UPR to restore ER homeostasis, the apoptotic cascade will be activated to eliminate stressed cells, leading to cell death and dropout (Paschen and Frandsen, 2001; Rao et al., 2004). Unresolved ER stress also activates pathological signaling pathways of oxidative stress, inflammation and immune responses, and dysregulated angiogenesis, and is implicated in a plethora of human diseases, such as neurodegenerative diseases (e.g., Alzheimer’s disease), cardiovascular and peripheral vascular diseases, and diabetes.
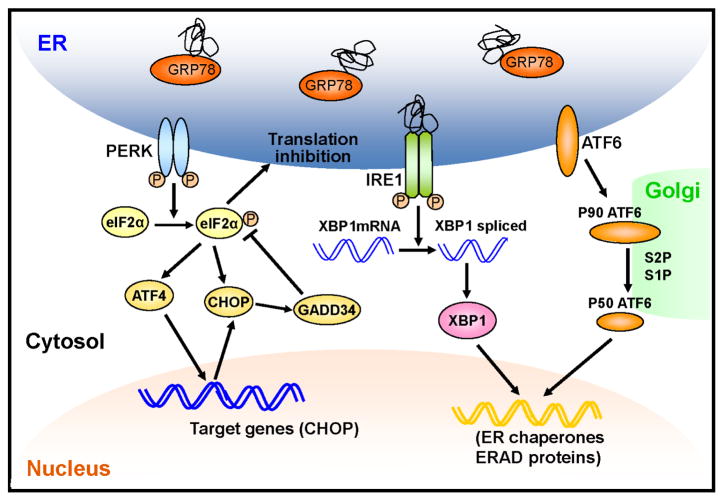
Figure 1. Activation of the UPR during ER stress.
Upon accumulation of unfolded or misfolded proteins in the ER lumen, GRP78 dissociates from ER stress transducers including IRE1, PERK and ATF6. Loss of GRP78 binding allows oligomerization and autophosphorylation of IRE1. In addition, unfolded proteins can directly bind to IRE1 resulting in its activation. Activated IRE1 splices XBP1 mRNA through its RNase activity, generating spliced XBP1 encoding a transcription factor that upregulates ER chaperones and UPR genes involved in the ER-associated protein degradation (ERAD). PERK is activated in a similar manner to IRE. Activated PERK phosphorylates eIF2α, leading to a global attenuation of protein synthesis and a concomitant increase in ATF4 translation. ATF4 binds to the UPR response element (UPRE) and induces its target genes such as CHOP. Enhanced eIF2α phosphorylation further increases CHOP protein level by facilitating its translation. CHOP, in turn, suppresses eIF2 phosphorylation by upregulating GADD34 resulting in the recovery of protein synthesis. After the dissociation of GRP78, ATF6 translocates to Golgi apparatus, where it is activated by proteolysis. Activated ATF6 transcriptionally induces ERAD and other UPR target genes.
The retina is a very thin, stratified neural tissue lining the posterior pole of the eye, composed of a highly organized network of neurons (photoreceptors, interneurons and ganglion cells), glia and astrocytes, and blood vessels. Irreversible damage to retinal neurons causes retinal dysfunction, degeneration, and cell death, resulting in acute or chronic loss of visual function, such as is manifest in age-related macular degeneration (AMD), retinitis pigmentosa (RP), retinal detachment, and glaucomatous retinopathy. In diabetic retinopathy (DR), injury of retinal vascular cells resulting in retinal edema and ischemia also causes retinal neuronal death and blindness. Although many advances and breakthroughs in the basic and clinical research of retinal diseases have been made over the past several years, the mechanisms underlying key pathophysiological events that cause retinal degeneration are not fully understood. Intriguingly, a number of studies have demonstrated that ER stress plays a role in neuronal cell injury in the retina, and these findings have raised the possibility that inhibition of ER stress may provide a novel and effective therapeutic approach in the treatment of retinal diseases (Adachi et al., 2011; Gorbatyuk et al., 2010; Inokuchi et al., 2009; Li et al., 2009; Li et al., 2011; Zhong et al., 2012a; Zhong et al., 2012c). In this article, we review recent progress on the study of ER stress in the retina, discuss the mechanisms and therapeutic promise of targeting ER stress in retinal degeneration, and highlight the importance of the adaptive UPR in protecting retinal cells in pathological and stress conditions.
2. The unfolded protein response (UPR)
At present it remains unclear how these ER membrane proteins sense ER stress and activate the UPR. One widely accepted model implicates the ER chaperone GRP78 (glucose-regulated protein 78), also known as Bip (immunoglobulin heavy chain-binding protein) (reviewed in Ron and Walter, 2007). It has been shown that each of these three ER membrane proteins contains an ER luminal domain that sequesters GRP78 when concentrations of misfolded proteins are low in the ER lumen. Binding of GRP78 to ER stress transducers keeps these proteins in an inactive state in unstressed cells (Bertolotti et al., 2000; Ng et al., 1992). During ER stress, GRP78 dissociates from the ER membrane proteins and binds to misfolded or unfolded proteins and facilitates their ATP-dependent protein folding. Loss of GRP78 binding allows oligomerization and autophosphorylation of PERK and IRE1, resulting in activation of the corresponding UPR pathways (Haze et al., 2001). However, this model is challenged by the evidence that dissociation of GRP78 from IRE1 is not sufficient to activate IRE1 (Kimata et al., 2004). Further, recent work by Gardner and Walter shows that unfolded proteins per se may be the activating ligands for IRE1, whereas the GRP78/BiP association only plays a role in fine-tuning of IRE1-mediated signaling (Gardner and Walter, 2011). Thus, more complex mechanisms may be involved in the initiation of ER stress response or UPR in mammalian cells under distinct stress conditions. Nevertheless, activation of the UPR pathways by the three major ER stress sensors, IRE1, PERK and ATF6, plays a pivotal role in remaining the function and homeostasis of the ER and has also been implicated in a vast variety of cellular processes. Major molecular components of the UPR are summarized in Table 1.
Table 1.
ER chaperones and molecular components of the UPR.
| Protein/Gene | Function(s) | Reference |
|---|---|---|
| ER stress sensors | ||
| Bip (GRP78)/HSPA5 | Dissociation with ER stress sensors activates the UPR | (Bertolotti et al., 2000; Ng et al., 1992) |
| IRE1/ERN1 | Splices the mRNA encoding XBP1 to activate XBP1 Mediates degradation of ER-targeted mRNA (RIDD) Binds to TRAF2 and activates pathways of Ask1/JNK and IKK/NF-κB |
(Hollien et al., 2009; Urano et al., 2000; Yoshida et al., 2001) |
| PERK/EIF2AK3 | Phosphorylates eIF2α to downregulate global protein translation | (Harding et al., 2000) |
| ATF6/ATF6 | Activated in Golgi apparatus and upregulates ER-targeted genes | (Chen, 2002) |
| Transcription factors | ||
| XBP1/XBP1 | Induces ER chaperones (Bip, p58IPK, ERdj4, HEDJ, PDI-P5, EDEM, etc) Regulates other UPR genes (ATF6 and CHOP) Regulates genes in ER biogenesis Regulates oxidative stress, immune response and lipogenesis |
(Glimcher, 2010; Lee, 2002; Reimold, 2000; Zhong et al., 2012) |
| ATF6/ATF6 | Regulates UPR genes (XBP1, BIP/GRP78, CHOP) Regulates proteins involved in ERAD |
(Yamamoto et al., 2007; Yoshida et al., 2000) |
| ATF4/ATF4 | Induces pro-apoptotic gene CHOP Regulates stress response genes Regulates genes in oxidative stress and angiogenesis |
(Harding et al., 2003; Lange et al., 2008; Roybal et al., 2004) |
| CHOP/DDIT3 | Regulates pro- and anti-apoptotic genes and initiates apoptosis Induces eIF2α dephosphorylation via upregulation of GADD34 |
(Marciniak SJ, 2004; McCullough et al., 2001) |
| CREBH/CREBH | Activates acute-phase genes and regulates inflammatory response | (Zhang et al., 2006) |
| Regulator of protein translation | ||
| eIF-2α/EIF2A | Inhibits eIF2B to arrest general protein translation Increases translation of ATF4 and CHOP during ER stress |
(Harding et al., 1999; Ron and Walter, 2007) |
| ER Chaperones | ||
| Bip (GRP78)/HSPA5 | Binds unfolded or misfolded proteins and promotes protein folding/refolding | (Ting and Lee, 1988) |
| Calnexin/CANX | Recognizes oligosaccharide and promotes glycosylated protein folding | (Ware et al., 1995) |
| Calreticulin/CALR | promotes glycosylated protein folding and quality control Binds to calcium and functions as calcium buffering chaperone in ER |
(Michalak et al., 1999) |
| PDI/PDIA2 | Catalyzes disulfide bond formation | (Hatahet and Ruddock, 2009) |
| ERdj5/ERdj5 | Functions as a disulfide reductase to degrade misfolded proteins in the ER | (Ushioda et al., 2008) |
The IRE1 pathway
The IRE1 (inositol-requiring enzyme 1) pathway is the most evolutionarily conserved UPR branch, from yeast to humans, and has been shown to play a critical role in protecting stressed cells from injury and cell death (Lin et al., 2007). In mammalian cells, there are two functional homologs of IRE1p: IRE1α and IRE1β. IRE1α is ubiquitously expressed, whereas IRE1β expression is restricted primarily to intestinal epithelial cells (Tirasophon et al., 1998a; Wang et al., 1998). Both proteins localize to the ER and are capable of transducing the signal across the ER membrane and activating downstream signaling events associated with ER stress (Patil and Walter, 2001). During ER stress, IRE1 is activated by oligomerization followed by activation of its cytoplasmic kinase and endoribonuclease domains (see Walter and Ron, 2011). The latter splices the mRNA of X-box binding protein 1 (XBP1) resulting in a frameshift in the coding sequence and generation of a new protein, named spliced XBP1 (XBP1s). XBP1s is a potent basic leucine zipper (bZIP) transcription factor that functions as a master regulator of the adaptive response to ER stress through induction of ER chaperones. XBP1-deficient cells are susceptible to oxidative stress and inflammation-induced cell death (Lee et al., 2002; Reimold et al., 2000; Zhong et al., 2012b). It also plays a role in ER biogenesis, fetal development, lipogenesis, adipogenesis, and other important cellular processes (Glimcher, 2010).
In addition to its prominent function in XBP1 splicing, IRE1 mediates the cleavage and degradation of ER-targeted mRNAs to reduce the burden from newly synthesized proteins on the stressed ER (Hollien and Weissman, 2006; Hollien et al., 2009). This new branch of the UPR was named regulated Ire1-dependent decay (RIDD). RIDD also modulates the generation and/or degradation of small non-coding RNA molecules (microRNAs) and regulates target proteins at post-transcriptional level. For example, sustained activation of IRE1α cleaves microRNAs 17, 34a, 96, and 125b, resulting in enhanced translation of caspase-2 mRNA and elevated protein level of this proapoptotic protein during ER stress (Upton et al., 2012). Deletion of XBP1 increases IRE1 expression and activation, resulting in RIDD-mediated cleavage of cytochrome P450-encoding mRNA in the liver and the cleavage of the mRNA of secretory μ chains of IgM in plasma cells (Benhamron et al., 2013), suggesting a reciprocal regulation between XBP1 and RIDD. Interestingly, a recent study using a Drosophila model shows that RIDD is required for photoreceptor differentiation and rhabdomere morphogenesis and this effect is XBP1-independent (Coelho et al., 2013). Future studies on the function and potential implications of RIDD in retinal neuronal development and protection are consequently of great interest.
The PERK pathway
PERK (PKR-like endoplasmic reticulum kinase) is a serine/threonine protein kinase located on the ER membrane. Like IRE1, PERK is activated by ER stress via dimerization and autophosphorylation. Once activated, PERK phosphorylates the eukaryotic initiation factor eIF2α, which in turn inhibits the guanine nucleotide exchange factor eIF2B, resulting in down-regulation of global translation initiation and protein biosynthesis (Harding et al., 2000b). While phosphorylation of eIF2α reduces general protein translation, some genes, e.g. activating transcription factor 4 (ATF4), with upstream open reading frames (uORFs) in their 5′ untranslated region could escape from the eIF2α-initiated translational attenuation (Harding et al., 2003). ATF4 is a bZIP transcription factor belonging to the activating transcription factor/cAMP response element binding (ATF/CREB) protein family. It binds to cAMP response element (CRE) and CCAAT/enhancer binding protein (C/EBP)/ATF response element (CARE) and regulates genes involved in cellular stress response, hematopoiesis, lens and skeletal development, inflammation, angiogenesis, oxidative stress, and apoptosis (Lange et al., 2008; Masuoka and Townes, 2002; Roybal et al., 2004; Zhang et al., 2011b).
The most well-studied target gene of ATF4 during persistent ER stress is C/EBP homologous protein-10 (CHOP), also known as growth-arrest and DNA-damage-inducible gene 153 (GADD153). Induction of CHOP signals stressed cells to enter apoptosis. Although each of the three branches of the UPR is capable of activating CHOP, ATF4 is considered to be the major transcription factor inducing CHOP transcription. In addition, CHOP is regulated by eIF2α phosphorylation via a mechanism similar to that utilized by ATF4 resulting in enhanced translation of CHOP (Palam et al., 2011). In turn, CHOP promotes the dephosphorylation of eIF2α through induction of GADD34. As a consequence, eIF2α phosphorylation-mediated attenuation of translation is impaired, resulting in overwhelmed protein loading in the ER and apoptosis (Marciniak et al., 2004). CHOP also signals apoptosis through binding directly to the promoter of the TRB3 gene and inhibiting AKT activation, as well as through increasing calcium influx from the ER to the mitochondria, resulting in the activation of mitochondria-dependent apoptotic pathway (Giorgi et al., 2009).
The ATF6 pathway
ATF6 consists of the two closely related proteins, ATF6α and ATF6β, in mammalian cells, both of which are ER membrane-bound transcription factors activated during ER stress (Yamamoto et al., 2007). Unlike its fellow ER stress sensors IRE1 and PERK, ATF6 is activated through transportation to the Golgi, where it is cleaved by Site-1 (serine protease) and Site-2 (metalloprotease) proteases (Chen et al., 2002). Once ATF6 is cleaved, it is transported to the nucleus where it can play a role in ERAD and in apoptosis. ATF6 also can bind to XBP1 and increase expression of XBP1 and other molecular chaperons, such as GRP78. Studies from the Mori group demonstrated that mammalian ATF6α, but not ATF6β, is required for transcriptional induction of major ER chaperones and that ATF6α heterodimerizes with XBP1 for the induction of major ERAD components (Yamamoto et al., 2007). Mice deficient in ATF6α or ATF6β have normal embryonic/postnatal development, while double knockout of ATF6α and ATF6β is embryonic lethal (Yamamoto et al., 2007). A recent study further supports a role of ATF6 in maintaining ER function survival of stressed cells (Martindale et al., 2006). Using transgenic mice that overexpress ATF6 in the heart, the authors showed that enhancing ATF6 function up-regulated protective molecules such as GRP78, ameliorated myocardial injury, and reduced both apoptosis and necrosis in cardiac tissue from mice exposed to ischemia/reperfusion (I/R). These results suggest that activation of the ATF6 branch of the UPR may exert salutary and beneficial effects against ER stress-associated cellular injury.
3. ER stress and age-related macular degeneration
Age-related macular degeneration (AMD) is a complex, multifactoral disease associated with slow, progressive degeneration of photoreceptor cells in the central retina, resulting in decreased central visual acuity and function in the elderly (reviewed in Bressler et al., 1988; Alexander, 1993; Bird et al., 1995). It is the leading cause of blindness in people 65 years of age and older and affects more than 1.75 million individuals in the United States (The Eye Diseases Prevalence Research Group; see Friedman et al., 2004a). The pathogenic mechanism of AMD is complex and far from well understood. Although there is no direct evidence that suggests ER stress in human AMD, the close association of ER stress with inflammation, oxidative stress, apoptosis and angiogenesis predict an important role of ER stress in the development of AMD (Libby and Gould, 2010; Sauer et al., 2008). In addition, chronic ER stress has been linked to a variety of age-related neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease (Salminen et al., 2010). In a review article, Libby and Gould proposed that ER stress induced by misfolded proteins could lead to chronic oxidative stress and complement deregulation in the RPE and/or choroid, which causes damage to RPE cells and photoreceptor degeneration (Libby and Gould, 2010). Reduced levels of ERp29, an ER chaperone that promotes protein trafficking, were observed in the RPE from Ccl2−/−/Cr3x−/− mice (Ethen et al., 2006). However, the exact role of ERp29 and other ER chaperones in RPE cells and their implication in AMD remains to be investigated.
Inflammation plays a central role in the pathogenesis of AMD. Genetic and biochemical studies have identified strong association of AMD with variants in a number of inflammatory genes, such as complement factor H (CFH), complement factor B (CFB), complement component 2 (CC2) and complement component 3 (C3), pro-inflammatory cytokines (interleukin (IL)1b, IL6, and IL8) and anti-inflammatory cytokine IL10, chemokine (C-X3-C motif) receptor 1 (CX3CR1), Toll-like receptor 4 (TLR4), and major histocompatibility complex class I (HLA) (reviewed in Kanda et al., 2008; Anderson et al., 2010). Although aging-associated changes, environmental factors, as well as photo-oxidative stress, may act as the initial triggers of RPE and photoreceptor damage, dysregulated immune responses, evident by the presence of immune response proteins in drusen of post-mortem AMD retinas, may amplify the cellular damage leading to the progression of maculopathy towards advanced clinical features (Kanda et al., 2008; Anderson et al., 2010). Studies over the past two decades have implied ER stress in the regulation of inflammatory responses in a variety of cell types and tissues (Urano et al., 2000; Deng et al., 2004; Zhang and Kaufman, 2008). We have shown that ER stress induced by hyperglycemia, metabolic disturbance, and inflammatory cytokines contributes to endothelial and Müller cell inflammation in diabetic retinopaty (Li et al, 2009; Li et al, 2011; Chen et al, 2012, Zhong et al, 2012a; Zhong et al, 2012c). The role of ER stress in the regulation of inflammatory response in the RPE and photorecptors warrants future investigation.
Oxidative stress is believed to be a primary cause of age-related RPE damage. Multiple lines of evidence indicate that oxidative stress, resulting from excessive generation of reactive oxygen species (ROS) and/or decrease in cellular antioxidant defense mechanisms, contributes to pathogenesis of inflammation, apoptosis and cell death in various retinal diseases (Frey and Antonetti, 2011; Cai and McGinnis, 2012). Protein folding is considered a highly redox-dependent process. Protein oxidation leads to ROS generation during the formation of native disulphide bonds catalyzed by protein disulfide isomerase (PDI) and the process where PDI is recycled by thiol oxidase Ero1. On the other hand, aberrations of the redox status in the ER can negatively affect protein folding resulting in ER stress (for review, see Malhotra and Kaufman, 2007). Thus, ROS generation may act as a downstream target and as an upstream inducer of the UPR. Recent studies from our laboratory have revealed a role of UPR in controlling oxidative stress and cell survival in RPE cells (Zhong et al., 2012b; Chen et al., 2013). In one of the studies, we examined induction of ER stress in the retina and RPE/choroid complex from mice exposed to cigarette smoke. We observed a 4-fold increase in CHOP expression in the RPE/choroid complex after 2 wks of cigarette smoke exposure. In cultured human RPE cells, pharmacological inhibition of ER stress by chemical chaperones significantly alleviated apopotosis and cell death induced by various oxidants including hydroquinone, NaIO3 and cigarette smoke extract (CSE) (Chen et al., 2013; Huang et al., 2013). Inhibition of the PERK-eIF2α-CHOP pathway also protected RPE cells from oxidative injury and cell death (Fig. 2). These findings suggest oxidative stress activation of the the PERK/eIF2α/CHOP pathway may play a role in RPE cell damage.
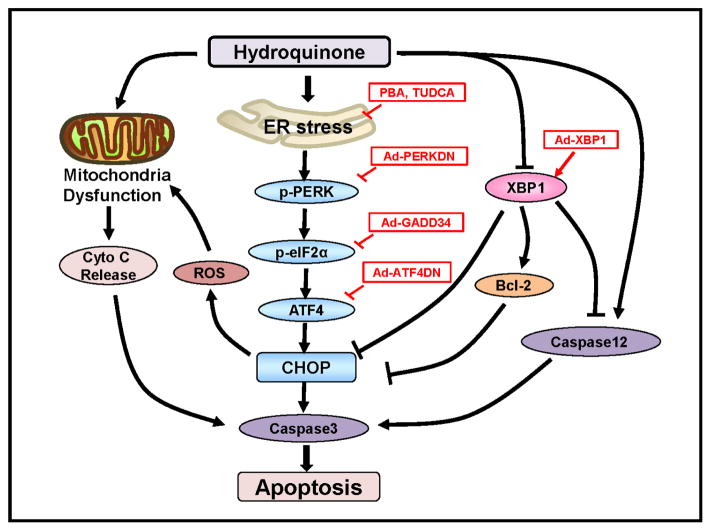
Figure 2. Role of ER stress and dysregulated UPR signaling in hydroquinone-induced RPE apopotosis.
Hydroquinone induces ER stress resulting in activation of the PERK-UPR branch and upregulation of CHOP expression. Inhibition of CHOP by means of chemical chaperones, Ad-PERKDN, and Ad-GADD34ΔN (expressing constitutively active GADD34) that target ER hydroquinone suppresses the adaptive UPR signaling mediated by XBP1 in RPE cells. Loss of XBP1 exacerbates hydroquinone-induced RPE apoptosis through regulation of Bcl-2, caspase12, and CHOP. In contrast, overexpressing XBP1 protects RPE cells and ameliorates hydroquinone-ER stress. Mitochondrial dysfunction-associated cytochrome C release and ER stress-mediated pro-apoptotic events converge on caspase-3 activation ultimately leading to apoptosis and cell death.
Intriguingly, our studies also suggest a differential role of the UPR pathways in regulation of RPE responses to oxidative stress. We found that in a rat model of light-induced retinal degeneration, expression of spliced (active) XBP1 and GRP78 was suppressed in the RPE, and these changes were coincident with increased oxidative stress and decreased expression of anti-oxidant genes (SOD2 and Nrf2) (Zhong et al., 2012b). Knockdown of XBP1 by siRNA resulted in reduced expression of SOD1, SOD2, catalase, and glutathione synthase in cultured human RPE cells and sensitized the cells to oxidative damage, implying a potential role of XBP1 in regulation of oxidative stress. To study the role of XBP1 in RPE cells, we generated conditional knockout mice that lack XBP1 only in RPE cells (Zhong et al., 2012b). Compare to wild type mice, XBP1-KO mice demonstrated lower leves of SOD1, SOD2, and catalase with enhanced oxidative stress in the RPE, and developed RPE apoptosis, loss of cone photoreceptors, reduced ONL thickness, and deficit in retinal function at ages of as early as 4–6 months. When challenged with intravenous injection of sodium iodate (NaIO3), XBP1-KO mice developed significantly increased RPE apoptosis and RPE atrophy, worsened photoreceptor loss, more severe disruption of the IS/OS junction, and increased number of inflammatory cells (Chen et al., 2013). These changes can be in part attributable to enhanced expression of CHOP, decreased expression of anti-apoptotic gene Bcl-2 and elevated activation of caspase-12 in XBP1-deficient RPE (Fig. 2). In cultured RPE cells, overexpression of XBP1 alleviated apoptosis and cell death induced by oxidative stress (Chen et al., 2013). These results suggest that XBP1 may function as a central coordinator of cellular response to oxidative and ER stresses and play an important role in cell survival. More interestingly, results from an ex vivo experiment indicate that the RPE isolated from old mice, when challenged with ER stress, only show low level of XBP1 activation while the RPE from yonger mice demonstrate a much stronger response (Zhong et al., 2012b). In addition, the protein level of active XBP1 was also reduced by the cigarette smoke component hydroquinone in cultured RPE cells (Chen et al., 2013). These observations led to our speculation that impaied function of the adaptive mechanism, e.g. mediated by XBP1 (Fig. 3), may partically contribute to age- and oxidative stress-related retinal injury. This hypothesis remained to be tested in future studies.
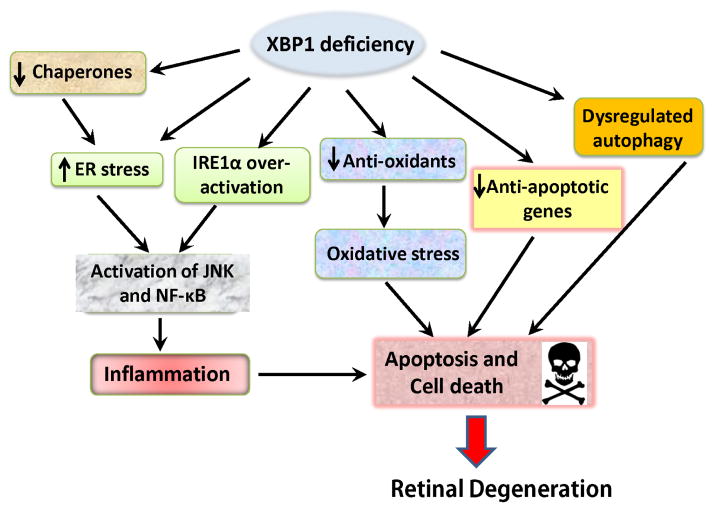
Figure 3. Potential mechanisms of XBP1 deficiency-related RPE cell injury.
XBP1 is a master regulator of the adaptive UPR in response to ER stress. Activation of XBP1 induces expression of ER-targeted genes including molecular chaperones such as GRP78, p58IPK, ERp29, and proteins involved in ER-associated degradation. Impaired expression and/or oactivation of XBP1 results in reduced ER folding capacity and increased ER stress. In addition, lack of XBP1 can lead to overactivation of IRE1 resulting in enhanced JNK and NF-kB activation and inflammation. As a transcription factor, XBP1 regulates a large number of non-ER-targeted genes. It also interacts with other signaling molecules and modulates vital cellular processes of oxidative stress, autophagy and apoptosis.
In addition to inducing apoptosis, ER stress may contribute to CNV formation by up-regulating VEGF expression and promoting angiogenesis (reviewed by (Salminen et al., 2010)). Additionally, ER stress down-regulates the anti-angiogenic factor pigment epithelium-derived factor (PEDF), tipping the balance toward pro-angiogenesis in the eye (Salminen et al., 2010). As to the mechnaim of ER stress promoting angiogenesis, all three branches of the UPR have been shown to play a role in VEGF regulation. Among the molecular components of the UPR, ATF4 has been extensively studied in RPE cells and is considered a key inducer of VEGF expression and secretion (for review, see (Zhang et al., 2011a)). More recently, a study showed that suppression of ER stress by a cyclopropene fatty acid (Sterculic acid) remarkably reduces 7-ketocholesterol-induced inflammatory response and mitigates laser-induced CNV formation. This finding implies a potential use of ER stress inhibitors in prevention and treatment of neovascular AMD (Huang et al., 2012).
4. ER stress and retinal degeneration
4.1. Retinal degeneration and protein misfolding
Retinal degeneration, such as rod-cone degenerations like retinitis pigmentosa (RP), is manifest when retinal (neuronal) cell functionality, viability, and structural integrity become compromised, resulting in progressive and irreversible neuronal cells loss in the retina and blindness. Hallmark features of retinal changes in RP include attenuated retinal vessels, “bone spicule” intraretinal pigmentation, mottling and granularity of the RPE, and optic nerve head pallor (Tzekov et al., 2011). RP is genetically transmitted from one generation to the next typically by one of three common inheritance modes: autosomal dominant, autosomal recessive, and X-linked; in addition, RP can occur as a result of idiopathic (simplex) random mutation. Mutations in the rod visual pigment rhodopsin (Rho), the most abundant protein in the photoreceptors (Hargave, 2001), are the most common causes of RP and account for 25–30% of the autosomal dominant forms of the disease (Sohocki et al., 2001). To date, more than 150 rhodopsin mutations have been reported (Kisselev, 2005) (see also RetNet™ https://sph.uth.tmc.edu/retnet/).
P23H Rho, a missense mutation at amino acid position 23 of rhodopsin that replaces a proline with a histidine, is the most common rhodopsin mutation; it prevents rhodopsin from binding its chromophoric prosthetic group, 11-cis-retinal, leading to autosomal dominant RP (Kaushal and Khorana, 1994; Liu et al., 1996). Using a transgenic Xenopus laevis model, Tam and Moritz demonstrated that the mutant P23H Rho causes protein misfolding, resulting in low levels of opsin protein expression and retention of misfolded proteins in the ER of inner segments; this, in turn, leads to ER stress contributing to rod photoreceptor cell death (Tam and Moritz, 2006). Consistent with these findings, several other studies have confirmed the role of ER stress in the pathogenesis of RP in a P23H Rho transgenic mouse model. Also, retinal levels of the ER chaperone GRP78 were found to be decreased in P23H Rho transgenic rats, while over-expressing GRP78 remarkably reduced ER stress-associated pro-apoptotic gene CHOP expression and attenuated apoptosis of photoreceptors (Gorbatyuk et al., 2010). Similarly, dark rearing of P23H Rho mutant mouse enhanced protein folding in the presence of 11-cis-retinal chromophore, which requires an intact 11-cis-retinal binding site within the mutant truncated rhodopsin, and completely or partially rescued rod photoreceptors from P23H mutantion-induced apoptosis (Tam and Moritz, 2007). Furthermore, because of the endogenous chaperone properties of 11-cis-retinal, attempts at using it as a visual system pharmacological agent have been made; however, it is not sufficiently stable to be suitable for clinical use. A recent study has suggested use of retinobenzaldehydes, a new class of stable, nonpolyene-type rhodopsin ligands with a low toxicity profile, as an alternative (Ohgane et al., 2010). This class of compounds exhibits the capacity to facilitate correct folding and intracellular trafficking of P23H Rho mutant proteins, and thus has the potential to be used in the treatment of RP in the future.
4.2. ER stress is a primary cause of photoreceptor death associated with rhodopsin mutations
Based on the role of ER stress in photoreceptor degeneration, a number of recent studies have actively pursued interesting approaches that target ER stress as a viable treatment option for RP. A recent study indicated that calnexin (Cnx), a lectin chaperon, binds preferentially to misfolded, mutant rhodopsin rather than the correctly folded rhodopsin (Noorwez et al., 2009). Targeting the Cnx-mutant rhodopsin complex, in the presence of the pharmacological chaperone 11-cis-retinal, results in the correct folding of the mutant rhodopsin. This makes pharmacological correction of mutant rhodopsin, as opposed to replacing the mutant rhodopsin by gene therapy, an interesting and potentially viable target for new drug trials. Perhaps of greater interest, a recent study identified that inactivation of VCP/ter94, a chaperone that extracts misfolded proteins from the ER and facilitates their degradation, actually suppresses retinal degradation in Drosophila Rh1P37H rhodopsin mutants (Griciuc et al., 2010). This raises the question of whether or not retrotranslocation and/or degradation of visual pigment is the primary cause of photoreceptor degeneration, and suggests yet another “druggable” approach to treating retinal degenerations, targeting ER stress (Griciuc et al., 2010). Further, a study by Lin and associates dissected what are the protective pathways in the UPR and when cells switch from the protective activation of the UPR to signaling cells to enter apoptosis (Lin et al., 2007). They discovered that in an animal model of RP, the IRE1-mediated protective pathway was induced, but declined rapidly after induction of ER stress. In contrast, the PERK pathway which leads to the activation of pro-apoptotic genes such as CHOP, persisted under prolonged ER stress (Lin et al., 2007). Furthermore, several recent studies evaluated ER stress in the aninmal models of retinal degeneration caused by genetic (i.e., rhodopsin mutations including S334ter, RCS, P23H-3, and hT17M) or environmental (i.e., intensive light exposure) factors (Kroeger et al., 2012; Kunte et al., 2012; Shinde et al., 2012). The authors observed significant increase in GRP78/Bip expression but only modest induction of CHOP accompanied by loss of photoreceptors in various models (Kroeger et al., 2012). Apart from the work in animal models, a recent exciting study revealed ER stress, e.g., increased GRP78 and CHOP expression, in rod cells derived from an RP patient with Rho mutation (Jin et al., 2012). This evidence further suggests an important role of ER stress and the UPR in photoreceptor loss in RP.
In addition, the fruit fly Drosophila has emerged as a valuable model for studying genetic and molecular mechanisms of retinal neurodegenerative diseases (reviewed in Wang and Montell, 2007; Knust, 2007; Cook et al., 2011). Using this model, Mendes and colleagues showed that, instead of inducing apoptosis, mild ER stress protects photoreceptor cells against cell death from various stimuli (Mendes et al., 2009). Furthermore, the authors showed that the anti-apoptotic and anti-oxidant effects of pre-treatment with mild ER stress are associated with the activation of the IRE1/XBP1 pathway, while low levels of PERK/eIF2α pathway activation are correlated with enhanced cell survival. These results corroborate several recent studies carried out in mouse models of RP, which demonstrated that activation of the IRE pathway, over-expression of ER chaperones such as GRP78, and/or enhancing the function of ERAD, protect photoreceptors from degeneration (Lin et al., 2007). A study of autosomal dominant RP in Drosophila suggests that ARFT72A, a homologue to the mammalian ARL1 located in the Golgi membranes of the photoreceptors cells, could play a role in protein quality control in the ER (Lee, 2011b). In the ninaE(D1) Drosophila model of autosomal dominant RP, loss of ARFT72A was able to rescue the ARFT72A membrane accumulation in the ER as well as the defect in rhodopsin maturation and suppress retinal degeneration. This study establishes an additional role for ARFT72A that has not previously been studied, implicating ARFT72A in the function of quality control machinery of the ER by distinguishing the cargoes destined for secretion from those destined for degradation. Together, the studies in Drosophila reinforce the case for targeting the UPR as a means of pharmacological intervention in RP pathogenesis (see Mendes et al., 2010; Lin and LaVail, 2010; Griciuc et al., 2011).
4.3. ER stress, CNG channel dysfunction, and other protein mutations in retinitis pigmentosa
Besides mutations of rhodopsin, disturbed homeostasis of several other proteins in photoreceptor cells, RPE cells and choriocapillaries also have shown cytotoxic effects associated with ER stress. For instance, defective trafficking of cone photoreceptor cyclic nucleotide-gated (CNG) channel subunits induces cell death of photoreceptor cells (Duricka et al., 2012). Compared with wild-type, 661W cells (a mouse cone photoreceptor-derived cell line) expressing CNGA3 subunits bearing the R563H and Q655X mutations exhibited lower cell viability associated with ER stress and activation of the pro-apoptotic UPR pathways. Pharmacological intervention of ER stress with chemical chaperones and the cGMP analogue CPT-cGMP [8-(4-chlorophenylthio)-cGMP] enhanced CNGA3 subunit maturation and promoted localization of the defective subunits to the plasma membrane, improving cell survival of photoreceptors. This suggests that ER stress induced by localization-defective CNG channels may contribute to photoreceptor degeneration. Interestingly, a recent study suggests that deficiency of the channel subunits CNGA3 and CNGB3 also results in ER stress and activation of the UPR, which activates ER-associated caspases (caspase-12 and caspase-7) leading to consequent cell death (Thapa et al., 2012).
A R14W mutation in the gene encoding carbonic anhydrase IV (CAIV), which is highly expressed in the choriocapillaris of the eye, has been shown to be responsible for an autosomal dominant form of RP (RP17) in a South African family (Rebello, 2004). Over-expressing the R14W mutant induces ER stress, which activates pro-apoptotic genes such as CHOP, resulting in endothelial cell apoptosis and ultimately leading to ischemia in the overlying retina and photoreceptor degeneration. In addition, two other mutations in the CAIV gene were identified and connected to an RP phenotype: R69H and R219S (Datta et al., 2009). Both mutations were capable of impairing CAIV trafficking to the cell surface. This causes abnormal intracellular retention of CAIV in the ER, leading to ER stress and increased cell death. As well, a novel missense mutation in the CLRN1 gene, previously associated with Usher’s syndrome, was recently linked to autosomal recessive RP (Khan et al., 2011). Through a case study of two consanguineous families, this mutation was found to cause attenuation of the retinal vessels, bone spicule pigmentation in the peripheral retina, and rod-cone pattern progression of the disease, which are associated with retention of the mutant protein in the ER and ER stress.
Notably, RP is not one disease; rather, it is a collection of many different genetic diseases that develop through a wide variety of genetic mutations, all of which lead to a common phenotype, i.e., progressive, irreversible dysfunction, death and loss of rod photoreceptor cells (reviewed in Phelan and Bok, 2000; Hartong et al., 2006). It, therefore, has become increasingly difficult to use gene therapy to target a signal mutant in the treatment of RP. However, one common feature in these diseases is the increased cellular burden of mutant proteins resulting in exaggerated ER stress, which causes apoptosis and loss of photoreceptors. Thus, enhancing the function of ER chaperones and improving the adaptive UPR pathways may provide an alternative approach for reducing sustained ER stress and preventing photoreceptor degeneration (Farrar et al., 2010; Griciuc et al., 2011). In addition, because most cases of RP are caused by mutations in rhodopsin, recent studies suggests that gene silencing may be a viable treatment option in this disease (Sinohara et al., 2008). The importance of this hypothesis is that choosing to eliminate the misfolded mutant protein rather than attempting to insert a functional protein will prevent UPR involvement and prolong ER stress activation. This would not work for all types of inherited degenerative disease; however, this treatment option would be theoretically ideal for cases like the S334ter-3 and S334ter-4 mutant transgenic rats that express normal and mutant isoforms of rhodopsin in a ratio of about 9:1 (Green et al., 2000). Getting rid of the mutant protein would allow the cell to continue functioning normally and prevent activation of the UPR.
4.4. ER stress in Stargardt disease and Leber congenital amaurosis
ER stress has also been linked to two not-so-commonly studied retinal degenerative diseases: Stargardt disease and Leber congenital amaurosis (LCA). Stargardt disease is an inherited macular degenerative disease that affects juveniles between the ages of six and twelve (reviewed in Shroyer et al., 1999; Donoso et al., 2001; Vasireddy et al., 2010). The symptoms of the disease are most similar to age-related macular degeneration, with rapid central vision loss, but the onset of the disease occurs much sooner in life. A rare form of autosomal dominant Stargardt disease is linked to the mutant ELOVL4 gene, which encodes an enzyme thought to be involved in the generation of very long-chain fatty acids (reviewed in Agbaga et al., 2010; Molday and Zhang, 2010). The ELOVL4 mutant protein does not buildup in the ER, but rather mislocalizes and forms aggregates; however, of most interest, the ELOVL4 mutant protein binds to and sequesters the wild-type protein into the aggregates as well as, and induces ER stress as indicated by an increase in GRP78 and CHOP levels in mutant-transfected cells (Karan et al., 2005). These findings suggest that sequestering of the wild-type ELOVL4 protein to non-ER compartments by the mutant ELOVL4 protein (i.e., a dominant-negative mechanism) plays a role in cellular dysfunction leading to the progression of this form of Stargardt disease. Thus, targeting the ELOVL4 mutant protein as a candidate for treatment of Stargardt disease is a viable option to restore the function of the endogenous wild-type ELOVL4 enzyme.
LCA is one of the most severe forms of inherited retinal degeneration caused by mutations of retinal pigment epithelium protein of 65 kDa (RPE65) or lecithin-retinol acyltransferase (LRAT), two key enzymes involved in visual cycle (reviewed in Cremers et al., 2002; Bennett, 2004). Like Stargardt disease, LCA preferentially affects children starting from infancy, but results in more severe retinal dystrophy, typically with poor visual prognosis. RPE65 is known as a retinoid isomerase that converts all-trans-retinyl esters to 11-cis-retinol, while LRAT catalyzes the esterification of all-trans retinol (vitamin A) to all-trans retinyl esters, providing substrates to RPE65. Deficiency of these enzymes results in disrupted recycling of 11-cis-retinal, the chromophore of the visual pigments rhodopsin in rod photoreceptors and cone opsins in cone photoreceptors that capture light to initiate the phototransduction cascade. Interestingly, a recent study suggests that, in addition to loss of their enzymatic functions, mutations of RPE65 or LRAT may also lead to photoreceptor degeneration through induction of ER stress (Zhang et al., 2011). Using a LRAT−/− mouse model, Zhang and colleagues demonstrated that mistrafficking of S-opsin (the short wavelength cone visual pigment) resulting in mislocalization and accumulation of S-opsin in LRAT−/− cones occurs before the onset of ventral/central cone degeneration. As these areas of retina express higher levels of S-opsin than the dorsal retina in mice, these results may explain why ventral and central cones degenerate more rapidly than do dorsal cones in Rpe65−/− and Lrat−/− LCA models. In addition, human blue opsin (S-opsin) and mouse S-opsin, but not mouse M-opsin or human red/green (L/M-) opsins, aggregated to form cytoplasmic inclusions in transfected cells, which may explain why blue cone function is lost earlier than red/green-cone function in patients with LCA (see Cideciyan, 2010). Further, over-expressing human blue opsin or mouse S-opsin induces activation of CHOP, which indicates an involvement of ER stress. Replacing rhodopsin with S-opsin in LRAT−/− rods also resulted in mislocalization and aggregation of S-opsin in the inner segment and the synaptic region of rods, accompanied by ER stress and accelerated degeneration of rods. In contrast, systemic administration of chemical chaperone TUDCA reduced ER stress and rescued cone degeneration in LRAT−/− mice, likely through enhancing the ER-associated protein degradation (ERAD) of cone membrane-associated proteins (Zhang et al., 2012). These results collectively suggest that ER stress play a critical role in photoreceptor cell death in LCA. Future studies are need to test the therapeutic potential of chemical chaperones in combination with gene therapies that replace mutant enzymes, to promote photoreceptor survival through enhancing protein trafficking and reducing ER stress.
5. ER stress and retinal neuronal injury in glaucoma
Glaucoma is a common eye disease in which the optic nerve suffers damage involving loss of retinal ganglion cells resulting in permanently damaged vision in the affected eye (reviewed in Allingham et al., 2010). The disease is often associated with increased intraocular pressure (IOP), but can also occur with normal pressures, a condition known as normal-tension glaucoma (NTG), or low tension glaucoma. Glaucoma can be divided into two main categories: open angle and closed angle. Open angle glaucoma is the slow progressive form of the disease where individuals may not notice they have lost vision until significant vision loss has occurred. However, closed angle glaucoma is a sudden, often painful onset of vision loss that progresses very quickly; often these individuals seek medical attention fast enough to avoid permanent damage to their eyes. A survey by the National Institutes of Health indicated that, as of 2004, 2 million individuals in the United States were affected with open-angle glaucoma, and, due to the rapid aging of the US population, this number will increase to 3 million by the year 2020 (The Eye Diseases Prevalence Research Group; see Friedman et al., 2004b).
Retinal ganglion cell death resulting in chronic glaucomatous optic neuropathy is a major cause of vision loss in glaucoma. In 2007, Shimazawa and colleagues reported that incubation of cultured RGC-5 cells, a transformed retinal neuronal cell line, with tunicamycin induced apoptotic cell death accompanied by ER stress (Shimazawa et al., 2007). However, the relevance of those findings to ganglion cell biology, either in vivo or in vitro, is now suspect: although originally thought to represent a transformed ganglion cell line, more recent evidence suggests that RGC-5 cells are, in reality, synonymous with 661W cells, an SV40-transformed, mouse photoreceptor-derived cell line (see Al-Ubaidi, 2014; Clark et al., 2013; Krishnamoorthy et al., 2013). At best, the results obtained by Shimazawa and coworkers may reflect, more broadly, the cell biological effects of tunicamycin on cultured retinal neurons, rather than on ganglion cells per se. Intravitreal injection of tunicamycin induced retinal ganglion cell loss and thinning of the inner plexiform layer in mice. Moreover, intravitreal injection of N-methyl-D-aspartate (NMDA), which binds to the NMDA receptor, which in turn results in excitotoxicity and neuronal death in the retina and retinal ischemia by increasing intraocular pressure, significantly increased ER stress in the retina of ERAI mice. These mice carry the F-XBP1-DBD-venus expression gene and were used to monitor ER-stress in vivo. In transverse cross-sections of retinas from ERAI mice, the fluorescence intensity was first increased in cells of the ganglion cell and inner plexiform layers at 12 and 24 h, respectively, after NMDA injection, and was localized to ganglion and amacrine cells at 12 and 24 h, respectively, and to microglial cells at 72 h. These results suggest that ganglion cells are sensitive to ER stress.
Doh and colleagues also examined the change of ER stress markers in the retina in a rat model of chronic glaucoma (Doh et al., 2010). In their study, glaucoma due to elevated IOP was induced by cauterizing three episcleral veins in adult male Sprague-Dawley rats and the high IOP was maintained in the cauterized eyes for 8 weeks. They found that expression of ER stress markers (e.g., Bip, CHOP and phospho-PERK) were significantly elevated at 1 or 2 weeks, compared to age-matched controls, and the increase persisted throughout the 8-week experiment in cauterized eyes. Moreover, immunohistochemistry showed a strong signal for both phospho-PERK and CHOP in ganglion cells, accompanied by TUNEL-positive cells and decreased number of ganglion cells after 8 weeks of IOP elevation. These results again suggest that increased ER stress and the PERK-p-eIF2alpha-CHOP pathway may play a role in ganglion cell loss in glaucoma.
In addition to the PERK pathway, activation of IRE1α and its downstream effectors, including apoptosis signal-regulating kinase 1 (ASK1), SAPK/ERK kinase 1 (SEK1) and JNK, also has been observed in retinal ganglion cells in a rat model of retinal ischemia-reperfusion (Hata et al., 2008). In this study, retinal ischemia was induced by clamping the ophthalmic artery within the dural sheath of the optic nerve. After 60 minutes, the clip was removed to allow reperfusion of the retinal vessels. Retinas were isolated and fixed for immunostaining after 6, 9, 12, 18, and 24 hours, and 2, 5, and 9 days of reperfusion. The results showed that immunoreactivity of IRE1α was significantly higher in ganglion cells in ischemic retinas than in controls. Moreover, the numbers of SEK1-, ASK1-, and JNK-positive cells were significantly increased in the GCL compared to those in control retinas. These results indicate that activation of the IRE1α–associated apoptotic pathway may contribute to ganglion cell death in ischemic retinal damage (retinal ischemia-reperfusion injury) including glaucomatous retinopathy.
While most studies to date have focused on the role of ER stress in glaucomatous retinal neurodegeneration, results from a few recent studies provide exciting evidence that ER stress is implicated in the pathogenesis of primary open angle glaucoma (POAG) (Zode et al., 2011). Mutations in the gene encoding myocilin (MYOC; also called TIGR), a secreted glycoprotein found in high amounts in the cells of the trabecular meshwork, ciliary body, iris, and sclera, but in low levels in retina and optic nerve (reviewed in Tamm, 2002), have been recognized as the most common genetic cause of POAG and are responsible for an estimated 4% of POAG and most cases of autosomal dominant juvenile-onset open-angle glaucoma. Approximately 70 different MYOC/TIGR mutations have been identified; the significance of these mutations is, however, largely unknown (reviewed in Polansky, 2003). To study the mechanism of MYOC mutation-induced POAG, Zode and colleagues developed a transgenic mouse model that express human MYOC containing the Y437H mutation in the iridocorneal angle and the sclera. These mice exhibit glaucoma phenotypes that closely resemble the clinical features seen in patients with POAG caused by the Y437H MYOC mutation, including elevated IOP, retinal ganglion cell death, and axonal degeneration. The authors observed that mutant myocilin was not secreted into the aqueous humor, but rather accumulated in the ER of the trabecular meshwork, resulting in ER stress and apoptosis. Reduction of ER stress with the chemical chaperone PBA prevented glaucoma phenotypes in Tg-MYOC(Y437H) mice by promoting the secretion of mutant myocilin into the aqueous humor and by decreasing intracellular accumulation of myocilin in the ER, thus preventing TM cell death. Similarly, using a natural osmolyte as an approach for the treatment of glaucoma, Jia and colleagues reported that TMAO was able to facilitate the folding and secretion of the mutant MYOC and successfully rescued trabecular meshwork cells from apoptosis (Jia et al., 2009). These studies demonstrate that ER stress is implicated in the pathogenesis of POAG, and that the use of chemical chaperons that inhibit ER stress may offer a new, efficacious approach for treatment of glaucoma patients.
6. Future Directions
The ER has been recognized as a principal stress sensor and signal transducer in the cell, and plays a central role in coordinating the cellular responses to various metabolic physiological changes in the cell. Consistent with this knowledge, the etiological role of ER stress has been explored in many human diseases in recent years. Relative to the advances in the areas of neurodegenerative diseases of the central nervous system, cardiovascular disease, cancer and diabetes, the study of ER stress in retinal diseases is still in its infancy. Also, it may be worth revisiting the interpretation of certain experimental results obtained before the UPR had been discovered. For example, three decades ago, Fliesler and colleagues reported that intravitreal injection of tunicamycin in frogs caused a progressive, photoreceptor-specific retinal degeneration (Fliesler et al., 1984). At the time, tunicamycin was thought to be a relatively selective inhibitor of protein N-glycosylation (see Elbein, 1984). However, its ability to provoke ER stress and the UPR had yet to be discovered (nearly a decade later). In retrospect, the retinal degeneration caused by intravitreal tunicamycin likely involved ER stress. In our recent studies, we showed that induction of ER stress by tunicamycin and other UPR stimulators was also sufficient to induce inflammation in retinal vascular and Müller cells, resulting in vascular leakage (Li et al., 2009; Zhong et al., 2012a). Intravitreal injection of tunicamycin was also able to induce loss of retinal ganglion cells and thinning of the inner plexiform layer in mice (Shimazawa et al., 2007). Furthermore, increased ER stress has been implicated in a number of retinal diseases, including retinal degenerations of diverse origins, AMD, diabetic retinopathy, and glaucomatous retinopathy. This highlights the need to explore further the role of ER stress in the pathogenesis of retinal cell injury, to understand the function of the UPR in retinal degeneration and vascular damage, and thereby to gain mechanism-based insights that will lead to the development of new therapeutic regimens to prevent and treat retinal diseases.
Importantly, several recent studies have shown that small molecule compounds which protect ER function in stressed cells, namely, pharmacological and chemical chaperones, may have great therapeutic promise in retinal diseases. These structurally distinct compounds promote protein folding and stabilize protein conformation, thus preserving protein structure and function and reducing stress in the ER (reviewed by Noorwez et al., 2008; Engin and Hotamisligil, 2011). For example, two chemical chaperones, i.e., PBA (Jeng et al., 2007; Duricka et al., 2012) and TUDCA (Boatright et al., 2006), have been used successfully to prevent retinal degeneration and ischemic injury in animal models. These compounds are approved by the U.S. Food and Drug Administration (FDA) for clinical use in humans for the treatment of urea cycle disorders (PBA) and cholestatic liver diseases (TUDCA) (Engin and Hotamisligil, 2011). In addition to developing small molecule chaperones as novel therapeutic approaches for retinal diseases, it is important to understand the molecular basis of ER stress-mediated cell damage in the retina and to identify key pathogenic genes that link ER stress with retinal pathologies. As an example, studies over the past decade have identified a number of signaling pathways that are implicated in ER stress-mediated apoptotic process. These pathways include CHOP induction, caspase-12 activation, mitochondria dysfunction, JNK activation, the Fas-FasL system, and others (reviewed in Jing et al., 2012). Blocking each of these pathways reduces or prevents ER stress-induced apoptosis to a certain extent; however, induction of an individual pro-apoptotic pathway may not be sufficient to induce apoptosis. This suggests that prolonged ER stress may activate multiple subthreshold pro-apoptotic pathways, and these pathways interact and regulate each other to execute apoptosis. Likewise, multiple signaling molecules and transcription factors have been identified as downstream targets of the UPR.
In conclusion, understanding the roles of the UPR and its target genes in retinal cells under normal and stressed conditions may provide a useful avenue to identify new therapeutic targets for the treatment of retinal deseases.
Highlights.
-
1
ER stress has implicated in various forms of retinal degeneration.
-
2
Normal function of the adaptive UPR is vital for maintaining protein homeostasis.
-
3
The UPR pathways differentially regulate survival signaling in retinal cells.
-
3
Prolonged ER stress activates pro-cell-death pathways to eliminate damaged cells.
-
5
Small molecule chaperones may benefit the treatment of retinal degeneration.
Acknowledgments
We would like to thank the past and current Zhang lab members for helpful discussions and contributions to the studies summarized in this review. Supported, in part, by NIH/NEI grants EY019949 (SXZ), EY007361 (SJF), by grants from the Oklahoma Center for the Advancement of Science and Technology, American Health Assistance Foundation, American Diabetes Association, (SXZ), by an Unrestricted Grant to the Department of Ophthalmology, SUNY-Buffalo, from Research to Prevent Blindness (SJF, SXZ), and by facilities and resources provided by the Veterans Administration Western New York Healthcare System (SJF). The views expressed herein do not necessarily reflect those of the Veterans Administration or the U.S. Government.
Abbreviations
- AMD
age-related macular degeneration
- ASK1
apoptosis signal-regulating kinase 1
- ATF
activating transcription factor
- Bip
immunoglobulin heavy chain-binding protein
- CHOP
C/EBP homologous protein-10
- CNV
choroidal neovascularization
- DR
diabetic retinopathy
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- GRP78
glucose-regulated protein 78
- IRE1
inositol-requiring enzyme 1
- LCA
Leber congenital amaurosis
- LRAT
lecithin-retinol acyltransferase
- PBA
4-phenylbutyric acid
- PEDF
pigment epithelium-derived factor
- PERK
PKR-like endoplasmic reticulum kinase
- RIDD
regulated Ire1-dependent decay
- ROS
reactive oxygen species
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- RPE65
retinal pigment epithelium protein of 65 kDa
- TMAO
trimethylamine-N-oxide
- TRAF2
tumor necrosis factor-associated factor 2
- TUDCA
tauroursodeoxycholic acid
- UPR
unfolded protein response
- XBP1
X-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, et al. Endoplasmic reticulum stress induces retinal endothelial permeability of extracellular-superoxide dismutase. Free Radical Research. 2011;45:1083–1092. doi: 10.3109/10715762.2011.595408. [DOI] [PubMed] [Google Scholar]
- Agbaga MP, et al. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res. 2010;51:1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, et al. Molecular biology of the cell. Garland Science; New York: 2002. [Google Scholar]
- Allingham RR, et al. Shields Textbook of Glaucoma. 6. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. p. 656. [Google Scholar]
- Al-Ubaidi MR. RGC-5: are they really 661W? The saga continues. Exp Eye Res. 2014;119:115. doi: 10.1016/j.exer.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamron S, et al. Regulated IRE-1 dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur J Immunol. 2013 doi: 10.1002/eji.201343953. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bennett J. Gene therapy for Leber congenital amaurosis. Novartis Found Symp. 2004;255:195–202. [PubMed] [Google Scholar]
- Bertolotti A, et al. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Boatright JH, et al. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis. 2006;12:1706–1714. [PubMed] [Google Scholar]
- Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Cai X, McGinnis JF. Oxidative stress: the achilles’ heel of neurodegenerative diseases of the retina. Front Biosci. 2012;17:1976–1995. doi: 10.2741/4033. [DOI] [PubMed] [Google Scholar]
- Chen C, et al. Role of UPR dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid Redox Signaling. 2013 doi: 10.1089/ars.2013.5240. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Activating transcription factor 4 mediates hyperglycaemia-induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1 diabetes. Diabetologia. 2012;55:2533–2545. doi: 10.1007/s00125-012-2594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, et al. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J of Biol Chem. 2002;277:13045–13052. doi: 10.1074/jbc.M110636200. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, et al. On the use of immortalized ocular cell lines in vision research: The unfortunate story of RGC-5. Exp Eye Res. 2013;116:433. doi: 10.1016/j.exer.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Coelho DS, et al. Xbp1-Independent ire1 signaling is required for photoreceptor differentiation and rhabdomere morphogenesis in drosophila. Cell Rep. 2013;5:791–801. doi: 10.1016/j.celrep.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T, et al. 800 facets of retinal degeneration. Prog Mol Biol Transl Sci. 2011;100:331–368. doi: 10.1016/B978-0-12-384878-9.00008-X. [DOI] [PubMed] [Google Scholar]
- Cremers FP, et al. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–1176. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- Datta R, et al. Pathogenesis of retinitis pigmentosa associated with apoptosis-inducing mutation in carbonic anhydrase IV. PNAS. 2009;106:3437–3442. doi: 10.1073/pnas.0813178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhubhghaill SSN, et al. The Pathophysiology of cigarette smoking and age-related macular degeneration. In: Anderson RE, Hollyfield JG, LaVail MM, editors. Retinal Degenerative Diseases. Springer; New York: 2010. pp. 437–446. [Google Scholar]
- Doh SH, et al. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Research. 2010;1308:158–166. doi: 10.1016/j.brainres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Donoso LA, et al. Autosomal dominant Stargardt-like macular dystrophy. Surv Ophthalmol. 2001;46:149–163. doi: 10.1016/s0039-6257(01)00251-x. [DOI] [PubMed] [Google Scholar]
- Duricka D, et al. Defective trafficking of cone photoreceptor CNG channels induces the unfolded protein response and ER-stress-associated cell death. Biochem J. 2012;441:685–696. doi: 10.1042/BJ20111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit Rev Biochem. 1984;16:21–49. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab. 2010;12(Suppl 2):108–115. doi: 10.1111/j.1463-1326.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- Ethen C, et al. The proteome of central and peripheral retina with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:2280–2290. doi: 10.1167/iovs.05-1395. [DOI] [PubMed] [Google Scholar]
- Farrar GJ, et al. Gene therapeutic approaches for dominant retinopathies. Curr Gene Ther. 2010;10:381–388. doi: 10.2174/156652310793180661. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, et al. Photoreceptor-specific degeneration caused by tunicamycin. Nature. 1984;311:575–577. doi: 10.1038/311575a0. [DOI] [PubMed] [Google Scholar]
- Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011;15:1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004a;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Friedman DS, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004b;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara M, et al. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS ONE. 2008;3:e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner BM, Walter P. Unfolded proteins are ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, et al. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher LH. XBP1: the last two decades. Ann Rheum Dis. 2010;69:i67–i71. doi: 10.1136/ard.2009.119388. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk MS, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Pro Natl Acad Sci U S A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E, et al. Characterization of rhodopsin mis-sorting and constituitive acvitvation in a transgenic rat model of retinitis pigmetosa. Invest Ophthalmology Vis Sci. 2000;41:1546–1553. [PubMed] [Google Scholar]
- Griciuc A, et al. Inactivation of VCP/ter94 supresses retinal pathology caused by misfolded rhodopsin in drosophila. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, et al. ER stress in retinal degeneration: a target for rational therapy? Trends Mol Med. 2011;17:442–451. doi: 10.1016/j.molmed.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Hartong DT, et al. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, et al. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hargave P. Rhodopsin structure, function, and topography--the Freidenwald lecture. Invest Ophthalmol Vis Sci. 2001;42:3–9. [PubMed] [Google Scholar]
- Hata N, et al. Increased expression of IRE1alpha; and stress-related signal transduction proteins in ischemia-reperfusion injured retina. Clin Ophthalmol. 2008;2:743–752. doi: 10.2147/opth.s3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signaling. 2009;11:2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- Haze K, et al. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355:19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Huang C, et al. Cigarette smoke and RPE injury: role of oxidative stress and ER stress. Invest Ophthalmol Vis Sci. 2013;54:ARVO E-abstract 3198. [Google Scholar]
- Huang JD, et al. Sterculic acid antagonizes 7-ketocholesterol-mediated inflammation and inhibits choroidal neovascularization. Biochim Biophys Acta (BBA) - Molecular and Cell Biology of Lipids. 2012;1821:637–646. doi: 10.1016/j.bbalip.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi Y, et al. Effect of an Inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest Ophthalmol Vis Sci. 2009;50:334–344. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
- Jeng YY, et al. Retinal ischemic injury rescued by sodium 4-phenylbutyrate in a rat model. Exp Eye Res. 2007;84:486–92. doi: 10.1016/j.exer.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Jia L, et al. Correction of the disease phenotpe of myocilin-causing glaucoma by a natural osmolyte. Invest Ophthalmol Vis Sci. 2009;50:3743–3749. doi: 10.1167/iovs.08-3151. [DOI] [PubMed] [Google Scholar]
- Jin ZB, et al. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Transl Med. 2012;1:503–509. doi: 10.5966/sctm.2012-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing G, et al. ER Stress and Apoptosis: A New Mechanism for Retinal Cell Death. Exp Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan G, et al. Loss of ER retention and sequestration of the wild-type ELOVL4 by Stargardt disease dominant negative mutants. Mol Vis. 2005;11:657–664. [PubMed] [Google Scholar]
- Kanda A, et al. Inflammation in the pathogenesis of age-related macular degeneration. Br J Ophthalmol. 2008;92:448–450. doi: 10.1136/bjo.2007.131581. [DOI] [PubMed] [Google Scholar]
- Kaushal S, Khorana HG. Structure and function in rhodopsin. 7 point mutation associated with autosomal dominant retinitis pigmentosa. Biochem. 1994;33:6121–6128. doi: 10.1021/bi00186a011. [DOI] [PubMed] [Google Scholar]
- Khan M, et al. CLRN1 mutations cause nonsyndromic retinitis pigmentosa. Opthamol. 2011:1444–1448. doi: 10.1016/j.ophtha.2010.10.047. [DOI] [PubMed] [Google Scholar]
- Kimata Y, et al. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J Cell Biol. 2004;167:445–456. doi: 10.1083/jcb.200405153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, et al. A Forensic Path to RGC-5 Cell Line Identification: Lessons Learned. Invest Ophthalmol Vis Sci. 2013;54:5712–5719. doi: 10.1167/iovs.13-12085. [DOI] [PubMed] [Google Scholar]
- Kroeger H, et al. Induction of endoplasmic reticulum stress genes, BiP and HOP, in genetic and environmental models of retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:7590–7599. doi: 10.1167/iovs.12-10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E. Photoreceptor morphogenesis and retinal degeneration: lessons from Drosophila. Curr Opin Neurobiol. 2007;17:541–547. doi: 10.1016/j.conb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kunte MM, et al. ER stress is involved in T17M rhodopsin-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:3792–3800. doi: 10.1167/iovs.11-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PS, et al. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, et al. Drosophila arf72A acts as an essential regulator of endoplasmic reticulum quality control and suppresses autosomal-dominant retinopathy. Int J Biochem Cell Biol. 2011;43:1392–1401. doi: 10.1016/j.biocel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Lee K, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583:1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J Biol Chem. 2011;286:4912–4921. doi: 10.1074/jbc.M110.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Gould DB. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Adv Exp Med Biol. 2010;664:403–409. doi: 10.1007/978-1-4419-1399-9_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944– 949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Lavail MM. Misfolded proteins and retinal dystrophies. Adv Exp Med Biol. 2010;664:115–121. doi: 10.1007/978-1-4419-1399-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, et al. Structure and function in rhodopsin: correct folding and misfolding in two point mutants in the intradiscal domain of rhodopsin identified in retinitis pigmentosa. Proc Natl Acad Sci U S A. 1996;93:4554–4559. doi: 10.1073/pnas.93.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale JJ, et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99:736–745. doi: 10.1182/blood.v99.3.736. [DOI] [PubMed] [Google Scholar]
- McCullough KD, et al. Gadd153 Sensitizes Cells to Endoplasmic Reticulum Stress by Down-Regulating Bcl2 and Perturbing the Cellular Redox State. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, et al. ER stress protects from retinal degernation. EMBO J. 2009;28:1269–1307. doi: 10.1038/emboj.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes HF, et al. Pharmacological manipulation of rhodopsin retinitis pigmentosa. Adv Exp Med Biol. 2010;664:317–323. doi: 10.1007/978-1-4419-1399-9_36. [DOI] [PubMed] [Google Scholar]
- Michalak M, et al. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344(Pt 2):281–292. [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res. 2010;49:476–492. doi: 10.1016/j.plipres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D, et al. Analysis in vivo of GRP78-BiP/substrate interactions and their role in induction of the GRP78-BiP gene. Mol Biol Cell. 1992;3:143–155. doi: 10.1091/mbc.3.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorwez SM, et al. A high-throughputhotra screening method for small-molecule pharmacologic chaperones of misfolded rhodopsin. Invest Ophthalmol Vis Sci. 2008;49:3224–3230. doi: 10.1167/iovs.07-1539. [DOI] [PubMed] [Google Scholar]
- Noorwez S, et al. Calnexin improves the folding efficiency of mutant rhodopsin in the presence of pharamological chaperone 11-cis-retinal. J Biol Chem. 2009;284:33333–33342. doi: 10.1074/jbc.M109.043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgane K, et al. Retinobenzaldhydes as a proper-trafficing inducers of folding defective P23H rhodopsin mutant responsible for retinitis pigmentosa. Bioorg Med Chem. 2010;18:7022–7028. doi: 10.1016/j.bmc.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Palam LR, et al. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Frandsen A. Endoplasmic reticulum dysfunction--a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem. 2001;79:719–725. doi: 10.1046/j.1471-4159.2001.00623.x. [DOI] [PubMed] [Google Scholar]
- Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- Phelan JK, Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol Vis. 2000;6:116–124. [PubMed] [Google Scholar]
- Polansky JR. Current perspectives on the TIGR/MYOC gene (Myocilin) and glaucoma. Ophthalmol Clin North Am. 2003;16:515–527. doi: 10.1016/s0896-1549(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Rao RV, et al. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Rebello G, et al. Apoptosis-inducing signal sequence mutation in carbonic anhydrase IV identified in patients with the RP17 form of retinitis pigmentosa. PNAS. 2004;101:6617–6622. doi: 10.1073/pnas.0401529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold A, et al. An essential role in liver development for transcription factor XBP1. Genes Dev. 2000;14:152–157. [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Roybal CN, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- Salminen A, et al. Endoplasmic Reticulum Stress in Age-Related Macular Degeneration: Trigger for Neovascularization. Mol Med. 2010;16:535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer T, et al. Unfolding the Therapeutic Potential of Chemical Chaperones for Age-related Macular Degeneration. Expert Rev Ophthalmol. 2008;3:29–42. doi: 10.1586/17469899.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Shimazawa M, et al. Involvement of ER stress in retinal cell death. Mol Vis. 2007;13:578–587. [PMC free article] [PubMed] [Google Scholar]
- Shinde VM, et al. ER stress in retinal degeneration in S334ter Rho rats. PLoS ONE. 2012;7:e33266. doi: 10.1371/journal.pone.0033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, et al. The rod photoreceptor ATP-binding cassette transporter gene, ABCR, and retinal disease: from monogenic to multifactorial. Vision Res. 1999;39:2537–2544. doi: 10.1016/s0042-6989(99)00037-1. [DOI] [PubMed] [Google Scholar]
- Sinohara T, et al. Silencing gene therapy for mutant membrane, secretory, and lipid proteins in retinitis pigmentosa (RP) Med Hypotheses. 2008;70:378–380. doi: 10.1016/j.mehy.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Sohocki M, et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat. 2001;17:45–51. doi: 10.1002/1098-1004(2001)17:1<42::AID-HUMU5>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam B, Moritz OL. Characterization of rhodospin P23H-induced retinal degeneration in a xenopus laevis model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006;47:3234–3241. doi: 10.1167/iovs.06-0213. [DOI] [PubMed] [Google Scholar]
- Tam B, Moritz OL. Dark rearing rescues P23H rhodopsin-induced retinal degeneration in a transgenic xenopus laevis model of retinitis pigmentosa: A chromophore-dependent mechanism characterized by production of N-terminally truncated mutant rhodopsin. J Neurosci. 2007;27:9043–9053. doi: 10.1523/JNEUROSCI.2245-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Thapa A, et al. Endoplasmic Reticulum Stress-associated Cone Photoreceptor Degeneration in Cyclic Nucleotide-gated Channel Deficiency. J Biol Chem. 2012;287:18018–18029. doi: 10.1074/jbc.M112.342220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J, Lee AS. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988;7:275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Tirasophon W, et al. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ, et al. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- Tzekov R, et al. Protein misfolding and retinal degeneration. Cold Spring Harb Perspect Biol Epub. 2011 doi: 10.1101/cshperspect.a007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JP, et al. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Ushioda R, et al. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science. 2008;321:569–572. doi: 10.1126/science.1159293. [DOI] [PubMed] [Google Scholar]
- Vasireddy V, et al. Genetics and molecular pathology of Stargardt-like macular degeneration. Prog Retin Eye Res. 2010;29:191–207. doi: 10.1016/j.preteyeres.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Wang XZ, et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware FE, et al. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, et al. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Cell Biol. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zhang SX, et al. Endoplasmic reticulum stress and inflammation: mechanisms and implications in diabetic retinopathy. J Ocul Biol Dis Infor. 2011;4:51–61. doi: 10.1007/s12177-011-9075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, et al. Cone opsin determins the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci USA. 2011;108:8879–8884. doi: 10.1073/pnas.1017127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, et al. Chemical chaperone TUDCA preserves cone photoreceptors in a mouse model of leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2012;53:3349–3356. doi: 10.1167/iovs.12-9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, et al. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Müller cell derived inflammatory cytokine production in diabetes. Diabetes. 2012a;61:492–504. doi: 10.2337/db11-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, et al. X-box binding protein 1 is essential for the anti-oxidant defense and cell survival in the retinal pigment epithelium. PLoS ONE. 2012b;7:e38616. doi: 10.1371/journal.pone.0038616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, et al. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes. Adv Exp Med Biol. 2012c;723:285–292. doi: 10.1007/978-1-4614-0631-0_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode G, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]