Abstract
Background
Although preoperative chemotherapy (cisplatin-etoposide) and radiation followed by surgery is considered a standard-of-care for superior sulcus (SS) cancers, treatment is rigorous and relapse limits long term survival. S0220 was designed to incorporate an active systemic agent, (docetaxel) as consolidation therapy.
Methods
Patients with histologically-proven and radiologically-defined T3-T4, N0-N1, M0 SS NSCLC underwent induction therapy with cisplatin-etoposide, concurrently with thoracic RT 45 Gy. Non-progressing patients underwent surgical resection within 7 weeks. Consolidation consisted of docetaxel every 3 weeks for 3 doses. The accrual goal was 45 eligible patients. The primary objective was feasibility.
Results
Of 46 patients registered, 44 were eligible and assessable; 38 (86%) completed induction, 29 (66%) underwent surgical resection, and 20 (45% of eligible, 69% surgical and 91% of those initiating consolidation therapy) completed consolidation docetaxel; 28/29 (97%) underwent a complete (R0) resection; 2 (7%) died of ARDS. In resected patients, 21/29 (72%) had a pathologic complete or near complete response. Known site of first recurrence was local (2), local-systemic (1) and systemic (10), 7 in the brain only. The 3-year progression-free survival is 56% and 3-year overall survival is 61%.
Conclusion
Although trimodality therapy provides excellent R0 and local control, only 66% of patients underwent surgical resection and only 45% completed the treatment regimen. Even in this subset, distant recurrence continues to be a major problem, particularly brain only relapse. Future strategies to improve treatment outcomes in this patient population must increase the effectiveness of systemic therapy and reduce the incidence of brain only metastases.
Introduction
Superior sulcus (SS) non-small cell lung cancer (NSCLC) is a form of locally advanced lung cancer that originates in the apex of the lung. Invasion of the chest wall and potentially mediastinal structures makes resection challenging. Surgery by itself is infrequently curative; a combined modality approach first adopted by Paulson and Shaw in the 1950’s resulted in 5-year survival rates of 25–30%.1 This became a standard-of-therapy until 2001 when the Southwest Oncology Group (SWOG)/North American Intergroup published the results of a prospective phase II clinical trial (SWOG 9416/Intergroup 0160), establishing induction chemoradiotherapy followed by surgical resection as the new standard-of-care, with an 80% surgical resection rate and a 44% 5-year overall survival.2,3 This result was mimicked by a phase II trial performed by the Japan Clinical Oncology Group (JCOG), protocol 9806, using a similar therapeutic approach, resulting in a 56% 5-year overall survival.4 Systemic failure was the major contributor to long-term mortality for both trials, present in approximately 80% of patients who recurred.
One strategy to control systemic recurrence is the administration of post-operative consolidation chemotherapy. SWOG 9416 planned for 2 cycles of additional etoposide and cisplatin after surgery. However, only 83% of the surgically-treated patients received the prescribed therapy. Others have attempted to deliver post-operative chemotherapy after induction chemoradiotherapy and surgery to NSCLC patients, including SS tumor patients, with limited success and questionable benefit.5 In 2001, when this study was conceived, docetaxel had been proven to be active in patients with NSCLC recurrent after platinum-based therapy, showing improved response and survival when compared with best supportive care.6 SWOG experience with docetaxel consolidation in stage IIIB NSCLC following definitive cisplatin, etoposide and concurrent thoracic radiotherapy suggested that this might be a more effective and better tolerated approach to further cisplatin-etoposide in the post-operative treatment of SS cancers.7 We designed a phase II trial to determine the feasibility of treating SS NSCLC with induction chemoradiotherapy and definitive resection followed by consolidation docetaxel.
Patients and Methods
Eligibility Criteria
Patients with a single, primary, previously untreated histologically or cytologically confirmed SS NSCLC, with selected stage IIB (T3N0), IIIA (T3N1), or IIIB (T4N0-1) NSCLC were eligible. SS cancer was defined as apical lung tumor with or without associated Pancoast syndrome (neurologic symptoms secondary to invasion of the inferior brachial plexus); or apical lung tumors with computed tomography (CT scan) or magnetic resonance imaging (MRI) evidence of invasion of upper chest wall, usually with involvement of ribs 1 or 2; upper thoracic vertebral bodies; or subclavian vessels. Lack of N2 nodal involvement was confirmed by negative mediastinoscopy or negative CT scan and negative positron emission tomography (PET) of the mediastinum. CT scan of the abdomen and bone scan or PET, and CT scan or MRI of the brain were used to assess for metastatic disease. Pulmonary function testing with diffusion capacity (PFTs) and routine laboratory testing was used to assess for fitness for planned treatment. Patients were required to have a Zubrod performance status of 0–2. The study protocol (ClinicalTrials.govIdentifier:NCT00062439; http://clinicaltrials.gov/) was approved by the institutional review boards at participating institutions. Patients were informed of the investigational nature of the study and provided written informed consent in accordance with institutional and federal guidelines.
Treatment Regimen
After informed consent, eligible patients received induction chemoradiotherapy with cisplatin 50 mg/m2 intravenously (IV) on days 1, 8, 29, and 36, and etoposide 50 mg/m2 IV days 1 through 5 and 29 through 33, initiated simultaneously with thoracic radiotherapy given daily Monday through Friday in 1.8 Gy fractions to a total dose of 45 Gy. Radiotherapy included computerized treatment planning and delivery to the primary tumor and ipsilateral supraclavicular region. Coverage of the mediastinum or hilum was not mandated.
Non-progressing patients underwent thoracotomy 3–7 weeks after completion of induction therapy. Resection was via upper lobectomy, bilobectomy or pneumonectomy, with involved areas of the chest wall and/or spine resected en bloc with the involved lung. Appropriate hilar and mediastinal lymph nodes were removed (levels 2R, 4R, 7, 8, 9, and 10R for right sided tumors, and levels 5, 6, 7, 8, 9, and 10L for left sided lesions). Patients who did not progress, but were deemed unfit for surgery, or who refused surgery, were continued on radiotherapy to a total dose of 61–61.2 Gy.
Both resection and full-dose radiotherapy who did not undergo surgery patients were to receive consolidation docetaxel 75 mg/m2 IV every 21 days for three doses beginning 3 to 8 weeks after completion of surgery or definitive radiotherapy.
Response Evaluations
Response was evaluated using RECIST 8 with assessments following 2–4 weeks after completion of induction chemoradiotherapy. Patients were restaged with CT scan of the chest and upper abdomen, CT scan or MRI of Brain, and PFTs (bone scans were repeated only if new symptoms or elevated alkaline phosphatase occurred). Patients were restaged at the completion of all therapy (4–6 weeks after completion of consolidation chemotherapy) and at 3-month intervals for 2 years and then 6-month intervals for 3 more years, for a total of 5 years or until death. At each of the follow-up visits, a routine history, physical examination, routine laboratory tests, a chest radiograph and chest and upper abdomen computed tomograms were performed. Other tests were obtained as necessary. Post-treatment brain scanning (contrast enhanced CT or MRI) was recommended at 6 and 12 months.
Toxicity Evaluation
All patients were monitored for toxicity from treatment, recorded using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.9
Statistical Methods
The primary objective of this phase II study was to assess the feasibility of treating patients with Stage IIB/IIIB SS NSCLC with a regimen of induction chemoradiotherapy, followed by surgical resection and consolidation docetaxel. Feasibility was defined by the percentage of patients completing all protocol treatment. The design specified that if the true rate was 55% or less, the regimen would not warrant further consideration, whereas if the rate were at least 75%, it would be promising. The accrual goal was 45 eligible patients and 30 to complete all protocol treatment. This design had an exact one-sided alpha level of 4% and exact power of 87%. With 45 patients accrued over 48 months and an additional 24 months of follow-up, this design also had 84% power to reject the null hypothesis of median survival time (MST) equal to 27 months in favor of MST of 45 months using a 1-sided −0.05 level test.
Overall survival (OS) and progression-free survival (PFS) were estimated using the method of Kaplan-Meier. Exact binomial 95% confidence intervals (CIs) were calculated for response rate (confirmed and unconfirmed, complete and partial responses) to induction chemoradiotherapy and the rate of complete resection (R0) following induction therapy. Estimation of loco-regional failure rates and their 95% CIs were calculated.10
For patients who underwent surgery, Kaplan-Meier estimates of recurrence-free survival and overall survival from the date of surgery were calculated. A logrank test was used to compare survival between patients who had achieved a pathologic CR (< 5% viable tumor in margins) and those who had less than a complete pathologic response.
Results
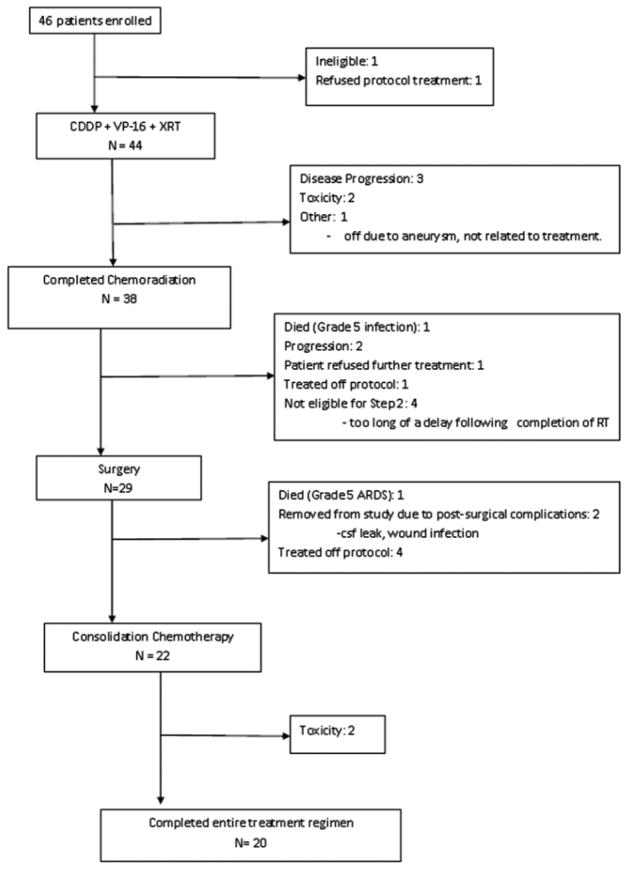
Accrual to the 3 phases of the trial is shown in Figure 1. Between July 1, 2003 and October 1, 2007 46 patients were registered. 44 patients were eligible, with 1 patient ineligible due to N3 disease and 1 patient refused protocol treatment. Table 1 shows baseline characteristics of the 44 eligible patients.
Figure 1.
Protocol Schema for Southwest Oncology Group 0220 Schema CDDP=cisplatin, VP-16=etoposide, XRT=radiation therapy, ARDS=Adult Respiratory Distress Syndrome, csf=cerebrospinal fluid
Table 1.
Baseline Patient Characteristics
| AGE | ||
| Median (Range) | 59 | (44 – 75) |
| SEX | ||
| Males | 32 | 73% |
| Females | 12 | 27% |
| RACE | ||
| White | 40 | 91% |
| Asian | 3 | 7% |
| Native American | 1 | 2% |
| PERFORMANCE STATUS | ||
| 0 | 15 | 34% |
| 1 | 29 | 66% |
| HISTOLOGY | ||
| Adenocarcinoma | 17 | 39% |
| Large Cell | 4 | 9% |
| Squamous | 10 | 23% |
| Other Non Small Cell | 13 | 30% |
| N STAGE | ||
| N0 | 39 | 89% |
| N1 | 5 | 11% |
| T STAGE | ||
| T3 | 32 | 73% |
| T4 | 12 | 27% |
Of the 44 patients, 29 (66%) underwent surgical resection. Twenty-two patients (50% of 44 eligible and 76% of the 29 resected) received some consolidation chemotherapy and of those, 20 (91%) completed all 3 cycles. Therefore, 45% (95% CI: 30% – 61%) of patients completed all protocol treatment.
Induction chemoradiotherapy
One of the 44 patients who received induction chemotherapy, 2% died due to infection with Grade 3–4 neutropenia. Seven additional patients experienced Grade 4 toxicity (16%), which included neutropenia (5 cases), and 1 case each of allergic reaction, dehydration, febrile neutropenia, lymphopenia, and thrombosis/embolism. Thirty-eight (86%) completed the induction regimen. Table 2 shows the number of patients with a given type and grade of toxicity during induction chemoradiotherapy.
Table 2.
Toxicity during Concurrent ChemoRadiotherapy in 44 Evaluable Patients
|
|
Grade
|
||
|---|---|---|---|
| ADVERSE EVENT | 3 | 4 | 5 |
|
|
|
||
| Allergic reaction | 0 | 1 | 0 |
| Dehydration | 1 | 1 | 0 |
| Diarrhea | 1 | 0 | 0 |
| Nausea | 1 | 0 | 0 |
| Vomiting | 2 | 0 | 0 |
| Thrombosis/embolism | 0 | 1 | 0 |
| Esophagitis | 1 | 0 | 0 |
| Mucositis | 1 | 0 | 0 |
| Fatigue | 2 | 0 | 0 |
| Hyponatremia | 1 | 0 | 0 |
| Hemoglobin | 3 | 0 | 0 |
| Infection w/≥ Grade 3 | |||
| Neutropenia | 1 | 0 | 1 |
| Neutrophils | 10 | 5 | 0 |
| Febrile neutropenia | 1 | 1 | 0 |
| Total | 11 | 7 | 1 |
Surgical Resection
Twenty-nine patients underwent surgical resection. Lobectomy was performed in 24(83%), segmentectomy in 2 (7%), bilobectomy in 2 (7%) and one (3%) patient had wedge resection. The 3 patients who underwent sublobar resection were protocol violations and were removed from study. Chest wall or adjacent organ resection was performed in 25 (86%) patients. Each had 1 or more of the following structures resected: chest wall (22), major vascular (1), pericardium (1), and other (10); and 7 patients had more than 1 adjacent structure resected. The median number of lymph node stations examined was 5 (range: 0–9), with 10% of the patients having no nodes examined. Table 3 summarizes the operative stage for the patients that underwent resection. Comparing the preclinical stage to the surgical stage, 4/29 (14%) were unchanged, 2 (7%) were upstaged and 23/29 (79%) were downstaged. The median operating room time, defined as the time from entrance to exit from the operating room, was 4.3 hours (range 1.4–9.5) and the median estimated blood loss was 0.5 L (range 0–7.5). Eleven patients (38%) had intra- and/or post-operative (to within 7 days of surgery) transfusions. Among these 11 patients the median number of units of transfusion was 2 (range 1–22). There were two surgical deaths (2/29, 7%), both due to adult respiratory distress syndrome (ARDS). Two additional patients experienced Grade 4 toxicity related to surgery, including one case each of ARDS, cerebrospinal fluid leak, chylothorax, hemorrhage, hypoxia, lung infection, respiratory insufficiency, renal failure and ventricular fibrillation. An intra- or post-operative complication occurred in 16 patients (55%), the most common of which was atrial arrhythmia (9 cases, 31%).
Table 3.
Surgical Stage in the 29 Operable Patients
| Clinical Stage at baseline | Pathologic Stage at Surgery | ||||||
|---|---|---|---|---|---|---|---|
| T0N0 | T1N0 | T2N0 | T3N0 | T4N0 | T4N3 | Total | |
| T3N0 | 6 | 7 | 1 | 4 | 1 | 0 | 19 |
| T3N1 | 1 | 2 | 0 | 0 | 0 | 0 | 3 |
| T4N0 | 1 | 1 | 0 | 3 | 0 | 1 | 6 |
| T4N1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Total | 8 | 11 | 1 | 7 | 1 | 1 | 29 |
Twenty-eight of 29 (97%) patients who underwent surgery had R0 resections; the remaining patient had an R1 resection and was treated with adjuvant radiation therapy. Post-resection pathology revealed no evidence of residual tumor (pCR) in 8 (28%), minimal residual tumor (<5%) in 13 (45%), and the remaining 8 (28%) had gross residual tumor (>5% viable tumor).
Consolidation docetaxel
Twenty of the 22 patients who initiated consolidation docetaxel, completed the planned treatment. There were no deaths related to consolidation therapy, but 6 patients experienced grade 4 adverse events which included five cases of neutropenia. Table 4 shows toxicity during consolidation chemotherapy.
Table 4.
Toxicity during Consolidation Chemotherapy in 22 Patients
|
|
Grade
|
||
|---|---|---|---|
| ADVERSE EVENT | 3 | 4 | 5 |
|
|
|
||
| Dehydration | 2 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 |
| Dyspnea | 2 | 0 | 0 |
| Fatigue | 1 | 0 | 0 |
| Nausea | 1 | 0 | 0 |
| Vomiting | 1 | 0 | 0 |
| Weight Loss | 1 | 0 | 0 |
| Hypoxia | 1 | 0 | 0 |
| Infection w/≥ Grade 3 | |||
| Neutropenia | 1 | 0 | 0 |
| Neutropenia | 3 | 5 | 0 |
| Total | 7 | 6 | 0 |
Response, PFS, and OS
Thirty-two eligible patients had measurable disease per RECIST at baseline and were included in the response analysis. Two patients went off treatment due to toxicity prior to their first follow-up disease assessment and are counted as non-responders. There were nine partial responses documented out of 32 patients for an estimated response rate of 28% (CI 14% – 47%).
The median follow-up among patients still alive (n=25) is 45 months (range: 2 – 75 months). The median PFS is 43 months (95% CI: 20 – 43) and the OS is 50 months (95% CI: 20 – 50). The 3-year PFS is 56% (CI: 40% – 70%) and the 3-year OS is 61% (CI: 44% –74%) (Figures 2). Figure 4 illustrates the cumulative incidence of PFS failure types. Three patients recurred locally first, 10 patients had a distant site of first recurrence and 7 patients died prior to documentation of disease recurrence. Seven patients had documented recurrence in the brain and each had no other site of recurrence.
Figure 2.

Progression-Free and Overall Survival from Date of Enrollment. A: Progression Free Survival; B: Overall Survival
Figure 4.
Cumulative Incidence of Progression-Free Survival Failure Types: Comparison of the Rate of Local Recurrence to Distant Relapse or Death
Comment
The current study failed to achieve its primary endpoint, feasibility. Twenty or 45% completed the planned therapy; this rate was not dissimilar from SWOG 9416. Through the SWOG 0220 design, we have defined the reasons for noncompletion of the trimodality induction protocol. Future trials must address the findings to increase the likelihood for protocol completion.
Although survival was not the primary endpoint, there appeared to be a survival benefit of the current regimen. With modest power of 84% to reject the null hypothesis, the observed median OS was 50 months from enrollment, greater than the 41 months that would be sufficient to conclude that the true median OS is greater than 27 months. (see Statistical Methods). Despite not reaching the accrual goal, this trial confirms the efficacy of the core therapy.
Treatment of non-metastatic SS cancers with a trimodality approach is the de facto standard-of-care.11–16 In a phase II clinical trial studying trimodality therapy (SWOG 8805) in 126 IIIA (N2) and IIIB NSCLC, of which Pancoast NSCLC is a subset, chemoradiotherapy cytoreduced the primary cancer, potentially downstaging patients.17 Based on these results and the poor outcomes of SS therapy, SWOG 9416 used the identical regimen as in SWOG 8805.2,3 With 110 eligible patients, this is the largest trial performed in SS cancers and employed meticulous surgical staging. It demonstrated the effectiveness of the trimodality approach, with 95% completing induction; median survival was 33 months and the overall 5-year survival of 44%. The R0 resection was 94% and, in that group, there was a median survival of 94 months and a 5-year survival of 54%. Although not a phase III trial, SWOG 9416 established induction chemoradiotherapy with cisplatin-etoposide and concurrent radiotherapy as a standard-of-care for SS NSCLC. Two major evidenced-based guidelines have now accepted this tri-modality approach as a standard-of-care for SS cancers.15,16,18
Perhaps the greatest hurdle to improving survival using this treatment regimen is the relatively high rate of brain-only metastasis seen following therapy. In a retrospective review of SWOG combined-modality lung cancer trials, Gaspar et al. demonstrated that 20% of patients relapse in brain-only and an additional 6% in brain plus other sites.19 In the current study, of the 13 patients for whom the first site of relapse was known, 7 patients recurred only in the brain. Efforts to evaluate the use of prophylactic cranial irradiation in locally advanced NSCLC in the clinical trial setting have been hampered by poor accrual and it is unlikely that a data-driven answer to this problem will be obtained. Fortunately, the availability of high quality imaging, minimally-invasive neurosurgical techniques, and stereotactic radiotherapy have improved the salvage rate for these patients, but strategies to mitigate this problem are still sorely needed.
Table 6 summarizes the SS cancer prospective clinical trial literature and provides us insight into the nature of the disease and treatment. All are phase II trials, 2 single institution and 3 multi-institution cooperative group trials. Approximately 75% of cases were T3 and the remainder T4 and approximately 70–90% were clinically N0. There was no consistent use of brain imaging, positron emission tomography (PET) scanning or surgical mediastinal staging. Four of the trials used induction chemoradiotherapy, while the Gomez trial used adjuvant chemoradiotherapy.20 The induction programs were all platinum-based. Etoposide was most commonly used as the additional chemotherapeutic agent. The dose of radiotherapy was 45 Gy concurrently with the chemotherapy, although there were some variations in using hyperfractionated radiotherapy and whether or not the mediastinum was included. The number of patients that were surgically resectable at the end of the induction program was 70–80% with the exception of the Marra trial, a single institutional trial, being 94%.21 All patients in the Gomez trial were resected. The pathologic complete response rate for the SWOG trials, 9416 and 0220, were between 28% and 36%. The Marra trial had a 45% and Kunitoh trial a 21% pathologic complete response. Two of the trials offered consolidation therapy, SWOG 9416 and SWOG 0220. The number of surgical patients that were able to receive consolidation therapy was similar, 67 and 76%. All 3 postoperative treatment trials, the 2 SWOG trials and the Gomez trial, had no deaths from the postoperative treatment phase. Postoperative chemotherapy was completed in 80–90%. The median overall survival for the SWOG trials was between 3–4 years. A survival advantage was conferred if there was a complete or near complete response and if the surgical margin was negative for malignancy. Finally, taken together these trials demonstrate excellent local control in resected patients, but relatively high failure rates in distant sites. Time to recurrence was within the 2–3 years after surgery and the most frequent location of recurrence was the brain.
Table 62–4, 20, 21.
Phase II Trials for the Treatment of Superior Sulcus NonSmall Cell Lung Cancer 2001-Present
|
1st Author/Insti tution or Group + Trial #/Publ Year |
Number of Patients (after exclusio ns) |
Inclusion Criteria |
Chemother apy Regimen |
Radiation Therapy Dose |
Grade 3 + 4 Toxicity |
Inductio n Deaths |
Inductio n Disease Progressi on |
Number Surgical ly Resecte d |
Number Completel y Resected (R0) |
pCR + Min Residual = Total |
Surgical Mortalit y |
Number Surgically Rx w Consolida tion Thx |
Planned Consolida tion Regimen |
Consolidatio n Deaths, Grade 3 + 4 Toxicity |
Number Surgically Rx Completin g Consolida tion that started it |
Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rusch/SWOG 9416/2001, 20072,3 | 110 | Histologically or Cytologically proven, cT3-4, cN0-1 NSCLC, Mediastinoscopy All, No Wedge Allowed, PS≤2, cT3 71%, cN0 not reported | CDDP/VP-16 × 2 | Concurrent 45 Gy to include ipsilateral supraclavicular nodes, not mediastinum or hilum | Combination not reported | 3(2.7%) | 9/110(8.2%) | 88(80%) | 83(94%) | 32/88(36 %) + 29/88(33 %)=61/8 8(69%) | 2/88(2.3%) | 59/88(67 %) | CDDP/VP-16 × 2 | Complications during this Phase Not Reported | 49/59(83 %) | MST 33 mos all 110 patients, 94 mos if R0; 2- yr OS 55%, 5-yr OS 44%, 54% for R0, if pCR+Min Res 45%; pCR better survival |
| Marra/University of Essen/200721 | 31 | Histologically or Cytologically proven cT3-4, cN0-3 NSCLC, Mediastinoscopy All, PS≤2, Wedge Allowed cT3 81%, cN0 67.7% | CDDP/VP-16 × 3 in first 22, remaining 9 PTX instead of VP-16 during Rad Rx | At 10th wk, Concurrent 45 Gy (1.5 BID) including ipsilateral supraclavicular nodes, if cN2-3 target mediastinum, IIIB treated w PCI | 32%, not graded according to CTCAE | 0 | Not Reported | 29(94%) | 27/29(93.5%) on final analysis | 13/29 (45%)+7/ 29 (24%)=2 0/29(69 %) | 2/29(7 %) | Not Applicable | Not Applicable | Not Applicable | Not Applicable | MST 54 mos, 2-yr OS 74%, 5-yr DFS 52%, 5-yr OS 46% |
| Kunitoh/JCOG 9806/20084 | 75 | Histologically or Cytologically proven cT3-4, cN0-1 NSCLC, CT criteria node < 1 cm, Mediastinoscopy to R/O Disease, No Wedge Allowed, ipsilateral N3 included, PS≤1, cT3 74%, cN0 78% | MMC/VND/CDDP × 2 | Concurrent 45 Gy (splitcourse 1-wk) includes ipsilateral supraclavicular nodes, not mediastinum or hilum | Combination not reported | 1(1.3%) | 5/75 (6.7%) | 57/75 (76%) | 51/57 (89%) | 12/57(21 %), min residual disease not reported | 2/57(3.5%) | Boost Radiotherapy to 66.6 Gy if not R0, no consolidation chemothx | Not Applicable | Not Applicable | Not Applicable | DFS 28 mos, OS not reached, DFS 3-yr 49%, 5-yr 45%; OS 3-yr 61%; 5-yr 56%; R0 patients OS 5-yr 70% |
| Gomez/MD Anderson/2 01220 | 32 | Histological cT3-4, cN0-2 NSCLC, No Wedge Allowed, PS≤70%, cT3 81%, cN0 84.5% | Postoperative CDDP/VP-16 × 5, first 2 cycles concurrent w Rad Rx, included hilum & mediastinum | Concurrent Postoperative 60 Gy (1.2 gy Fractions BID); if margin neg, 64.8 Gy if margin pos, 11 Rx Prophylactic PCI | NA | NA | NA | 32(100 %) | 23(72%) | NA | 0% | NA | Adjuvant Provided, Not Consolidation | No Deaths, Dysphagia 10, Pneumonitis 1, Lung Fibrosis 1, Leukopenia 1, Granulocytopenia 1 | 78% Complete d Protocol | DFS 2-yr 49%, 5-yr 45%, 10-yr 45%; OS 2-yr 72%, 5-yr 50%, 10-yr 45% |
| Kernstine/SWOG 0220/2012 | 44 | Histologically or Cytologically proven NSCLC cT3-4, N0-1 Negative mediastinum confirmed by mediastinoscopy or Negative PET+CT, PS ≤2, No Wedge Allowed, cT3 73%, cN0 89% | CDDP/VP-16 × 2 | Concurrent 45 Gy to include the ipsilateral supraclavicular area, Hilum & Mediastinum not mandated | 18 (41%) | 1 (2%) from neutropenic infection | 5/44 (11%) | 29 (66%) | 28(97%) | 8/29 (28%) + 13/29 (45%)= 21/29 (72%) | 2/29 (7%), Both from ARDS | 22/29(76 %) | Dox × 3 | 0%, 13/22(59%) | 20/22(91 %) | 1-yr PFS 76%, 3-yr PFS 56%, OS 61%. Median OS 4 yrs |
ARDS-Adult Respiratory Distress Syndrome; BID-Twice Daily; CDDP-Cisplatin; CTCAE-Common Terminology Criteria for Adverse Events; CT-Computed Tomogram; DFS-Disease Free Survival; Dox-Docetaxel; Gy-Gray radiation dose; JCOG-Japan Clinical Oncology Mitomycin C; mos-months; MST-median survival time; OS-Overall Survival; pCR-Pathologically Complete Response, no viable tumor in the resected specimen; PET-Positron Emission Tomography; PS-Performance Status (Zubrod); PTX-Paclitaxel; R0-complete clear of disease; Rad Rx-Radiation Therapy; SWOG-Southwest Oncology Group; VND-Vindesine; VP-16-Etoposide; Wk-Week
We conclude that induction chemoradiotherapy with etoposide and cisplatin provides an excellent rate of response and an excellent margin free (R0) rate of resection. Whether post-operative consolidation therapy of any type adds to efficacy remains unclear. Further studies designed to improve the complete response (pCR), as a surrogate for increasing survival, are warranted, as are investigations designed to prevent or reduce the incidence of brain relapse, the most common cause of distant failure.
Figure 3.
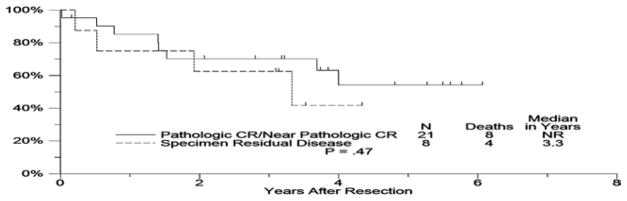
Overall Survival from Date of Surgery Pathologic CR/near Pathologic CR compared with Specimen Residual Disease
Table 5.
Outcomes by Clinical Stage at Baseline
| Clinical Stage at baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (N=44) | T3N0 (N=28) | T3N1 (N=4) | T4N0 (N=11) | T4N1 (N=1) | ||||||
| Completed Induction | 38 | 86% | 23 | 82% | 4 | 100 % | 10 | 91% | 1 | 100 % |
| Surgical Resection per protocol | 29 | 66% | 19 | 68% | 3 | 75% | 6 | 55% | 1 | 100 % |
| R0 resection | 28 | 64% | 18 | 64% | 3 | 75% | 6 | 55% | 1 | 100 % |
| Path CR | 21 | 48% | 14 | 50% | 3 | 75% | 3 | 27% | 1 | 100 % |
| with minimal residual disease | 13 | 30% | 8 | 29% | 2 | 50% | 2 | 18% | 1 | 100 % |
| no minimal residual disease | 8 | 18% | 6 | 21% | 1 | 25% | 1 | 9% | 0 | 0% |
| Completed Consolidation | 20 | 45% | 15 | 54% | 3 | 75% | 2 | 18% | 0 | 0% |
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA63848, CA76429, CA67575, CA20319, CA95860, CA46368, CA45377, CA35178, CA35176, CA46282, CA45808, CA42777, CA35431, CA86780, CA31946, CA14958, CA21115, CA25224 and in part Sanofi-Aventis. Funding agencies had no involvement in the study design, data collection, analysis and interpretation, or in the writing of the report and submission.
Abbreviations and Acronyms
- ARDS
Adult Respiratory Distress Syndrome
- BID
Twice per day
- CDDP
Cisplatin
- CT
Computed Tomogram
- CTCAE
Common Terminology Criteria for Adverse Events
- DFS
Disease Free Survival
- Dox
Docetaxel
- Gy
Gray, radiation dose
- IV
Intravenous
- JCOG
Japan Clinical Oncology Group
- L
Liter
- m2
square meter surface area
- mg
milligram
- Min
Minimum
- MMC
Mitomycin C
- MRI
Magnetic Resonance Imaging
- MST
Median Survival Time
- N0
No regional lymph node involvement
- N1
Regional Nodes, 1st Stage, American Joint Committee on Cancer, Cancer Staging Manual, 6th edition
- N2
Regional Nodes, 2nd Stage, American Joint Committee on Cancer, Cancer Staging Manual, 6th edition
- NSCLC
Non-small cell lung cancer
- OS
Overall Survival
- pCR
Pathological Complete Response
- PET
Positron Emission Tomography
- PFS
Performance Free Survival
- PFTs
Pulmonary Function Tests
- PS
Performance Status
- PTX
Paclitaxel
- R0
No residual cancer at the margin of resection
- R1
Microscopic disease at the margin of resection
- R2
Gross tumor at the margin of resection
- Rad Rx
Radiation Therapy
- RECIST
Response Evaluation Criteria In Solid Tumors
- SS
Superior Sulcus
- SWOG
Southwest Oncology Group
- T3
Primary Tumor, 3rd Stage, American Joint Committee on Cancer, Cancer Staging Manual, 6th edition
- T4
Primary Tumor, 4th Stage, American Joint Committee on Cancer, Cancer Staging Manual, 6th edition
- VND
Vindesine
- VP
16 Etoposide
- Wk
Week
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw RR, Paulson DL, Kee JL. Treatment of Superior Sulcus Tumor by Irradiation Followed by Resection. Ann Surg. 1961;154:29–40. doi: 10.1097/00000658-196107000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160) J Thorac Cardiovasc Surg. 2001;121:472–83. doi: 10.1067/mtc.2001.112465. [DOI] [PubMed] [Google Scholar]
- 3.Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160) J Clin Oncol. 2007;25:313–8. doi: 10.1200/JCO.2006.08.2826. [DOI] [PubMed] [Google Scholar]
- 4.Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol. 2008;26:644–9. doi: 10.1200/JCO.2007.14.1911. [DOI] [PubMed] [Google Scholar]
- 5.Felip E, Rosell R. New strategies in the treatment of resectable non-small cell lung cancer. Expert Rev Anticancer Ther. 2001;1:224–8. doi: 10.1586/14737140.1.2.222. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 7.Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–10. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 10.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Narayan S, Thomas CR., Jr Multimodality therapy for Pancoast tumor. Nat Clin Pract Oncol. 2006;3:484–91. doi: 10.1038/ncponc0584. [DOI] [PubMed] [Google Scholar]
- 12.Kraut MJ, Vallieres E, Thomas CR., Jr Pancoast (superior sulcus) neoplasms. Curr Probl Cancer. 2003;27:81–104. doi: 10.1016/s0147-0272(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 13.Garcia JA, Bhakta S, Fuller CD, Thomas CR., Jr Multidisciplinary approach to superior sulcus tumors. Cancer journal. 2005;11:189–97. doi: 10.1097/00130404-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Sanborn RE, Thomas CR. Current Status and Future Directions of Multimodality Therapy for Non-Small Cell Lung Cancer of the Superior Sulcus. Current Cancer Therapy Reviews. 2011;7:2–9. [Google Scholar]
- 15.Detterbeck FC, Jones DR, Kernstine KH, Naunheim KS. Lung cancer. Special treatment issues. Chest. 2003;123:244S–58S. doi: 10.1378/chest.123.1_suppl.244s. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed February 17, 2013];Non-Small Cell Lung Cancer. 2013 2013, at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 17.Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–92. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 18.Shen KR, Meyers BF, Larner JM, Jones DR. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:290S–305S. doi: 10.1378/chest.07-1382. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar LE, Chansky K, Albain KS, et al. Time From Treatment to Subsequent Diagnosis of Brain Metastases in Stage III Non–Small-Cell Lung Cancer: A Retrospective Review by the Southwest Oncology Group. Journal of Clinical Oncology. 2005;23:2955–61. doi: 10.1200/JCO.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Gomez DR, Cox JD, Roth JA, et al. A prospective phase 2 study of surgery followed by chemotherapy and radiation for superior sulcus tumors. Cancer. 2012;118:444–51. doi: 10.1002/cncr.26277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marra A, Eberhardt W, Pottgen C, et al. Induction chemotherapy, concurrent chemoradiation and surgery for Pancoast tumour. Eur Respir J. 2007;29:117–26. doi: 10.1183/09031936.00108205. [DOI] [PubMed] [Google Scholar]