Abstract
During embryonic development, uncommitted pluripotent cells undergo progressive epigenetic changes that lock them into a final differentiated state. Can mammalian cells change identity within the living organism? Direct lineage reprogramming of cells has attracted attention as a means to achieve organ regeneration. However, it is unclear whether cells in the CNS are endowed with the plasticity to reprogram. Neurons in particular are considered among the most immutable cell types, able to retain their class-specific traits for the lifespan of the organism. Here we focus on two experimental paradigms, glia-to-neuron and neuron-to-neuron conversion, to consider how lineage reprogramming has challenged the notion of CNS immutability, paving the way for the application of reprogramming strategies to reshape neurons and circuits in vivo.
Introduction
C. H. Waddington likened the process of cell differentiation to a marble rushing along a downward slope and ending up in one of many valleys surrounded by impassable hills. Despite long-held dogmas on the impossibility of overcoming epigenetic barriers and changing the identity of differentiated cells, it is increasingly accepted that it is possible to push the marble uphill to enter new valleys and thus convert one differentiated cell type directly into another. This process has been termed transdifferentiation or direct lineage reprogramming, and various cell types have been directly reprogrammed to acquire a new differentiated identity, across organ systems and in different species (reviewed in [1] and [2]).
Blood, heart, and pancreas were among the first tissues where successful lineage reprogramming was described in vivo [3–5]. In recent years similar strategies have been used to probe the reprogramming capabilities of the mammalian CNS, exposing previously unappreciated degrees of cellular plasticity and challenging the immutability of the programs that define neuronal and glia identity.
Glia-to-Neuron Reprogramming
The idea of reprogramming endogenous glia originated from the now over a decade-old notion that radial glia during development and adulthood is at the base of neuron-producing lineages [6–9]. These findings begged the question of whether parenchymal astroglia, a very abundant cell type in the brain, could be forced to turn into neurons upon expression of transcription factors that instruct neurogenesis during development. Indeed a series of in vitro studies set the stage by showing that forced expression of Pax6, Neurog2, Ascl1, or Dlx2 can reprogram astroglia from the early postnatal cortex into induced neurons with functional neuronal properties [10–12]. Notably, consistent with their respective roles in dorsal and ventral telencephalic development [13–16], Neurog2 or Ascl1/Dlx2 direct the conversion of astrocytes into glutamatergic and GABAergic neurons [10,11]. Thus young astroglia is capable of differentially interpreting neurogenic cues resulting in distinct neuronal terminal features.
The cellular context is a major player in determining what terminal features are acquired. Ascl1 a central player in converting fibroblasts and other cell types into neuronal cells in vitro can induce the generation of different neuronal fates, from glutamatergic to dopaminergic neurons, depending on synergism with other factors, starting cell type and timing of expression. The importance of the cellular context in affecting the outcome of reprogramming transcription factors, is also exemplified by the fact that while endogenous Ascl1 is normally required for oligodendrogliogenesis [17,18], its expression in none of the in vitro reprogramming paradigms resulted in the genesis of oligodendroglia. Notably, however, forced expression of Ascl1 in adult neural stem cells of the dentate gyrus, rather than promoting neurogenesis, diverts these to oligodendrogliogenesis [19,20].
In addition to astroglia, some of the earliest studies into the reprogramming capabilities of CNS cells showed that oligodendrocyte precursor cells (OPCs, often referred to as NG2 cells) can revert back into neural stem cell-like cells following sequential exposure to growth factors [21]. NG2 cells continue to proliferate in the adult brain [22] and may thus represent an interesting target for in vivo direct conversion. Whether turning these cells into neurons is to be deemed bona fide reprogramming or rather reflects an altered programming process depends on how “terminal” one considers the differentiation of NG2 cells. In fact, while it has been reported that these cells might retain multipotency, there is also accruing evidence that they exert important glial functions in their own right [23]. Moreover, there is considerable heterogeneity in the differentiation potential observed among NG2 cells of different brain regions [24]. Notwithstanding a final verdict on these issues, NG2 cells certainly are a natural target to consider for generating neurons.
Finally, it is critical to consider that the adult brain also contains a source of non-neural cells that may be ideal starting populations for reprogramming into neurons. In a first demonstration of this concept, Karow et al. showed that pericytes, cells normally found juxtaposed to blood vessels, can be isolated from the adult human brain and converted into functional induced neurons upon forced expression of Sox2 and Ascl1 [25].
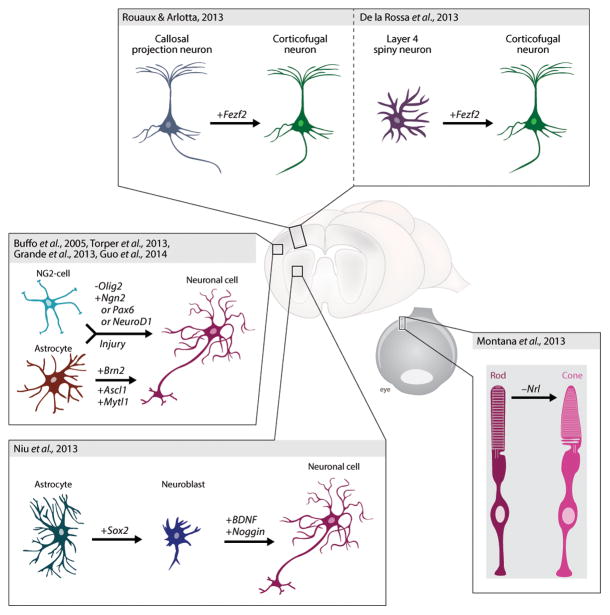
Collectively, these studies set the stage for subsequent work aimed at reprogramming resident cells into neurons within the vastly more complex context of the living brain (Figure 1). First attempts at in vivo reprogramming of endogenous glia utilized retroviruses encoding Pax6 to target cells that proliferate in response to brain injury [26]. Encouragingly, DCX-positive cells were produced. However, they quickly disappeared suggesting abortive neurogenesis. Almost a decade later, these attempts have now been repeated with novel factors at hand. Work from several laboratories utilized retro- or lentiviral vectors to express different neurogenic fate determinants in brain-resident cells. Torper et al conditionally expressed into striatal astrocytes the cocktail of transcription factors first described to reprogram fibroblasts into neurons [27]. Despite the caveat of potentially unwanted targeting of additional cell types due to promoter leakage, this led to the generation of morphologically and neurochemically identifiable neurons, suggesting that glia can be reprogrammed into neurons in the adult brain [27]. This conclusion is supported by studies in neocortical and striatal lesion models, where retrovirus-mediated expression of Neurog2 was found to induce neurogenesis, albeit the identity of the starting cells remained unknown [28]. However, neither of these studies reported on the functionality of the neurons. Employing a stab wound injury model Guo et al. found that the gene encoding the basic helix loop helix transcription factor NeuroD1 can reprogram both reactive astroglia and NG2 cells into neurons with a surprising degree of efficiency [29]. Recordings from brain slices showed that these neurons were functional in that they received synaptic inputs and were capable of repetitively firing action potentials [29]. Another study succeeded in obtaining electrophysiologically functional neurons from astrocytes in vivo by a two-step process. Forced expression of Sox2 in striatal astrocytes led to the generation of proliferating, DCX-positive neuroblasts, which could only be turned into functional neurons upon further treatment with brain-derived neurotrophic factor (BDNF) and the bone-morphogenetic protein (BMP) inhibitor Noggin [30]. What is particularly interesting in this second approach is that the starting number of astrocytes could be amplified by transitioning through a proliferating neuroblast stage.
Figure 1. Direct lineage reprogramming of glia and neurons in vivo.
Astrocytes and other glial cells have been converted into neuron-like cells within the parenchyma of the brain. Similarly, young postmitotic neurons in the neocortex and the retina have been directed to acquire new neuronal class-specific identities.
Neuron-to-neuron reprogramming
As a distinct alternative to reprogramming of non-neuronal resident cells of the brain, replacement of specific classes of neurons may also be achieved via reprogramming of neurons themselves, from a class into another. Once generated during development central neurons become permanently postmitotic and do not change their identity over time. However, it is unclear whether under the influence of appropriate signals neurons could be instructed to acquire signature features of a different cell type.
It remains largely unknown how neuronal class-specific features are maintained after development, which makes it difficult to predict whether neurons can be lineage reprogrammed. Work in invertebrates indicates that expression of key developmental transcription factors need to be maintained into adulthood in order for neurons to keep class-specific properties [31,32]. Initial evidence suggests that vertebrate neurons may have some of the same strategies in place. For example, the transcription factor Nurr1 is necessary for midbrain dopaminergic neurons to maintain terminal features such as dopaminergic identity and expression of some lineage specific transcription factors [33]. These experiments suggest that terminal neuronal identity is at least partly maintained via “active” mechanisms of transcriptional regulation. In agreement, nuclei of some neurons when exposed to the most powerful reprogramming environment known, i.e. the cytoplasm of the egg, appear able to support the development of an entire mouse. In 2004 the nucleus of an olfactory epithelial neuron, a unique class of sensory neurons that continue to be produced in the adult, was transferred into an enucleated oocyte via somatic cell nuclear transfer, and live mice were successfully obtained [34,35]. Using a similar approach, viable mice were subsequently produced using the nuclei of postmitotic neurons from the cerebral cortex of juvenile, but not adult, mice [36–38]. This work has provided some proof-of-principle evidence that no irreversible genetic or epigenetic changes have likely taken place during neuronal development that preclude the acquisition of a new cellular identity. Intriguingly, in response to oncogenes neurons of the cerebral cortex appear able to dedifferentiate to a progenitor state and initiate glioblastoma formation [39], although rare targeting of progenitors cannot be excluded in any of these studies.
Should neurons retain the capability of reprogramming their identity, could neurons then be converted from one class into another within the CNS? Could this in turn result in re-wiring of circuitry and gaining of new function? Given that neurons share some pan-neuronal features, conversion of closely-related neuron subtypes may facilitate the generation of highly specialized neurons and circuits for therapeutic benefit. This field is only emerging, but some studies are exploring this possibility. Similar to the work on reprogramming non-neuronal cells, master selector genes able to drive the acquisition of class-specific neuronal identity can be powerful tools to instruct a neuronal class-switch in vivo. In a first application to neurons of the cerebral cortex, the transcription factor Fezf2, a master gene capable of instructing multiple features of identity of corticospinal motor neurons (CSMN) [40,41], was used to turn other cortical neurons into CSMN within the brain. Despite neurons being postmitotic, Fezf2 is sufficient to directly reprogram embryonic and postnatal callosal projection neurons (CPN) into corticofugal neurons, including CSMN. Reprogrammed callosal neurons acquire molecular properties of CSMN, and change their axonal connectivity to corticofugal projections directed below the cortex towards the spinal cord [42]. Interestingly, electrophysiological properties and afferent/efferent connectivity of layer V neurons develop when Fezf2 is expressed in layer IV stellate neurons [43]. The data indicate that neurons of the neocortex can undergo a change of identity postmitotically when exposed to cell autonomous signals instructive of a different neuronal lineage (Figure 1). In the case of callosal neurons, postmitotic neuronal identity could be changed as late as P3 and P6, approximately ten days after the neurons have become postmitotic. The data indicate that the postmitotic nature of neurons does not per se preclude reprogramming. This is in agreement with prior cell fusion experiments that could instruct reprogramming without cell division [44] and with direct reprogramming in the pancreas, where exocrine cells direct conversion into beta cells does not require cell division [5]. However neuronal nuclear plasticity progressively declines over the first postnatal week and reprogramming capabilities in response to Fezf2 have exhausted by P21 [42]. This is likely not a phenomenon unique to cortical neurons, as “complete” reprogramming of rod into cone photoreceptors quickly turns into “partial” reprogramming over a similar time window [45]. In this study, conditional removal of the fate-specifying transcription factor Nrl from adult rods directly reprogrammed rods into cones. Although the cells retained some rod-specific traits, the work demonstrates that partial conversion of rods into cones is feasible in adults. Notably, this study tested the potential therapeutic relevance of such conversion showing that adult rods normally sensitive to retinitis pigmentosa not only became disease-resistant upon conversion into cones, but that as a result, endogenous cones were spared from the secondary death that normally affects them when rods are allowed to degenerate.
Collectively, these studies begin to challenge the concept that neurons may be irreversibly differentiated cells, yet also highlight how fundamental obstacles and limitations remain. Studies are needed to extend the critical period of postmitotic neuron reprogramming to the adult brain. The mechanisms that preclude reprogramming of adult neurons are not understood and this goes hand in hand with a lack of knowledge of how neuronal class-specific identity is kept unchanged during lifetime. Some evidence exists for active maintenance of neuronal identity, but this alone could be a problem for long-lived cells as it could become deregulated with time. It is likely that epigenetic and genetic changes may also be in place to passively “lock-in” identity, even if not necessarily in an irreversible manner. In support for the existence of epigenetic blocks to neuronal reprogramming in vivo, failure of reprogramming of adult rods into cone photoreceptors may at least partly be due to DNA methylation at key, class-specific loci [45].
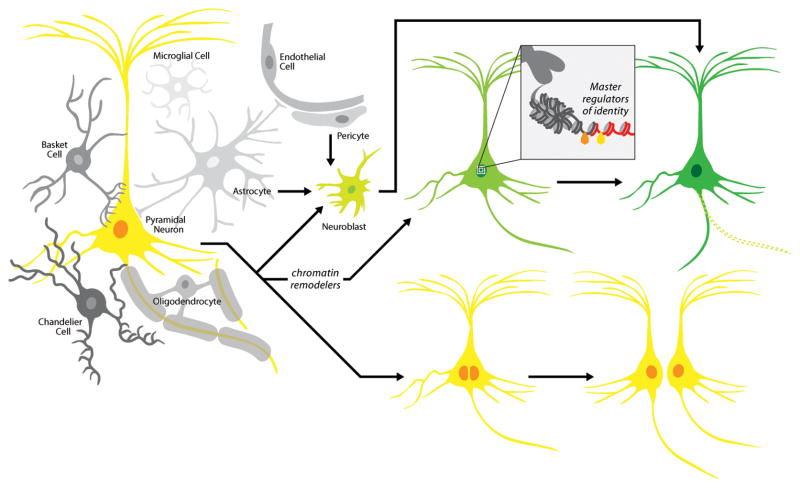
Exploiting the power of using invertebrate systems, Oliver Hobert’s group identified C. elegans lin-53, a chromatin remodeler, as a major inhibitor of the reprogramming of germ cells into gustatory neurons by the selector gene, CHE-1 [46]. This work put forward the guiding principle that combining chromatin modification with overexpression of fate specifying transcription factors may be a common way to facilitate reprogramming across species. It will be interesting to determine whether this molecular strategy will be successful to change the identity of cells refractory to reprogramming, like adult neurons (Figure 2).
Figure 2. Theoretical ways of generating new neurons via reprogramming in vivo.
Within the multicellular, complex environment of the adult CNS, astrocytes and other resident cell types, like pericytes, may be instructed to convert into plastic cellular intermediates (e.g. neuroblasts), which in turn may be receptive to instructive signals and become neurons. Neurons may transition to a more plastic cell state by undergoing epigenetic remodeling, which in turn could make them more permissive to fate specifying transcription factors able to induce the acquisition of a new neuronal class-specific identity. They also could revert to a more plastic cellular intermediate (e.g. neural stem cells, neuroblasts etc.). An alternative route to neuronal replacement could include the scenario of neuronal cell division. This process may also contribute to reset some of the epigenetic barriers normally blocking reprogramming.
Outlook
While the prospect of reprogramming the CNS is exciting, there remain conceptual and practical barriers for in vivo reprogramming to result in function-promoting outcomes or circuit modification for therapeutic benefit. Most of the direct reprogramming scenarios entail the loss of a cell of a specific identity and function. While a minor loss of cells could be tolerated, ideal strategies should allow for the replacement of the reprogrammed cell or for a local expansion of the newly generated neurons. One possibility would be the generation of an expandable intermediate cell. Should “reprogramming” of the postmitotic state of adult neurons be possible, as it has been shown for postmitotic cardiomyocytes [47], cell division may provide the additional benefit of facilitating the reprogramming process by erasing some of its epigenetic constrains.
Another crucial aspect concerns the correct incorporation of newly generated neurons into the pre-existing circuit. This is of utmost significance not only for the functional replacement of degenerated neurons, but also to avoid aberrant connections that may be detrimental to normal circuit function. The highly regulated step-by-step integration of new neurons in the adult neurogenic niches [48] can provide important lessons on the challenges a neuron derived through reprogramming must surmount in order to functionally integrate.
Finally, the greatest of all challenges would entail the regeneration of an entire circuitry consisting of distinct types of neurons and glia. Would reprogramming the identity of one neuron type result in the reorganization of the local circuitry? It is clear that in order to establish a complex nervous tissue a 1-to-1 reprogramming strategy is bound to fail and that sufficient capacity of self-organization will be required. While there is currently no concrete strategy on how to achieve this, the fact that pluripotent stem cells can generate organoids containing neurons of different regional identities in a seemingly orderly arrangement akin to real brains [49], gives hope that there will eventually be a way through this bottleneck.
A long way lays ahead for this exciting new field and it is likely that a concrete functional application of reprogramming strategies in the brain will require addressing fundamental questions regarding the way the CNS maintains its identity and the mechanisms that preclude cell conversion in vivo. Investigation of the mechanisms that control integration of new neurons into functional circuitry will be necessary to truly unfold the therapeutic value of these studies.
Highlights.
We review experimental evidence for the feasibility of direct reprogramming within the brain.
First we discuss achievements in the attempt of glia-to-neuron reprogramming in vivo.
This is followed by reviewing the state of the art of neuron-to-neuron reprogramming.
The challenges ahead of in vivo lineage reprogramming are discussed.
Acknowledgments
We thank Ryoji Amamoto for insightful discussions and comments on the manuscript and Dennis Sun for drawing of illustrations. We apologize to colleagues whose work we could not include due to space limitations. B.B. is supported by the German Research Foundation (DFG), the Federal Ministry of Education and Research (BMBF) and the Bavarian State Ministry of Sciences, Research, and the Arts. P.A. is supported by grants from the U.S. National Institute of Health, the ALS Association and the Harvard Stem Cell Institute. P.A. is a New York Stem Cell Foundation-Robertson Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ladewig J, Koch P, Brustle O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat Rev Mol Cell Biol. 2013;14:225–236. doi: 10.1038/nrm3543. [DOI] [PubMed] [Google Scholar]
- 2.Amamoto R, Arlotta P. Development-inspired reprogramming of the mammalian central nervous system. Science. 2014;343:1239882. doi: 10.1126/science.1239882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian L, Huang Y, Spencer C, Foley A, Vedantham V, Liu L, Conway S, Fu J-d, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton D. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 7.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- 9.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 10.Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Gotz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Sanchez R, Tiedt S, Schroeder T, Gotz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. This study shows that one and the same astrocyte population can be reprogrammed into distinct subtypes of induced neurons by forced expression of different transcription factors: Neurog2 reprograms astrocytes into glutamatergic neurons, while Dlx2/Ascl1 expression instructs generation of GABAergic neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Gotz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 13.Mattar P, Langevin LM, Markham K, Klenin N, Shivji S, Zinyk D, Schuurmans C. Basic helix-loop-helix transcription factors cooperate to specify a cortical projection neuron identity. Mol Cell Biol. 2008;28:1456–1469. doi: 10.1128/MCB.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, Klenin N, Brown C, Langevin LM, Seibt J, Tang H, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parras CM, Schuurmans C, Scardigli R, Kim J, Anderson DJ, Guillemot F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parras CM, Hunt C, Sugimori M, Nakafuku M, Rowitch D, Guillemot F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci. 2007;27:4233–4242. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun SM, Machado RA, Jessberger S. Temporal Control of Retroviral Transgene Expression in Newborn Cells in the Adult Brain. Stem Cell Reports. 2013;1:114–122. doi: 10.1016/j.stemcr.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 22.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 24.Vigano F, Mobius W, Gotz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16:1370–1372. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- **25.Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascon S, Khan MA, Lie DC, Dellavalle A, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. This study provides the first proof that the adult human brain harbors cells that can be reprogrammed in vitro into induced neurons. Moreover, by showing that these cells are pericyte-derived, the authors identify an unexpected target cell population for lineage reprogramming in the brain. [DOI] [PubMed] [Google Scholar]
- 26.Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. This is the first study to provide evidence for direct lineage conversion of striatal astrocytes into neurons in vivo by using the transcription factor combination Ascl1/Brn2/Myt1l, originally employed for fibroblast reprogramming into induced neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grande A, Sumiyoshi K, Lopez-Juarez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell. 2013 doi: 10.1016/j.stem.2013.12.001. Using glia-specific promoters the authors demonstrate that both reactive astrocytes and NG2 glia can be reprogrammed by forced expression of a single transcription factor, NeuroD1, into functional neurons within the cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. This work shows that forced expression of Sox2 can convert striatal astrocytes into proliferative neuroblasts, which in turn can be differentiated into functional neurons when exposed to BDNF and noggin. The finding that Sox2 is capable of inducing a neuronal identity comes somewhat as a surprise, as Sox2 is believed to oppose neuronal differentiation by proneural genes during development. Indeed, the authors report that the effect depends on transient expression of Sox2 while continuous expression of this transcription factor is detrimental to successful reprogramming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eade KT, Fancher HA, Ridyard MS, Allan DW. Developmental transcriptional networks are required to maintain neuronal subtype identity in the mature nervous system. PLoS Genet. 2012;8:e1002501. doi: 10.1371/journal.pgen.1002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hobert O. Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol. 2011;27:681–696. doi: 10.1146/annurev-cellbio-092910-154226. [DOI] [PubMed] [Google Scholar]
- 33.Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, Muramatsu S, Sumi-Ichinose C, Nomura T, Metzger D, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggan K, Baldwin K, Tackett M, Osborne J, Gogos J, Chess A, Axel R, Jaenisch R. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44–49. doi: 10.1038/nature02375. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Ishii T, Feinstein P, Mombaerts P. Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature. 2004;428:393–399. doi: 10.1038/nature02433. [DOI] [PubMed] [Google Scholar]
- 36.Makino H, Yamazaki Y, Hirabayashi T, Kaneko R, Hamada S, Kawamura Y, Osada T, Yanagimachi R, Yagi T. Mouse embryos and chimera cloned from neural cells in the postnatal cerebral cortex. Cloning and stem cells. 2005;7:45–61. doi: 10.1089/clo.2005.7.45. [DOI] [PubMed] [Google Scholar]
- 37.Osada T, Tamamaki N, Song S-Y, Kakazu N, Yamazaki Y, Makino H, Sasaki A, Hirayama T, Hamada S, Nave K-A, et al. Developmental pluripotency of the nuclei of neurons in the cerebral cortex of juvenile mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8368–8374. doi: 10.1523/JNEUROSCI.1591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamazaki Y, Makino H, Hamaguchi-Hamada K, Hamada S, Sugino H, Kawase E, Miyata T, Ogawa M, Yanagimachi R, Yagi T. Assessment of the developmental totipotency of neural cells in the cerebral cortex of mouse embryo by nuclear transfer. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14022–14026. doi: 10.1073/pnas.231489398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Friedmann-Morvinski D, Bushong E, Ke E, Soda Y, Marumoto T, Singer O, Ellisman M, Verma I. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science (New York, NY) 2012;338:1080–1084. doi: 10.1126/science.1226929. This study reports that glioblastomas can originate from a variety of differentiated cells including postmitotic neurons of the cerebral cortex. An oncogenic trigger (increased Ras mitogenic signal combined with downregulation of p53) was restricted to neurons using two transgenic Cre mouse lines (Syn1-Cre and CamK2-Cre). The work suggests that neurons are capable of dedifferentiation and generation of dividing cells that in turn maintain tumor progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouaux C, Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nature neuroscience. 2010;13:1345–1347. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- **42.Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nature cell biology. 2013;15:214–221. doi: 10.1038/ncb2660. This study demonstrates that the identity of postmitotic callosal projection neurons can be reprogrammed to that of corticofugal projection neurons in vivo. Reprogramming of callosal neuron class-specific identity occurs in response to ectopic and restricted expression of the transcription factor Fezf2. Neurons can reprogram postnatally within a newly defined window of “nuclear” neuronal plasticity that closes by P21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.De la Rossa A, Bellone C, Golding B, Vitali I, Moss J, Toni N, Lüscher C, Jabaudon D. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nature neuroscience. 2013;16:193–200. doi: 10.1038/nn.3299. This study shows that development of input and output connectivity of layer IV cortical neurons changes to that of layer V neurons upon expression of Fezf2. In line with reference [42], the work shows that Fezf2 can instruct the generation of layer V neurons and suggests that developmental circuit wiring is sensitive to the nature of the target neurons. [DOI] [PubMed] [Google Scholar]
- 44.Cowan C, Atienza J, Melton D, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science (New York, NY) 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- *45.Montana CL, Kolesnikov AV, Shen SQ, Myers CA, Kefalov VJ, Corbo JC. Reprogramming of adult rod photoreceptors prevents retinal degeneration. Proc Natl Acad Sci U S A. 2013;110:1732–1737. doi: 10.1073/pnas.1214387110. This study demonstrates that conditional removal of the Nrl gene from adult rod photoreceptors, reprograms them into cone-like cells. This work is notable because conversion of rods into cones leads to rescue of retina degeneration in a model of retinitis pigmentosa by preserving endogenous cone function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tursun B, Patel T, Kratsios P, Hobert O. Direct conversion of C. elegans germ cells into specific neuron types. Science (New York, NY) 2011;331:304–308. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 48.Bergami M, Berninger B. A fight for survival: the challenges faced by a newborn neuron integrating in the adult hippocampus. Dev Neurobiol. 2012;72:1016–1031. doi: 10.1002/dneu.22025. [DOI] [PubMed] [Google Scholar]
- 49.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]