Abstract
Alzheimer’s disease (AD) is the most prevalent cause of dementia, affecting more than 25 million people worldwide. Current models of the pathophysiological mechanisms of AD suggest that the accumulation of soluble oligomeric forms of amyloid-β (Aβ) peptides causes early loss of excitatory synapses and impairs synaptic plasticity. The signaling pathways mediating Aβ oligomer-induced impairment of synaptic plasticity and loss of excitatory synapses are only beginning to be unraveled. Here, we review recent evidence supporting the critical contribution of conserved ‘stress-response’ kinase pathways in AD progression.
Introduction
There is currently no effective treatment for Alzheimer’s disease (AD) and the steady increase in human life span adds to the considerable burden that this devastating neurodegenerative disease imposes on our society. AD is characterized by the deposition in the brain of extracellular amyloid plaques composed of aggregated amyloid-β (Aβ) peptide, and of intracellular neurofibrillary tangles composed of aggregates of hyperphosphorylated protein Tau.
Loss of synapses in the hippocampus and neocortex is a cardinal feature that occurs at an early clinical stage of the disease, and strongly correlates with the degree of cognitive impairment [1]. In mouse models of AD, severe changes in synaptic function and maintenance can occur well before the appearance of amyloid plaques, supporting the amyloid hypothesis which suggests that soluble oligomers of Aβ are causal to synaptic toxicity [2], trigger synaptic dysfunction, synapse loss and impaired long-term potentiation (LTP) [3]. The exact nature of the Aβ species (dimers, trimers, Aβ*56, protofibrils) responsible for this early synaptotoxicity is still under investigation [4]. Most experimental designs use a mixture of various forms of synthetic Aβ oligomers, or natural Aβ oligomers isolated from the brain of AD subjects, to induce rapid loss of excitatory synapses in hippocampal and cortical neurons in vitro [5–10]. Transgenic mouse models of AD engineered to overexpress human mutant forms of Amyloid Precursor Protein (hAPP), with or without overexpression of mutant presenilins (PS), produce high levels of Aβ1-40 and Aβ1–42 peptides and oligomers, recapitulate the reduction of excitatory synapses, exhibit neuronal network dysfunction and cognitive deficits in spatial learning [6,11].
Current evidence suggests that the excitatory post-synaptic compartment represents the main target of Aβ toxicity [8], and several candidate receptors for Aβ oligomers have recently emerged, such as cellular prion protein PrPC [12**] and EphB2 [13**]. Other cell surface receptors display altered function or expression in various AD mouse models, such as α7-nicotinergic acetylcholine receptor [14], the receptor for advanced glycation endproducts (RAGE) [15], metabotropic glutamate receptor mGluR5 [16], ionotropic glutamate NMDA and AMPA receptors [5,7,14,17]. Aβ oligomer binding to the post-synaptic compartment impairs the expression and function of these receptors, leading to altered synaptic plasticity and maintenance, and ultimately spine loss. The identification of the downstream signaling pathways mediating Aβ oligomer-dependent synaptotoxicity has considerable relevance for our understanding of the pathophysiology of AD and for the development of new therapeutic strategies. Here we review recently identified molecular pathways that contribute to Aβ oligomerinduced synaptotoxicity. Glycogen synthase kinase-3 (GSK-3) and CDK5 are not discussed in the present review because of space limitation and several recent reviews have addressed their potential contribution in AD [18–20].
PrPC-mGluR5-Fyn versus EphB2
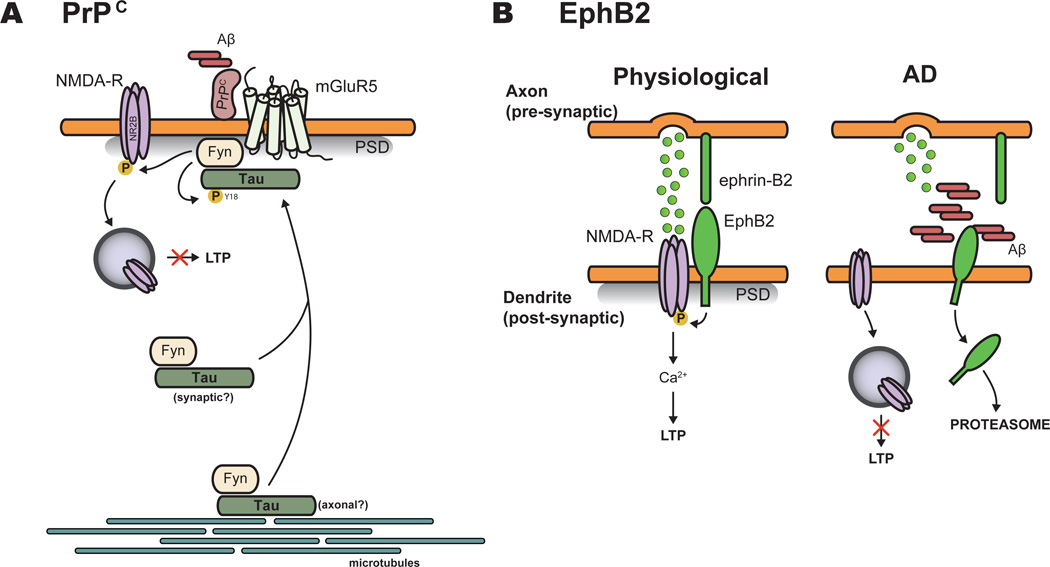
PrPC has been identified through an unbiased genome-wide screen as a potential highaffinity receptor for Aβ oligomers [12**] (Figure 1A), and the binding of Aβ species to PrPC has been confirmed in human AD brains [4,21*]. Experiments using genetic deletion or immunodepletion of PrPC in hAPP mouse models support PrPC as an essential mediator of Aβ oligomer-induced impairment of synaptic plasticity and memory, and synapse loss (reviewed in [22]), although this does not appear to be the case in every genetic backgrounds such as the J20 model [23]. The binding of synthetic or natural Aβ oligomers to PrPC at the post-synaptic density activates the Src kinase Fyn [4,21*], which in turn phosphorylates NR2B subunit, leading to increased surface NMDA receptor and excitotoxicity, followed by spine and receptor loss [21*]. Interestingly, Tau targets Fyn to the post-synapse to induce Aβ toxicity, and Tau deletion in hAPP mice, which causes disruption of post-synaptic targeting of Fyn, prevents Aβ-induced NMDA receptor-mediated excitotoxicity [24*]. Aβ-dependent PrPC-Fyn signaling induces Tau missorting and hyperphosphorylation at Y18 [4]. However, the requirement and functional role of this Fyn-dependent phosphorylation site on Tau for mediating Aβ toxicity at the synapse has not been investigated yet. Furthermore, mGluR5 is required for coupling Aβ oligomers/PrPC complex with Fyn to induce dendritic spine loss and memory impairment [22]. mGluR5 antagonist 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) blocks these deficits in APP/PS1dE9 and 3×Tg mouse models, suggesting that preventing mGluR5 activation by Aβ oligomers/PrPC complex may be therapeutically relevant for treating AD [22].
Figure 1.
Involvement of the PrPC-mGluR5-Fyn and EphB2-NMDA pathways in Aβ oligomerinduced synaptic pathology. (A) PrPC is a functional receptor of Aβ oligomers. Upon binding, Aβ/PrPC complexes activate Fyn. mGluR5 receptor seems required for coupling Aβ oligomers/PrPC complexes to Fyn. Tau plays an important role in signal transduction by tethering Fyn post-synaptically where it phosphorylates NR2B subunit and Tau at Y18. The origin of Tau/Fyn complexes, either axonal and/or postsynaptic, is currently unknown. Phosphorylation of NMDA receptors by Fyn leads to increased surface NMDA receptor (NMDA-R) and excitotoxicity, followed by spine (and receptor) loss. (B) EphB2 is another functional receptor for Aβ oligomers. In physiological conditions, EphB2/ephrin-B2 complexes regulate NMDA receptor function via NR1 and/or NR2B subunit phosphorylation. In AD, Aβ oligomer binding to EphB2 receptor induces EphB2 internalization and degradation in the proteasome. Decreased EphB2 receptor alters NMDA receptor function, resulting in LTP impairment. This figure is adapted from [13**]. PSD: postsynaptic density.
EphB2 tyrosine kinase receptor has been recently identified as another high-affinity receptor for Aβ oligomers (Figure 1B) and EphB2 is known to play an important role in regulating the synaptic localization and function of NMDA receptors [25]. AD patients and hAPP transgenic mouse models of AD have reduced level of EphB2 in the hippocampus [26]. Recent studies revealed that Aβ oligomers can bind EphB2 to trigger EphB2 degradation in the proteasome, and knockdown of EphB2 impairs NMDA receptor signaling, leading to defective LTP [13**]. Remarkably, virus-mediated expression of EphB2 in the dentate gyrus of hAPP J20 mouse model blocks synaptic and memory deficits, suggesting that restoring EphB2 expression locally in the dentate gyrus may be an effective therapeutic strategy [13**].
Calcineurin-NFAT / GSK3-NFAT
Aβ oligomers dysregulate calcium homeostasis through a mechanism involving NMDA receptors (reviewed in [27]). Aβ-induced calcium elevation activates the calcium-dependent phosphatase B calcineurin, which in turn promotes nuclear translocation and activation of the transcriptional nuclear factor of activated T cells (NFAT) [28,29]. Both calcineurin and NFAT activity are increased in AD brains [28], and activation of the calcineurin-NFAT cascade in rodents results in dystrophic neurites, dendritic simplification and dendritic spine loss in vitro and in vivo [28,29]. Strategies aimed at blocking calcineurin or NFAT activation prevent spine loss and dendritic dystrophy in the Tg2576 transgenic mouse model of AD, supporting calcineurin- NFAT contribution to Aβ synaptotoxicity [28,29]. Furthermore, NFAT-dependent apoptotic pathway is activated by inhibition of GSK-3 [30], potentially explaining the conflicting outcomes in preclinical and clinical trials using GSK-3 inhibitors for treating AD and questioning the single therapeutic strategy of inhibiting GSK-3 [18].
Centaurin-α1-Ras-Elk-1
Centaurin-α1 (also named p42/IP4) is an ADP ribosylation factor (Arf) GTPase-activating protein (GAP) that is required for normal dendritic development [31]. It interacts with Ras and activates Ras-E26-like kinase 1 (Elk-1). Both centaurin-α1 and Elk-1 can associate with the mitochondrial permeability transition pore complex to regulate its function [32]. Centaurin-α1 protein level is increased in the brain of AD patients and hAPP J20 mouse model [33], and increased association of Elk-1 with mitochondria is also observed in the J20 mice [34]. Recent studies demonstrated that Aβ1–42 oligomers increase the expression level of centaurin-α1 in cultured neurons, which induces a Ras-dependent association of Elk-1 with mitochondria, leading to mitochondrial and synaptic dysfunction in organotypic hippocampal slices [34]. Blockade of the centaurin-α1-Ras-Elk-1 pathway rescues Aβ-induced dendritic spine loss, spine structural plasticity, and mEPSC (miniature Excitatory Post-synaptic Currents) amplitude and frequency, suggesting the contribution of this pathway in neuronal dysfunction in early stage of AD. Further studies are required to determine the relevance of this pathway in vivo. Interestingly, extensive experimental evidence from the cancer field has demonstrated that the Ras-MAPK pathway is tightly connected to the AMPK (AMP-activated Kinase) and mTOR (mammalian target of rapamycin) pathways [35,36] which have been recently shown to play a critical role in the early synaptoxic effects of Aβ oligomers (see below).
mTOR
The serine/threonine kinase mTOR plays a central role in various cellular processes, including cell size, cell proliferation through regulation of protein synthesis, and also negatively regulates autophagy (reviewed in [37]). In neurons, mTOR plays an important role in long-term synaptic plasticity, axon pathfinding and regeneration, dendrite arborization and spine morphology (reviewed in [38]). mTOR signaling has been shown to be up-regulated in mouse models and human cases of AD, although data appears to be conflicting, and increased mTOR signaling seems to be caused by Aβ (reviewed in [39]). Therefore, Aβ-mediated hyperactivation of mTOR may contribute to early cognitive defects in Alzheimer’s disease. Accordingly, inhibition of mTOR by rapamycin treatment improves cognitive deficits and rescues both Aβ and Tau pathologies in AD mouse models [40,41]. However, given the central role of mTOR in learning and memory, further studies may be required to clarify its potential relevance to treat AD.
MARK kinases
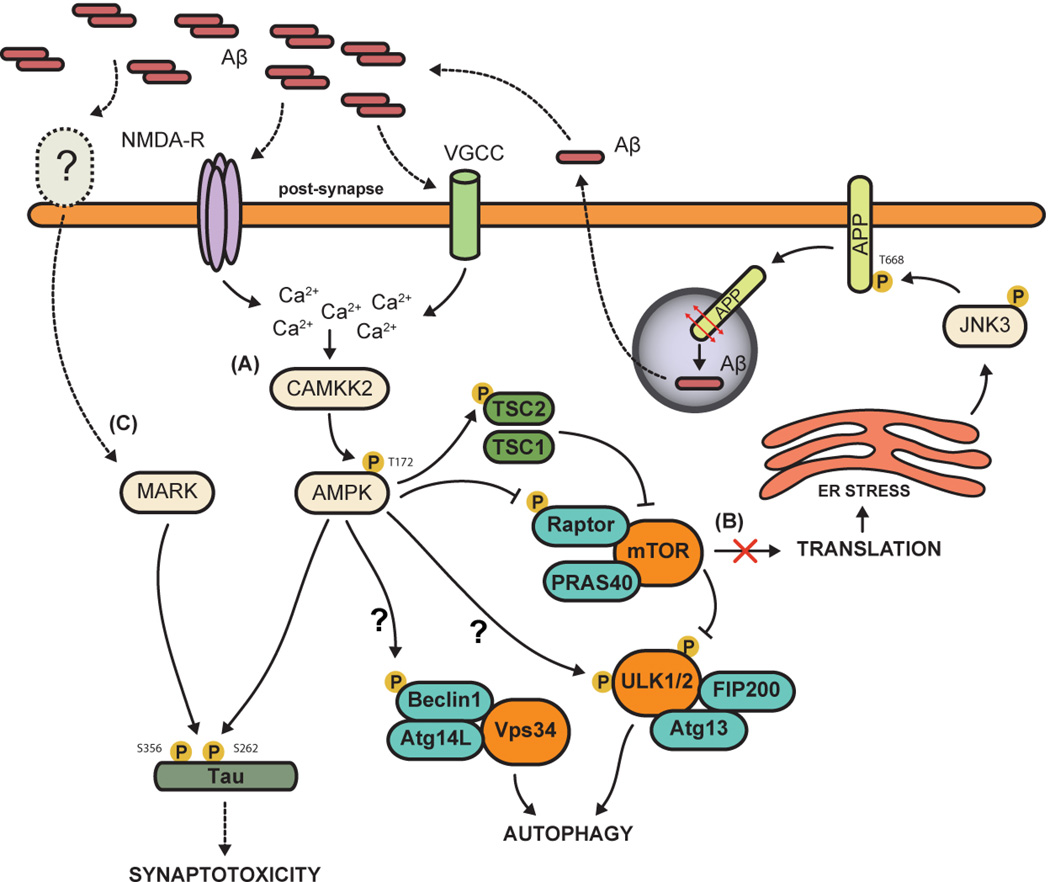
The serine/threonine microtubule-affinity regulating kinases (MARK) belong to the AMPK-like family of kinases, and consist of four members (MARK1 to MARK4) in mammals. MARKs are the closest orthologs of the fruit fly partition defective-1 (PAR-1) which plays an important role in regulating cell polarity [42]. MARK1 and MARK2 were originally identified as regulators of microtubule stability [43]. Through their ability to phosphorylate Tau on KXGS motifs in the microtubule binding domain, MARKs cause Tau detachment from microtubules and microtubule destabilization (reviewed in [44,45]). Genome-wide association studies suggest a potential link of MARK4 to late onset AD [46], and recent functional studies suggest that MARK kinases participate to the synaptotoxic effects of Aβ in vitro [47,48]. Overexpression of MARK4 in hippocampal neurons leads to Tau hyperphosphorylation (Figure 2), reduced expression of synaptic markers, loss of dendritic spines and synapses, while an inhibitor of all MARK family members abrogated the toxic effects of Aβ oligomers on dendritic spines and synapses, assayed at the morphological and electrophysiological levels [47]. However, conflicting studies reported that overexpression of MARK2 prevents Aβ oligomer-induced synaptotoxicity, whereas pharmacological inhibition of the kinase results in spine loss in presence or absence of Aβ [48]. Future studies will have to define Aβ-dependent signaling pathway leading to activation of MARKs, and use loss- and gain-of-function approaches in hAPP mouse models to better understand the role of these group of kinases in mediating the toxic effects of Aβ.
Figure 2.
Aβ oligomers activate several ‘stress-response’ kinase pathways in neurons. (A) Oligomeric Aβ as well as increased intracellular calcium flux, induced either by NMDA receptor activation or membrane depolarization through opening of voltage-gated calcium channels (VGCC), can activate the CAMKK2-AMPK pathway. AMPK over-activation leads to increased phosphorylation of Tau at S262 which results in dendritic spine loss. Furthermore, AMPK promotes autophagy, which is abnormally activated in AD, by acting on three main pathways: (1) indirectly, by inhibiting mTORC1 (mTOR complex 1, which inhibits autophagy) through phosphorylation of Raptor and/or TSC2 (Tuberous Sclerosis Complex 2); (2) directly, by activating ULK1/2 (Unc-51 Like Kinases), the mammalian orthologs of yeast ATG1, an upstream regulator of the autophagy pathway; and (3) via Beclin1 phosphorylation which promotes its assembly with class III PI3-kinase (PIK3c3; also named Vps34) and ATG14L. The two question marks represent ways whereby AMPK has been shown to regulate autophagy in non-neuronal cells but not in neurons yet. (B) mTOR inhibition further results in a protein translation block which causes widespread ER stress, leading to JNK3 activation. In turn, JNK3 phosphorylates APP at T688, promoting its endocytosis and processing by secretases, consequently increasing Aβ40/42 biogenesis. (C) The AMPK-related kinases MARKs can also phosphorylate Tau at ADrelevant epitopes (KXGS motifs) to mediate the synaptotoxic effects of Aβ oligomers, although the upstream pathway leading to their activation in mammalian neurons is currently unclear, and could be either LKB1 or CAMKK2 [53**].
Role of the CAMKK2-AMPK pathway in neurodegenerative diseases
Calcium/Calmodulin-dependent Kinase Kinase 2 (CAMKK2) is a serine/threonine protein kinase whose activity is increased upon Ca2+ influx through Ca2+/calmodulin binding. It is the upstream activator of calcium/calmodulin kinases CAMKI and CAMKIV, and of the metabolic sensor AMPK [49]. CAMKK2 is mainly expressed in the central nervous system, where it plays important roles, presumably through via CAMKI, in axon and synaptic development, synaptic plasticity and memory (reviewed in [50]).
AMPK is a serine/threonine protein kinase composed of three subunits, including a catalytic subunit (α1 or α2 in mammals), a β adaptor subunit (β1 or β2), and a γ subunit (γ1, γ2 or γ3) that is allosterically activated upon binding to AMP or ADP (reviewed in [35,36,51]). Phosphorylation of the threonine residue T172 within the activation loop of the α subunit is required for catalytic activation. Several kinases can act as direct upstream activators of AMPK through phosphorylation of T172, including CAMKK2 and LKB1. AMPK is instrumental at restoring proper ATP level through a myriad of effectors, converging on repression of the mTOR pathway and promotion of autophagy [35,36,51], which are both deregulated in AD mouse models and human patients (reviewed in [39,52]). In neurons, AMPK can be activated by increased intracellular Ca2+ (through depolarization and opening of voltage-gated calcium channels or through activation of ionotropic glutamate receptors such as NMDA receptors) and therefore is not only a metabolic sensor but also reports changes in neuronal activity [53**,54*].
In the brain, AMPK activity is increased by metabolic stresses associated with ischemia, hypoxia or glucose-deprivation [55,56], and is abnormally elevated in several human neurodegenerative disorders including AD and other tauopathies, amyotrophic lateral sclerosis, and Huntington’s disease [57–59]. However, whether over-activation of AMPK in these different pathological contexts plays a role in the disease progression remained controversial (see [63] for example). Activated AMPK seems strongly enriched in tangle- and pre-tangle bearing neurons in AD patients, suggesting that AMPK might participate to AD progression by modulating Tau phosphorylation [59]. Recent reports indicated that AMPK over-activation might have deleterious outcomes by increasing Aβ generation through β-secretase transcriptional up-regulation [64] and inhibition of mTOR-dependent protein synthesis [65**], and by aberrantly promoting autophagosome formation through RAGE-CAMKK2 pathway [66].
The CAMKK2-AMPK pathway was recently shown to mediate the early synaptotoxic effects of Aβ oligomers, in part through Tau phosphorylation [53**] (Figure 2). We found that the CAMKK2-AMPK pathway is robustly activated by acute application of Aβ42 oligomers in vitro confirming a previous report [54*], and that AMPK activation is elevated in the cortex and hippocampus of the J20 hAPP-overexpressing mouse model at 4 and 12 month-old [53**]. More importantly, we found that abolishing CAMKK2 or AMPK catalytic activity or expression protects hippocampal and cortical neurons from the synaptotoxic effects of Aβ42 oligomers in vitro as well as the loss of dendritic spines observed in the J20 mouse model in vivo [53**].
AMPK, BRSK1/2 and MARK1-4 can robustly phosphorylate Tau on S262 in the KXGS motif of the microtubule-binding domain, and preventing phosphorylation on this site blocks the synaptotoxic effects induced by Aβ oligomers or by overexpression of these kinases [47,48,53**,54*,67]. Tau aggregation and clearance seems to be regulated by a balance between phosphorylation and acetylation at the KXGS motifs [68]. The relevance of AMPK and MARK kinases over-activation and Tau phosphorylation in AD pathogenesis requires further in vivo evidence.
AMPK-mTOR-JNK3
A recent report suggests that c-Jun N-terminal kinase 3 (JNK3) activation, which is abnormally increased in the brain of human AD cases and hAPP mouse models, is central to the development of AD pathology by maintaining a positive feedback loop that leads to continued production of Aβ [65**] (Figure 2). Accordingly, genetic deletion of JNK3 in a hAPP mouse model (5XFAD) results in dramatic reduction in Aβ42 levels and Aβ plaque loads, and improved cognition. Interestingly and in accordance with the recent report discussed above [53**], in vitro studies suggest that Aβ42 oligomer-dependent activation of AMPK inhibits mTOR, resulting in a translational block responsible for endoplasmic reticulum (ER) stress which activates JNK3. In turn, JNK3 phosphorylates APP at T688, facilitating its endocytosis and processing, thereby increasing Aβ production in vitro. This study contrasts with other studies who proposed to inhibit mTOR as a therapeutic approach for AD [40,41].
The microtubule-binding protein Tau as a critical effector of Aβ-induced synaptotoxicity
Remarkably, the adverse effects of Aβ on neuronal degeneration and cognitive dysfunction appear to depend in large part on Tau (reviewed in [69–71]). For example, synthetic Aβ oligomers or Aβ dimers isolated from the brain of AD subjects induce hyperphosphorylation of Tau at AD-relevant epitopes in hippocampal neurons, Tau mislocalization into the somatodendritic compartment, disrupt the microtubule cytoskeleton and cause neuritic degeneration [9,72]. Knocking down endogenous Tau does not appear to be phenotypic in neurons but instead fully prevents neuritic changes, whereas overexpressing human Tau accelerated them [9,48]. In vivo, genetic deletion of Tau in the hAPP J20 mouse model prevents behavioral deficits without altering Aβ burden, and protects both transgenic and non-transgenic mice against excitotoxicity [73,74**].
Conclusion
Many kinase pathways have been implicated in AD progression. However, it is presently unclear which pathways are mediating the synaptotoxic effects of Aβ oligomers and which are activated in response to impairment of synaptic homeostasis and/or neuronal viability. Furthermore, there is increasing evidence that Aβ oligomers can trigger the over-activation of ‘stress-response’ pathways including the CAMKK2-AMPK pathway [53**,54*] and the downstream mTOR-ER stress-JNK3 pathway [65**]. These pathways seem to play important roles in the early steps of AD progression, inducing loss of excitatory synapses and reduction of synaptic plasticity in hippocampal and cortical circuits. Future investigations need to determine how these ‘stress-response’ kinase pathways interact functionally during AD progression, and whether preventing their over-activation could represent a new therapeutic avenue to prevent the cognitive decline characterizing AD progression. This study provides the first in vivo evidence that genetic deletion of tau prevents cognitive deficits in a hAPP transgenic mouse model without altering Aβ burden.
-
-
Several ‘stress-response’ kinase pathways are over-activated in Alzheimer’s disease
-
-
Aβ oligomers activate the CAMKK2-AMPK pathway which phosphorylates Tau on Serine 262
-
-
Recent results involve mTOR inhibition, protein synthesis block and ER stress in AD progression
-
-
Blocking these ‘stress-response’ pathways prevents the synaptotoxic effects of Aβ oligomers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson M, Sherman MA, Amar F, Nuvolone M, Schneider JA, Bennett DA, Aguzzi A, Lesne SE. The complex PrP(c)-Fyn couples human oligomeric Abeta with pathological tau changes in Alzheimer's disease. J Neurosci. 2012;32:16857a–16871a. doi: 10.1523/JNEUROSCI.1858-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid {beta}-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nature Neuroscience. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. In this study, the authors performed an unbiased genome-wide screen to identify proteins that have high affinity for Aβ oligomers, and suggest that PrPC is a potential receptor. While initially challenged by other groups, follow-up studies strongly support Aβ oligomers/PrPC interplay in AD pathophysiology (see [4,21] for example).
- 13. Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. EphB receptors play an important role in controlling NMDA receptor physiology, and both are decreased in AD pathology. This study reveals that Aβ oligomers target EphB2 receptor, leading to EphB2 receptor degradation and reduced synaptic NMDA receptors and LTP. The authors further provide multiple ways to selectively interfere with this pathway to overcome cognitive deficits in a hAPP mouse model of AD.
- 14.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 15.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 16.Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei P, Ayton S, Bush AI, Adlard PA. GSK-3 in Neurodegenerative Diseases. Int J Alzheimers Dis. 2011;2011:189246. doi: 10.4061/2011/189246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avila J, Wandosell F, Hernandez F. Role of glycogen synthase kinase-3 in Alzheimer's disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev Neurother. 2010;10:703–710. doi: 10.1586/ern.10.40. [DOI] [PubMed] [Google Scholar]
- 20.Shukla V, Skuntz S, Pant HC. Deregulated Cdk5 activity is involved in inducing Alzheimer's disease. Arch Med Res. 2012;43:655–662. doi: 10.1016/j.arcmed.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci. 2012;15:1227–1235. doi: 10.1038/nn.3178. A follow-up study on Aβ/PrPC functional interaction. The authors provide compelling evidence that Aβ/PrPC complexes activate the kinase Fyn which subsequently phosphorylates NR2B subunit, resulting in loss of synaptic NMDA receptors.
- 22.Um JW, Strittmatter SM. Amyloid-beta induced signaling by cellular prion protein and Fyn kinase in Alzheimer disease. Prion. 2013;7:37–41. doi: 10.4161/pri.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cisse M, Sanchez PE, Kim DH, Ho K, Yu GQ, Mucke L. Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice. J Neurosci. 2011;31:10427–10431. doi: 10.1523/JNEUROSCI.1459-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. The authors demonstrate that endogenous Tau is present in dendritic spines in physiological conditions, where it tethers the kinase Fyn to PSD-95 and NMDA receptor signaling complexes. Blocking Fyn/NMDA receptor interaction by preventing Tau localization in dendrites mitigates Aβ toxicity at the post-synapse.
- 25.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 26.Simon AM, de Maturana RL, Ricobaraza A, Escribano L, Schiapparelli L, Cuadrado-Tejedor M, Perez-Mediavilla A, Avila J, Del Rio J, Frechilla D. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer's disease. J Alzheimers Dis. 2009;17:773–786. doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- 27.Paula-Lima AC, Brito-Moreira J, Ferreira ST. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer's disease. J Neurochem. 2013;126:191–202. doi: 10.1111/jnc.12304. [DOI] [PubMed] [Google Scholar]
- 28.Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudry E, Wu HY, Arbel-Ornath M, Hashimoto T, Matsouaka R, Fan Z, Spires-Jones TL, Betensky RA, Bacskai BJ, Hyman BT. Inhibition of the NFAT pathway alleviates amyloid beta neurotoxicity in a mouse model of Alzheimer's disease. J Neurosci. 2012;32:3176–3192. doi: 10.1523/JNEUROSCI.6439-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Sintes R, Lucas JJ. NFAT/Fas signaling mediates the neuronal apoptosis and motor side effects of GSK-3 inhibition in a mouse model of lithium therapy. J Clin Invest. 2010;120:2432–2445. doi: 10.1172/JCI37873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore CD, Thacker EE, Larimore J, Gaston D, Underwood A, Kearns B, Patterson SI, Jackson T, Chapleau C, Pozzo-Miller L, et al. The neuronal Arf GAP centaurin alpha1 modulates dendritic differentiation. J Cell Sci. 2007;120:2683–2693. doi: 10.1242/jcs.006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galvita A, Grachev D, Azarashvili T, Baburina Y, Krestinina O, Stricker R, Reiser G. The brain-specific protein, p42(IP4) (ADAP 1) is localized in mitochondria and involved in regulation of mitochondrial Ca2+ J Neurochem. 2009;109:1701–1713. doi: 10.1111/j.1471-4159.2009.06089.x. [DOI] [PubMed] [Google Scholar]
- 33.Reiser G, Bernstein HG. Neurons and plaques of Alzheimer's disease patients highly express the neuronal membrane docking protein p42IP4/centaurin alpha. Neuroreport. 2002;13:2417–2419. doi: 10.1097/00001756-200212200-00008. [DOI] [PubMed] [Google Scholar]
- 34.Szatmari EM, Oliveira AF, Sumner EJ, Yasuda R. Centaurin-alpha1-Ras-Elk-1 signaling at mitochondria mediates beta-amyloid-induced synaptic dysfunction. J Neurosci. 2013;33:5367–5374. doi: 10.1523/JNEUROSCI.2641-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- 37.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- 39.Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 40.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, Oddo S. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies. Aging Cell. 2013;12:370–380. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- 43.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 44.Matenia D, Mandelkow EM. The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci. 2009;34:332–342. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi K, Suzuki A, Ohno S. PAR-1/MARK: a kinase essential for maintaining the dynamic state of microtubules. Cell Struct Funct. 2012;37:21–25. doi: 10.1247/csf.11038. [DOI] [PubMed] [Google Scholar]
- 46.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu W, Polepalli J, Wagh D, Rajadas J, Malenka R, Lu B. A critical role for the PAR-1/MARK-tau axis in mediating the toxic effects of Abeta on synapses and dendritic spines. Hum Mol Genet. 2012;21:1384–1390. doi: 10.1093/hmg/ddr576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM. Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013 doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Racioppi L, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J Biol Chem. 2012;287:31658–31665. doi: 10.1074/jbc.R112.356485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: Modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 53. Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron. 2013;78:94–108. doi: 10.1016/j.neuron.2013.02.003. This work complements other studies [54*,65**] by providing in vitro and in vivo evidence that over-activation of the CAMKK2-AMPK pathway by Aβ oligomers plays a critical role in the early loss of excitatory synapses through Tau phosphorylation at S262.
- 54. Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to beta-amyloid exposure. Biochem J. 2011;15:503–12. doi: 10.1042/BJ20101485. This study provided the first biochemical demonstration that Aβ oligomers can activate the CAMKK2-AMPK pathway in neurons, and increases the phosphorylation of Tau at AD-relevant epitopes.
- 55.Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, et al. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 57.Ju TC, Chen HM, Lin JT, Chang CP, Chang WC, Kang JJ, Sun CP, Tao MH, Tu PH, Chang C, et al. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington's disease. J Cell Biol. 2011;194:209–227. doi: 10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim MA, Selak MA, Xiang Z, Krainc D, Neve RL, Kraemer BC, Watts JL, Kalb RG. Reduced activity of AMP-activated protein kinase protects against genetic models of motor neuron disease. J Neurosci. 2012;32:1123–1141. doi: 10.1523/JNEUROSCI.6554-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vingtdeux V, Davies P, Dickson DW, Marambaud P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies. Acta Neuropathol. 2010 doi: 10.1007/s00401-010-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caberlotto L, Lauria M, Nguyen TP, Scotti M. The Central Role of AMP-Kinase and Energy Homeostasis Impairment in Alzheimer's Disease: A Multifactor Network Analysis. PLoS One. 2013;8:e78919. doi: 10.1371/journal.pone.0078919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer's disease. J Neurochem. 2011;118:460–474. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]
- 62.Cai Z, Yan LJ, Li K, Quazi SH, Zhao B. Roles of AMP-activated protein kinase in Alzheimer's disease. Neuromolecular Med. 2012;14:1–14. doi: 10.1007/s12017-012-8173-2. [DOI] [PubMed] [Google Scholar]
- 63.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yoon SO, Park DJ, Ryu JC, Ozer HG, Tep C, Shin YJ, Lim TH, Pastorino L, Kunwar AJ, Walton JC, et al. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron. 2012;75:824–837. doi: 10.1016/j.neuron.2012.06.024. The authors report that Aβ oligomers activate a stress-response kinase pathway, including AMPK, mTOR and JNK3, which promotes a positive feedback loop resulting in increased production of Aβ.
- 66.Son SM, Jung ES, Shin HJ, Byun J, Mook-Jung I. Abeta-induced formation of autophagosomes is mediated by RAGE-CaMKKbeta-AMPK signaling. Neurobiol Aging. 2012;33(1006):e1011–e1023. doi: 10.1016/j.neurobiolaging.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida H, Goedert M. Phosphorylation of microtubule-associated protein tau by AMPK-related kinases. J Neurochem. 2012;120:165–176. doi: 10.1111/j.1471-4159.2011.07523.x. [DOI] [PubMed] [Google Scholar]
- 68.Cook C, Carlomagno Y, Gendron TF, Dunmore J, Scheffel K, Stetler C, Davis M, Dickson D, Jarpe M, Deture M, et al. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum Mol Genet. 2014;23:104–116. doi: 10.1093/hmg/ddt402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 70.Pooler AM, Noble W, Hanger DP. A role for tau at the synapse in Alzheimer's disease pathogenesis. Neuropharmacology. 2014;76(Pt A):1–8. doi: 10.1016/j.neuropharm.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roberson ED, Scearce-Levie K, Palop JJ, Yan FR, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. This study provides the first in vivo evidence that genetic deletion of tau prevents cognitive deficits in a hAPP transgenic mouse model without altering Aβ burden.