Abstract
There are multiple barriers to axonal growth after CNS injury. Myelin-associated inhibitors represent one group of barriers extrinsic to the injured neurons. Nogo, MAG and OMgp are three prototypical myelin inhibitors that signal through multiple neuronal receptors to exert growth inhibition. Targeting myelin inhibition alone modulates the compensatory sprouting of uninjured axons but the effect on the regeneration of injured axons is limited. Meanwhile, modulating sprouting, a naturally occurring repair mechanism, may be a more attainable therapeutic goal for promoting functional repair after CNS injury in the near term.
1. Introduction
It is notorious that after injury of the adult mammalian central nervous system (CNS), damaged axons cannot regenerate to a significant extent, leading to major functional impairments in patients of spinal cord injury (SCI). Because the peripheral nervous system (PNS) has a remarkable ability to regenerate axons, extensive efforts have been focusing on understanding the differences between the PNS and the CNS. The key observation that CNS axons can regenerate in a PNS environment [1] prompted the notion that the environment in the PNS, but not the CNS, is conducive to axon regeneration. One major distinction between the CNS and the PNS is the origin of the myelin and its composition. This led to the hypothesis that CNS myelin is inhibitory to axon regeneration. The production of the IN-1 antibody against an inhibitory activity from CNS myelin [2], the identification of Nogo [3], other myelin-associated inhibitors (MAIs) and their receptors, and the many in vitro and in vivo studies since have contributed much to our understanding of the molecular regulation of axonal growth after CNS injury. It is now widely recognized that both neuron-intrinsic and extrinsic mechanisms contribute to the lack of CNS axon regeneration. Here we discuss the role of the prototypical myelin inhibitors in the context of recent development in the field of axon growth and repair after CNS injury.
2. Definition of regeneration and sprouting
The literature on MAIs in axonal repair is abundant, mostly aimed at addressing the key question: can the manipulation of the MAIs and their receptors promote axon regeneration in vivo? The short answer is: yes and no. Indeed, the answer depends on the definition of regeneration. There are many different terms used to describe axon growth after injury: regeneration, sprouting, regenerative sprouting, or even axonal plasticity. Use of inaccurate or ambiguous terminology has been a major issue in the field, leading to confusion and disagreement. This is partly due to the continuous evolution of scientific concepts and partly to the limitations of the experimental tools available at any given time.
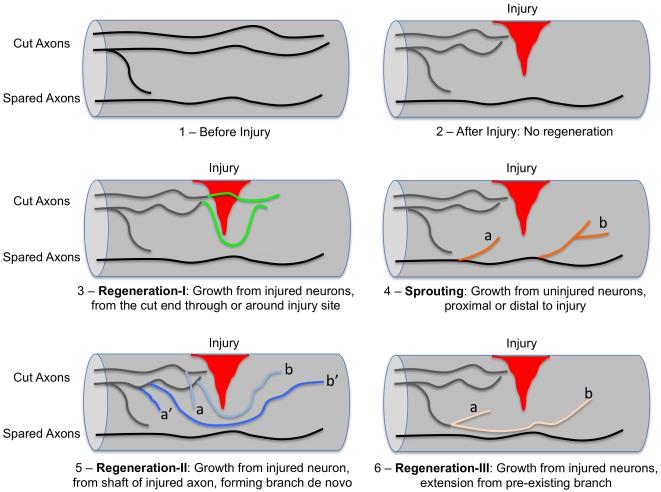
To allow for a meaningful discussion, here we provide one way to define regeneration and sprouting. In this definition, whether any axonal growth after injury is regeneration or sprouting depends solely on whether or not a neuron has been injured in the first place. Regeneration is axonal growth from injured neurons, while sprouting is axonal growth from uninjured neurons (Fig. 1). Under this definition, there are three typical scenarios for regeneration. First, regeneration can originate from the cut end (or tip) of injured axons (Fig. 1.3), which is the most classic type of regeneration. In the literature regenerating axons often have to grow beyond (either through or around) the injury site and towards their original targets to be considered significant or relevant. However, this may not be necessary if neurons proximal to the injury can relay information from regenerated axons [4]. Second, regeneration can originate from the shaft of injured axons, forming new branches de novo (Fig. 1.5). In this scenario, regeneration can initiate close to the injury site or at a distance, and the growth can cover a short or long distance (Fig. 1.5). Third, regeneration can be extension from pre-existing, non-injured axonal branches of injured neurons (Fig. 1.6). In contrast, as axonal growth from uninjured neurons, sprouting generally occurs as a compensatory response to injury of other neurons. Just as regeneration, sprouting may also initiate at different locations (proximal or distal, close or distant) relative to the injury site, and the growth can also be for short or long distances (Fig. 1.4).
Figure 1. Axon regeneration versus axon sprouting after injury in the spinal cord.
1) Axons in the non-injured spinal cord. 2) After a partial injury, injured axons normally do not regenerate. 3) Regeneration scenario I: injured axons grow from the cut end (i.e. injured axonal tip), through or around the injury site. This is the typical definition of regeneration. 4) Sprouting is any new axonal growth from uninjured neurons. This occurs in response to injury of other neurons. It can occur proximal (a) or distal (b) to the injury site. 5) Regeneration scenario II: axonal growth from the shaft of injured axons, forming new branches de novo. The growth can originate close to the injury site (a, b) or at a distance (a’, b’); it can be for a short (a, a’) or long (b, b’) distance. 6) Regeneration scenario III: axonal extension from pre-existing branches of injured neurons. It can be for a short (a) or long (b) distance. The common theme for all scenarios of regeneration here is that axonal growth is from injured neurons.
It should be noted that even though regeneration and sprouting can be strictly defined conceptually, it is not always technically straightforward to distinguish the different types of axonal growth depicted in Fig. 1. For instance, axonal growth represented in Fig. 1.5 (a, a’) and 1.6 (a) are often collectively referred to as “regenerative sprouting” in the literature. Note that in all these three cases, growth is from injured neurons, thus the term “regenerative sprouting” contradicts with the definition of sprouting as growth from uninjured neurons and could be confusing. It is therefore always advisable to describe in great detail the axon growth phenotype one observes in spinal cord injury models.
Distinguishing regeneration from sprouting based on the injury status of the neurons will be useful in investigating the molecular mechanisms because injured and uninjured neurons are likely to be differentially regulated in their axon growth abilities [5]. Using more defined terms to describe axonal growth also has important bearing on clinical applications. A treatment that promotes sprouting but not regeneration can be efficacious for anatomically incomplete but not complete injuries. Targeting the appropriate cohort of patients would be critical for the success of clinical trials.
3. Multiple ligands and multiple receptors involved in axon growth inhibition
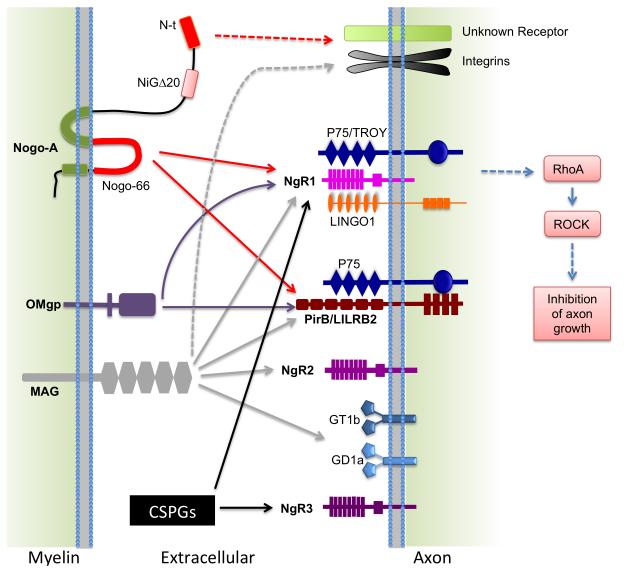
There are three prototypical MAIs: Nogo, MAG and OMgp, all of which have potent inhibitory activity on neurite growth in vitro. These MAIs signal through multiple neuronal receptors and co-receptors to effect cytoskeleton rearrangement and neurite inhibition through a signaling pathway involving Rho and Rho-associated kinase (ROCK) (Fig. 2). There are other potential MAIs expressed by myelin and oligodendrocytes. Here we focus on the prototypical myelin inhibitors and their receptors.
Figure 2. Interaction of the prototypical myelin-associated inhibitors with their receptors.
Nogo, MAG and OMgp all bind to NgR1 and PirB receptors. NgR1 forms a complex with LINGO-1 and p75NTR (or TROY) to signal growth inhibition in the axons. PirB can also bind to p75NTR. In addition, MAG can bind NgR2 and gangliosides GD1a and GT1b. N-terminal (N-t) Nogo and MAG may also signal through an integrin-based mechanism. Other unknown receptors may exist. Chondroitin sulfate proteoglycans (CSPGs), the glial scar-derived inhibitors, can bind to NgR1 and NgR3. Thus, myelin inhibitors and CSPGs share some receptors.
3.1. Multiple Ligands
MAG was the first MAI characterized molecularly [6]. It is a transmembrane glycoprotein (Fig. 2) produced by myelinating glial cells: oligodendrocytes in the CNS and Schwann cells in the PNS, with a higher expression level in the CNS. MAG functions in the maintenance of myelinated axons [7]. Its effects on axon growth are bi-modal: MAG promotes axon growth from young neurons and inhibits growth from older neurons, a switch that is age and neuron type dependent [6]. MAG has been widely used as an inhibitory substrate for neurite growth assays using postnatal and adult neurons. However, relatively few studies addressed its function in axonal growth after injury in vivo. In genetic studies, targeting MAG alone did not improve axon regeneration [8,9••,10••]. Interestingly, both genetic deletion and intrathecal delivery of sialidase to interfere MAG binding to sialoglycans (gangliosides GD1a and GT1b) enhanced serotonergic (5-HT) axon sprouting [10••,11]. Surprisingly, genetically deleting MAG reduced corticospinal tract (CST) axon sprouting [10••]. The CST is a functionally important tract that controls voluntary movements in humans, and has been extensively studied in rodent models of spinal cord injury [12]. Thus, MAG may have opposing roles – growth inhibitory on some neurons but promoting on other neurons - even in the adult CNS. Moreover, MAG may mediate axon stability and integrity, and protect axons under pathological conditions [13,14•]. Genetically deleting MAG led to accelerated axonal loss in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis [15•]. Together, these studies indicate that MAG has divergent roles in axonal response to injury and disease: in addition to its well-publicized role in growth inhibition, MAG may promote axonal growth and/or protect axons from further degeneration in the adult CNS.
OMgp is a glycosylphosphatidylinositol (GPI)-linked protein (Fig. 2). Originally found in CNS myelin extract, it is expressed not only by oligodendrocytes but also by neurons, including adult CNS neurons [16,17]. Its role in developmental axon sprouting is still poorly understood [18]. Interestingly, OMgp is involved in the regulation of synaptic plasticity and activity-dependent synaptic strength [19], and it may influence axonal target specification during the development of thalamocortical projections [20]. In three independent studies, genetically deleting OMgp did not promote CST axon regeneration [9••,10••,21]. While deleting OMgp similarly did not lead to 5-HT axon regeneration [9••,10••], it enhanced 5-HT axon sprouting [10••,21]. Thus, OMgp inhibits axon sprouting after CNS injury in vivo.
Nogo (or Rtn4) is the most extensively studied MAI, with Nogo-A being the isoform most abundantly expressed by oligodendrocytes [22]. Alternative splicing and alternative promoter usage generate two other isoforms: Nogo-B and Nogo-C. Nogo-A is a transmembrane protein expressed in the endoplasmic reticulum but may reach cell surface. An extracellular 66 amino acid loop named Nogo-66 in the C-terminus (shared among the 3 isoforms) induces growth cone collapse and inhibits neurite growth [3] (Fig. 2). A Nogo-A specific region is also inhibitory to neurite growth independently of Nogo-66 [23]. Interestingly, Nogo deficient mice display a delayed closure of the critical period for ocular dominance plasticity, implicating a role for Nogo in regulating experience dependent plasticity [24].
Multiple different Nogo knockout mice have been analyzed for axon growth and repair after injury [25-28]. The different Nogo knockout lines all lack Nogo-A but have different effects on Nogo-B and C [29••]. Consistent among the different studies, CNS myelin preparations made from various Nogo mutant mice all exhibited a reduced inhibitory effect on neurite growth in vitro, indicating that Nogo has a substantial contribution to myelin-associated growth inhibition [25-27]. In vivo data were rather different, ranging from extensive [25], suggestive (i.e. with a non-significant trend)[26] to no enhanced CST regeneration [27] in various Nogo mutant mice. Different factors have been proposed to explain these discrepancies, such as the definition of regeneration/sprouting, the type and severity of the lesion, the age of the mice at the time of the lesion, the genetic background or the configuration of the gene disruption [29••]. It was found later that the evidence for the most extensive CST regeneration in a Nogo-A,B gene trap mutant appeared to have risen from an inadvertent axon labeling artifacts, providing a cautionary tale for detailed anatomical analyses with spinal injury models [30]. When the Nogo-A,B gene trap mutant was re-assessed by a different group under conditions to minimize axon labeling artifacts, CST regeneration was no longer observed [28]. Together, these studies indicate that the effect of deleting Nogo on CST regeneration after experimental spinal cord injury is limited at best.
In contrast, most studies in the literature agree on a role for Nogo in CST axon sprouting. In this regard, it should be noted that any partial injury that spare even a minor population of CST axons may allow for CST sprouting that gives the appearance of regeneration [31]. Indeed, the earlier studies using the IN-1 antibodies [32], and later more specific Nogo-A antibodies in rodent models [33] and in primate models [34] could be reconciled with genetic studies if sprouting had been the emphasis. In genetic studies, an increase of CST axon sprouting was consistently reported in Nogo mutant mice by different labs, independent of the mutation analyzed and the strain background as long as the CST was not completely severed [9••,10••,26,35]. It is interesting to note that chondroitin sulfate proteoglycans (CSPGs), the astroglia-derived axon growth inhibitors, also appear to exert their effect on CNS repair primarily through modulating axon sprouting rather than regeneration [36•,37••].
Distinguishing sprouting from regeneration is not only important in investigating the underlying mechanisms, it is also important in translational effort: a therapy that is designed to improve functional recovery primarily based on enhanced sprouting is unlikely to have any chance of success for anatomically complete injuries. Sprouting occurs spontaneously without any treatment after injury. It is the body’s natural repair mechanism for the CNS that can be modulated by targeting glia-derived growth inhibitors. Sprouting axons do not have to travel far to reach appropriate targets while regenerating axons may have to travel long distance in order to make functional connections. For these reasons, modulating sprouting, rather than regeneration, might be a more attainable therapeutic goal in the near term to promote functional recovery. The mechanisms by which sprouting leads to functional recovery remain to be extensively investigated.
3.2. Multiple Receptors
NgRs (NgR1, NgR2 and NgR3) are a family of three leucine-rich repeat GPI-linked proteins that have been shown to bind axon growth inhibitors. The first MAI receptor discovered was Nogo receptor 1 (NgR1 or Nogo-66 receptor) because of its binding to Nogo-66 [38]. Later on it was found that NgR1 binds to MAG [39] and OMgp [40] as well, despite the three MAIs not sharing structural similarities (Fig. 2). NgR2 also binds to MAG, with even a higher affinity than NgR1 [41]. Unexpectedly, NgR1 along with NgR3 bind to CSPGs [42••], highlighting potential functional redundancy and crosstalk between the two different classes of inhibitors (Fig. 2).
NgR1 forms a complex with co-receptors, LINGO-1 and P75NTR or TROY, to initiate intracellular signaling [43-46]. One working model is that the formation of ligand/receptor/co-receptors complex promotes proteolysis of p75NTR (via α- and γ-secretase), which activates protein kinase C and the small GTPase RhoA/ROCK/Cofilin pathway, thereby promoting actin depolymerization in the growth cones and blocking neurite extension [47]. However, whereas NgR1 is required to promote growth cone collapse from Nogo-66, it is not for required for its longer-term effect on neurite extension [48-50]. Physiologically, NgR1 has a role in activity-dependent synaptic strength and plasticity [24,51] and is involved, as are NgR2/3, in restricting synapse formation during development [52•]. It will be interesting to find out whether (and if so, how) these physiological functions relate to their function in CNS repair.
Among NgRs, NgR1 is the most extensively studied for its function in vivo after injury. A peptide blocking Nogo66-NgR1 interaction had mixed results in CST regeneration and functional recovery [53-55,56••]. Genetic studies by two independent labs found no enhancement in CST regeneration in two different NgR1 knockout mouse lines [48,49]. In line with this, deleting p75NTR, a co-receptor for NgR1, also did not enhance CST axon regeneration [49,57]. In NgR1 knockout mice, even 5-HT axons did not exhibit enhanced regeneration after a complete spinal transection [58]. However, deleting NgR1 enhanced CST axon sprouting across the midline following a unilateral lesion [35], consistent with a role for myelin-mediated inhibition in axon sprouting after injury. Further studies are required to substantiate the robustness of such enhanced sprouting and associated functional benefit.
PirB. Paired immunoglobulin-like receptor B (PirB, LILRB2 in human) is another major MAI receptor that binds to Nogo, MAG and OMgp (Fig. 2) [59]. MAI binding triggers PirB’s interaction with p75NTR [60], leading to the recruitment of phosphatases (SHP-1 and SHP-2), which then modulate tropomyosin-receptor kinase phosphorylation and associated signal transduction pathways [61••]. Interestingly, PirB is associated with ocular-dominance plasticity [62], just as Nogo, NgR1 and CSPGs [24,63]. However, deleting PirB did not enhance axon regeneration after optic nerve crush [61••], traumatic brain lesion [64] or spinal cord injury [56••]. Furthermore, even blocking NgR1 with NEP1-40 in PirB knockout mice did not enhance CST regeneration [56••]. Interestingly, however, SHP-1/2 knockdown promoted optic nerve regeneration [61••].
Other receptors. Beside NgRs and PirB, other receptors or signaling mediators have been proposed for MAIs. For example, both MAG and amino-Nogo can signal through an integrin-based mechanism [65,66] (Fig. 2). However, a direct interacting partner for amino-Nogo remains to be identified.
4. Combined effects of targeting multiple growth inhibitors
The presence of multiple myelin inhibitors along with multiple receptors prompted the question of functional redundancy among the different myelin inhibitors. Two independent groups generated and characterized Nogo/MAG/OMgp triple knockout mice [9••,10••]. A detailed discussion of the different mutations and genetic background used can be found in a previous review [29••]. The results are summarized here. Using in vitro neurite growth assays, both studies found a substantial contribution from Nogo to the inhibitory activity of CNS myelin [9••,10••]. In vivo results were more divergent. In one study, deleting Nogo-, MAG, OMgp or all three inhibitors together did not promote CST regeneration after dorsal hemisection; deleting all three together did not promote 5-HT regeneration after complete transection [10••]; deleting Nogo promoted CST sprouting after pyramidotomy while deleting MAG or OMgp promoted 5-HT sprouting after lateral hemisection. In no case was any additive or synergistic effect seen on axon sprouting, implicating a potential ceiling effect of manipulating myelin inhibitors. What was most surprising was that deleting MAG reduced CST sprouting, as discussed above [10••]. Again, this study emphasizes the importance of understanding the in vivo role of myelin-associated inhibitors – or, perhaps more correctly, myelin-associated axon growth modulators – before targeting these molecules in therapeutic development. No functional recovery was reported in this study.
In the other study, the investigators confirmed their previous finding that deleting Nogo alone promoted CST regeneration [9••]. Deleting MAG or OMgp alone did not lead to CST regeneration but in combination with Nogo deletion led to more CST regeneration than Nogo deletion alone [9••]. Targeting Nogo or all three inhibitors also promoted 5-HT sprouting with a partial lesion model [9••]. Taken together, these two studies reinforce the notion that manipulating MAIs has more consistent and reproducible effect on axon sprouting than regeneration. The complexity involving molecule and tract specific effects on axonal growth after injury remains to be fully elucidated. Again, it is extremely important to understand the in vivo roles of individual MAIs under physiological and pathophysiological conditions before targeting them in therapies. Moving therapies forward without a clear understanding of the in vivo function of the intended molecular targets will lead to unnecessary failure in translational effort.
Functional redundancy may also exist between the myelin inhibitors and CSPGs. Using NEP1-40 and chondroitinase treatment, two different groups did not see a synergistic effect on axon growth in a slice culture and organotypic co-culture system respectively [67,68]. In contrast, acute treatment with Nogo-A antibody and delayed Chondroitinase treatment have been reported to promote CST growth additively and combining the two treatments was more effective in promoting functional recovery when applied together with a rehabilitation scheme [69••]. The authors made an interesting observation that the diameters of CST axons affected by the two treatments are different, with targeting Nogo promoting growth of larger diameter axons while targeting CSPGs promoting the growth of finer processes with varicosities. The molecular mechanism underlying this phenomenon warrants further investigation.
5. Concluding remarks
Myelin-Associated Inhibitors (MAIs) are molecules present in the CNS myelin that modulate axon growth. Most evidence in the literature is consistent with a role for MAIs in axon sprouting, reproducible with a variety of injury models, axonal tracts and across different labs. Axon regeneration, however, remains limited by targeting these molecules alone. Both regeneration and sprouting can contribute to functional recovery. Distinguishing these two forms of injury-induced axonal growth is important not only to the understanding of the underlying molecular regulation but also to the development of effective therapeutic strategies to treat CNS injuries and other neurological conditions. Indeed, promoting sprouting could be as functionally important, if not more, as regeneration. Indeed, it may be more realistic to target sprouting than frank regeneration in the near term. The roles of MAIs in axon sprouting are complex. Once the neuron-intrinsic growth state is elevated, extrinsic axon growth modulators including MAIs are more likely to stand out as the next barrier for regeneration. Regardless of sprouting or regeneration, the anatomical substrate provided by enhanced growth is only likely to be useful for functional gains with additional, activity-dependent mechanisms such as that provided by rehabilitation or training.
Highlights.
-
-
Regeneration and sprouting are two forms of injury-induced axonal growth
-
-
Myelin inhibitors modulate axonal sprouting after CNS injury
-
-
Regeneration elicited intrinsically can be further modulated by myelin inhibitors
-
-
Promoting sprouting to restore function may be a more attainable near-term goal
Acknowledgement
We apologize to those researchers whose original work we were not able to cite due to space limitations. Work in the authors’ lab has been funded by grants from NIH/NINDS (R01NS054734), California Institute for Regenerative Medicine (RB3-02143), the Craig H. Neilsen Foundation, Wings for Life Spinal Cord Research Foundation, Roman Reed Spinal Cord Injury Research Fund of California, the Dana Foundation, Christopher and Dana Reeve Foundation and International Spinal Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 2.Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- 3.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 4.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 7.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 9.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. •• This is one of the two independent Nogo/MAG/OMgp triple knockout studies on spinal axon growth after injury. The authors reported that deleting MAG and OMgp further enhances corticospinal and serotonergic axon growth and functional recovery in Nogo-A,B knockout mice.
- 10.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. •• This is one of the two independent Nogo/MAG/OMgp triple knockout studies on spinal axon growth after injury. The authors used four different injury models to study the regeneration or sprouting of two different axonal tracts (corticospinal and serotonergic axons). They reported no enhanced regeneration in triple knockout mice but a complex pattern of effects on axon sprouting including inhibitor-specific and tract-specific effects.
- 11.Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci U S A. 2010;107:11561–11566. doi: 10.1073/pnas.1006683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen T, Mehta NR, Conant K, Kim KJ, Jones M, Calabresi PA, Melli G, Hoke A, Schnaar RL, Ming GL, et al. Axonal protective effects of the myelin-associated glycoprotein. J Neurosci. 2009;29:630–637. doi: 10.1523/JNEUROSCI.5204-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinter J, Lazzati T, Schmid D, Zeis T, Erne B, Lutzelschwab R, Steck AJ, Pareyson D, Peles E, Schaeren-Wiemers N. An essential role of MAG in mediating axon-myelin attachment in Charcot-Marie-Tooth 1A disease. Neurobiol Dis. 2012;49C:221–231. doi: 10.1016/j.nbd.2012.08.009. • The authors reported elevated MAG expression in patients of Charcot-Marie-Tooth disease type 1A (CMT1A), a hereditary demyelinating peripheral neuropathy, and in a mouse model for CMT1A. MAG deletion exacerbated disease phenotypes in CMT1A mice, leading to loss of axon-glia interaction. The data implicates MAG in sustaining axonal integrity in CMT1A.
- 15.Jones MV, Nguyen TT, Ewaleifoh O, Lebson L, Whartenby KA, Griffin JW, Calabresi PA. Accelerated axon loss in MOG35-55 experimental autoimmune encephalomyelitis (EAE) in myelin-associated glycoprotein-deficient (MAGKO) mice. J Neuroimmunol. 2013;262:53–61. doi: 10.1016/j.jneuroim.2013.06.008. • Following on their previous studies, the authors provided evidence for an axon protective role for MAG early in EAE, an animal model of multiple sclerosis.
- 16.Vourc’h P, Andres C. Oligodendrocyte myelin glycoprotein (OMgp): evolution, structure and function. Brain Res Brain Res Rev. 2004;45:115–124. doi: 10.1016/j.brainresrev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Lee JK, Case LC, Chan AF, Zhu Y, Tessier-Lavigne M, Zheng B. Generation of an OMgp allelic series in mice. Genesis. 2009;47:751–756. doi: 10.1002/dvg.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang KJ, Susuki K, Dours-Zimmermann MT, Zimmermann DR, Rasband MN. Oligodendrocyte myelin glycoprotein does not influence node of ranvier structure or assembly. J Neurosci. 2010;30:14476–14481. doi: 10.1523/JNEUROSCI.1698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil V, Bichler Z, Lee JK, Seira O, Llorens F, Bribian A, Morales R, Claverol-Tinture E, Soriano E, Sumoy L, et al. Developmental expression of the oligodendrocyte myelin glycoprotein in the mouse telencephalon. Cereb Cortex. 2010;20:1769–1779. doi: 10.1093/cercor/bhp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji B, Case LC, Liu K, Shao Z, Lee X, Yang Z, Wang J, Tian T, Shulga-Morskaya S, Scott M, et al. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol Cell Neurosci. 2008;39:258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 26.Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 27.Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Chan AF, Luu SM, Zhu Y, Ho C, Tessier-Lavigne M, Zheng B. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci. 2009;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JK, Zheng B. Role of myelin-associated inhibitors in axonal repair after spinal cord injury. Exp Neurol. 2012;235:33–42. doi: 10.1016/j.expneurol.2011.05.001. •• In depth review on the role of MAIs in injury-induced axonal growth, including detailed discussion on the divergent results obtained by different groups regarding the regeneration, or the lack of it, in different Nogo knockout mice.
- 30.Steward O, Zheng B, Banos K, Yee KM. Response to: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187-199. Neuron. 2007;54:191–195. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Steward O, Zheng B, Ho C, Anderson K, Tessier-Lavigne M. The dorsolateral corticospinal tract in mice: an alternative route for corticospinal input to caudal segments following dorsal column lesions. J Comp Neurol. 2004;472:463–477. doi: 10.1002/cne.20090. [DOI] [PubMed] [Google Scholar]
- 32.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 33.Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 34.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 35.Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2012;235:5–17. doi: 10.1016/j.expneurol.2011.08.008. • Interesting review describing the roles of chondroitin sulphate proteoglycans and presenting evidences that targeting CSPGs can alter plasticity of the adult CNS to restore function after injury.
- 37.Starkey ML, Bartus K, Barritt AW, Bradbury EJ. Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur J Neurosci. 2012;36:3665–3678. doi: 10.1111/ejn.12017. •• In this paper, the authors demonstrated that chondroitinase ABC treatment can enhance compensatory sprouting from uninjured neurons and that this growth is associated with some level of functional recovery.
- 38.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 39.Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 40.Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 41.Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. •• In this interesting study, the authors reported that the glial scar-derived inhibitors, CSPGs, can also bind to NgR1 and NgR3. NgR1 and NgR3 double mutant showed an increase of optic nerve regeneration, further enhanced by co-deletion of RPTPσ, a previously identified CSPG receptor. These data suggest shared receptor mechanisms for the two classes of glia-derived axon growth inhibitors, MAIs and CSPGs.
- 43.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 44.Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 45.Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 46.Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 47.Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 48.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 2007;27:7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wills ZP, Mandel-Brehm C, Mardinly AR, McCord AE, Giger RJ, Greenberg ME. The nogo receptor family restricts synapse number in the developing hippocampus. Neuron. 2012;73:466–481. doi: 10.1016/j.neuron.2011.11.029. • This report shows that NgR1 inhibits the formation of new excitatory synapses through TROY, RhoA and ROCK. This may be relevant to how NgR limits synaptic plasticity and how blocking NgR1 can enhance functional connectivity.
- 53.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steward O, Sharp K, Yee KM, Hofstadter M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp Neurol. 2008;209:446–468. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura Y, Fujita Y, Ueno M, Takai T, Yamashita T. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J Biol Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. •• This is the first paper to report the effect of PirB deletion on CNS axon regeneration in vivo. The authors demonstrated that mice lacking PirB do not exhibit improved corticospinal axon regeneration. Morever, adminstering NEP1-40, a NgR antagonist, in PirB knockout mice did not enhance regeneration.
- 57.Song XY, Zhong JH, Wang X, Zhou XF. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci. 2004;24:542–546. doi: 10.1523/JNEUROSCI.4281-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JK, Chow R, Xie F, Chow SY, Tolentino KE, Zheng B. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J Neurosci. 2010;30:10899–10904. doi: 10.1523/JNEUROSCI.2269-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 60.Fujita Y, Takashima R, Endo S, Takai T, Yamashita T. The p75 receptor mediates axon growth inhibition through an association with PIR-B. Cell Death Dis. 2011;2:e198. doi: 10.1038/cddis.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujita Y, Endo S, Takai T, Yamashita T. Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO J. 2011;30:1389–1401. doi: 10.1038/emboj.2011.55. •• In this article, the authors describe the signaling pathway of PirB-mediated growth inhibition. They showed that MAG binding to PirB leads to the recruitment of SHP-1 and SHP-2, which in turn inhibit Trk activity. This work identified SHP-1 and SHP-2 as new molecular targets in the myelin inhibitory pathway.
- 62.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 63.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 64.Omoto S, Ueno M, Mochio S, Takai T, Yamashita T. Genetic deletion of paired immunoglobulin-like receptor B does not promote axonal plasticity or functional recovery after traumatic brain injury. J Neurosci. 2010;30:13045–13052. doi: 10.1523/JNEUROSCI.3228-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goh EL, Young JK, Kuwako K, Tessier-Lavigne M, He Z, Griffin JW, Ming GL. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain. 2008;1:10. doi: 10.1186/1756-6606-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28:1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mingorance A, Sole M, Muneton V, Martinez A, Nieto-Sampedro M, Soriano E, del Rio JA. Regeneration of lesioned entorhino-hippocampal axons in vitro by combined degradation of inhibitory proteoglycans and blockade of Nogo-66/NgR signaling. FASEB J. 2006;20:491–493. doi: 10.1096/fj.05-5121fje. [DOI] [PubMed] [Google Scholar]
- 68.Nakamae T, Tanaka N, Nakanishi K, Kamei N, Sasaki H, Hamasaki T, Yamada K, Yamamoto R, Izumi B, Ochi M. The effects of combining chondroitinase ABC and NEP1-40 on the corticospinal axon growth in organotypic co-cultures. Neurosci Lett. 2010;476:14–17. doi: 10.1016/j.neulet.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 69.Zhao RR, Andrews MR, Wang D, Warren P, Gullo M, Schnell L, Schwab ME, Fawcett JW. Combination treatment with anti-Nogo-A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. Eur J Neurosci. 2013;38:2946–2961. doi: 10.1111/ejn.12276. •• The authors examined the combination of acutely applied anti-Nogo-A antibody, delayed chondroitinase ABC treatment and delayed rehabilitation on forelimb functional recovery after a partial cervical injury. They showed that the combination of either anti-Nogo-A or chondroitinase with rehabilitation improved functional recovery, but the combination of all three was more effective. They also provided intriguing evidence for differential regulation of axonal growth by Nogo-A and CSPGs.