Abstract
Phenol oxidase (PO) was isolated as a proenzyme (pro-phenol oxidase, pro-PO) from the hemolymph of Manduca sexta larvae and purified to homogeneity. Pro-PO exhibits a M(r) of 130,000 on gel filtration and two bands with an apparent M(r) of approximately 100,000 on SDS/PAGE, as well as size-exclusion HPLC. Activation of pro-PO was achieved either by specific proteolysis by a cuticular protease or by the detergent cetylpyridinium chloride at a concentration below the critical micellar concentration. A cDNA clone for M. sexta pro-PO was obtained from a larval hemocyte cDNA library. The clone encodes a polypeptide of approximately 80,000 Da that contains two copper-binding sites and shows high sequence similarity to POs, hemocyanins, and storage proteins of arthropods. The M. Sexta pro-PO, together with other arthropod pro-POs, contains a short stretch of amino acids with sequence similarity to the thiol ester region of alpha-macroglobulins and complement proteins C3 and C4.
Full text
PDF

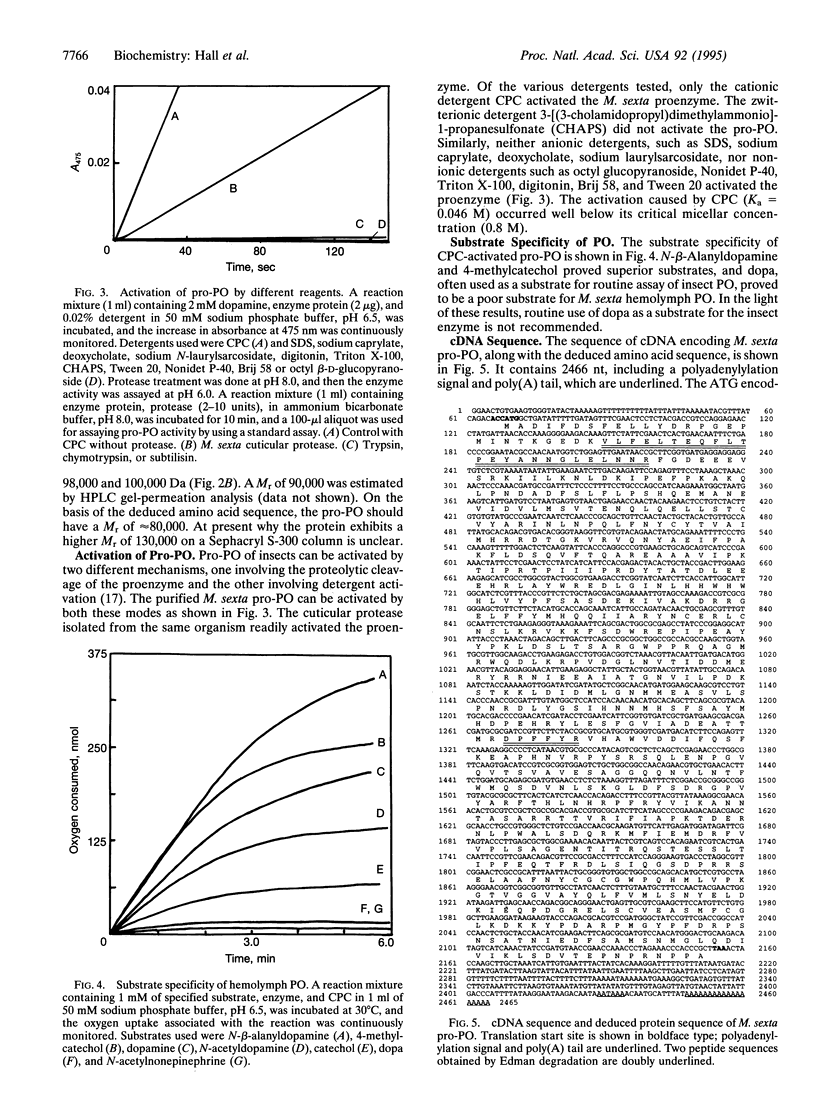


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida M. Purification and characterization of pre-phenoloxidase from hemolymph of the silkworm Bombyx mori. Arch Biochem Biophys. 1971 Jun;144(2):749–762. doi: 10.1016/0003-9861(71)90383-3. [DOI] [PubMed] [Google Scholar]
- Aspán A., Huang T. S., Cerenius L., Söderhäll K. cDNA cloning of prophenoloxidase from the freshwater crayfish Pacifastacus leniusculus and its activation. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):939–943. doi: 10.1073/pnas.92.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beintema J. J., Stam W. T., Hazes B., Smidt M. P. Evolution of arthropod hemocyanins and insect storage proteins (hexamerins). Mol Biol Evol. 1994 May;11(3):493–503. doi: 10.1093/oxfordjournals.molbev.a040129. [DOI] [PubMed] [Google Scholar]
- Durrant H. J., Ratcliffe N. A., Hipkin C. R., Aspan A., Söderhäll K. Purification of the pro-phenol oxidase enzyme from haemocytes of the cockroach Blaberus discoidalis. Biochem J. 1993 Jan 1;289(Pt 1):87–91. doi: 10.1042/bj2890087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enghild J. J., Thøgersen I. B., Salvesen G., Fey G. H., Figler N. L., Gonias S. L., Pizzo S. V. Alpha-macroglobulin from Limulus polyphemus exhibits proteinase inhibitory activity and participates in a hemolytic system. Biochemistry. 1990 Oct 30;29(43):10070–10080. doi: 10.1021/bi00495a009. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Okino N., Kawabata S., Iwanaga S., Ohnishi E. Nucleotide sequence of the cDNA encoding the proenzyme of phenol oxidase A1 of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7769–7773. doi: 10.1073/pnas.92.17.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M., Söderhäll K., Sottrup-Jensen L. Amino acid sequence around the thiolester of alpha 2-macroglobulin from plasma of the crayfish, Pacifastacus leniusculus. FEBS Lett. 1989 Aug 28;254(1-2):111–114. doi: 10.1016/0014-5793(89)81019-1. [DOI] [PubMed] [Google Scholar]
- Hara T., Miyoshi T., Tsukamoto T. Comparative studies of larval and pupal phenoloxidase of the housefly, Musca domestica L. Comp Biochem Physiol B. 1993 Oct;106(2):287–292. doi: 10.1016/0305-0491(93)90302-l. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991 Nov;5(14):2902–2909. [PubMed] [Google Scholar]
- Heyneman R. A. Final purification of a latent phenolase with mono- and diphenoloxidase activity from Tenebrio molitor. Biochem Biophys Res Commun. 1965 Oct 26;21(2):162–169. doi: 10.1016/0006-291x(65)90103-8. [DOI] [PubMed] [Google Scholar]
- Kawabata T., Yasuhara Y., Ochiai M., Matsuura S., Ashida M. Molecular cloning of insect pro-phenol oxidase: a copper-containing protein homologous to arthropod hemocyanin. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7774–7778. doi: 10.1073/pnas.92.17.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Pau R. N., Eagles P. A. The isolation of an o-diphenal oxidase from third-instar larvae of the blowfly Calliphora erythrocephala. Biochem J. 1975 Sep;149(3):707–712. doi: 10.1042/bj1490707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts D., Sugumaran M. 1,2-dehydro-N-beta-alanyldopamine as a new intermediate in insect cuticular sclerotization. J Biol Chem. 1994 Sep 2;269(35):22217–22221. [PubMed] [Google Scholar]
- Saul S. J., Dali H., Sugumaran M. Quinone and quinone methide as transient intermediates involved in the side chain hydroxylation of N-acyldopamine derivatives by soluble enzymes from Manduca sexta cuticle. Arch Insect Biochem Physiol. 1991;16(2):123–138. doi: 10.1002/arch.940160205. [DOI] [PubMed] [Google Scholar]
- Saul S. J., Sugumaran M. 4-alkyl-o-quinone/2-hydroxy-p-quinone methide isomerase from the larval hemolymph of Sarcophaga bullata. I. Purification and characterization of enzyme-catalyzed reaction. J Biol Chem. 1990 Oct 5;265(28):16992–16999. [PubMed] [Google Scholar]
- Saul S. J., Sugumaran M. N-acetyldopamine quinone methide/1,2-dehydro-N-acetyl dopamine tautomerase. A new enzyme involved in sclerotization of insect cuticle. FEBS Lett. 1989 Sep 25;255(2):340–344. doi: 10.1016/0014-5793(89)81117-2. [DOI] [PubMed] [Google Scholar]
- Saul S. J., Sugumaran M. o-quinone/quinone methide isomerase: a novel enzyme preventing the destruction of self-matter by phenoloxidase-generated quinones during immune response in insects. FEBS Lett. 1989 Jun 5;249(2):155–158. doi: 10.1016/0014-5793(89)80614-3. [DOI] [PubMed] [Google Scholar]
- Saul S., Sugumaran M. A novel quinone: quinone methide isomerase generates quinone methides in insect cuticle. FEBS Lett. 1988 Sep 12;237(1-2):155–158. doi: 10.1016/0014-5793(88)80191-1. [DOI] [PubMed] [Google Scholar]
- Spycher S. E., Arya S., Isenman D. E., Painter R. H. A functional, thioester-containing alpha 2-macroglobulin homologue isolated from the hemolymph of the American lobster (Homarus americanus). J Biol Chem. 1987 Oct 25;262(30):14606–14611. [PubMed] [Google Scholar]
- Sugumaran M., Giglio L., Kundzicz H., Saul S., Semensi V. Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch Insect Biochem Physiol. 1992;19(4):271–283. doi: 10.1002/arch.940190406. [DOI] [PubMed] [Google Scholar]
- Sugumaran M., Semensi V., Kalyanaraman B., Bruce J. M., Land E. J. Evidence for the formation of a quinone methide during the oxidation of the insect cuticular sclerotizing precursor 1,2-dehydro-N-acetyldopamine. J Biol Chem. 1992 May 25;267(15):10355–10361. [PubMed] [Google Scholar]
- Söderhäll K. Prophenoloxidase activating system and melanization - a recognition mechanism of arthropods? A review. Dev Comp Immunol. 1982 Fall;6(4):601–611. [PubMed] [Google Scholar]
- Thompson D. C., Thompson J. A., Sugumaran M., Moldéus P. Biological and toxicological consequences of quinone methide formation. Chem Biol Interact. 1993 Feb;86(2):129–162. doi: 10.1016/0009-2797(93)90117-h. [DOI] [PubMed] [Google Scholar]
- Thøgersen I. B., Salvesen G., Brucato F. H., Pizzo S. V., Enghild J. J. Purification and characterization of an alpha-macroglobulin proteinase inhibitor from the mollusc Octopus vulgaris. Biochem J. 1992 Jul 15;285(Pt 2):521–527. doi: 10.1042/bj2850521. [DOI] [PMC free article] [PubMed] [Google Scholar]