Abstract
Diabetic retinopathy (DR) is one of the most common complications of diabetes mellitus. Vision loss in DR principally occurs due to breakdown of the blood-retinal barrier (BRB), leading to macular edema, retinal detachment and inner retinal and vitreous hemorrhage. Several growth factors have been shown to play crucial role in the development of these vascular changes; however, the cellular and molecular mechanisms of DR are not yet fully revealed. In the current study we investigated the role of bone morphogenetic protein-2 (BMP2) in DR. We examined the changes in the protein levels of BMP2 in human vitreous and retina in addition to the mouse retina of streptozotocin-induced diabetes. To detect the source of BMP2 during diabetes, human retinal endothelial cells (hRECs) were subjected to high glucose (HG) for 5 days and levels of BMP2 protein were analyzed in conditioned media of these cells relative to control. We also evaluated the effect of BMP2 on the levels of VEGF in cultured rat Müller cells (rMC1). In addition, we tested the pro-inflammatory effects of BMP2 by examining its effect on leukocyte adhesion to cultured hRECs, and levels of adhesion molecules and cytokines production. Finally, the effect of different concentrations of BMP2 on permeability of confluent monolayer of hRECs was evaluated using FITC-Dextran flux permeability assay and by measuring Transcellular Electrical Resistance (TER) using Electric Cell-substrate Impedance Sensing (ECIS).
Our results show, for the first time, the up-regulation of BMP2 in diabetic human and mouse retinas in addition to its detection in vitreous of patients with proliferative DR (72±7 pg/ml). In vitro, hRECs showed upregulation of BMP2 in HG conditions suggesting that these cells are a potential source of BMP2 in diabetic conditions.
Furthermore, BMP2 induced VEGF secretion by Müller cells in-vitro; and showed a dose response in increasing permeability of cultured hRECs. Meanwhile, BMP2 pro-inflammatory effects were recognized by its ability to induce leukocyte adhesion to the hRECs, intercellular adhesion molecule-1 (ICAM-1) and upregulation of interleukin-6 and 8 (IL-6 and IL-8). These results show that BMP2 could be a contributing growth factor to the development of microvascular dysfunction during DR via enhancing both pro-angiogenic and inflammatory pathways. Our findings suggest BMP2 as a potential therapeutic target to prevent/treat DR.
Keywords: Diabetic retinopathy, BMP 2, VEGF, Inflammation, Retinal endothelial cells, Leukocyte adhesion, Blood-retinal barrier, Müller cells
1. Introduction
Diabetic retinopathy (DR) is the most common cause of blindness in persons aged 25-74 years and is a major socioeconomic burden in the United States (Zhang et al., 2010). Vascular injury during DR is characterized by an early stage of inflammatory response, leukostasis, hyperpermeability and capillary degeneration followed by pathological retinal neovascularization (RNV) (Zhang et al., 2011). Vision loss in DR principally occurs due to breakdown of the blood-retinal barrier (BRB), resulting in macular edema, retinal detachment and inner retinal and vitreous hemorrhage (Wilkinson-Berka et al., 2013). Several growth factors have been shown to play crucial role in the development of these vascular changes such as vascular endothelial growth factor (VEGF) (Al-Shabrawey et al., 2011), Angiopoietin (Rangasamy et al., 2011), insulin like growth factor (Smith et al., 1997). However, the underlying cellular and molecular mechanisms of DR are not yet fully elucidated.
Bone Morphogenetic Proteins (BMPs) comprise an extensive group of conserved growth factors of which over 30 members have been identified to date and constitute the largest subgroup of the Transforming Growth Factor Beta (TGFβ) superfamily (Ducy and Karsenty, 2000; Guo and Wu, 2012). BMPs were first detected in extracts of bone with abilities to direct ectopic bone formation (thus the name) (Urist, 1965; Wozney et al., 1988). Nowadays, they are known to be involved in several developmental processes, including critical paracrine roles relevant to vascular physiology and pathology, therefore, several investigators have suggested changing the name to Body Morphogenetic Proteins (Guo and Wu, 2012; Reddi, 2005; Shao et al., 2007; Wagner et al., 2010).
BMP2 could be considered the most investigated and clinically relevant member of the BMPs subgroup. It is a secreted dimeric protein that has been studied extensively in osteogenesis, whether during bone development or repair (Long, 2012; Tsuji et al., 2006). It has been shown that BMP2, 4 and 7 and their receptors (BMPRs) play essential roles during eye development (Du et al., 2010; Dudley and Robertson, 1997). Some investigators suggested that BMP signaling might play a role in retinal vascular homeostasis (Mathura et al., 2000) as well as diabetes induced-vascular dysfunction such as atherosclerosis (Csiszar et al., 2009; Derwall et al., 2012; Pi et al., 2012). The vascular effect of BMP signaling pathway has been correlated to its ability to induce oxidative stress, inflammatory and angiogenic responses (Akeel et al., 2012; Csiszar et al., 2006; Csiszar et al., 2009; Liberman et al., 2011). The relevance of BMPs in angiogenesis is illustrated by the discovery of BMP endothelial cell precursor derived regulator (BMPER), which is an extracellular regulator of BMPs required for proper BMP signaling (Kelley et al., 2009; Moser et al., 2003). Recently, the effect of BMPER has been studied in an oxygen-induced retinopathy (OIR) mouse model highlighting the role of this extracellular regulator of BMPs in modulating BMP signaling at the protein level after angiogenic stimuli (Moreno-Miralles et al., 2011). However, despite these evidences suggesting an important role of BMPs in the induction and/or maintenance of vascular inflammation and angiogenesis in pathological conditions, such as DR, the underlying mechanism still remains relatively unclear. In addition, the role of BMP2 in the pathogenesis of DR has not been yet characterized.
In the current study we investigated the role of BMP2 in DR. We examined the changes in the protein levels of BMP2 in diabetic human vitreous and retina samples, diabetic mouse retinas, in addition to, conditioned media of hRECs treated with HG. We also evaluated the effect of BMP2 on the levels of VEGF in cultured Müller cells. Finally, we assessed the effect of BMP2 on the barrier function of hRECs, on the adhesion of leukocytes to cultured hRECs and the levels of ICAM-1 in the same cells.
2. Materials and Methods
2.1. Human samples
2.1.1. Retina samples
Human retina and retinal sections were obtained from the Cooperative Human Tissue Network Hospital (CHTN) of the University of Pennsylvania and Capital Bioscience (Rockville, MD).
2.1.2. Vitreous samples
Undiluted vitreous fluid samples (0.3 - 0.6 ml) were obtained from 11 patients with proliferative diabetic retinopathy (PDR). The indications for vitrectomy in patients with PDR were traction retinal detachment, and/or non-clearing vitreous hemorrhage. Vitreous samples were collected undiluted by manual suction into a syringe through the aspiration line of vitrectomy, before opening the infusion line. The samples were centrifuged (500 rpm for 10 min, 4°C) and the supernatants were aliquoted and frozen at −80°C until assay. This study was approved and conducted by the Research Centre, College of Medicine, King Saud University; in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki), and informed consents were obtained from all patients.
2.2.Experimental animals
All animal experiments followed the guidelines established by the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Georgia Regents University. C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME), weighing between 20-25 grams were used for the in vivo experiments.
Experimental diabetes was developed in 6-8 weeks old mice by intra-peritoneal injection of 55mg/kg Streptozotocin (STZ) dissolved in sterile water for 3 consecutive days as described previously. Mice were considered diabetic when their plasma glucose level exceeded 250 mg/dL. Diabetic mice (10-12 weeks from the onset of diabetes) and age matched control mice (normal) were sacrificed using carbon dioxide (CO2) inhalation and all efforts were made to minimize suffering. Retinas from both groups were processed for quantification of the levels of BMP2 protein in retinal homogenate and retinal frozen section.
2.3.Cell culture
2.3.1. Human Retinal Endothelial Cells (hRECs)
Cells (hRECs, Cell Systems, Kirkland, Washington) from passages 6-8 were grown on gelatin-coated dishes and maintained in CSC Complete Serum Free Media (Catalog #SF-4Z0-500-R, Cell Systems, Kirkland, Washington) supplemented with 10% Fetal Bovine Serum (FBS, Atlanta Biologicals, Atlanta, GA), 1% Bacc Off® (Ciprofloxacin, Catalog #4Z0-643, Cell Systems, Kirkland, Washington) and 2% recombinant human growth factor (RocketFuel Catalog #SF-4ZR-500-R, Cell Systems, Kirkland, Washington). After the cells were 80-90% confluent, they were serum starved (1% FBS) overnight, then treated with BMP2 (50, 10 and in some experiments 5 ng/ml) or high glucose (HG) (D-Glucose, 30nM). The osmlarity of the control group, in the HG experiment, was adjusted to the same level of HG group using L-Glucose (30 mM). Experiment was terminated after 24 hours of BMP2 treatment for analysis of barrier function and leukocyte adhesion. Cell lysates and conditioned media were used for Western Blot analysis of ICAM1 and multiplex assay of various cytokines respectively. Meanwhile, the HG experiment was terminated 5 days after initiation of treatment and the conditioned media was collected and analyzed for its BMP2 content using ELISA.
2.3.2. Rat Müller cells (rMC1)
These cells (passages 6-8) were a generous gift from Dr/Vijay Sarthy (NorthWestern University, Chicago, Illinois) grown on collagen-coated dishes and maintained in M199 media supplemented with 4.5gm/l glucose, 2 mmol/L L-glutamine, 1% penicillin/streptomycin, and 10% FBS. After the cells formed complete confluent layer, the cells were serum starved (1% FBS) overnight, then treated with BMP2 (10ng/ml). Experiment was terminated after 24 hours and the conditioned media was collected at 12 and 24 hours for analysis of VEGF levels.
2.4.Immuno-histochemical analysis
Retinal paraffin sections of human subjects with or without DR were fixed in 10% neutral buffered formalin (HT50-1-128; Sigma, St Louis, MO). Following rehydration of the paraffin section and two washes in PBS, endogenous peroxidase activity was blocked using hydrogen peroxide diluted 1:10 with distilled water for 10 min. Sections were treated with Proteinase K (S3020; Dako, Carpinteria, CA) for 10 min and washed twice in PBS. All preparations were then treated with universal blocking reagent (HK085-5K; Biogenex, Fremont CA) for 8 min according to the manufacturer’s instructions. Excess reagent was removed with a quick rinse with PBS. Sections were incubated with rabbit polyclonal BMP2 antibody (1:100 dilutions in PBS) for 2 h. Following two washes in PBS, biotinylated anti-rabbit immunoglobulin (HK336-9R; Biogenex, Fremont CA) were added to all slides for 30 min, and then peroxidase-conjugated streptavidin (HK330-9K; Biogenex, Fremont CA) was added for an additional 30 min. Excess reagent was removed, and the slides were washed in PBS and incubated with chromogen (liquid AEC; HK121-5K; Biogenex, Fremont CA) for 10-30 min until the desired color appeared. All preparations were counterstained with hematoxylin (7221; Richard-Allan Scientific, Kalamazoo, MI) for 30 s and mounted in aqueous mounting medium (Faramount aqueous S3025; Dako, Carpinteria, CA).
2.5.Immunofluorescence
The expression of BMP2 in the mouse retina was examined with immuno-fluorescence using BMP2 antibody (Santa Cruz Biotechnology, Dallas, Texas) and isolectin-B4 as a vascular marker (Vector Laboratories, Burlingame, CA). Briefly, frozen retinal sections from normal and diabetic mice were fixed in paraformaldehyde (4%) for 10 min followed by incubation in 3% normal goat serum for 1 h in a humidified container. The sections were then incubated in primary antibody (1:100), isolectin-B4 (15 μg/ml) and NGS (1:50) overnight in a humidified container at 4 °C. In next day the sections were incubated in avidin-conjugated Texas red 25ug/mL (Vector Laboratories, Burlingame, CA) and Oregon green-labeled anti-rabbit antibody (1:500) (Molecular Probes, Eugene, OR). Sections were covered using 4′, 6diamidino-2-phenylindole (DAPI) mounting medium (Vector Laboratories, Burlingame, CA), and images were obtained with confocal microscopy (LSM 510; Carl Zeiss, Thornwood, NY).
2.6.Western blotting (WB)
Western blotting was used to assess the expression BMP2 in human and mouse retinas and ICAM1 in BMP2-treated hRECs cell lysate from different experimental groups. Retinal samples were homogenized in a modified RIPA buffer (20mM Tris-HCl, 2.5 mM ethylenediaminetetraacetic acid, 50mM NaF, 10mM Na4P2O7, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride). Homogenates (30-50 μg protein) and cell lysates were separated by electrophoresis on a precast Tris-HCl 4-20% gradient gel, and transferred to nitrocellulose membrane. Retina homogenates were reacted with primary antibodies against BMP2 (1:200, (Santa Cruz Biotechnology, Dallas, Texas) and hRECs cell lysates were tested for ICAM-1 (1:250, (Santa Cruz Biotechnology, Dallas, Texas). Protein was detected by utilizing horseradish peroxidase-linked secondary antibodies and enhanced chemiluminescence (Amersham, Pittsburgh, PA). Membranes were stripped and re-probed for β-actin 1:2000 (Abcam, Cambridge, MA) to demonstrate equal loading and the results were quantified by densitometry analysis.
2.7.ELISA for BMP2 and VEGF
The quantification of human BMP2 and VEGF in the undiluted human vitreous samples and the conditioned media of BMP2-treated rMC1, respectively, were done using ELISA kits according to the manufacturer’s instruction. All reagents and standards were prepared as directed by the manufacturer (R&D Systems, Minneapolis, MN). First, Assay Diluent was added to each well (100 μl of Assay Diluent RD1-19, 50 μl of Assay Diluent RD1W; for BMP2 and VEGF respectively). Then, the standards and samples were added in each well (50 μl and 200 μl; for BMP2 and VEGF respectively). Plates were covered with adhesive strips and incubated for 2 hours at room temperature. Wells were aspirated and washed by filling each well with Wash Buffer (400 μl). Washing was repeated 3 times. After the last wash, inverting the plate and blotting it against clean paper towels was done to remove any remaining Wash Buffer. Conjugates (Monoclonal antibody specific for BMP-2/VEGF and conjugated to horseradish peroxidase) were then added (200 μl) to each well. The plate was covered with a new adhesive strip and incubated for 2 hours at room temperature. The aspiration/wash step was repeated. Substrate Solution was added (200 μl) to each well and protected from light. Incubation with substrate was done at room temperature (30 and 20 minutes; for BMP2 and VEGF respectively). Finally, 50 μl of Stop Solution was added to each well. The optical density was detected in each sample (well) within 30 minutes, using a microplate reader set to 450 nm and corrected at 540 nm.
2.8 Multiplex Cytokine Assay
For simultaneous quantitation of Interleukin-1β (IL-1β), Interleukin-4 (IL-4), Interleukin-8 (IL-8), Interleukin-6 (IL-6), Granulocyte-colony stimulating factor (G-CSF), interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α), a Bio-Plex human cytokine assay was used according to the manufacture protocol (Bio-rad, Hercules, CA). Briefly, the premixed standards were reconstituted in 0.5 ml of culture medium, generating a stock concentration of 50,000 pg/ml for each cytokine. The standard stock was serially diluted in the same culture medium to generate 8 points for the standard curve. The assay was performed in a 96-well filtration plate supplied with the assay kit. Premixed beads (50 μl) coated with target capture antibodies were transferred to each well of the filter plate (5,000 beads per well per cytokine) and washed twice with Bio-Plex wash buffer. Premixed standards or samples (50 μl) were added to each well containing washed beads. The samples were used directly without further dilution. The plate was shaken for 30 sec and then incubated at room temperature for 30 min with low-speed shaking (300 rpm). After incubation and washing, premixed detection antibodies (50 μl, final concentration of 2 μg/ml) were added to each well. The incubation was terminated after shaking for 10 min at room temperature. After 3 washes, the beads were re-suspended in 125 μl of Bio-Plex assay buffer. Beads were read on the Bio-Plex suspension array system (Bio-rad, Hercules, CA), and the data were analyzed using Bio-Plex Manager™ software version 3.0 (Bio-rad, Hercules, CA).
2.9 In-Vitro Leukocyte adhesion using Myeloperoxidase (MPO) assay
Human leukocytes were purchased from Sanguine Biosceince (Valencia, CA). The viability of the leukocytes were assessed by the trypan blue exclusion test and was always >90%. hRECs were grown to confluence in 6 well plates then were treated with BMP2 (50, 10 and 5 ng/ml) or 10 μg/ml of the endotoxin E. coli lipopolysaccharide (LPS, Sigma Aldrich, St. Louis, MO) for 24 hrs. Following the treatment, leukocytes (300,000-400,000 cells/well) were added to confluent monolayer hRECs and incubated for 90 minutes at 37°C. Immediately before the assay, non-adherent cells were removed by washing three times with RPMI-1640 medium (Life Technology, Carlsbad, CA) before adding 200μl of lysis buffer (1X Triton-X homogenization buffer) to each well then 50μl of homogenates of different groups were transferred to 96-well black wall plate. Myeloperoxidase activity was evaluated using Fluro MPO Myeloperoxidase Detection Kit (Catalog# MPO100-3, Cell Technology, Mountain View, CA) according to manufacturer instructions. Detection of fluorescent MPO fluorescence was performed using a fluorescence plate reader at an excitation wavelength of 540 nm and an emission wavelength of 595 nm. Blanked results were plotted as fold change/control.
2.10 FITC-Dextran flux permeability assay
Assessment of retinal endothelial cell barrier function was done using FITC-Dextran permeability assay as previously described (Monaghan-Benson and Wittchen, 2011; Othman et al., 2013). hRECs were seeded on collagen/fibronectin coated membranes with 0.4 mm pores (Transwell; Corning Costar, Tewksbury, MA), in normal glucose media. After becoming completely confluent cells were shifted to 1% serum media overnight then were treated by 10ng/ml BMP2. VEGF (100 ng/ml) was used as positive control. FITC-dextran (1mg/ml of 70,000 kDa) was then added to the upper chambers followed by obtaining aliquots from the lower and upper chambers at different time points (1, 3 and 5 hours) then measuring the fluorescence intensity with a plate reader. The FITC-dextran that passed across the hRECs monolayer was corrected to the fluorescence reading of samples from the upper chamber.
2.11 Transcellular Electrical Resistance (TER) using Electric Cell-substrate Impedance Sensing (ECIS)
Normalized TER was measured using ECIS in hRECs treated with 50, 10 and 5 ng/ml BMP2 in addition to control and VEGF 50ng/ml (positive control) as previously described (Othman et al., 2013). Briefly, 8W10E+ arrays were used. These arrays were coated with cysteine for 30 minutes then with gelatin for 30 minutes before seeding the hRECs at a density of 105/well in serum starved media. Cells were left undisturbed until fully attached forming a confluent monolayer to arrays indicated by capacitance below 10 Ohm. Different treatment were prepared and added without removing the existing media covering the confluent monolayer of hRECs. The electric currents, passing through the confluent monolayer of hRECs, were measured and recorded independently in each well by the Electrical Cell-Substrate Impedance Sensing (ECIS from Applied Biophysic, Inc., Troy, NY.). TER was measured at current frequency 4000 and recorded over the experimental time course (24 hours). Resistance values for each chamber were normalized as the ratio of measured resistance at each time point (1 hour) to baseline resistance (normalized resistance) and plotted as a function of time.
2.12 Statistical Analysis
Experimental data were collected in triplicate except where noted. The results are expressed as the mean ± SEM. The statistical analysis was performed using Student’s paired t-test or one-way ANOVA with Tukey Kramer post-hoc test for multiple group comparisons. A confidence level of P<0.05 was considered statistically significant.
3. Results
3.1.Diabetes up-regulates retinal BMP2 both in vivo and in vitro
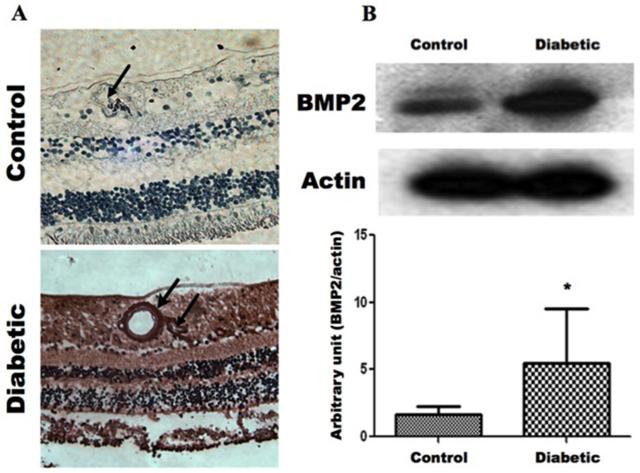
We evaluated the changes in BMP2 levels of retina and vitreous samples of diabetic human subjects in addition to experimental diabetic mice using immunohistochemistry, ELISA and Western blotting. Immunohistochemistry of human retina demonstrated marked increase of the retinal BMP2 in diabetic subjects. There is noticeable increase in the expression of BMP2 in relation to retinal vasculatures (Fig.1A). This finding was confirmed by Western blot analysis of retina samples, which demonstrated a significant increase of BMP2 in retinal homogenate from diabetic subjects (Fig.1B). Moreover, ELISA assessment of BMP2 in the vitreous samples from patients with PDR showed detectable amount of BMP-2 (72 pg/ml ±7).
Figure 1. Increased BMP2 Expression in Human Retina During Diabetic Retinopathy.
A) Immunohistochemistry of BMP2 in normal and diabetic human retina. There is marked increase in the expression of BMP2 in DR compared to the normal retina (Control). BMP2 is increased different layers (brown stain) particularly in relation to retinal vessels (Arrow). B) Western blot analysis of BMP2 in normal and diabetic human retina. Showing up-regulation of BMP2 in retina of diabetic (D) subjects. (*: pValue < 0.05 versus control group, n=4)
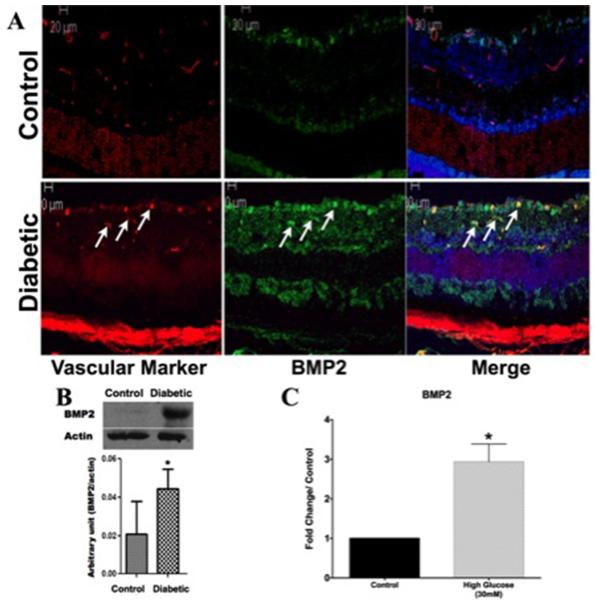
Furthermore, we evaluated the impact of diabetes on retinal levels of BMP2 in experimental diabetic mice. Similar to human retina, we noticed marked increase in the levels of BMP2 in diabetic mice in comparison to the control (Fig. 2A). Immuno-localization in which retinal sections were reacted to vascular marker (Isolectin B4) and BMP2 antibody also showed marked increase in BMP2 immuno-reactivity, which was localized mainly in relation to retinal vessels. These results were supported by Western blot analysis of retina samples, which demonstrated a significant increase of BMP2 in retinal homogenate from diabetic versus control mice (Fig.2B). BMP2 was also upregulated in conditioned media of hRECs treated with HG (30 mM D-glucose) in comparison to the control which was treated by (30mM L-glucose). There was a 2.9 (±0.4) fold increase in HG treated group versus the control (pvalue: 0.02, n=4).
Figure 2. Increased BMP2 expression in Retina of Diabetic mice.
A) Immunofluorescence of BMP2 in normal and diabetic mouse retina. There is marked increase in the expression of BMP2 (Green) in diabetic mouse retina compared to the normal retina (Control). BMP2 is localized mainly in relation to retinal vasculature (Red) (Arrows). B) Western blot analysis of BMP2 in normal and diabetic mice retina showing upregulation of BMP2 in retina of diabetic mice compared to control group (n=5). (C) BMP2 ELISA of conditioned media of high glucose (30mM) treated hRECs for 5 days. Results are plotted as fold change/control and show a significant upregulation of BMP2 as a result of HG (2.9 ±0.4, pvalue: 0.02, n=4) (*:pValue < 0.05 versus control group).
3.2.Effect of BMP2 on leukocyte/endothelial cell interaction
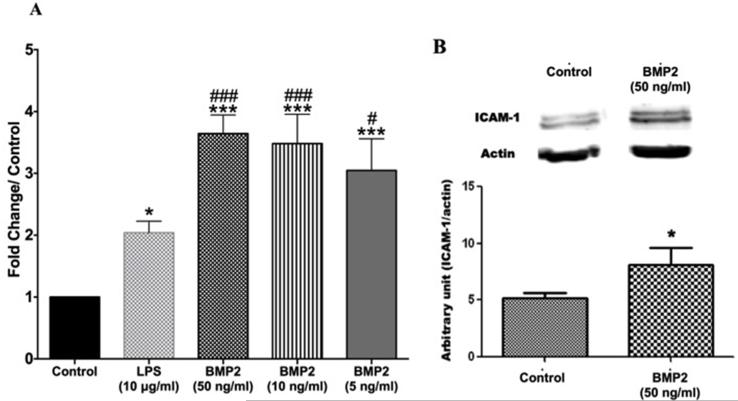
To examine the pro-inflammatory role of BMP2, we assessed its effect on leukocyte adhesion to the hRECs using different concentrations (50, 10 and 5 ng/ml). Our experiment demonstrated significant increases in leukocyte adhesion in BMP2-treated compared to the non-treated cells. Interestingly, the effect of BMP2 was significantly higher than the effect of the pro-inflammatory endotoxin, LPS (Fig 3A). Although our experiment demonstrated no significant difference between the different concentrations of BMP2, we noticed a dose response patter for the effect of BMP2 on leukostasis in which the 50ng/ml elicited the highest effect and the 5ng/ml the lowest effect (MPO activity fold change/control was 2.1 ±0.1, 3.6 ±0.1, 3.5 ±2, 3.0 ±2 in LPS, BMP2 50ng/ml, 10ng/ml and 5ng/ml respectively). In addition, BMP2-induced leukostasis was associated with a significant up-regulation of the adhesion molecule ICAM-1 in hRECs (Fig.3B).
Figure 3. BMP2 effect on leukocyte adhesion to hRECs and expression of adhesion molecule ICAM-1.
(A) Bar graph representing fold change/control of myeloperoxidase activity (indicator of leukocyte adhesion) showing that the used BMP2 concentrations (50, 10 and the 5ng/ml) showed increased leukocyte adhesion to hRECs. These effects were significantly higher than both control and to LPS (10μg/ml) (n=4). (B) Western blot of ICAM-1 in hRECs treated with BMP2 showing up-regulation of ICAM-1 hRECs treated with BMP2 (50ng/ml) versus control group (n=3). (*: pvalue< 0.05 versus control, ***: pvalue< 0.001 versus control, ###: pvalue< 0.001 versus LPS, #: pvalue< 0.05 versus LPS).
3.3. Effect of BMP2 on cytokines levels in hRECS
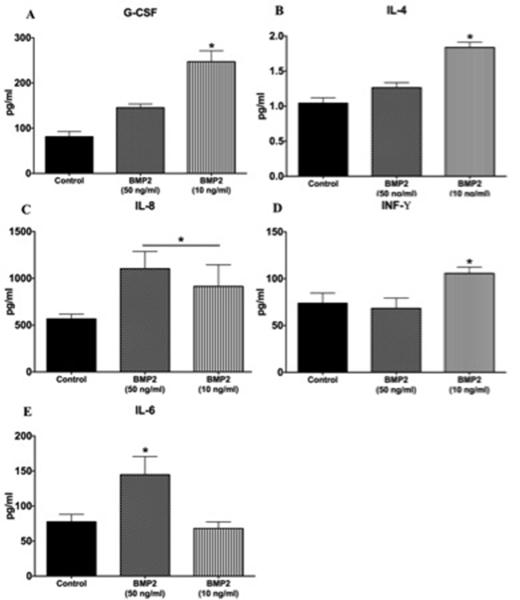
To further investigate the pro-inflammatory effect of BMP2, we tested the effect of BMP2 on cytokine production by hRECs. The analyzed cytokines included some pro-inflammatory cytokines involved in the pathogenesis of DR (IL-8, INF-γ, IL-1β, TNF-α and IL-6) and others with protective anti-inflammatory effects (G-CSF and IL4). Two of the tested cytokines (IL-1β and TNF-α) were not detected in the tested samples upon stimulation of hRECs by both BMP2 concentrations. However, BMP2 at lower concentration (10 ng/ml) induced both anti-and pro-inflammatory cytokine productions by hRECs. G-CSF and IL-4 were significantly higher than both the control and the 50ng/ml groups (G-CSF: 81.2 ±5.7 pg/ml, 247.2 ±24.2 pg/ml and 145.3 ±4.8 pg/ml for control group, BMP2 10ng/ml and 50 ng/ml respectively; IL-4: 1.0 ±0.08 pg/ml, 1.8 ±0.07 pg/ml and 1.2 ±0.07pg/ml for control group, BMP2 10ng/ml and 50 ng/ml respectively) (Figure 4A and B). On the other hand, pro-inflammatory cytokines were also induced by the same concentration of BMP2 (10ng/ml) including IL-8 and INF-γ (IL-8: 568 ±49.7 pg/ml, 1139 ±65 pg/ml and 1104 ±183 pg/ml for control group, BMP2 10ng/ml and 50 ng/ml respectively; INF-γ: 73.8 ±5.4 pg/ml, 105.6 ±6.7 pg/ml and 68.3 ±6.3 pg/ml for control group, BMP2 10ng/ml and 50 ng/ml respectively) (Figure 4C and D). On the other hand, BMP2 at higher concentration (50ng/ml) did not show significant changes in the levels of anti-inflammatory cytokines (G-CSF or IL-4) and induced significant increase in the production of the pro-inflammatory cytokines, IL-8 (Figure 4C) and IL-6 (IL-6: 77.6 ±5.3 pg/ml, 68.1 ±9.3 pg/ml and 144.7 ±15 pg/ml for control group, BMP2 10ng/ml and 50 ng/ml respectively) (Figure 4E).
Figure 4. Multiplex results of.
(A) G-CSF, (B) IL-4, (C) IL-8, (D) INF-γ and (E) IL-6 measured in conditioned media of hRECs treated with 10 and 50 ng/ml BMP2. (*: pValue < 0.01 versus other groups, n=4).
3.4.Effect of BMP2 on VEGF expression in rat Müller cells
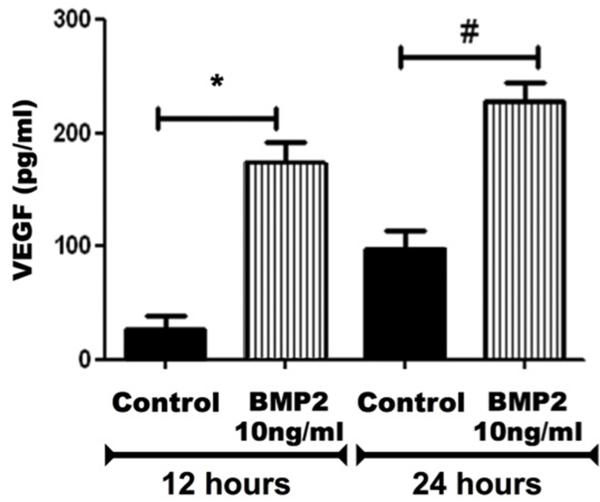
Since VEGF is a crucial factor in mediating the retinal vascular injury during DR, we examined whether BMP2 impacts VEGF expression in cultured Müller cells, the main source of retinal VEGF (Reichenbach and Bringmann, 2013). ELISA assay was performed to assess the levels of VEGF in rMC1-conditioned media collected after 12 and 24 hours from the beginning of the treatment. VEGF was significantly increased in BMP2-treated rMC1 in comparison to the control (174 ±31 pg/ml versus 28 ±20 pg/ml after 12 hours and 229 ±29 pg/ml versus 97 ±29 pg/ml after 24 hours) (Fig.5).
Figure 5. ELISA of VEGF expression in cultured rat Müller cells.
The levels of VEGF were evaluated after 12 and 24 hours from the beginning of BMP2 (10ng/ml) treatment. (*: pValue < 0.05 versus control group, n=5).
3.5.Effect of BMP2 on retinal endothelial cell permeability
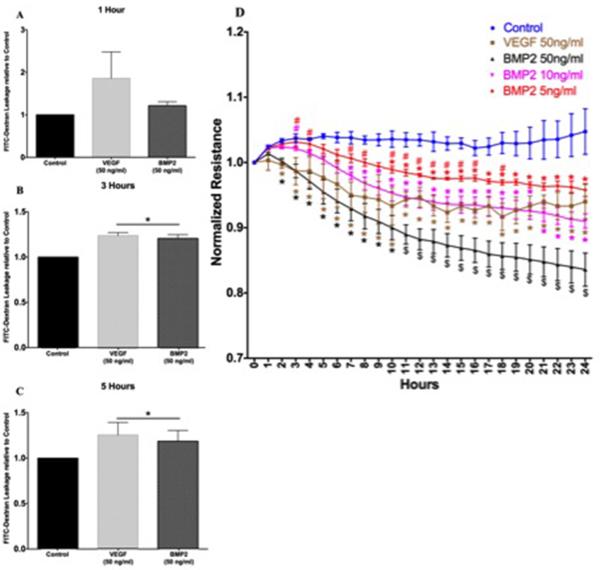
One of the early features of DR is breakdown of blood retinal barrier and vascular hyper-permeability, which has been shown to be VEGF-mediated process. Therefore, we evaluated whether BMP2 has any effect on retinal endothelial barrier function. We assessed hRECs permeability using FITC-Dextran flux assay at different time points, (1, 3 and 5 hours). Interestingly BMP2 induced similar effect to VEGF on the REC barrier. There were no significant changes in the leakage of FITC-dextran by either BMP2 or VEGF after one hour (Figure 6A). Both BMP2 and VEGF demonstrated 1.2 and 1.5 fold increase in the leakage of FITC-dextran after 3 and 5 hours respectively (Fig.6B and C).
Figure 6. Effect of BMP2 on retinal endothelial cell barrier function.
FITC-Dextran leakage relative to control at 1 hour (A), 3 hours (B) and 5 hours (C) time points. Both BMP2 and VEGF demonstrated 1.2 and 1.5 fold increase in the leakage of FITC-dextran after 3 and 5 hours respectively. (D) Normalized TER resistance measured using ECIS: showing a dose response effect of BMP2 on hRECs normalized resistance where the significant decrease in resistance versus control started at 2 Hours, 7 Hours and 10 Hours time points for the 50, 10 and 5ng/ml BMP2 respectively (*: pValue < 0.05 versus control group, #: pValue < 0.05 versus VEGF group, $: pValue < 0.05 versus all other groups; group n=3).
We also tested the impact of different concentrations of BMP2 (50, 10 and 5 ng/ml) on the hRECs barrier function by measuring the changes in TER using ECIS (Fig.6D). The 50ng/ml BMP2 concentration induced a significant decrease in TER compared to the control starting from the 2nd hour after initiation of the treatment; similar to the effect of VEGF (50ng/ml). However, this concentration of BMP2 sustained the decrease in TER during the whole course of the experiment (24 hrs) and showed more decrease in the TER compared to the effect of the VEGF starting from the 11th hour. Lower concentrations of BMP2 (10 and 5ng/ml) also induced significant decrease in the TER which was comparable to the effect of VEGF. However, their effect was delayed several hours than the effect of the higher concentration of BMP2 (50ng/ml). Furthermore, there was concentration dependent effect of BMP2 on the TER as shown in Figure 6D.
4. Discussion
To the best of our knowledge, this is the first report of the up-regulation of BMP2 in retinas of diabetic human subjects and experimental mice. In addition, BMP2 was detected in the vitreous fluid of patients with inactive and active proliferative DR. The increase in the levels of BMP2 in diabetic retina suggests a potential role in the pathogenesis of DR. This notion is supported by the retinal endothelial hyperpermeability effect and enhanced secretion of VEGF from Müller cells by BMP2 treatment. In addition, the pro-inflammatory effect of BMP2 was established also in-vitro, by its ability to induce leukocyte adhesion to hRECs with upregulation of the adhesion molecule ICAM-1 in these cells and the production of pro-inflammatory cytokines. Taken together, our in vivo and in vitro data suggest that BMP2 contributes to the pathogenesis of microvascular dysfunction during DR probably through enhanced secretion of VEGF from Müller cells and activating retinal endothelial cells as evidenced by increased leukocyte adhesion and hyperpermeability.
The number of people with diabetes mellitus in the United States is expected to triple by 2050, indicating a major health problem in the coming decades. Thus, management of complications due to diabetes is a growing source of health care expenses. Meanwhile, despite the advances in the field of metabolic control of diabetic patients, the prevalence of diabetic retinopathy (DR) remains as high as 40% (Bandello et al., 2013; Heng et al., 2013). These facts reflect the significance of better understanding the pathogenesis of DR to offer new treatment options to avoid one of the most common complication of DM (Stitt et al., 2013).
Hyperglycemia is considered the main factor implicated in the structural and functional changes related to DR (Bandello et al., 2013). In our study, the consistent association of BMP2 with retinal blood vessels suggests that retinal ECs are the source of BMP2 within the retina and vitreous fluid of diabetic patients and experimental mice. ECs have been known as a source of BMP2 in different experimental models (Bouletreau et al., 2002; Csiszar et al., 2005; Hussein et al., 2012; Matsubara et al., 2012). The fact that hyperglycemia can induce microvascular ECs to secrete BMP2 has been suggested by Zhang et al (Zhang et al., 2008). They reported that high glucose induced the transcription of BMP2 in human umbilical vein endothelial cells HUVECs in-vitro. Of note, our in vivo and in vitro results strongly support these findings.
Vascular Endothelial Growth Factor (VEGF) has always been regarded as a key player in the pathogenesis and progression of DR due to its potent pro-angiogenic and pro-inflammatory effects (Bandello et al., 2013; Reichenbach and Bringmann, 2013; Wang et al., 2010). Müller cells are considered one of the major sources of VEGF in the retina (Bandello et al., 2013; Reichenbach and Bringmann, 2013; Wang et al., 2010). In our study, we tested the effect of BMP2 on the VEGF production by Müller cells. At both time points, the used BMP2 concentration (10 ng/ml) induced a significant up-regulation of VEGF in Müller cells. We previously reported the same effect of BMP2 on VEGF production in pre-osteoblasts (Akeel et al., 2012). VEGF has been described as a mediator of neo-vascularization in ischemic retinal diseases such as diabetic retinopathy (Aiello et al., 1994; Zhang et al., 2009). Furthermore, strong correlation exists between increases in intraocular VEGF concentration and the development of PDR (Goto et al., 2008). In addition to its angiogenic effects, VEGF is considered a predominant mediator of vascular permeability (Bandello et al., 2013; Zhang et al., 2009). The pro-inflammatory effect of VEGF has been previously confirmed by its ability to induce conformational changes in the tight junctions of endothelial cells and is associated with the up-regulation of ICAM-1, an endothelial adhesion molecule, leading to further endothelial cell damage and leukostasis (Wang et al., 2010). Thus BMP2 might have indirect pro-angiogenic and pro-inflammatory effects through the induction of VEGF secretion by Müller cells. An interesting fact is worth mentioning here is that VEGF is also known to induce BMP2 production in microvascular endothelial cells (Bouletreau et al., 2002). In addition to its indirect effect on angiogenesis (through induction of secretion of VEGF), BMP2 is known to have direct effects on microvascular ECs (Langenfeld and Langenfeld, 2004). That’s why we tested the effect of BMP2 on hRECs. Our in-vitro permeability results showed a dose response effect of BMP2 on hRECs barrier dysfunction as evidenced by the stronger and sustained effect of BMP2 50ng/ml throughout the course of experiment in comparison to the 5 and 10 ng/ml concentrations. These findings suggest that BMP2 might contribute to vascular permeability through both paracrine and autocrine effects by regulating VEGF expression in Müller cells and impacting the hRECs barrier, respectively.
To evaluate the direct pro-inflammatory effects of BMP2 on hRECs, we evaluated its effect on leukocyte adhesion to retinal endothelial cells. The previously mentioned hyperpermeability effect of BMP2 was found to be coupled with a dose dependent increase in leukocyte adhesion that was higher than the effect of the inflammatory endotoxin, LPS. In addition, the 50ng/ml BMP2 induced an up-regulation of ICAM-1 adhesion molecule. Of note, we previously linked the pathogenesis of vascular injury during DR to ECs/leukocyte interaction (Al-Shabrawey et al., 2008). Our new results, where BMP2 is shown to directly increase both hyperpermeability and leukocyte adhesion in retinal ECs, add a significant threat on the BRB integrity; the driving force leading to macular edema (Bandello et al., 2013; Wang et al., 2010).
To further confirm the pro-inflammatory effect of BMP2 on hRECs, we tested the effect of two BMP2 concentrations (10 and 50ng/ml) on cytokines production by these cells. Although both concentrations induced significant increase in leukocyte adhesion to hRECs, there was a differential effect on the levels of different cytokines. While the 10ng/ml BMP2 treatment showed a significant increase in INF-γ, the IL-6 was the main cytokine induced by the 50 ng/ml. In addition, both concentrations showed a similar increase in IL-8 production. In patients with PDR, IFN-γ is considered an inflammatory cytokine with an indirect angiogenic effect through the activation of VEGF (Paine et al., 2012a). IL-8 is known to be a pro-inflammatory cytokine that positively regulates angiogenesis (Juel et al., 2012; Paine et al., 2012a). IL-6 is known to be one of the potent pro-inflammatory cytokines involved in the pathogenesis of DR by mediating retinal VEGF expression, leukostasis, and vascular remodeling (Gustavsson et al., 2013; Rojas et al., 2010). This cytokine is particularly important in accelerating BRB breakdown and retinal vascular endothelial dysfunction (Paine et al., 2012b).
During inflammation, anti-inflammatory cytokines are usually produced in an attempt to modulate the inflammatory process (Cilensek et al., 2011). The disturbance in balance between pro-inflammatory and anti-inflammatory mechanisms will lead to destructive changes within the retina like that seen in DR (Juel et al., 2012). In our experiment, although both concentrations induced pro-inflammatory cytokine production, interestingly, the 10ng/ml induced also anti-inflammatory protective responses, including, G-CSF and IL-4. G-CSF is known, not only, to have a neuro-protective effect on retinal photoreceptor cells (Oishi et al., 2008), but also, it has an anti-apoptotic effect on hRECs in-vitro and thus protected the retina from oxidative stress-induced damage (Kojima et al., 2011). In addition, IL-4 is an anti-inflammatory cytokine that inhibits the production of pro-inflammatory cytokines (Cilensek et al., 2011). In contrast, when hRECs were stimulated by a higher BMP2 concentration (50ng/ml) only pro-inflammatory cytokines were triggered with no effect on the tested anti-inflammatory cytokines (G-CSF and IL-4).
In addition to the cytokines induced by different BMP2 concentrations, IL-1β and TNF-α were not detected in our study. IL-1β and TNF-α are two of the main cytokines involved in the pathogenesis of DR and are known to be present in the vitreous and serum of patients with PDR and PVR (Demircan et al., 2006; Gustavsson et al., 2013). Both were shown to be produced by the resident inflammatory cells within the retina; microglial cells (Juel et al., 2012; Kohno et al., 2013).
The mechanism by which hyperglycemia stimulates hRECs to secrete BMP2 and the mechanism by which BMP2 activates hRECs and induce DR phenotype as well as the crosstalk between the BMP2/SMAD signaling system and VEGF signaling pathway are attractive areas for our future research directions. However, taken together, our observations suggest that BMP2 contributes to vascular injury during DR through direct activation of retinal endothelial cells and via indirect pathway that involves VEGF secretion from Müller cells. These findings suggest that BMP2 could be a potential therapeutic target to prevent the development and/or progression of microvascular dysfunction associated with DR. However, further investigations are required to dissect the exact mechanism of BMP2 regulation and function in DR.
Highlights.
BMP2 is upregulated in diabetic human retinas and retinas of diabetic mice.
Retinal endothelial cells are a source of BMP2 in response to high glucose treatment.
BMP2 induces hyper-permeability, leukocyte adhesion and pro-inflammatory cytokines in human retinal endothelial cells
BMP2 regulates VEGF expression in Muller cells
These findings suggest that BMP2 could be a potential therapeutic target to prevent the development and/or progression of microvascular dysfunction associated with DR
Acknowledgment
This study was funded by the National Eye Institute (1R01EY023315-01) and Qatar National Research Fund (NPRP 4-1046-3-284) to the corresponding author. We also thank Dr.Vijay Sarthy, Northwestern University for providing us with the rMC1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. VASCULAR ENDOTHELIAL GROWTH-FACTOR IN OCULAR FLUID OF PATIENTS WITH DIABETIC-RETINOPATHY AND OTHER RETINAL DISORDERS. New England Journal of Medicine. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Akeel S, El-awady A, Hussein K, El-Refaey M, Elsalanty M, Sharawy M, Al-Shabrawey M. Recombinant bone morphogenetic protein-2 induces upregulation of vascular endothelial growth factor and interleukin 6 in human preosteoblasts: Role of reactive oxygen species. Archives of Oral Biology. 2012;57:445–452. doi: 10.1016/j.archoralbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY, Gurel Z, Sheibani N, Maddipati KR. Increased Expression and Activity of 12-Lipoxygenase in Oxygen-Induced Ischemic Retinopathy and Proliferative Diabetic Retinopathy Implications in Retinal Neovascularization. Diabetes. 2011;60:614–624. doi: 10.2337/db10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Investigative Ophthalmology & Visual Science. 2008;49:3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandello F, Lattanzio R, Zucchiatti I, Del Turco C. Pathophysiology and treatment of diabetic retinopathy. Acta Diabetologica. 2013;50:1–20. doi: 10.1007/s00592-012-0449-3. [DOI] [PubMed] [Google Scholar]
- Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Implications for fracture healing. Plastic and Reconstructive Surgery. 2002;109:2384–2397. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- Cilensek I, Hercegovac A, Starcevic JN, Vukojevic K, Babic MS, Zivin AM. Polymorphisms of interleukin-4,-10 and 12B genes and diabetic retinopathy. Central European Journal of Biology. 2011;6:558–564. [Google Scholar]
- Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao O, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. American Journal of Pathology. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Lehoux S, Ungvari Z. Hemodynamic Forces, Vascular Oxidative Stress, and Regulation of BMP-2/4 Expression. Antioxidants & Redox Signaling. 2009;11:1683–1697. doi: 10.1089/ars.2008.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells - Role of nuclear factor-kappa B activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (London, England) 2006;20:1366–1369. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of Bone Morphogenetic Protein Signaling Reduces Vascular Calcification and Atherosclerosis. Arteriosclerosis Thrombosis and Vascular Biology. 2012;32:613–U168. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Xiao Q, Yip HK. Regulation of Retinal Progenitor Cell Differentiation by Bone Morphogenetic Protein 4 Is Mediated by the Smad/Id Cascade. Investigative Ophthalmology & Visual Science. 2010;51:3764–3773. doi: 10.1167/iovs.09-4906. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney International. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Developmental Dynamics. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Goto H, Nishikawa T, Sonoda K, Kondo T, Kukidome D, Fujisawa K, Yamashiro T, Motoshima H, Matsumura T, Tsuruzoe K, Araki E. Endothelial MnSOD overexpression prevents retinal VEGF expression in diabetic mice. Biochemical and Biophysical Research Communications. 2008;366:814–820. doi: 10.1016/j.bbrc.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine & Growth Factor Reviews. 2012;23:61–67. doi: 10.1016/j.cytogfr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Gustavsson C, Agardh C-D, Agardh E. Profile of intraocular tumour necrosis factor- and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmologica. 2013;91:445–452. doi: 10.1111/j.1755-3768.2012.02430.x. [DOI] [PubMed] [Google Scholar]
- Heng LZ, Comyn O, Peto T, Tadros C, Ng E, Sivaprasad S, Hykin PG. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetic Medicine. 2013;30:640–650. doi: 10.1111/dme.12089. [DOI] [PubMed] [Google Scholar]
- Hussein KA, Zakhary IE, Elawady AR, Emam HA, Sharawy M, Baban B, Akeel S, Al-Shabrawey M, Elsalanty ME. Difference in Soft Tissue Response Between Immediate and Delayed Delivery Suggests a New Mechanism for Recombinant Human Bone Morphogenetic Protein 2 Action in Large Segmental Bone Defects. Tissue Engineering Part A. 2012;18:665–675. doi: 10.1089/ten.TEA.2011.0148. [DOI] [PubMed] [Google Scholar]
- Juel HB, Faber C, Udsen MS, Folkersen L, Nissen MH. Chemokine expression in retinal pigment epithelial ARPE-19 cells in response to coculture with activated T cells. Invest Ophthalmol Vis Sci. 2012;53:8472–8480. doi: 10.1167/iovs.12-9963. [DOI] [PubMed] [Google Scholar]
- Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. Journal of Cell Biology. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H, Chen Y, Kevany BM, Pearlman E, Miyagi M, Maeda T, Palczewski K, Maeda A. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. The Journal of biological chemistry. 2013;288:15326–15341. doi: 10.1074/jbc.M112.448712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Otani A, Oishi A, Makiyama Y, Nakagawa S, Yoshimura N. Granulocyte colony-stimulating factor attenuates oxidative stress-induced apoptosis in vascular endothelial cells and exhibits functional and morphologic protective effect in oxygen-induced retinopathy. Blood. 2011;117:1091–1100. doi: 10.1182/blood-2010-05-286963. [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Langenfeld J. Bone Morphogenetic Protein-2 stimulates angiogenesis in developing tumors. Molecular Cancer Research. 2004;2:141–149. [PubMed] [Google Scholar]
- Liberman M, Johnson RC, Handy DE, Loscalzo J, Leopold JA. Bone morphogenetic protein-2 activates NADPH oxidase to increase endoplasmic reticulum stress and human coronary artery smooth muscle cell calcification. Biochemical and Biophysical Research Communications. 2011;413:436–441. doi: 10.1016/j.bbrc.2011.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nature Reviews Molecular Cell Biology. 2012;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- Mathura JR, Jafari N, Chang JT, Hackett SF, Wahlin KJ, Della NG, Okamoto N, Zack DJ, Campochiaro PA. Bone morphogenetic proteins-2 and-4: Negative growth regulators in adult retinal pigmented epithelium. Investigative Ophthalmology & Visual Science. 2000;41:592–600. [PubMed] [Google Scholar]
- Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51:168–180. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan-Benson E, Wittchen ES. In vitro analyses of endothelial cell permeability. Methods in molecular biology (Clifton, N.J.) 2011;763:281–290. doi: 10.1007/978-1-61779-191-8_19. [DOI] [PubMed] [Google Scholar]
- Moreno-Miralles I, Ren R, Moser M, Hartnett ME, Patterson C. Bone Morphogenetic Protein Endothelial Cell Precursor-Derived Regulator Regulates Retinal Angiogenesis In Vivo in a Mouse Model of Oxygen-Induced Retinopathy. Arteriosclerosis Thrombosis and Vascular Biology. 2011;31:2216–U2166. doi: 10.1161/ATVBAHA.111.230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu YX, Aitsebaomo J, Ren RQ, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Molecular and Cellular Biology. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi A, Otani A, Sasahara M, Kojima H, Nakamura H, Yodoi Y, Yoshimura N. Granulocyte Colony-Stimulating Factor Protects Retinal Photoreceptor Cells against Light-Induced Damage. Investigative Ophthalmology & Visual Science. 2008;49:5629–5635. doi: 10.1167/iovs.08-1711. [DOI] [PubMed] [Google Scholar]
- Othman A, Ahmad S, Megyerdi S, Mussell R, Choksi K, Maddipati KR, Elmarakby A, Rizk N, Al-Shabrawey M. 12/15-Lipoxygenase-Derived Lipid Metabolites Induce Retinal Endothelial Cell Barrier Dysfunction: Contribution of NADPH Oxidase. Plos One. 2013:8. doi: 10.1371/journal.pone.0057254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine SK, Basu A, Mondal LK, Sen A, Choudhuri S, Chowdhury IH, Saha A, Bhadhuri G, Mukherjee A, Bhattacharya B. Association of vascular endothelial growth factor, transforming growth factor beta, and interferon gamma gene polymorphisms with proliferative diabetic retinopathy in patients with type 2 diabetes. Molecular Vision. 2012a;18:2749–2757. [PMC free article] [PubMed] [Google Scholar]
- Paine SK, Sen A, Choudhuri S, Mondal LK, Chowdhury IH, Basu A, Mukherjee A, Bhattacharya B. ASSOCIATION OF TUMOR NECROSIS FACTOR alpha, INTERLEUKIN 6, AND INTERLEUKIN 10 PROMOTER POLYMORPHISM WITH PROLIFERATIVE DIABETIC RETINOPATHY IN TYPE 2 DIABETIC SUBJECTS. Retinathe Journal of Retinal and Vitreous Diseases. 2012b;32:1197–1203. doi: 10.1097/IAE.0b013e31822f55f3. [DOI] [PubMed] [Google Scholar]
- Pi X, Lockyer P, Dyer LA, Schisler JC, Russell B, Carey S, Sweet DT, Chen Z, Tzima E, Willis MS, Homeister JW, Moser M, Patterson C. Bmper Inhibits Endothelial Expression of Inflammatory Adhesion Molecules and Protects Against Atherosclerosis. Arteriosclerosis Thrombosis and Vascular Biology. 2012;32:2214–+. doi: 10.1161/ATVBAHA.112.252015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A Potential Role for Angiopoietin 2 in the Regulation of the Blood-Retinal Barrier in Diabetic Retinopathy. Investigative Ophthalmology & Visual Science. 2011;52:3784–3791. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi AH. BMPs: From bone morphogenetic proteins to body morphogenetic proteins. Cytokine & Growth Factor Reviews. 2005;16:249–250. doi: 10.1016/j.cytogfr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- Rojas M, Zhang W, Lee DL, Romero MJ, Nguyen DT, Al-Shabrawey M, Tsai N-T, Liou GI, Brands MW, Caldwell RW, Caldwell RB. Role of IL-6 in Angiotensin II-Induced Retinal Vascular Inflammation. Investigative Ophthalmology & Visual Science. 2010;51:1709–1718. doi: 10.1167/iovs.09-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JS, Al Aly Z, Lai CF, Cai S, Huang E, Behrmann A, Towler DA. Vascular Bmp-Msx2-Wnt signaling and oxidative stress in arterial calcification. In: Zaidi M, editor. Skeletal Biology and Medicine, Pt B: Disease Mechanisms and Therapeutic Challenges. 2007. pp. 40–50. [DOI] [PubMed] [Google Scholar]
- Smith LEH, Kopchick JJ, Chen W, Knapp J, Kinose F, Daley D, Foley E, Smith RG, Schaeffer JM. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clinical Science. 2013;125:1–17. doi: 10.1042/CS20120588. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nature Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science (New York, N.Y.) 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Wagner DO, Sieber C, Bhushan R, Boergermann JH, Graf D, Knaus P. BMPs: From Bone to Body Morphogenetic Proteins. Science Signaling. 2010:3. doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu X, Elliott MH, Zhu M, Le Y-Z. Müller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Rana I, Armani R, Agrotis A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clinical Science. 2013;124:597–615. doi: 10.1042/CS20120212. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. NOVEL REGULATORS OF BONE-FORMATION - MOLECULAR CLONES AND ACTIVITIES. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhou S.-h., Zhao S, Li X.-p., Liu L-P, Shen X-Q. Pioglitazone can downregulate bone morphogenetic protein-2 expression induced by high glucose in human umbilical vein endothelial cells. Pharmacology. 2008;81:312–316. doi: 10.1159/000119118. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. Journal of cardiovascular disease research. 2011;2:96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao S, Hambly BD, Gillies MC. Vascular endothelial growth factor-A: A multifunctional molecular player in diabetic retinopathy. International Journal of Biochemistry & Cell Biology. 2009;41:2368–2371. doi: 10.1016/j.biocel.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Saaddine JB, Chou C-F, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BEK, Klein R. Prevalence of Diabetic Retinopathy in the United States, 2005-2008. Jama-Journal of the American Medical Association. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]