Abstract
Cancer is a multistep process resulting in uncontrolled cell division. It results from aberrant signaling pathways that lead to uninhibited cell division and growth. Various recent epidemiological studies have indicated that consumption of cruciferous vegetables such as garden cress, broccoli, etc., reduces the risk of cancer. Isothiocyanates (ITC) have been identified as major active constituents of cruciferous vegetables. ITCs occur in plants as glucosinolate and can readily be derived by hydrolysis. Numerous mechanistic studies have demonstrated the anti-cancer effects of ITCs in various cancer types. ITCs suppress tumor growth by generating reactive oxygen species or by inducing cycle arrest leading to apoptosis. Based on the exciting outcomes of pre-clinical studies, few ITCs have advanced to the clinical phase. Available data from pre-clinical as well as available clinical studies suggests ITCs to be one of the promising anti-cancer agents available from natural sources. This is an up-to-date exhaustive review on the preventive and therapeutic effects of ITCs in cancer.
Keywords: BITC, PEITC, Sulforaphane, AITC, Isothiocyanate, cancer
1. Introduction
Cancer is the leading cause of deaths worldwide, accounting for 7.6 million deaths according to recent statistics. The number of deaths due to cancer is projected to increase to 13.1 million in 2030. These figures implicate marginal efficacy of present standard available therapies to cancer patients, implying the urgent need to identify new strategies/agents that can be included in cancer preventive or therapeutic regimen.
Historical evidence purports nature being a prodigious source of many drugs and drug leads for various ailments, including cancer [1]. Several epidemiological studies have been published over the past few decades that indicate a strong correlation between intake of fruits & vegetables and reduced risk of cancer [2–4]. Basic benefits of using bioactive dietary agents are low cost, well known applications in traditional medicinal system, accessibility and minimal or no toxicity.
Epidemiological and case-control studies continue to support the notion that consumption of cruciferous vegetables reduces the risk of developing various types of cancers such as pancreatic, prostate, ovarian and breast [5–11]. Isothiocyanates (ITCs) occur in cruciferous vegetables as glucosinolates and are converted to ITCs by the action of the enzyme myrosinase. ITCs from these vegetables are also released by cutting or chewing or by intestinal micro flora present in humans [12] (Figure 1). ITCs have been shown to have substantial chemopreventive activity against various human malignancies [13, 14]. Some of the widely studied ITCs that have potent anti-cancer effects are Allyl isothiocyanate (AITC), Benzylisothiocyanate (BITC), Phenethylisothiocyanate (PEITC) and Sulforaphane (SFN). Unless stated, most of the studies mentioned in this article used 95–98% pure ITCs for evaluating anti-cancer effects. This exhaustive review highlights the specificity of ITCs against various targets in cancer.
Figure 1.
Mechanism of cellular uptake of ITCs
2. Chemoprevention by ITCs
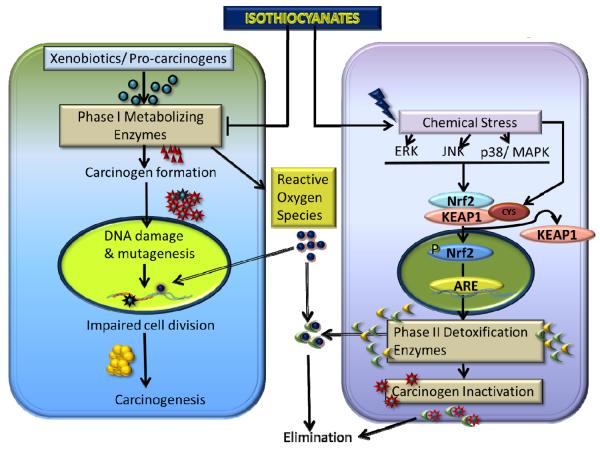
An individual's susceptibility to cancer is determined by numerous factors including maintenance of a critical balance between phase I and phase II enzymes. Phase I primarily consists of cytochrome P450 enzymes, which play an important role in metabolizing the xenobiotics and carcinogens. However, in this process, several chemicals or pro-carcinogens are activated or converted into highly reactive electrophilic metabolites. The generated electrophiles can disturb the genomic stability by causing DNA damage. Chemopreventive effects of ITCs are exerted by inhibition of the bio-activation of carcinogens by phase I drug metabolizing enzymes [15–18]. The mechanistic studies by Morse et. al. and others indicate that administration of ITCs prevents the tumor promoting effects of various chemical carcinogens in different animal models [19–21]. PEITC has been shown to possess significant chemopreventive properties against tobacco-induced carcinogens in rodent models of lung and esophageal cancers [22, 23]. AITC also inhibits NNK (a tobacco derived carcinogen) induced tumors in rats [24]. Similar to other ITCs, AITC induces phase II detoxifying enzymes quinone reductase and glutathione S-transferase in the animal tissues [25]. Cytochrome P450E1 and N-dimethylnitrosoamine demethylase (NDMAd) are major enzymes that cause bio-activation of tobacco specific nitrosoamines. These enzymes can be inhibited by glutathione conjugates of ITCs [26]. ITCs also inhibit various isoforms of CYP450 directly; for example BITC suppresses cytochrome P450 2E1 while sulforaphane inhibits cytochrome P450 1A2 [27, 28]. Sulforaphane also has been shown to inhibit steroid and xenobiotic receptor (SXR), a nuclear hormone receptor that regulates expression of CYP3A4 [29]. Zhou et. al. showed specific antagonism by sulforaphane to inhibit drug clearance due to SXR-induced activity of CYP3A4.
Phase II enzymes like glutathione-S-transferase (GST), NADPH quinine oxidoreductase and UDP-glucuronosyltransferases play an important role in detoxifying carcinogens as well as xenobiotics. ITCs are known to induce phase II enzymes, which further explain the cancer chemo-preventive activity of ITCs [28, 30–34]. GST catalyzes the conjugation of glutathione with electrophilic compounds making them more water soluble and facilitating their removal from the body [35, 36]. It is well known that ITC-GSH conjugate is exported out by MRPs [37]. As a result of continuous conjugation and efflux of the conjugate, intracellular GSH level drops significantly within 3h of ITC treatment. This time also coincides with the induction of GST and MAPK [38]. Due to non-availability of GSH, ITCs bind with other vital cellular proteins causing their thiocarbamoylation [37]. Although being electrophilic, no studies have reported direct binding of ITCs to cellular DNA [39]. In addition PEITC has been shown to de-methylate the promoter region of GSTP1 to induce the expression of GSTP1 [40]. ITCs also induce GSTs which scavenges ROS [41]. The action of phase II enzymes is primarily regulated by the antioxidant or electrophile response element (ARE/EpRE). The latter can be activated by the transcription factors such as the basic leucine zipper (bZIP) Nrf2, which heterodimerizes with Maf G/K to exhibit its effects. ITCs induce the Nrf2 transcription factor to activate ARE, which in-turn translates into the activation of mitogen activated protein kinase (MAPK) ERK/JNK, PI3K and PKC [41–44]. SFN induces epigenetic modifications by inhibition of HDAC 1, 4, 5 and 7. In addition, SFN induced de-methylation at the promoter region of Nrf2 causes enhanced expression of Nrf2 in the TRAMP mice model for prostate cancer [45]. SFN's chemopreventive effects mainly depend on induction of phase II enzymes through the activation of antioxidant response elements like Keap1/Nrf2 [31, 46, 47]. SFN mediated induction of Nrf2 was found to be through the activation of heme oxygenase 1 and inhibition of p38 in hepatoma cells [42]. Furthermore, several studies have shown induction of thioredoxin reductase as well its substrate thioredoxin by SFN in various cancer cell lines [48–50]. Inhibition of key survival pathway such as NF-kB and AP-1 by ITCs also contributes to the chemopreventive effects of ITCs [44].
ITCs thus modulate phase I and II enzymes to reduce the bio-activation of carcinogens as well as enhanced detoxification. This dual mechanism leads to reduced binding of carcinogens with the DNA and hence less mutagenic or carcinogenic effects.
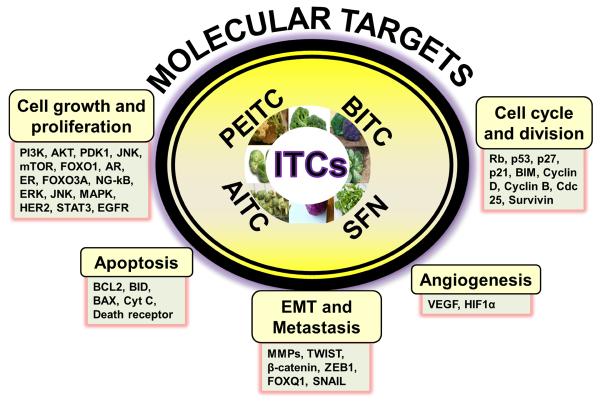
These studies suggest existence of mutually distinct mechanisms of chemo-preventive and chemo-therapeutic effects of ITCs. Specific targets have been identified that mediate chemotherapeutics effects of different ITCs against human cancers [30, 41, 44, 51–53]. These targets might vary with the structural variations amongst ITCs as well as the nature and origin of cancer. Several studies demonstrate that ITCs modulate cancer cell signaling by acting on multiple targets to suppress growth and progression of cancer cells [41, 53].
3. Uptake of ITC by Cancer Cells
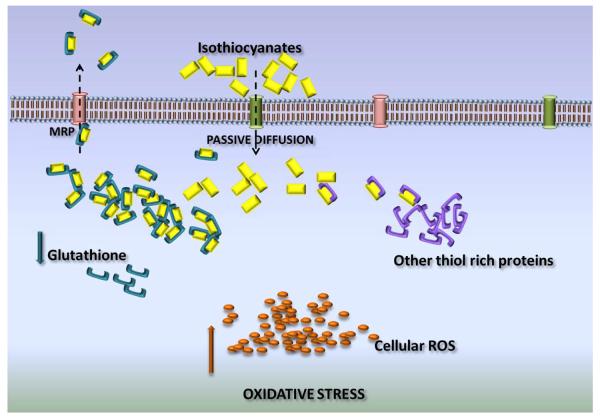
The uptake of anti-cancer agents is an important limiting factor for efficacy. Most of the ITCs can be taken up by the cells through passive diffusion. The cellular uptake of ITCs correlates with the induction of phase II detoxifying enzymes important for chemo-preventive activity. It was observed that the intracellular concentrations of ITCs can reach up to 100–200 folds higher than the extracellular concentrations. For example when hepatoma cells were incubated with 100μM SFN for about 30 minutes, the intracellular concentrations reached about 6.4mM [38]. The magnification of intracellular concentration was due to the formation of dithiocarbamates, as ITCs rapidly conjugate with thiols, particularly GSH. Uptake of ITCs in cancer cells was GSH dependent. The uptake was reduced if GSH concentration was increased. The ITC-GSH conjugate being the substrate of MRPs is transported out of the cells. This mechanism of uptake and cellular accumulation can be vital in designing the dose regimens of these ITCs. The dose will require the adjustment for high accumulation as well as to compensate for the rapid export through transport proteins likes MRPs [54]. The shuttling of ITC-GSH causes prompt depletion of intracellular GSH, resulting in the perturbation of cellular redox homeostasis. This could be one plausible mechanism of reactive oxygen species (ROS) generation by ITCs.
4. Chemotherapeutic Targets
A. Benzylisothiocyanate (BITC)
BITC occurs in cruciferous vegetables like cabbage, mustard, watercress, cauliflower and horseradish that constitute a significant proportion of our daily diet. Accumulating evidence suggests the anti-cancer effects of BITC through suppression of initiation, growth and metastasis of human cancers in various mouse models [55–61]. BITC induces apoptosis selectively in cancer cells through multiple mechanisms [55, 60, 62]. Major anti-cancer effects of BITC are due to the generation of reactive oxygen species. BITC causes cell cycle arrest as well as disruption of mitochondrial membrane potential to initiate mitochondrial pathway of apoptosis [57, 63]. Studies from our laboratory have demonstrated the anti-cancer effect of BITC against pancreatic tumor growth via inhibition of key molecules overexpressed in cancer such as AKT, STAT3, HDAC and NF-kB (Table 1) [7, 55, 56, 59]. The targets of BITC can be divided as per their role against cancer promoting mechanisms.
Table 1.
Chemo preventive effects of ITCs
| Compounds | Efficacy | Organ | Mechanism | Dose/Duration | Cell line | References | |
|---|---|---|---|---|---|---|---|
| BITC | Anti-angiogenesis | Pancreas | ↓VEGF, MMP-2, HIF-a | In vitro | 5, 10,20 μM/24 h | BxPC-3,PanC-1 | [77] |
| ↑RhoB | |||||||
| ↓STAT-3 phosphorylation (Tyr-705), HIF-a, VEGFR-2, VEGF, MMP-2, CD31, RhoC. | In vivo | 12 μmol/40 days | |||||
|
| |||||||
| BITC | Anti-angiogenesis | Breast | ↓CD31, VEGF, VEGFR | In vitro | 2.5, 5, 7.5μuM/24 & 48h | MDA-MB-231 | [78] |
| In vivo | 6 & 9 μmol/14days | ||||||
|
| |||||||
| BITC | Apoptosis | Pancreas | ↓PI3K, AKT, PDK1, mTOR, FOXO1, FOXO3a | In vitro | 5, 10, 20 μM/24h | BxPC-3,PanC-1 | [55] |
| ↑Bim, p27, and p21 | In vivo | 12 μmol/45 days | |||||
|
| |||||||
| BITC | Apoptosis | Breast | ↓mTOR, FOXO1 | In vitro | 2.5, 5μM/6 & 9h | MDA-MB-231, MCF-7, MDA-MB-468, BT-474, BRI-JM04 | [68] |
|
| |||||||
| BITC | Apoptosis | Pancreas | ↓NF-kappaB, cyclin D1, ↑HDAC1, and HDAC3 | In vitro | 10 μM/24h | BxPC-3, Capan-2 | [56] |
|
| |||||||
| BITC | Apoptosis | Pancreas | ↑ERK, JNK, P38, ROS | In vitro | 10 μM/24h | Capan-2, MiaPaCa-2 | [57] |
|
| |||||||
| BITC | Apoptosis | pancreas | ↑ATR (Ser-428), Chk2 (Thr-68), Cdc25C (Ser-216), Cdk-1 (Tyr-15)and induction of p21 Waf1/Cip1 | In vitro | 2.5, 5, 10 μM/24, 48 h | BxPC-3, PanC-1 | [58] |
| ↓NF-kappa B | |||||||
|
| |||||||
| BITC | Apoptosis | pancreas | ↓STAT-3, Mcl-1, Bcl-2 | In vitro | 5–40 μM/24 h | BxPC-3, AsPC-1, Capan-2, MiaPaCa-2, Panc-1 | [59] |
| In vivo | 60 μmol/wk/6 weeks | ||||||
|
| |||||||
| BITC | Apoptosis, Cell cycle arrest | Brain | ↑ROS | In vitro | 10, 20μM/24h | U87MG | [82] |
|
| |||||||
| BITC | Apoptosis | pancreas | ↑H2A.x, p21, Chk2 | In vivo | 10 μmol/L/24 h | Capan-2 | [60] |
| ↓CyclinB1, Cdc2, Cdc25C | |||||||
|
| |||||||
| BITC | Apoptosis, Cell cycle arrest | pancreas | ↓Cdk1, cyclin B1, Cdc25B, NF-kappaB | In vitro | 5, 10 μM/24 h | BxPC-3 | [61] |
| ↑IkappaBa | |||||||
|
| |||||||
| BITC | Apoptosis | Ovary | ↓Bcl-2, ERK1/2 and Akt | In vitro | 10 μM/48 h | SKOV-3, KLE, SW954, SW756, HL60 | [62] |
| ↑caspase-3, −9, Bax, JNK1/2 and p38 | |||||||
|
| |||||||
| BITC | Apoptosis, cell cycle arrest | Bone | ↓cyclin A, cyclin B1, ↑Chkl, p53, caspase-9 and −3, ROS | In vitro | 7.5 μM/0, 12, 18, and 24 h | U-2 OS | [79] |
|
| |||||||
| BITC | Anti-metastasis | Lung | ↓MMP-2, Twist, betacatenin, Akt and NFkappaB | In vitro | 5 μM/24 h | L9981 | [80] |
| ↑ROS | |||||||
|
| |||||||
| BITC | Anti-tumorigenesis | Leukocyte | ↓hydrogen peroxide, ROS | In vitro | 1, 10, 100 μM/24 h | HL-60 | [81] |
| In vivo | 81 or 810 nmol/20 weeks | ||||||
|
| |||||||
| PEITC | Apoptosis | Breast | ↓HER2, EGFR, STAT-3, BCL-XL, XIAP, ROS | In vitro | 10 μM/24 h | MDA-MB-231, MCF-7 | [95] |
| ↑Bax, Bim, HER2 | In vivo | 12μmol/33 days | |||||
| ↓HER2, EGFR, STAT-3 | |||||||
|
| |||||||
| PEITC | Apoptosis | Prostate | ↓Bcl-2, XIAP | In vitro | 1 μM/24 h | PC-3, DU145 | [97] |
| ↑Bax, Bak | In vivo | 9 μmol/38 days | |||||
|
| |||||||
| PEITC | Apoptosis | Leukemia | ↑ROS, NO | In vitro | 10 μM/ 1–6 h | HL-60 | [98] |
| ↓GSH | |||||||
|
| |||||||
| PEITC | Apoptosis | Breast | ↑ROS, caspases 9 and 3 | In vitro | 20 μM/6 h | MDA-MB-231, MCF-7 | [99] |
| ↓GSH, Bax | |||||||
|
| |||||||
| PEITC | Apoptosis | T-cell | ↑JNK | In vitro | 20μM/24h | Jurkat | [101] |
|
| |||||||
| PEITC | Apoptosis | Lung | ↑JNK, p38, Erk1/2, AP-1 | In vivo | 15 micromol/g/140 days | [102] | |
|
| |||||||
| PEITC | Apoptosis | Colon | ↓SOS-1, PKC, ERK1/2, Rho A, MMP-2 and -9, GRB2, NF-κB, iNOS, COX-2 | In vitro | 2.5 μM/24, 48 h | HT29 | [103] |
|
| |||||||
| PEITC | Anti-metastasis | Liver | ↓MMP-2,MMP-9 | In vitro | 0.1–5 μM/24 h | SK-Hep1 | [104] |
| ↑TIMP1,2 | |||||||
|
| |||||||
| PEITC | Anti-angiogenesis | Prostate | ↑VEGF, VEGF receptor 2, Akt, EGF, G-CSF | In vitro | 4 μM/24 h | HUVEC, PC-3 | [105] |
|
| |||||||
| PEITC | Anti-angiogenesis | Breast | ↓HIF1alpha, CAIX, GLUT1, BNIP3, VEGF-A, 4E-BP1 | In vitro | 16 μM/24h | MCF7 | [106] |
|
| |||||||
| PEITC | Apoptosis, cell cycle arrest | Prostate | ↑p21WAF-1/Cip-1 and p27Kip1 | In vivo | 8 μmol/g/9 weeks | BALB/c male mice | [122] |
| ↓cyclins D and E, Rb | |||||||
|
| |||||||
| PEITC | Apoptosis, cell cycle arrest | Oral squamous cell | ↑p53, p21, pl7, Bax, Bid | In vitro | 5 μM/72 h | HSC-3 | [116] |
| ↓cyclin E, CDK2, Bcl-2 | |||||||
|
| |||||||
| PEITC | Apoptosis | multiple myeloma | ↑PARP, caspases-3 and -9, c-jun, HSP27 | In vitro | 2.5, 5 and 10 μM/12, 24, 48 h | MM. 1S | [51] |
| ↓Mcl-1, X-IAP, c-IAP and survivin | |||||||
|
| |||||||
| PEITC | Apoptosis | Breast | ↑p57 Kip2, p53, BRCA2, IL-2, and ATF-2 | In vitro | 3 μM/48 h | MCF-7 | [124] |
|
| |||||||
| PEITC | Apoptosis, Cell cycle arrest | Prostate | ↑p53, WEE1, caspase-3, -8, -9 | In vitro | 20 μM/24,48 h | DU 145 | [117] |
| ↓CDC25C | |||||||
|
| |||||||
| PEITC | Cell cycle arrest | Lung | ↓cell growth | In vitro | 3, 6, 9 μM/24, 48, 72 h | A549, H1299 | [125] |
|
| |||||||
| PEITC | Apoptosis | Ovary | ↓EGFR, AKT, p-GSK | In vitro | 40 μM/24 h | SKOV-3, OVCAR-3 , TOV-21G | [109] |
| ↓Tumor growth | In vivo | 12 μmol/42 days | |||||
|
| |||||||
| PEITC | Apoptosis | Oral cancer | ↑caspase-3,-8, BID, DR5 | In vitro | 10 μM/48 h | HN22 | [113] |
|
| |||||||
| PEITC | Apoptosis | Cervical cancer | ↑DR4, DR5, caspase-3, PARP | In vitro | 5 μM/48 h | HEp-2, KB | [114] |
| ↓ERK1/2 | |||||||
|
| |||||||
| PEITC | Apoptosis | Breast | ↑caspases 7 and 9, PARP | In vitro | 3–30 μM/24h | MCF-7 | [118] |
| ↓Bcl-2, XIAP | |||||||
|
| |||||||
| PEITC | Apoptosis | Breast | ↓ER-α36, ERK 1/2 | In vitro | 10 μM/48 h | MCF7, H3396, MDA-MB-231,SK-BR-3 | [110] |
|
| |||||||
| SFN | Apoptosis | Liver | ↑TrxR1 | In vitro | 12 μM/24 h | HepG2 | [49] |
|
| |||||||
| SFN | Apoptosis | Breast | ↑TrxR1 | In vitro | 3, 6 μM/24 h | MCF-7 | [50] |
|
| |||||||
| SFN | Apoptosis | Prostate | ↑Ac-histone H4, Bax, P21, ↓HDAC | In vitro | 15 μM/24 h | BPH-1, LnCaP, PC-3 | [133] |
|
| |||||||
| SFN | Apoptosis, Cell cycle arrest | Bladder | ↑p27 | In vitro | 5–20 μM | T24 | [147] |
|
| |||||||
| SFN | Apoptosis, Cell cycle arrest | Colon | ↑cyclins A and B1, bax, cytochrome c | In vitro | 15 μM/24 h | HT29 | [148] |
|
| |||||||
| SFN | Apoptosis, Cell cycle arrest | lymphoblastic leukemia | ↑caspases 3, 8, and 9, p21 | In vitro | 7.5 μM/24 h | LCL, Nalm-6, Jurkat, KOPTK1 | [149] |
| ↓Cdc2/Cyclin B1, AKT, mTOR | |||||||
|
| |||||||
| SFN | Apoptosis | Bladder | ↓tumor volume | In vivo | 12 mg/kg/5 weeks | athymic mice | [135] |
| ↑caspase 3 and cytochromec | |||||||
|
| |||||||
| SFN | Apoptosis | Prostate | ↓p65, VEGF, cylcin D1, and Bcl-X | In vitro | 20 and 30 μM/24 h | PC-3 | [136] |
|
| |||||||
| SFN | Cell cycle arrest | Colon | ↑ERK,JNK,p38,p21 | In vitro | 6.25, 12.5, 25, 50 and 100 μM/−24h | HT-29 | [150] |
| ↓cyclin D1 | |||||||
|
| |||||||
| SFN | Apoptosis | Colon | ↑ERKl/2 and Akt | In vitro | 20, 30 μM/24 h | Caco-2 | [151] |
|
| |||||||
| SFN | Apoptosis | Breast | ↑caspase-8, caspase-3, cytochrome c | In vitro | 0, 5, 15, or 25 μmol/L for 48 h | MDA-MB-231,MDA-MB-468, MCF-7, T47D | [137] |
| ↓Bcl-2, HDAC, ERalpha, EGFR, EGFR2 | |||||||
|
| |||||||
| SFN | Apoptosis | Prostate | ↓IAP, cIAP1, cIAP2, XIAP, p65 | In vitro | 20 μM/ 24 h | LNCaP, PC3 | [143] |
| ↑Apaf-1, E2F1 | |||||||
|
| |||||||
| SFN | Anti-angiogenesis, anti-metastasis | Pancreas stem cell | ↓Nanog, Oct-4, VEGF, PDGFRα, Zeb-1 | In vivo | 0–20 mg/kg/6 weeks | NOD/SCID/IL2Rgamma mice | [144] |
|
| |||||||
| SFN | Apoptosis | prostate | ↑ROS, Fas, caspase-8, Bid | In vitro | 40μM/4h | PC-3 | [145] |
|
| |||||||
| SFN | Anti-metastasis | Oral carcinoma | ↓MMP-1, -2 | In vitro | 1μM/24h | YD8, YD10B, YD15 | [152] |
|
| |||||||
| SFN | Apoptosis | Breast | ↓tumor growth | In vitro | 25 or 50 mg/kg/24 days | KPL-1 athymic mice | [153] |
| In vivo | |||||||
|
| |||||||
| SFN | Anti-metastasis | Bladder | ↓ZEB1, Snail | In vitro | 0–20μM/24h | T24 | [154] |
| ↑E-cadherin | |||||||
|
| |||||||
| AITC | Apoptosis, Cell cycle arrest | Prostate | ↓Bcl-2, cyclinBl, Cdc25B, Cdc25C | In vivo | 10μmol/26 days | Nude mice | [168] |
| ↑BID | |||||||
|
| |||||||
| AITC | Anti-proliferation | Colon | ↓mitosis | In vitro | 12 μM/24 h | HT-29 | [164] |
|
| |||||||
| AITC | Apoptosis | Prostate | ↑AP-1, ERK1/2, JNK1/2, Elk-1 and c-Jun | In vitro | 50 μM/24 h | PC-3 | [165] |
|
| |||||||
| AITC | Apoptosis, Cell cycle arrest | Bladder | ↓ α- and β-tubulin | In vitro | 30 μM/24 h | UM-UC-3 | [167] |
| ↑cytochrome c, caspase-9,-3, Bcl-2, JNK | |||||||
|
| |||||||
| AITC | Anti-metastasis | Liver | ↓MMP-2/-9 | In vitro | 0.1–5 μM/24 h | SK-Hep 1 | [169] |
A.1. Cell Proliferation and Growth
PI3K/AKT pathway has been shown to be activated in about 59% of the pancreatic tumors, and it also promotes cell division in other cancer forms [64, 65]. PI3K stimulation results in the phosphorylation of AKT at Thr-308 and Ser-473 through PDK1 activation [66]. Studies by Boreddy et. al. have shown that BITC inhibits PI3K/AKT signaling. BITC prevents the phosphorylation of AKT at both Thr-308 and Ser-473 along with suppression of PI3K (Tyr-458), PDK1 (Ser-241), mTOR (Ser-2448) [55]. The inhibition of mTOR signaling by BITC was also observed in human prostate cancer cells [67]. These studies showed that BITC had negligible effect on normal human pancreatic ductal epithelial (HPDE-6) cells, suggesting the specificity of BITC towards cancer cells [55]. These results also showed up-regulation of pro-apoptotic proteins like Bim, p21 and p27 due to nuclear accumulation of Forkhead Box Protein 1 (FOXO1). Inhibition of phosphorylation of FOXO1 (Ser-256) and Forkhead Box Protein 3a (FOXO3a) by BITC was due to the de-phosphorylation of AKT in pancreatic cancer cells [55]. Interestingly, BITC also reduced acetylation of FOXO proteins by reducing the level of CREB-binding protein (CBP) protein [55]. FOXO1 suppression was also shown to be responsible for BITC initiated cell-death in breast cancer cells [68].
NF-kB is a transcription factor that regulates cellular inflammation, immunity and proliferation [69, 70]. Batra et. al. showed that BITC-mediated downregulation of HDAC1 and HDAC3 expression was associated with the acetylation of NF-kB in pancreatic cancer cells [56]. BITC treatment significantly suppressed the phosphorylation of NF-kB at Ser-276 and Ser-536 in BxPC-3 and Capan-2 cells in a dose and time dependent manner [56, 61]. BITC reduced NF-kB protein expression in BxPC-3 cells but not in Capan-2 cells, indicating that BITC acts differentially on different cell lines [56]. The Capan-2 cells have wild type p53, whereas BxPC-3 cells harbor mutated p53, hence the role of p53 in BITC mediated down-regulation of NF-kB expression cannot be ruled out and remains to be explored further. The mechanistic studies revealed that neither IkB phosphorylation nor expression levels were altered by BITC, whereas IKK expression was down-regulated. Hence, down-regulation of IKK by BITC treatment could be the reason for inhibition of NF-kB phosphorylation (Ser-536) [56, 61].
Signal Transducer and Activator of Transcription 3 (STAT3) is hyper-activated in significant number of malignancies like breast cancer, pancreatic cancer, gastric cancer and head & neck cancer as well as in cancer stem cells where it enhances tumor aggressiveness and progression [71–73]. Sahu and Srivastava have shown that BITC suppresses the phosphorylation (Tyr-405 & Ser-727) and expression of STAT3 in pancreatic cancer cells lines such as BxPC-3, PanC-1, Capan-2 and MIA PaCa-2 [59]. The role of STAT3 in the anti-cancer effects of BITC was confirmed by STAT3α overexpression or through activation by Interleukin-6 (IL-6), which abrogates the effects of BITC (Table 1) [59].
A.2.Angiogenesis
The growing tumors are nourished through processes such as angiogenesis and neovascularization. Angiogenesis is mainly promoted by hypoxia inducible factor (HIF-1α) and vascular endothelial growth factor (VEGF) [74]. STAT-3 has been shown to be a positive regulator of VEGF and HIF-1α [75, 76]. Boreddy et al. demonstrated that BITC inhibits angiogenesis in chicken chorioallantoic membrane (CAM) and rat aortic ring assay [77]. This clearly indicates the anti-angiogenic potential of BITC. BITC-mediated suppression breast cancer xengrafts was associated with inhibition of critical angiogenic factors like CD31 and VEGF [78]. Furthermore, BITC down-regulated the expression of HIF1-α, VEGFR-2, MMP-2, Rho A, Rho C and RAC1, 2 and 3 in pancreatic, but the inhibition of VEGF, HIF-1α and MMP-2 was not observed in STAT3 overexpressing BxPC-3 cells [77]. This undoubtedly suggests that inhibition of tumor growth and angiogenesis by BITC correlates with STAT3 inhibition.
A.3.Mitochondrial Cell-death
Generation of reactive oxygen species (ROS) is an important mechanism to induce cell death, specifically in cancer cells. As shown by us and others, BITC significantly induced ROS generation in pancreatic cancer cells and glioma as well as other cancer models [57, 79–82]. ROS generation leads to disruption of mitochondrial membrane potential and release of pro-apoptotic molecules resulting in activation of caspase-mediated cell death [63, 81, 83]. Furthermore, BITC-mediated down-regulation of MCL-1 in human leukemia cells was also found to be correlated with the mitochondrial pathway of apoptosis [84]
A.4.Cell-cycle Arrest
ROS induced by BITC also DNA damage and G2/M cell cycle arrest as detected through increased phosphorylation of H2A.X (Ser-139) and ChK2 (Thr-68) [57, 60, 61]. Antioxidants block the effects of BITC confirming the role of ROS in cell cycle arrest [57]. BITC treatment increased the phosphorylation of the MAP kinases, such as ERK (Thr202/Thy204), JNK (Thr183/Tyr185) as well as p38 (Thr180/Tyr182) in a dose-dependent fashion [57, 62]. It was later found that BITC-induced cell cycle arrest was executed only through ERK, while the other MAP kinases were playing role in the induction of apoptosis [57].
A.5.Invasion and Metastasis
Metastasis is initiated by key regulators like matrix-metalloproteinases, Twist and β-catenin. A study showed that BITC treatment inhibited cell migration and invasion in lung cancer cells. This was accompanied with reduced expression of MMP-2, Twist and β-catenin [80]. Another study showed that oral administration of 5 and 10mg/kg BITC suppressed the expression of MMP-2& 9 in the sera and lungs of mice injected with 4T1 breast cancer cells [85]. BITC also inhibits the process of epithtelial to mesenchymal transition through FOXQ1 suppression in breast cancer cells, leading to reduced metastatic potential [86]. The data available for anti-metastatic effects of BITC is insufficient to prove the anti-metastatic efficacy. Hence, additional elaborate studies are required to establish the role of BITC in metastasis.
A. 6. In vivo Studies
Our in vivo studies indicated that BITC is well tolerated at a dose of 12 μmol/day (72mg/kg) in mice. Interestingly, in vivo tumor growth was markedly arrested by BITC treatment in athymic nude mice as compared to controls [59]. These results showed that after 6 weeks of 12 μmol/day BITC treatment by oral gavage, average tumor volume in BITC-treated mice was about 48% less as compared to the control group [59]. LC-MS analysis showed that after 46 days of BITC (12 μmol/day) treatment, mean concentration of 6.5±0.1 μmol/L (39mg/L) (n=10) & 7.5±0.3 μmol/g (45mg/g) (n=10) BITC was observed in the plasma and tumors of treated mice respectively [55]. These results suggest a reasonable bioavailability of BITC and also that the therapeutic concentration could be achieved in vivo by oral administration. No untoward side effect or change in body weight was observed, the suggesting that 12μmol/day BITC was relatively safe. Furthermore, suppression of in vivo angiogenesis by 12 μmol/day (72mg/kg) treated mice was observed by reduction of hemoglobin content by 76% in matrigel plugs implanted in the mice as well by 61% in the excised tumor xenografts, as compared to respective controls [77]. These results signify the potential anti-tumor and anti-angiogenic effects of BITC. The molecular targets of BITC have been described in detail in Table 1. Interestingly, dietary BITC also suppressed the growth of cancer stem cell in MMTV-neu breast cancer transgenic mice model along with inhibition of major stem cell markers like Oct4, SOX-2 and Nanog [87]. In contrast, the activation of NOTCH2 signaling by BITC was found to impede the therapeutic benefits of BITC [88]. A recent study from our group showed that the absorption and bioavailability of BITC can be enhanced by making the nanoemulsion of BITC [89].
A. 7. Toxicity studies
No major evidence of BITC side effect exists for the doses that are commonly used for anti-cancer studies. A study has shown that oral administration of BITC (0, 50, 100 and 200mg/kg) for 4 weeks caused reduction in body weight and reduced food consumption only at highest doses [90]. In addition, the study revealed that BITC treatment caused increase in serum cholesterol and decrease in triglycerides, accompanied with renal dysfunction. Furthermore, in this study BITC treatment reduced the weight of almost all the organs except the adrenals, where the weight was increased. Some transitory hematological changes like reduced hemoglobin and lymphocyte count with increased platelets, eosinophils and neutrophils were observed in BITC treated rats. It is pertinent to note that no significant signs of toxicity were observed at the dose of 50mg/kg. Although these changes were observed at higher doses (100 and 200mg/kg) of BITC, no mortality was reported [90]. The high doses like 100–200mg/kg BITC, which were associated with some side effects, are unlikely to be used for anti-tumor effects. So far the therapeutic doses of BITC, which suppresses in vivo tumor growth are much lower and not associated with any side effects and hence can be considered relatively safe.
B. Phenethylisothiocyanate (PEITC)
PEITC is another isothiocyanate that occurs conjugated with glucosinolate in many cruciferous plants. PEITC is abundantly present in plants such as watercress, garden cress and in some non-cruciferous plants like turnips and radishes [30, 91]. Watercress is the most prolific source of PEITC, which can release approximately 2–6 mg PEITC/ounce (0.07 to 0.21mg of PEITC/g) in humans [91, 92].
The effective concentrations of PEITC vary from 0.12μM to 14μM [93, 94]. Like BITC, PEITC also induces ROS generation selectively in cancer cells [95, 96]. Mechanistic studies have shown that PEITC disrupts mitochondrial electron transport chain (ETC) by inhibiting Complex I and III activity and reduces oxygen consumption rate in prostate cancer cells [97, 98]. Furthermore, PEITC is known to inhibit ROS-detoxifying mechanisms to enhance ROS-mediated cytotoxicity [96–98]. This was further proven in cells with varying levels of anti-ROS mechanisms that showed differential sensitivity towards PEITC [99, 100].
Two general mechanisms that have been identified for the anti-cancer activity of PEITC include cell cycle arrest and apoptosis induction [44, 101, 102]. Few studies also suggest anti-angiogenic and anti-metastatic effects of PEITC by mechanisms similar to BITC [80, 103–106]. PEITC has been shown to act on about 30 different targets present in cancer cells [107]. Mi et al. have shown that PEITC alters the function of critical amino acids of proteins and peptides through covalent interactions [107].
B.1.Cell Proliferation and Growth
Studies suggest that PEITC has multiple targets like AKT, EGFR and HER2 in cancer cells, which promote anti-apoptotic mechanisms in cancer cells. As discussed earlier, AKT (Protein kinase B) is frequently overexpressed in cancers and regulated by oncogenes like EGFR and HER2 [108]. Our studies demonstrated that PEITC inhibits EGFR and HER2 in ovarian and breast cancer cells [95, 109]. PEITC caused significant inhibition of activated EGFR (Tyr1068) to suppress the growth of ovarian cancer cells. Furthermore, PEITC reduced the phosphorylation of AKT and mTOR expression [109]. In this study PEITC also disrupted the complex of Raptor and Rictor with mTORC1 and mTORC2 [109]. In another study we observed inhibition of HER2 and AKT in breast cancer cells. These observations suggest that PEITC inhibits AKT activation by suppressing EGFR and HER2 expressions to suppress anti-apoptotic signaling in cancer cells (Table 1). Furthermore, PEITC also inhibits HDACs, the major epigenetic regulators resulting in the inhibition of androgen receptor in prostate cancer cells [40].
B.2.Angiogenesis
Similar to BITC, PEITC also inhibits vascular endothelial growth factor (VEGF), a major promoter of angiogenesis. Xiao and Singh showed suppression of VEGF by PEITC, which was later shown to be mediated through suppression of HIF1α [105, 110–112]. Based on the evidence provided in these studies, it can be suggested that PEITC inhibits angiogenesis mainly by inhibiting VEGF.
B.3.Mitochondrial Cell-death
Accumulating evidence from several studies showed induction of apoptosis signaling by PEITC. PEITC has been shown to activate death receptors and Fas-mediated extrinsic apoptotic pathway in oral and cervical cancer cells [113–115]. PEITC treatment also resulted in the activation of intrinsic pathway of apoptosis. PEITC modulates mitochondrial proteins like BCL2, BID and BAX, causing the release of cytochrome c into cytosol to induce intrinsic apoptosis pathway [94, 116–119]. However, the release of cytochrome c by PEITC treatment into cytosol to induce apoptosis was contradicted by a study conducted by Wu et. al. [120]. Further in-depth studies are thus required to delineate the exact mechanism of PEITC.
B.4.Cell-cycle Arrest
PEITC as well as its N-acetyl cysteine conjugate causes activation of Retinoblastoma (Rb) protein in prostate cancer cells, leading to attenuation of cell cycle progression [39, 121]. Furthermore, a G0/G1 phase cell cycle arrest by PEITC was associated with activation of p53 in oral squamous carcinoma cells, in multiple myeloma, osteogenic sarcoma and breast cancer cells and G2/M cell cycle arrest in prostate cancer cells [51, 52, 116, 117, 122, 123]. Interestingly, lung carcinoma cells expressing mutated p53 were shown to be more sensitive to PEITC as compared to cells with wild type p53 expression [107, 124].
B.5.Invasion and Metastasis
PEITC inhibits cancer cell invasion by inhibiting matrix metalloproteinases (MMP) and suppresses activity of ERK and NF-kB to inhibit metastasis [103, 104]. We recently demonstrated in vivo anti-metastatic potential of PEITC using a unique mouse model of breast cancer metastasis [94]. This model utilizes MDA-MB-231-Luc2 brain-seeking breast cancer cells that lodge in the brain from blood circulation when injected into the left ventricle of mouse heart. These cells later grow to form metastatic tumors in brain. Oral administration of 10 μmol PEITC (65mg/kg) for 10 days significantly prevented the seeding of breast cancer cells into the brain in this model. We also observed that PEITC administration suppressed the growth of metastasized tumor in the brain and enhanced the survival of mice bearing tumors in the brain [94]. This was the first evidence of in vivo anti-metastatic effects of PEITC in breast cancer model, but further studies are required to establish similar efficacy in other cancer forms. The molecular targets of PEITC have been described in detail in Table 1.
B.6. In vivo Studies
PEITC mediated inhibition of anti-apoptotic pathways was observed in the preclinical mouse model studies [125]. PEITC has a dose dependent bioavailability of about 70 – 110% by oral administration, which is a probable reason for in vivo efficacy [126]. Treatment of brain metastatic breast cancer has always been a problem due to the presence of blood brain barrier. Organ distribution study has revealed a fair availability of PEITC in brain suggesting better chances of PEITC to cross blood brain barrier [127]. This could be the reason for the anti-metastatic effects of PEITC [94].These studies indicate a high anti-tumor efficacy of PEITC in all organs including brain by oral administration. Orally administered PEITC causes significant inhibition of major oncogenic pathways like EGFR, HER2 and AKT in various in vivo cancer models leading to tumor growth suppression [94, 95, 109, 128]. These results clearly re-enforce potential for in vivo efficacy of PEITC.
B.7.Toxicity Studies
In addition to the beneficial effects, it is also essential to evaluate the probable side effects of PEITC. It was observed that i.p. administration of 80 and 160mg/kg PEITC caused increase in body weight of mice but reduction in the weights of liver and spleen [129]. Interestingly, preventive effects of PEITC were observed on acetaminophen induced hepatotoxicity and mortality [130]. These mutually contradicting observations make it important to establish a well-defined toxicity profile of PEITC using appropriate controls and population size.
B.8.Clinical Studies
Three clinical studies are currently under progress to test anti-cancer effects in humans. A phase I lung cancer study with PEITC conducted at MD Anderson Cancer Center was recently completed; however, the findings have not yet been published. Another phase I clinical study at the same institution has been planned to test the anti-leukemic effects of PEITC. Notably, a recent phase I clinical trial (NCI CN-55120) reported that 10μM PEITC can be achieved in the plasma after intake of 200 mg PEITC orally in human volunteers [131]. A phase II trial is also under progress in lung cancer patients at the Masonic Cancer Center, University of Minnesota in collaboration with the National Cancer Institute. The outcomes of these studies will provide data on the efficacy and toxicity of PEITC in humans.
C. Sulforaphane (SFN)
SFN is an isothiocyanate mainly present in broccoli and Brussels sprouts. Studies have shown that SFN is highly effective in blocking carcinogenesis. SFN inhibits HDAC activity to promote cell cycle arrest and apoptosis in Nrf2−/− cells suggesting Nrf2 independent mechanism of SFN [132, 133].
C.1.Cell proliferation and Growth
SFN acts on certain molecular targets like survivin and NF-kB that are vital for cancer cell survival [134, 135]. SFN induces apoptosis in breast cancer cells by the inhibition of Estrogen receptor (ER), EGFR1 and HER2, which are particularly important for the growth of breast cancer [136]. Recently SFN was shown to cause DNA damage through enhanced acetylation of DNA repair proteins. This effect was shown to be specific for cancer cells as there were no epigenetic changes or DNA damage observed in non-cancer cells [137]. Interestingly, based on the methylation of DNA and Cyclin D2 by SFN, a clinical trial (NCT01265953) has also been initiated at Portland, VA Medical Center [138]. Studies suggest significant epigenetic changes induced by SFN in various cancer models.
C.2.Angiogenesis
Very few studies have reported the anti-angiogenic effects of SFN. The suppression of VEGF and MMP-2 has been shown by SFN treatment [139, 140]. Another study indicated that VEGF suppression was mediated through inhibition of FOXO1/AKT pathway [141]. However, no further evidence exists for the anti-angiogenic effects of SFN. Due to the lack of sufficient evidence, anti-angiogenic activity cannot be considered as a critical mechanism of SFN.
C.3.Mitochondrial Cell-death
Another important mechanism of action of SFN was inactivation of inhibitors of apoptosis proteins (IAPs) [142]. SFN-mediated IAP inhibition was associated with BCL-2 inhibition suggesting activation of intrinsic apoptosis pathway [143]. SFN also causes generation of mitochondrial ROS in cancer cells that further leads to release of cytochrome c into cytosol augmenting cell apoptosis [144]. Interestingly, a ROS independent activation of MEK/ERK pathway was shown to lead to caspase dependent apoptosis in neuroblastoma cells [145].
C.4.Cell-cycle Arrest
SFN was shown to induce p27-mediated G0/G1 phase cell cycle arrest [146]. In addition SFN causes irreversible cell cycle arrest in G2/M phase followed by caspase-mediated apoptosis [147]. Recent studies have shown that SFN induces G2/M arrest through the activation of p21 (CIP1/WAF1) and inhibition of Cdc2/Cyclin B1 complex independent of p53 [148]. This study showed that apoptosis following G2/M arrest was induced by caspase and PARP activation in leukemia cells [148]. Specific activation of MAP kinases like ERK, JNK and p38 in response to SFN treatment was shown to be involved in inducing cell cycle arrest [149, 150].
C.5.Invasion and Metastasis
SFN exhibits potent anti-metastatic effects by suppressing cell migration and invasion. Jee et. al. observed that the anti-cell migratory effect of SFN was associated with MMP suppression [151]. Recently EMT was shown to be an important mechanism of SFN to inhibit cell migration and metastasis in different cancer types [143, 151-153]. Li et. al. have shown that SFN modulates Sonic hedgehog pathway to suppress self-renewal capacity of the pancreatic cancer stem cells and reduce EMT characteristics [143]. Significant suppression of SNAIL and ZEB-1 marked by the re-expression of E-cadherin was observed by SFN treatment that lead to reversal of EMT [153]. EMT prevention by SFN was also associated with induction of miR-200c and re-expression of the estrogen receptor [154]. The details of molecular targets of SFN have been described in Table 1.
C.6.In vivo Studies
Kanematsu et. al. demonstrated the in vivo efficacy of SFN against tumor growth and metastasis in breast cancer [152]. Pharmacokinetic studies show good bioavailability of SFN after oral administration. A concentration of 20μM in plasma was achieved after oral administration of 50 μmol SFN/rat (35 mg/kg) [155]. In a human study it was shown that after consumption of 200 μmol SFN (35.5mg), about 2 pmol/mg (0.355 ng/mg) SFN was detected in the breast tissue suggesting its availability at the tumor site [156]. The cumulative concentration of SFN in the small intestine was shown to be sufficient to inhibit tumor growth in the colonic tissue [157]. These studies clearly indicate bioavailability and favorable pharmacokinetic profile of SFN which can be instrumental for future development of SFN as an anti-cancer agent.
C.7.Toxicity Studies
Along with the anti-cancer activity of SFN, it is important to study its toxicity to assess to benefit to risk ratio. An increase in hepatoxicity indicators AST, ALT and LDH in plasma was observed with SFN (1.6mg/mouse/day (64mg/kg) for 14 weeks) administration in mouse bearing Benzo(a)pyrene [B(a)P] (100mg/kg b.wt.) induced lung cancer [158]. Interestingly, opposite findings were reported in another study. The rats were pre-treated with 3mg/kg SFN by intra-peritoneal injection. One hour later an intestinal ischemia/reperfusion surgery was performed to induce toxicity. It was observed that SFN administration increased the SOD levels along with reduction of myeloperoxidase, ALT and AST levels in serum [159]. Both the studies used significantly different concentrations of SFN, which can explain the opposite observations. However, due to the lack of confirmatory evidence, overall no conclusion can be drawn about the toxicity of SFN.
D. Allylisothiocyanate (AITC)
AITC is an aliphatic isothiocyanate derived from sinigrin and is excreted as NAC conjugates in the urine [160]. A recent study demonstrated a short term reversible DNA damage when AITC was provided in the diet [161]. Cancer cells in general are more susceptible to DNA damage leading to cell death. This explains the enhanced sensitivity of cancer cells towards AITC. The cytotoxic effects of AITC were shown to be specific to cancer cells [162]. Smith et. al. demonstrated apoptosis induction by AITC in colorectal cancer cells [163].
D.1.Cell Proliferation and Growth
AITC targets specific signaling molecules to suppress cancer cell growth. ERK and JNK signaling were involved in the activation of AP-1 by AITC to suppress cancer cell growth [164, 165].
D.2.Mitochondrial Cell-death
Geng et. al. observed that AITC resulted in the phosphorylation of BCL-2 to induce apoptosis, whereas mutated BCL-2 abrogated the cytotoxic effects of AITC [166].
D.3.Cell-cycle Arrest
Srivastava et. al. demonstrated the in vivo efficacy of AITC in prostate cancer [167]. This study indicated that cell growth arrest in G2/M phase by AITC was associated with the inhibition of cyclin B1, cell division cycle (Cdc)25B and Cdc25C.
D.4.Invasion and Metastasis
The anti-metastatic effects of AITC have been demonstrated through suppression of cell migration and invasion. It was observed that AITC inhibits MMP2/9 to exhibit anti-metastatic effects in hepatoma cells [168]. Furthermore, AITC exerts anti-angiogenic effects to suppress tumor growth by down-regulating angiogenic factors like nitric oxide and tumor necrosis factor α (TNFα) (Table 1).
D.5.In vivo Studies
AITC was shown to inhibit tumor and ascites formation from Ehrlich ascites tumor cells in mice. This study also revealed enhanced survival of ascites-bearing mice with AITC treatment [169]. Furthermore, i.p administration of 25μg AITC/animal (1 mg/kg) in mice inhibited tumor-directed capillary formation suggesting inhibition of angiogenesis. AITC treatment also reduced serum nitric oxide and TNFα levels indicating reduction in inflammatory markers by AITC [170]. These studies suggest a good in vivo efficacy of AITC. Nonetheless, more studies are required to confirm the in vivo activity against contemporary targets in cancer.
D.6.Toxicity Studies
Pre-clinical studies have demonstrated some toxicity induced by AITC. Significant hematological changes were observed with AITC treatment. Subcutaneous administration of 20 mg/kg AITC reduced WBC counts by 25% along with marked reduction of lymphocytes and monocytes. In addition, increase in neutrophil and corticosteroid levels were observed indicating stress induced by AITC. The AITC treatment caused reduction in thymus weights while increasing the weights of adrenals [171]. These observations suggest significant effect of AITC on blood profile and organ weights. Interestingly in another study, i.p administration of 25μg AITC/animal every day for 5 consecutive days showed reduced WBC count at the 9th day after starting the treatment [172]. Perhaps the differences between these observations could be due to different doses and the time points of analysis after AITC administration. Another study showed increased AST levels at high doses of AITC (100–150 mg/kg), but no change was observed at lower dose (50 mg/kg) suggesting dose dependent toxicity induced by AITC [173]. Interestingly, oral administration of AITC resulted in bladder toxicity in rats. This was found due to free AITC cleaved from urinary metabolites [174]. Taken together studies suggest that AITC exhibits toxic side effects, cautioning its use. Further in-depth studies are required to establish the toxicity profile of AITC so that the dose for anti-cancer effects can be titrated effectively.
5. Potential for Combination Therapy
Cancer cells contain multiple aberrant signaling pathways which lead to drug resistance and therapy failure in many patients. Combination therapy is known to kill cancer cells more effectively through diverse mechanisms simultaneously. ITCs exhibit a diverse range of cellular targets for anti-cancer effect. This property of ITCs makes them highly desirable for combinatorial therapeutic approaches. Several combination strategies have been tested in pre-clinical studies by combining ITCs amongst themselves or with conventional or new anti-cancer therapies (Table 2) [58, 175-182].
Table 2.
Effects of combinations of ITC with other anti-cancer agents
| BITC | Concentrations and treatment time of ITCs | Combination with other agents | Concentrations | Cancer Type | Targets affected by combination treatment | Effect of combined treatment | References |
|---|---|---|---|---|---|---|---|
| BITC | 5μM (18h) | TRAIL | 10ng/ml (6h) | Pancreatic | ↑ Caspase cleavage, ↓XIAP, ↑ BID cleavage, ↑ PARP cleavage | Synergistic | [192] |
| 20μM (1h) | Cisplatin | 15, 30, 45μM (48h) | Lung | ↓ β-Tubulin | Sensitization | [193]. | |
| 10μM (3days) | SFN | 10μM (3days) | Pancreatic | ↓ STAT3 | Synergistic | [202] | |
| 9, 12μmol(10 week) | PEITC (10 week) | 12μmol | Lung | ↓ Chemically induced tumorigenesis | Synergistic | [203] | |
| 2.5μM (24h) | Radiation | 5Gy (24–48h) | Pancreatic | ↑ Caspase cleavage, ↑ G2/M cell cycle Arrest, ↑ ATR, ↑ Chk2, ↑ Cdc25c, ↑ Cdk-1, ↑ p21Wafl/Cip1 | Synergistic | [58] | |
| PEITC | 5, 10μM (8, 24h) | Cisplatin | 5, 10μM (8, 24h) | Cervical | ↑ ERK, ↑ JNK, ↑ p38, ↑ MAPK | Synergistic | [181] |
| 20μM (1h) | Cisplatin | 15, 30, 45μM (48h) | Lung | ↓ β-Tubulin | Sensitization | [193] | |
| 0.1, 0.5, 2.5, 5μM (24h) | Adriyamycin | 20, 25μM (24h) | Cervical | ↓ pkc, ↓ Telomerase | Synergistic | [195] | |
| 0.1, 0.5, 2.5, 5μM (24h) | Etoposide | 20, 25μM (24h) | Cervical | ↓ pkc, ↓ Telomerase | Synergistic | [195] | |
| 5μM (48h) | Paclitaxel | 10nM (48h) | Breast | ↑ G2/M cell cycle Arrest | Synergistic | [196] | |
| 5μM (24h), 25mg/kg (50 days) | Platinum agents | 40 μM (24h), 5mg/kg (50 days) | Lung | ↓ GSH mediated export, ↑ ROS, ↑ DNA damage | Sensitization | [177] | |
| 5μM (24h) | Metformin | 8mM (24h) | Ovarian (Cisplatin resistant) | ↑ ROS | Synergistic | [178] | |
| 2.5μM (6h) | Vorinostat | 2μM (18h) | Leukemia | ↑ ROS | Synergistic | [179] | |
| 2μM (24h) | Docetaxel | 1nM (24h) | Prostate | ↑ XIAP, ↓ BCL-2 | Synergistic | [97] | |
| 0.025% in diet (10 and 16 weeks) | Curcumin | 1% in diet (10 and 16 weeks) | Prostate | ↓ PDK-1/AKT | Synergistic | [208] | |
| 2.5μmol (28 days) | Curcumin | 3μmol (28 days) | Prostate | ↓ AKT | Synergistic | [209] | |
| 10μM (24h) | Curcumin | 25μM (24h) | Prostate | ↓ EGFR, ↓ AKT, ↓ NFkB | Synergistic | [210] | |
| 2μM (24h) | SFN | 0.4μM (24h) | Leukemia | ↓ iNOS, ↓ COX-2, ↓ prostaglandin E2 ↓ tumor necrosis factor (TNF), ↓ interleukin-1 (IL-1) | Synergistic | [206] | |
| 0.008% in diet (17 weeks) | d-limonene, Indole-3carbinol | 0.63%, 0.18% in diet (17 weeks) | Lung | ↓ Chemically induced tumorigenesis | Synergistic | [183] | |
| SFN | 20μM (24, 48h) | Radiation | 2Gy (24, 48h) | Osteosarcoma | ↑ Caspase cleavage, ↓ ERK, ↓ AKT, ↓ G2/M cell cycle arrest | Synergistic | [176] |
| 10μM (0, 12, 24, 48, 72h) | Radiation | 4Gy (0, 12, 24, 48, 72h) | Head and Neck Cancer | ↓ AKT, ↓ MCL-1 | Synergistic | [184] | |
| 3μM (24h) | Arsenic trioxide | 1μM (24h) | Multiple Myeloma | ↑ ER stress, ↑ HSP90, ↑ PERK, ↑ eIF2α, ↑ Unfolded protein response, ↑ ROS | Synergistic | [185] | |
| 20–30μM (16h) | 5-FU | 45–60μM (16h) | Salivary gland adenoid cystic carcinoma | ↓ NFkB | Synergistic | [182] | |
| 25μM (24h) | Adriyamycin | 0.6–10μM (24h) | Adriyamycin resistant mouse fibroblasta with mutant p53 | ↑p53 | Sensitization | [194] | |
| 1–20μM (6, 24h) | Oxaliplatin | 100nM – 10μM (6, 24h) | Colorectal Cancer | ↑ DNA fragmentation | Synergistic | [197] | |
| 10μM (72h) 3mg/kg (3 days) |
Sorafenib | 20μM (48h) 60mg/kg (3 days) |
Pancreatic cancer stem cells | ↓ALDH1, ↓ NFkB | Synergistic | [180] | |
| 25μM (24h) | Resveratrol | 25μM (24h) | Glioma cells | ↓ LDH, ↓ AKT, ↑ Caspase 3 cleavage | Synergistic | [186] | |
| 20μM (48, 72h) | Diindolylmethane | 20μM (48, 72h) | Colon | ↑ G2/M cell cycle arrest | Antagonistic (At low concentrations) Synergistic (At higher concentrations) | [201] | |
| Quercetin | Pancreatic cancer stem cells | ↓ BCL-2, ↓ XIAP, ↑ Caspase 3 cleavage | Synergistic | [187] | |||
| 10, 20μM (16h) | Quercetin | 25, 50μM (16h) | Melanoma | ↓ MMP-9 | Synergistic | [188] | |
| 10μM (6days) | Epigallocatechin gallate | 20μM (6days) | Ovarian | ↓ hTERT, ↓ BCL-2 | Synergistic | [213] | |
| 25μM (24h) 45mg/kg (3, 12h) |
Epigallocatechin gallate | 20, 100μM (24h) 100mg/kg (3, 12h) |
Prostate | ↓ Nrf2, ↓ AP-1 | Synergistic | [212] |
Radiation therapy is an important intervention for majority of cancers. Radiation has been shown to activate some important cancer cell survival signaling molecules like AKT, ERK and MCL-1 which lead to reduced efficacy. Our studies have shown that when BITC was combined with radiation therapy, a 2.8 fold increase in apoptosis and cleavage of caspase-3 was achieved in pancreatic cancer cells [58]. In addition to increased apoptosis, inhibition of NF-kB and activation of p38 was also observed with the combination of BITC and radiation therapy [58]. The combination of BITC or SFN with the radiation therapy caused increased G2/M cell cycle arrest [58, 175]. Combination of SFN with radiation therapy also showed inhibition of activation of critical molecules like AKT, ERK and MCL-1 along with induction of endoplasmic reticulum stress, explaining its efficacy [175, 183-187].
TNF-related apoptosis-inducing ligand (TRAIL) is a potential chemotherapeutic agent. Interestingly, TRAIL death receptors are highly expressed on cancer cells but not on normal cells making the cancer cells more susceptible to TRAIL-induced apoptosis as compared to normal cells [188, 189]. However, resistance to TRAIL is reported in many cancer cells [189, 190]. Our studies showed that BITC sensitized pancreatic cancer cells to TRAIL-induced apoptosis by activating both intrinsic and extrinsic pathway [191].
Accumulating evidence shows that combination of ITCs with conventional chemotherapeutics improves the efficacy against resistant cancer cells. Studies suggest synergistic activity of ITCs with common anti-cancer agents like cisplatin, adriamycin, etoposide, paclitaxel, metformin, vorinostat and docetaxel [176, 181, 192-194]. Both BITC and PEITC increased the apoptotic effects of cisplatin through depletion of beta-tubulin, but the combination did not affect DNA platination [180, 192]. Furthermore, reversal of the resistance to cisplatin was observed with PEITC, which was mediated by depletion of cellular GSH [176]. The combination of Metformin and PEITC also showed high efficacy in cisplatin resistant cancer cells [177]. PEITC and SFN caused inhibition of anti-apoptotic proteins like protein kinase C (α, β, ε and ς) and telomerase, while increasing pro-apoptotic protein kinase Cб to enhance the apoptosis caused by adriamycin and etoposide [194]. Also, the combination of adriamycin with SFN-induced sensitivity in resistant cancer cells by the effect of adriyamycin independent of p53 [193]. An HDAC inhibitor, vorinostat induced ROS to increase resistance in cancer cells. PEITC treatment suppressed the cytoprotective antioxidant response through depletion of cellular ROS, to reverse the resistance in leukemia cells [178]. The efficacy of taxanes was also enhanced by PEITC in different forms of cancer [97, 195]. The combination of SFN with oxaliplatin caused increased DNA fragmentation, suggesting synergism through oxaliplatin dependent mechanism [196]. NF-kB is a known target of SFN [197]. NF-kB inhibition by SFN mediated synergism with sorafenib and 5-fluorouracil (5-FU) to inhibit pancreatic cancer stem cell survival and salivary gland adenoid cystic carcinoma respectively [179, 181]. These observations suggest that ITCs can utilize the mechanisms of action of conventional agents or can induce independent effects to exhibit synergism.
Although most of the combinations exhibited synergistic effects in cancer cells, a combination of 5-FU with SFN showed antagonistic activity in the normal cells by modulating G2/M cell cycle phase [198]. This suggests that ITCs protect normal cells from the toxic effects of conventional therapeutic agents. Another study showed that the combination of cisplatin with ITCs was selectively effective in cancer cells [180]. Although the mechanism of selectivity remains to be elucidated, these observations clearly suggest an urgent need for clinical testing of the combination therapies of ITCs with conventional anti-cancer chemotherapeutics.
ITCs have been shown to offer synergism amongst themselves and other anti-cancer compounds. 3, 3'-diindolylmethane (DIM) is an important constituent of cruciferous vegetables and exhibits anti-cancer effects [199]. DIM synergizes with SFN leading to enhanced cell cycle arrest in colon cancer cells [200]. Amongst other ITCs, combination of BITC with SFN or PEITC was more effective in preventing pancreatic and lung cancer than the individual treatment [201, 202]. Curcumin is a well-known dietary agent with remarkable anti-cancer activity [203, 204]. The combination of curcumin with ITCs caused significant reduction in the levels of inflammatory markers. These observations advocate the possible synergistic or additive effect of curcumin in combination with ITCs [205, 206]. Several other studies re-enforce the enhanced anti-cancer effect of PEITC with curcumin through inhibition of pro-survival pathways like AKT, EGFR and NF-kB [207–209]. Epigallocatechin gallate (EGG), a green tea agent, has significant anti-cancer potential [210]. The chemo-preventive effects of the combination of SFN with EGG were successfully shown in transgenic model of prostate cancer through the induction of Nrf2 and AP-1 in Nrf2-deficient mice [211]. Furthermore, the combined treatment of SFN with EGG enhanced apoptosis in paclitaxel-resistant cancer cells by inhibiting hTERT and BCL-2 expression, showing therapeutic anti-cancer potential [212]. Taken together, it is clear from the above the studies that ITCs can be used for combination therapeutics in cancer treatment, especially for the resistant cancers. The combinations of ITCs with various anti-cancer agents and their prime mechanism of action have been summarized in Table 2.
6. Conclusion
Current epidemiological studies have certain limitations, such as differential exposure of the populations leading to misclassification, improper controls and possibility of recall bias. Hence, better designed studies are required to establish the role of ITCs as neutraceuticals for cancer prevention and treatment. Furthermore, better designed studies along with detailed mechanistic studies can provide us with an opportunity to use ITCs as the lead for synthesis of more potent and safe drugs through chemical modifications. It is important to note that some studies were done using extracts of ITCs from the vegetables. Few studies have shown that ITCs are susceptible to hydrolytic degradation at high temperatures and basic conditions [213, 214]. Thus the observations made by extracts of ITCs could be questionable especially if the extraction procedure was not appropriate or standard. These observations require further confirmation using pure forms of ITCs.
Recent studies have revealed many novel cancer targets. Specifically targeting these can enhance the efficacy of new as well as conventional therapies. Hence, it is important to test the efficacy of ITCs against new targets. Current preclinical evidence presented in the review provides an insight into potential anti-cancer mechanisms of action of the ITCs as well as their selectivity towards the cancer cells. Some clinical studies have been initiated already for some ITCs. Nonetheless, further detailed studies are required to establish the safety and efficacy profiles of these agents based on which they can be streamlined for further human studies. Based on the current data, it is evident that ITCs possess highly potential anti-cancer activity, but further detailed toxicity and clinical studies are required to warrant their future clinical benefits.
Figure 2.
Chemopreventive effects of ITCs
Figure 3.
Chemotherapeutic targets of ITCs
Acknowledgements
Sanjay K. Srivastava is currently an International Scholar at Kyung Hee University, Seoul, South Korea. This work was supported in part by R01 grants CA106953 and CA129038 (to Sanjay K. Srivastava) awarded by National Cancer Institute, NIH, and MRC grant 2007-0054931 (to S.H.K). Critical reading of the manuscript by Shari Morris is greatly appreciated.
Grant support: Supported in part by R01 grants CA129038 (to S.K.S) awarded by the National Cancer Institute, NIH and MRC grant 2007-0054931 (to S.H.K)
List of Abbreviations
- ITC
Isothiocyanate
- BITC
Benzylisothiocyanate
- AITC
Allyl isothiocyanate
- SFN
Sulforaphane
- PEITC
Phenethyl isothiocyanate
- GST
Glutathione-S-transferase
- VEGF
Vascular Endothelial Growth Factor
- STAT3
Signal Transducer and Activator of Transcription 3
- IL-6
Interleukin 6
- FOXO
Forkhead Box Protein
- MAPK
Mitogen-activated Protein Kinase
- Rb
Retinoblastoma protein
- ROS
Reactive Oxygen Species
- EMT
Epithelial to mesenchymal transition
- EGG
Epigallocatechin gallate
- SXR
Steroid and Xenobiotic receptor
- CYP
Cytochrome P450
- ER
Estrogen receptor
- EGFR
Epidermal growth factor receptor
Footnotes
Although we tried to include most of the published papers related to the objective of this review, it is possible that by mistake we may have missed a few papers. For this we would like to apologize to those authors.
In order to maintain consistency, we converted all the doses of ITCs into metric units which initially appeared in different forms in the literature. The conversion was made assuming the average weight of mice as 25g and average weight of rats as 250g. We would like to apologize for any deviation that might have occurred during unit conversion from the dose used in the actual study. We therefore included the units reported by the authors as well as values converted into metric units by us in parenthesis.
References
- [1].Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacological Research. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boggs DA, Palmer JR, Wise LA, Spiegelman D, et al. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women's Health Study. American Journal of Epidemiology. 2010;172:1268–1279. doi: 10.1093/aje/kwq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutrition and Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- [5].Tang L, Zirpoli GR, Guru K, Moysich KB, et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Palmer S. Diet, nutrition, and cancer. Progress in Food & Nutrition Science. 1985;9:283–341. [PubMed] [Google Scholar]
- [7].Boreddy SR, Srivastava SK. Pancreatic cancer chemoprevention by phytochemicals. Cancer Letters. 2012 doi: 10.1016/j.canlet.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fowke JH, Chung FL, Jin F, Qi D, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Research. 2003;63:3980–3986. [PubMed] [Google Scholar]
- [9].Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, et al. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. The Journal of Nutrition. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- [10].Bosetti C, Negri E, Franceschi S, Pelucchi C, et al. Diet and ovarian cancer risk: a case-control study in Italy. International Journal of Cancer. 2001;93:911–915. doi: 10.1002/ijc.1422. [DOI] [PubMed] [Google Scholar]
- [11].Zhang M, Yang ZY, Binns CW, Lee AH. Diet and ovarian cancer risk: a case-control study in China. British Journal of Cancer. 2002;86:712–717. doi: 10.1038/sj.bjc.6600085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:501–508. [PubMed] [Google Scholar]
- [13].Stoner GD, Morse MA. Isothiocyanates and plant polyphenols as inhibitors of lung and esophageal cancer. Cancer Letters. 1997;114:113–119. doi: 10.1016/s0304-3835(97)04639-9. [DOI] [PubMed] [Google Scholar]
- [14].Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fimognari C, Lenzi M, Hrelia P. Interaction of the isothiocyanate sulforaphane with drug disposition and metabolism: pharmacological and toxicological implications. Current Drug Metabolism. 2008;9:668–678. doi: 10.2174/138920008785821675. [DOI] [PubMed] [Google Scholar]
- [16].Gross-Steinmeyer K, Stapleton PL, Liu F, Tracy JH, et al. Phytochemical-induced changes in gene expression of carcinogen-metabolizing enzymes in cultured human primary hepatocytes. Xenobiotica. 2004;34:619–632. doi: 10.1080/00498250412331285481. [DOI] [PubMed] [Google Scholar]
- [17].Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metabolism and Disposition. 2001;29:1110–1113. [PubMed] [Google Scholar]
- [18].Yoshigae Y, Sridar C, Kent UM, Hollenberg PF. The Inactivation of Human CYP2E1 by Phenethyl Isothiocyanate, a Naturally Occurring Chemopreventive Agent, and Its Oxidative Bioactivation. Drug Metabolism and Disposition. 2013;41:858–869. doi: 10.1124/dmd.112.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Morse MA, Amin SG, Hecht SS, Chung FL. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Research. 1989;49:2894–2897. [PubMed] [Google Scholar]
- [20].Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. Journal of the National Cancer Institute. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- [21].Adam-Rodwell G, Morse MA, Stoner GD. The effects of phenethyl isothiocyanate on benzo[a]pyrene-induced tumors and DNA adducts in A/J mouse lung. Cancer Letters. 1993;71:35–42. doi: 10.1016/0304-3835(93)90094-p. [DOI] [PubMed] [Google Scholar]
- [22].Conaway CC, Wang CX, Pittman B, Yang YM, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Research. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- [23].Hecht SS. Approaches to chemoprevention of lung cancer based on carcinogens in tobacco smoke. Environmental Health Perspectives. 1997;105(Suppl 4):955–963. doi: 10.1289/ehp.97105s4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morse MA, Wang CX, Amin SG, Hecht SS, Chung FL. Effects of dietary sinigrin or indole-3-carbinol on O6-methylguanine-DNA-transmethylase activity and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA methylation and tumorigenicity in F344 rats. Carcinogenesis. 1988;9:1891–1895. doi: 10.1093/carcin/9.10.1891. [DOI] [PubMed] [Google Scholar]
- [25].Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. Journal of Agricultural and Food Chemistry. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- [26].Jiao D, Conaway CC, Wang MH, Yang CS, et al. Inhibition of N-nitrosodimethylamine demethylase in rat and human liver microsomes by isothiocyanates and their glutathione, L-cysteine, and N-acetyl-L-cysteine conjugates. Chemical Research in Toxicology. 1996;9:932–938. doi: 10.1021/tx9502094. [DOI] [PubMed] [Google Scholar]
- [27].Moreno RL, Kent UM, Hodge K, Hollenberg PF. Inactivation of cytochrome P450 2E1 by benzyl isothiocyanate. Chemical Research in Toxicology. 1999;12:582–587. doi: 10.1021/tx9900019. [DOI] [PubMed] [Google Scholar]
- [28].Steinkellner H, Rabot S, Freywald C, Nobis E, et al. Effects of cruciferous vegetables and their constituents on drug metabolizing enzymes involved in the bioactivation of DNA-reactive dietary carcinogens. Mutation Research. 2001;480–481:285–297. doi: 10.1016/s0027-5107(01)00188-9. [DOI] [PubMed] [Google Scholar]
- [29].Zhou C, Poulton EJ, Grun F, Bammler TK, et al. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Molecular Pharmacology. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]
- [30].Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. The AAPS Journal. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:949–954. [PubMed] [Google Scholar]
- [32].Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicology Letters. 1995;82–83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Research. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- [34].Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metabolism Reviews. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- [35].Mannervik B. The isoenzymes of glutathione transferase. Advances in Enzymology and Related Areas of Molecular Biology. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- [36].Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical Reviews in Biochemistry and Molecular Biology. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- [37].Thornalley PJ. Isothiocyanates: mechanism of cancer chemopreventive action. Anticancer Drugs. 2002;13:331–338. doi: 10.1097/00001813-200204000-00001. [DOI] [PubMed] [Google Scholar]
- [38].Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- [39].Beklemisheva AA, Feng J, Yeh YA, Wang LG, Chiao JW. Modulating testosterone stimulated prostate growth by phenethyl isothiocyanate via Sp1 and androgen receptor down-regulation. The Prostate. 2007;67:863–870. doi: 10.1002/pros.20472. [DOI] [PubMed] [Google Scholar]
- [40].Wang LG, Chiao JW. Prostate cancer chemopreventive activity of phenethyl isothiocyanate through epigenetic regulation (review) International Journal of Oncology. 2010;37:533–539. doi: 10.3892/ijo_00000702. [DOI] [PubMed] [Google Scholar]
- [41].Kong AN, Yu R, Hebbar V, Chen C, et al. Signal transduction events elicited by cancer prevention compounds. Mutation Research. 2001;480–481:231–241. doi: 10.1016/s0027-5107(01)00182-8. [DOI] [PubMed] [Google Scholar]
- [42].Keum YS, Yu S, Chang PP, Yuan X, et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Research. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- [43].Prawan A, Keum YS, Khor TO, Yu S, et al. Structural influence of isothiocyanates on the antioxidant response element (ARE)-mediated heme oxygenase-1 (HO-1) expression. Pharmaceutical Research. 2008;25:836–844. doi: 10.1007/s11095-007-9370-9. [DOI] [PubMed] [Google Scholar]
- [44].Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutation Research. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- [45].Zhang C, Su ZY, Khor TO, Shu L, Kong AN. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochemical Pharmacology. 2013;85:1398–1404. doi: 10.1016/j.bcp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutation Research. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- [47].Brigelius-Flohe R, Banning A. Part of the series: from dietary antioxidants to regulators in cellular signaling and gene regulation. Sulforaphane and selenium, partners in adaptive response and prevention of cancer. Free Radical Research. 2006;40:775–787. doi: 10.1080/10715760600722643. [DOI] [PubMed] [Google Scholar]
- [48].Bacon JR, Plumb GW, Howie AF, Beckett GJ, et al. Dual action of sulforaphane in the regulation of thioredoxin reductase and thioredoxin in human HepG2 and Caco-2 cells. Journal of Agricultural and Food Chemistry. 2007;55:1170–1176. doi: 10.1021/jf062398+. [DOI] [PubMed] [Google Scholar]
- [49].Zhang J, Svehlikova V, Bao Y, Howie AF, et al. Synergy between sulforaphane and selenium in the induction of thioredoxin reductase 1 requires both transcriptional and translational modulation. Carcinogenesis. 2003;24:497–503. doi: 10.1093/carcin/24.3.497. [DOI] [PubMed] [Google Scholar]
- [50].Wang W, Wang S, Howie AF, Beckett GJ, et al. Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. Journal of Agricultural and Food Chemistry. 2005;53:1417–1421. doi: 10.1021/jf048153j. [DOI] [PubMed] [Google Scholar]
- [51].Jakubikova J, Cervi D, Ooi M, Kim K, et al. Anti-tumor activity and signaling events triggered by the isothiocyanates, sulforaphane and phenethyl isothiocyanate, in multiple myeloma. Haematologica. 2011;96:1170–1179. doi: 10.3324/haematol.2010.029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huang C, Ma WY, Li J, Hecht SS, Dong Z. Essential role of p53 in phenethyl isothiocyanate-induced apoptosis. Cancer Research. 1998;58:4102–4106. [PubMed] [Google Scholar]
- [53].Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong AN. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Research. 1998;58:402–408. [PubMed] [Google Scholar]
- [54].Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. The Biochemical Journal. 2002;364:301–307. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clinical Cancer Research. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Batra S, Sahu RP, Kandala PK, Srivastava SK. Benzyl isothiocyanate-mediated inhibition of histone deacetylase leads to NF-kappaB turnoff in human pancreatic carcinoma cells. Molecular Cancer Therapeutics. 2010;9:1596–1608. doi: 10.1158/1535-7163.MCT-09-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sahu RP, Zhang R, Batra S, Shi Y, Srivastava SK. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30:1744–1753. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sahu RP, Epperly MW, Srivastava SK. Benzyl isothiocyanate sensitizes human pancreatic cancer cells to radiation therapy. Frontiers in Biosciences. 2009;1:568–576. doi: 10.2741/e55. [DOI] [PubMed] [Google Scholar]
- [59].Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. Journal of the National Cancer Institute. 2009;101:176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang R, Loganathan S, Humphreys I, Srivastava SK. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. The Journal of Nutrition. 2006;136:2728–2734. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]
- [61].Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- [62].Kalkunte S, Swamy N, Dizon DS, Brard L. Benzyl isothiocyanate (BITC) induces apoptosis in ovarian cancer cells in vitro. Journal of Experimental Therapeutics & Oncology. 2006;5:287–300. [PubMed] [Google Scholar]
- [63].Nakamura Y, Kawakami M, Yoshihiro A, Miyoshi N, et al. Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. The Journal of Biological Chemistry. 2002;277:8492–8499. doi: 10.1074/jbc.M109760200. [DOI] [PubMed] [Google Scholar]
- [64].Finkielsztein A, Kelly GM. Altering PI3K-Akt signalling in zebrafish embryos affects PTEN phosphorylation and gastrulation. Biology of the Cell. 2009;101:661–678. doi: 10.1042/BC20090034. [DOI] [PubMed] [Google Scholar]
- [65].Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. British Journal of Cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- [67].Lin JF, Tsai TF, Liao PC, Lin YH, et al. Benzyl isothiocyanate induces protective autophagy in human prostate cancer cells via inhibition of mTOR signaling. Carcinogenesis. 2013;34:406–414. doi: 10.1093/carcin/bgs359. [DOI] [PubMed] [Google Scholar]
- [68].Xiao D, Bommareddy A, Kim SH, Sehrawat A, et al. Benzyl isothiocyanate causes FoxO1-mediated autophagic death in human breast cancer cells. PLoS One. 2012;7:e32597. doi: 10.1371/journal.pone.0032597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annual Review of Immunology. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- [70].Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- [71].Darnell JE. Validating Stat3 in cancer therapy. Nature Medicine. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- [72].Wei D, Le X, Zheng L, Wang L, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- [73].Fouse SD, Costello JF. Cancer Stem Cells Activate STAT3 the EZ Way. Cancer Cell. 2013;23:711–713. doi: 10.1016/j.ccr.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Current Opinion in Cell Biology. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- [75].Xu Q, Briggs J, Park S, Niu G, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- [76].Jung JE, Lee HG, Cho IH, Chung DH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB Journal. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- [77].Boreddy SR, Sahu RP, Srivastava SK. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-alpha/VEGF/Rho-GTPases: pivotal role of STAT-3. PLoS One. 2011;6:e25799. doi: 10.1371/journal.pone.0025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Warin R, Xiao D, Arlotti JA, Bommareddy A, Singh SV. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Molecular Carcinogenesis. 2010;49:500–507. doi: 10.1002/mc.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wu CL, Huang AC, Yang JS, Liao CL, et al. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. Journal of Orthopaedic Research. 2011;29:1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- [80].Wu X, Zhu Y, Yan H, Liu B, et al. Isothiocyanates induce oxidative stress and suppress the metastasis potential of human non-small cell lung cancer cells. BMC Cancer. 2010;10:269. doi: 10.1186/1471-2407-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Miyoshi N, Takabayashi S, Osawa T, Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25:567–575. doi: 10.1093/carcin/bgh051. [DOI] [PubMed] [Google Scholar]
- [82].Zhu Y, Zhuang JX, Wang Q, Zhang HY, Yang P. Inhibitory effect of benzyl isothiocyanate on proliferation in vitro of human glioma cells. Asian Pacific Journal of Cancer Prevention. 2013;14:2607–2610. doi: 10.7314/apjcp.2013.14.4.2607. [DOI] [PubMed] [Google Scholar]
- [83].Kawakami M, Harada N, Hiratsuka M, Kawai K, Nakamura Y. Dietary isothiocyanates modify mitochondrial functions through their electrophilic reaction. Bioscience, Biotechnology, and Biochemistry. 2005;69:2439–2444. doi: 10.1271/bbb.69.2439. [DOI] [PubMed] [Google Scholar]
- [84].Zhou T, Li G, Cao B, Liu L, et al. Downregulation of Mcl-1 through inhibition of translation contributes to benzyl isothiocyanate-induced cell cycle arrest and apoptosis in human leukemia cells. Cell Death & Disease. 2013;4:e515. doi: 10.1038/cddis.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kim EJ, Hong JE, Eom SJ, Lee JY, Park JH. Oral administration of benzylisothiocyanate inhibits solid tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. Breast Cancer Research and Treatment. 2011;130:61–71. doi: 10.1007/s10549-010-1299-8. [DOI] [PubMed] [Google Scholar]
- [86].Sehrawat A, Kim SH, Vogt A, Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. 2013;34:864–873. doi: 10.1093/carcin/bgs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kim SH, Sehrawat A, Singh SV. Dietary chemopreventative benzyl isothiocyanate inhibits breast cancer stem cells in vitro and in vivo. Cancer Prevention Research. 2013;6:782–790. doi: 10.1158/1940-6207.CAPR-13-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kim SH, Sehrawat A, Singh SV. Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration. Breast Cancer Research and Treatment. 2012;134:1067–1079. doi: 10.1007/s10549-012-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Qhattal HS, Wang S, Salihima T, Srivastava SK, Liu X. Nanoemulsions of cancer chemopreventive agent benzyl isothiocyanate display enhanced solubility, dissolution, and permeability. Journal of Agricultural and Food Chemistry. 2011;59:12396–12404. doi: 10.1021/jf202612b. [DOI] [PubMed] [Google Scholar]
- [90].Lewerenz HJ, Bleyl DW, Plass R. Subacute oral toxicity study of benzyl isothiocyanate in rats. Die Nahrung. 1992;36:190–198. doi: 10.1002/food.19920360213. [DOI] [PubMed] [Google Scholar]
- [91].Hecht SS, Chung FL, Richie JP, Jr., Akerkar SA, et al. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiology, Biomarkers & Prevention. 1995;4:877–884. [PubMed] [Google Scholar]
- [92].Chung FL, Morse MA, Eklind KI, Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiology, Biomarkers & Prevention. 1992;1:383–388. [PubMed] [Google Scholar]
- [93].Guo Z, Smith TJ, Wang E, Eklind KI, et al. Structure-activity relationships of arylalkyl isothiocyanates for the inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism and the modulation of xenobiotic-metabolizing enzymes in rats and mice. Carcinogenesis. 1993;14:1167–1173. doi: 10.1093/carcin/14.6.1167. [DOI] [PubMed] [Google Scholar]
- [94].Gupta P, Adkins C, Lockman P, Srivastava S. Metastasis of breast tumor cells to brain is suppressed by phenethyl isothiocyanate in a novel in vivo metastasis model. PLoS One. 2013;8:e67278. doi: 10.1371/journal.pone.0067278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gupta P, Srivastava SK. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Medicine. 2012;10:80. doi: 10.1186/1741-7015-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Trachootham D, Zhou Y, Zhang H, Demizu Y, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [97].Xiao D, Singh SV. Phenethyl isothiocyanate sensitizes androgen-independent human prostate cancer cells to docetaxel-induced apoptosis in vitro and in vivo. Pharmaceutical Research. 2010;27:722–731. doi: 10.1007/s11095-010-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen G, Chen Z, Hu Y, Huang P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by beta-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxidants & Redox Signaling. 2011;15:2911–2921. doi: 10.1089/ars.2011.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Syed Alwi SS, Cavell BE, Donlevy A, Packham G. Differential induction of apoptosis in human breast cancer cell lines by phenethyl isothiocyanate, a glutathione depleting agent. Cell Stress & Chaperones. 2012;17:529–538. doi: 10.1007/s12192-012-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Powolny AA, Singh SV. Differential response of normal (PrEC) and cancerous human prostate cells (PC-3) to phenethyl isothiocyanate-mediated changes in expression of antioxidant defense genes. Pharmaceutical Research. 2010;27:2766–2775. doi: 10.1007/s11095-010-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. The Journal of Biological Chemistry. 1998;273:1769–1775. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- [102].Yang YM, Conaway CC, Chiao JW, Wang CX, et al. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Research. 2002;62:2–7. [PubMed] [Google Scholar]
- [103].Lai KC, Hsu SC, Kuo CL, Ip SW, et al. Phenethyl Isothiocyanate Inhibited Tumor Migration and Invasion via Suppressing Multiple Signal Transduction Pathways in Human Colon Cancer HT29 Cells. Journal of Agricultural and Food Chemistry. 2010 doi: 10.1021/jf102384n. [DOI] [PubMed] [Google Scholar]
- [104].Hwang ES, Lee HJ. Phenylethyl isothiocyanate and its N-acetylcysteine conjugate suppress the metastasis of SK-Hep1 human hepatoma cells. The Journal of Nutritional Biochemistry. 2006;17:837–846. doi: 10.1016/j.jnutbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [105].Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Research. 2007;67:2239–2246. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- [106].Wang XH, Cavell BE, Syed Alwi SS, Packham G. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochemical Pharmacology. 2009;78:261–272. doi: 10.1016/j.bcp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- [107].Mi L, Hood BL, Stewart NA, Xiao Z, et al. Identification of potential protein targets of isothiocyanates by proteomics. Chemical Research in Toxicology. 2011;24:1735–1743. doi: 10.1021/tx2002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Suzuki S, Dobashi Y, Minato H, Tajiri R, et al. EGFR and HER2-Akt-mTOR signaling pathways are activated in subgroups of salivary gland carcinomas. Virchows Archiv. 2012;461:271–282. doi: 10.1007/s00428-012-1282-3. [DOI] [PubMed] [Google Scholar]
- [109].Loganathan S, Kandala PK, Gupta P, Srivastava SK. Inhibition of EGFR-AKT axis results in the suppression of ovarian tumors in vitro and in preclinical mouse model. PLoS One. 2012;7:e43577. doi: 10.1371/journal.pone.0043577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kang L, Wang ZY. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-alpha36. Journal of Cellular and Molecular Medicine. 2010;14:1485–1493. doi: 10.1111/j.1582-4934.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Syed Alwi SS, Cavell BE, Telang U, Morris ME, et al. In vivo modulation of 4E binding protein 1 (4E-BP1) phosphorylation by watercress: a pilot study. The British Journal of Nutrition. 2010;104:1288–1296. doi: 10.1017/S0007114510002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Izzotti A, Larghero P, Balansky R, Pfeffer U, et al. Interplay between histopathological alterations, cigarette smoke and chemopreventive agents in defining microRNA profiles in mouse lung. Mutation Research. 2011;717:17–24. doi: 10.1016/j.mrfmmm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- [113].Huong LD, Shin JA, Choi ES, Cho NP, et al. beta-Phenethyl isothiocyanate induces death receptor 5 to induce apoptosis in human oral cancer cells via p38. Oral Diseases. 2012;18:513–519. doi: 10.1111/j.1601-0825.2012.01905.x. [DOI] [PubMed] [Google Scholar]
- [114].Huong le D, Shim JH, Choi KH, Shin JA, et al. Effect of beta-phenylethyl isothiocyanate from cruciferous vegetables on growth inhibition and apoptosis of cervical cancer cells through the induction of death receptors 4 and 5. Journal of Agricultural and Food Chemistry. 2011;59:8124–8131. doi: 10.1021/jf2006358. [DOI] [PubMed] [Google Scholar]
- [115].Pullar JM, Thomson SJ, King MJ, Turnbull CI, et al. The chemopreventive agent phenethyl isothiocyanate sensitizes cells to Fas-mediated apoptosis. Carcinogenesis. 2004;25:765–772. doi: 10.1093/carcin/bgh063. [DOI] [PubMed] [Google Scholar]
- [116].Chen PY, Lin KC, Lin JP, Tang NY, et al. Phenethyl Isothiocyanate (PEITC) Inhibits the Growth of Human Oral Squamous Carcinoma HSC-3 Cells through G(0)/G(1) Phase Arrest and Mitochondria-Mediated Apoptotic Cell Death. Evidence-based Complementary and Alternative Medicine. 2012;2012:718320. doi: 10.1155/2012/718320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tang NY, Huang YT, Yu CS, Ko YC, et al. Phenethyl isothiocyanate (PEITC) promotes G2/M phase arrest via p53 expression and induces apoptosis through caspase- and mitochondria-dependent signaling pathways in human prostate cancer DU 145 cells. Anticancer Research. 2011;31:1691–1702. [PubMed] [Google Scholar]
- [118].Lee JW, Cho MK. Phenethyl isothiocyanate induced apoptosis via down regulation of Bcl-2/XIAP and triggering of the mitochondrial pathway in MCF-7 cells. Archives of Pharmacal Research. 2008;31:1604–1612. doi: 10.1007/s12272-001-2158-2. [DOI] [PubMed] [Google Scholar]
- [119].Xiao D, Lew KL, Zeng Y, Xiao H, et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–2234. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- [120].Wang X, Di Pasqua AJ, Govind S, McCracken E, et al. Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. Journal of Medicinal Chemistry. 2011;54:809–816. doi: 10.1021/jm101199t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Chiao JW, Wu H, Ramaswamy G, Conaway CC, et al. Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis. 2004;25:1403–1408. doi: 10.1093/carcin/bgh136. [DOI] [PubMed] [Google Scholar]
- [122].Saw CL, Cintron M, Wu TY, Guo Y, et al. Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of Nrf2-mediated phase II drug metabolizing and antioxidant genes and synergism with isothiocyanates. Biopharmaceutics & Drug Disposition. 2011;32:289–300. doi: 10.1002/bdd.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Moon YJ, Brazeau DA, Morris ME. Dietary phenethyl isothiocyanate alters gene expression in human breast cancer cells. Evidence-based Complementary and Alternative Medicine. 2011;2011:462525. doi: 10.1155/2011/462525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Pawlik A, Szczepanski MA, Klimaszewska A, Gackowska L, et al. Phenethyl isothiocyanate-induced cytoskeletal changes and cell death in lung cancer cells. Food and Chemical Toxicology. 2012;50:3577–3594. doi: 10.1016/j.fct.2012.07.043. [DOI] [PubMed] [Google Scholar]
- [125].Sakao K, Desineni S, Hahm ER, Singh SV. Phenethyl isothiocyanate suppresses inhibitor of apoptosis family protein expression in prostate cancer cells in culture and in vivo. Prostate. 2012;72:1104–1116. doi: 10.1002/pros.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ji Y, Kuo Y, Morris ME. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharmaceutical Research. 2005;22:1658–1666. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- [127].Conaway CC, Jiao D, Kohri T, Liebes L, Chung FL. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metabolism and Disposition. 1999;27:13–20. [PubMed] [Google Scholar]
- [128].Gao N, Budhraja A, Cheng S, Liu EH, et al. Phenethyl isothiocyanate exhibits antileukemic activity in vitro and in vivo by inactivation of Akt and activation of JNK pathways. Cell Death Dis. 2011;2:e140. doi: 10.1038/cddis.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tsou MF, Tien N, Lu CC, Chiang JH, et al. Phenethyl isothiocyanate promotes immune responses in normal BALB/c mice, inhibits murine leukemia WEHI-3 cells, and stimulates immunomodulations in vivo. Environmental Toxicology. 2013;28:127–136. doi: 10.1002/tox.20705. [DOI] [PubMed] [Google Scholar]
- [130].Li Y, Wang EJ, Chen L, Stein AP, et al. Effects of phenethyl isothiocyanate on acetaminophen metabolism and hepatotoxicity in mice. Toxicology and Applied Pharmacology. 1997;144:306–314. doi: 10.1006/taap.1997.8134. [DOI] [PubMed] [Google Scholar]
- [131].Mi L, Xiao Z, Hood BL, Dakshanamurthy S, et al. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. The Journal of Biological Chemistry. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: from cells to mice to man. Seminars in Cancer Biology. 2007;17:363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wang F, Shan Y. Sulforaphane retards the growth of UM-UC-3 xenographs, induces apoptosis, and reduces survivin in athymic mice. Nutrition Research. 2012;32:374–380. doi: 10.1016/j.nutres.2012.03.014. [DOI] [PubMed] [Google Scholar]
- [135].Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- [136].Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Molecular Cancer Therapeutics. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- [137].Rajendran P, Kidane AI, Yu TW, Dashwood WM, et al. HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates. Epigenetics. 2013;8:612–623. doi: 10.4161/epi.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hsu A, Wong CP, Yu Z, Williams DE, et al. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clinical Epigenetics. 2011;3:3. doi: 10.1186/1868-7083-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Jackson SJ, Singletary KW, Venema RC. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vascular Pharmacology. 2007;46:77–84. doi: 10.1016/j.vph.2006.06.015. [DOI] [PubMed] [Google Scholar]
- [140].Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Molecular Cancer Therapeutics. 2006;5:575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- [141].Davis R, Singh KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncology Reports. 2009;22:1473–1478. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- [142].Choi S, Lew KL, Xiao H, Herman-Antosiewicz A, et al. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28:151–162. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- [143].Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Molecular and Cellular Biochemistry. 2013;373:217–227. doi: 10.1007/s11010-012-1493-6. [DOI] [PubMed] [Google Scholar]
- [144].Singh SV, Srivastava SK, Choi S, Lew KL, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. The Journal of Biological Chemistry. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- [145].Hsu YC, Chang SJ, Wang MY, Chen YL, Huang TY. Growth inhibition and apoptosis of neuroblastoma cells through ROS-independent MEK/ERK activation by sulforaphane. Cell Biochemistry and Biophysics. 2013;66:765–774. doi: 10.1007/s12013-013-9522-y. [DOI] [PubMed] [Google Scholar]
- [146].Shan Y, Sun C, Zhao X, Wu K, et al. Effect of sulforaphane on cell growth, G(0)/G(1) phase cell progression and apoptosis in human bladder cancer T24 cells. International Journal of Oncology. 2006;29:883–888. [PubMed] [Google Scholar]
- [147].Gamet-Payrastre L, Li P, Lumeau S, Cassar G, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Research. 2000;60:1426–1433. [PubMed] [Google Scholar]
- [148].Suppipat K, Park CS, Shen Y, Zhu X, Lacorazza HD. Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS One. 2012;7:e51251. doi: 10.1371/journal.pone.0051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Shen G, Xu C, Chen C, Hebbar V, Kong AN. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemotherapy and Pharmacology. 2006;57:317–327. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- [150].Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochemical Pharmacology. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- [151].Jee HG, Lee KE, Kim JB, Shin HK, Youn YK. Sulforaphane inhibits oral carcinoma cell migration and invasion in vitro. Phytotherapy Research. 2011;25:1623–1628. doi: 10.1002/ptr.3397. [DOI] [PubMed] [Google Scholar]
- [152].Kanematsu S, Yoshizawa K, Uehara N, Miki H, et al. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncology Reports. 2011;26:603–608. doi: 10.3892/or.2011.1311. [DOI] [PubMed] [Google Scholar]
- [153].Shan Y, Zhang L, Bao Y, Li B, et al. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. The Journal of Nutritional Biochemistry. 2013;24:1062–1069. doi: 10.1016/j.jnutbio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- [154].Gerhauser C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Curr Opin Clin Nutr Metab Care. 2013;16:405–410. doi: 10.1097/MCO.0b013e328362014e. [DOI] [PubMed] [Google Scholar]
- [155].Hu R, Hebbar V, Kim BR, Chen C, et al. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. The Journal of Pharmacology and Experimental Therapeutics. 2004;310:263–271. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]
- [156].Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- [157].Hu R, Khor TO, Shen G, Jeong WS, et al. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- [158].Priya DKD, Gayathri R, Gunassekaran GR, Murugan S, Sakthisekaran D. Inhibitory Effect of Sulforaphane against Benzo(a)pyrene Induced Lung Cancer by Modulation of Biochemical Signatures in Female Swiss Albino Mice. Asian Journal of Biochemistry. 2011;6:395–405. [Google Scholar]
- [159].Zhao HD, Zhang F, Shen G, Li YB, et al. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World Journal of Gastroenterology. 2010;16:3002–3010. doi: 10.3748/wjg.v16.i24.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Jiao D, Ho CT, Foiles P, Chung FL. Identification and quantification of the N-acetylcysteine conjugate of allyl isothiocyanate in human urine after ingestion of mustard. Cancer Epidemiology, Biomarkers & Prevention. 1994;3:487–492. [PubMed] [Google Scholar]
- [161].Charron CS, Clevidence BA, Albaugh GA, Kramer MH, et al. Assessment of DNA damage and repair in adults consuming allyl isothiocyanate or Brassica vegetables. The Journal of Nutritional Biochemistry. 2013;24:894–902. doi: 10.1016/j.jnutbio.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Smith T, Musk SR, Johnson IT. Allyl isothiocyanate selectively kills undifferentiated HT29 cells in vitro and suppresses aberrant crypt foci in the colonic mucosa of rats. Biochemical Society Transactions. 1996;24:381S. doi: 10.1042/bst024381s. [DOI] [PubMed] [Google Scholar]
- [163].Smith TK, Lund EK, Parker ML, Clarke RG, Johnson IT. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis. 2004;25:1409–1415. doi: 10.1093/carcin/bgh149. [DOI] [PubMed] [Google Scholar]
- [164].Xu C, Shen G, Yuan X, Kim JH, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–445. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- [165].Tsai SC, Huang WW, Huang WC, Lu CC, et al. ERK-modulated intrinsic signaling and G(2)/M phase arrest contribute to the induction of apoptotic death by allyl isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. International Journal of Oncology. 2012;41:2065–2072. doi: 10.3892/ijo.2012.1640. [DOI] [PubMed] [Google Scholar]
- [166].Geng F, Tang L, Li Y, Yang L, et al. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via Bcl-2 protein phosphorylation. The Journal of Biological Chemistry. 2011;286:32259–32267. doi: 10.1074/jbc.M111.278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Srivastava SK, Xiao D, Lew KL, Hershberger P, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- [168].Hwang ES, Lee HJ. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Experimental Biology and Medicine. 2006;231:421–430. doi: 10.1177/153537020623100408. [DOI] [PubMed] [Google Scholar]
- [169].Kumar A, D'Souza SS, Tickoo S, Salimath BP, Singh HB. Antiangiogenic and proapoptotic activities of allyl isothiocyanate inhibit ascites tumor growth in vivo. Integrative Cancer Therapies. 2009;8:75–87. doi: 10.1177/1534735408330716. [DOI] [PubMed] [Google Scholar]
- [170].Thejass P, Kuttan G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-alpha (TNF-alpha) production. Nitric Oxide. 2007;16:247–257. doi: 10.1016/j.niox.2006.09.006. [DOI] [PubMed] [Google Scholar]
- [171].Imaizumi K, Sato S, Sakakibara Y, Mori S, et al. Allyl isothiocyanate-induced changes in the distribution of white blood cells in rats. The Journal of Toxicological Sciences. 2010;35:583–589. doi: 10.2131/jts.35.583. [DOI] [PubMed] [Google Scholar]
- [172].Manesh C, Kuttan G. Effect of naturally occurring isothiocyanates on the immune system. Immunopharmacology and Immunotoxicology. 2003;25:451–459. doi: 10.1081/iph-120024512. [DOI] [PubMed] [Google Scholar]
- [173].Lewerenz HJ, Plass R, Macholz R. Effect of allyl isothiocyanate on hepatic monooxygenases and serum transferases in rats. Toxicology Letters. 1988;44:65–70. doi: 10.1016/0378-4274(88)90130-0. [DOI] [PubMed] [Google Scholar]
- [174].Masutomi N, Toyoda K, Shibutani M, Niho N, et al. Toxic effects of benzyl and allyl isothiocyanates and benzyl-isoform specific metabolites in the urinary bladder after a single intravesical application to rats. Toxicologic Pathology. 2001;29:617–622. doi: 10.1080/019262301753385942. [DOI] [PubMed] [Google Scholar]
- [175].Sawai Y, Murata H, Horii M, Koto K, et al. Effectiveness of sulforaphane as a radiosensitizer for murine osteosarcoma cells. Oncology Reports. 2013;29:941–945. doi: 10.3892/or.2012.2195. [DOI] [PubMed] [Google Scholar]
- [176].Wu WJ, Zhang Y, Zeng ZL, Li XB, et al. beta-phenylethyl isothiocyanate reverses platinum resistance by a GSH-dependent mechanism in cancer cells with epithelialmesenchymal transition phenotype. Biochemical Pharmacology. 2013;85:486–496. doi: 10.1016/j.bcp.2012.11.017. [DOI] [PubMed] [Google Scholar]
- [177].Chan DK, Miskimins WK. Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures. Journal of Ovarian Research. 2012;5:19. doi: 10.1186/1757-2215-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Hu Y, Lu W, Chen G, Zhang H, et al. Overcoming resistance to histone deacetylase inhibitors in human leukemia with the redox modulating compound beta-phenylethyl isothiocyanate. Blood. 2010;116:2732–2741. doi: 10.1182/blood-2009-11-256354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rausch V, Liu L, Kallifatidis G, Baumann B, et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Research. 2010;70:5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- [180].Wang X, Govind S, Sajankila SP, Mi L, et al. Phenethyl isothiocyanate sensitizes human cervical cancer cells to apoptosis induced by cisplatin. Molecular Nutrition & Food Research. 2011;55:1572–1581. doi: 10.1002/mnfr.201000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Wang XF, Wu DM, Li BX, Lu YJ, Yang BF. Synergistic inhibitory effect of sulforaphane and 5-fluorouracil in high and low metastasis cell lines of salivary gland adenoid cystic carcinoma. Phytotherapy Research. 2009;23:303–307. doi: 10.1002/ptr.2618. [DOI] [PubMed] [Google Scholar]
- [182].el-Bayoumy K, Upadhyaya P, Desai DH, Amin S, et al. Effects of 1,4-phenylenebis(methylene)selenocyanate, phenethyl isothiocyanate, indole-3-carbinol, and dlimonene individually and in combination on the tumorigenicity of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Anticancer Research. 1996;16:2709–2712. [PubMed] [Google Scholar]
- [183].Kotowski U, Heiduschka G, Brunner M, Czembirek C, et al. Radiosensitization of head and neck cancer cells by the phytochemical agent sulforaphane. Strahlentherapie und Onkologie. 2011;187:575–580. doi: 10.1007/s00066-011-2218-6. [DOI] [PubMed] [Google Scholar]
- [184].Doudican NA, Wen SY, Mazumder A, Orlow SJ. Sulforaphane synergistically enhances the cytotoxicity of arsenic trioxide in multiple myeloma cells via stress-mediated pathways. Oncology Reports. 2012;28:1851–1858. doi: 10.3892/or.2012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Jiang H, Shang X, Wu H, Huang G, et al. Combination treatment with resveratrol and sulforaphane induces apoptosis in human U251 glioma cells. Neurochemical Research. 2010;35:152–161. doi: 10.1007/s11064-009-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Frontiers in Biosciences. 2011;3:515–528. doi: 10.2741/e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Pradhan SJ, Mishra R, Sharma P, Kundu GC. Quercetin and sulforaphane in combination suppress the progression of melanoma through the down-regulation of matrix metalloproteinase-9. Experimental and Therapeutic Medicine. 2010;1:915–920. doi: 10.3892/etm.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sheridan JP, Marsters SA, Pitti RM, Gurney A, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- [189].Koschny R, Walczak H, Ganten TM. The promise of TRAIL--potential and risks of a novel anticancer therapy. Journal of Molecular Medicine. 2007;85:923–935. doi: 10.1007/s00109-007-0194-1. [DOI] [PubMed] [Google Scholar]
- [190].Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resistance Updates. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- [191].Wicker CA, Sahu RP, Kulkarni-Datar K, Srivastava SK, Brown TL. BITC Sensitizes Pancreatic Adenocarcinomas to TRAIL-induced Apoptosis. Cancer Growth and Metastasis. 2010;2009:45–55. [PMC free article] [PubMed] [Google Scholar]
- [192].Di Pasqua AJ, Hong C, Wu MY, McCracken E, et al. Sensitization of non-small cell lung cancer cells to cisplatin by naturally occurring isothiocyanates. Chemical Research in Toxicology. 2010;23:1307–1309. doi: 10.1021/tx100187f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Fimognari C, Lenzi M, Sciuscio D, Cantelli-Forti G, Hrelia P. Combination of doxorubicin and sulforaphane for reversing doxorubicin-resistant phenotype in mouse fibroblasts with p53Ser220 mutation. Annals of the New York Academy of Sciences. 2007;1095:62–69. doi: 10.1196/annals.1397.008. [DOI] [PubMed] [Google Scholar]
- [194].Mukherjee S, Dey S, Bhattacharya RK, Roy M. Isothiocyanates sensitize the effect of chemotherapeutic drugs via modulation of protein kinase C and telomerase in cervical cancer cells. Molecular and Cellular Biochemistry. 2009;330:9–22. doi: 10.1007/s11010-009-0095-4. [DOI] [PubMed] [Google Scholar]
- [195].Liu K, Cang S, Ma Y, Chiao JW. Synergistic effect of paclitaxel and epigenetic agent phenethyl isothiocyanate on growth inhibition, cell cycle arrest and apoptosis in breast cancer cells. Cancer Cell International. 2013;13:10. doi: 10.1186/1475-2867-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kaminski BM, Weigert A, Brune B, Schumacher M, et al. Sulforaphane potentiates oxaliplatin-induced cell growth inhibition in colorectal cancer cells via induction of different modes of cell death. Cancer Chemotherapy and Pharmacology. 2011;67:1167–1178. doi: 10.1007/s00280-010-1413-y. [DOI] [PubMed] [Google Scholar]
- [197].Kallifatidis G, Rausch V, Baumann B, Apel A, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- [198].Milczarek M, Misiewicz-Krzeminska I, Lubelska K, Wiktorska K. Combination treatment with 5-fluorouracil and isothiocyanates shows an antagonistic effect in Chinese hamster fibroblast cells line-V79. Acta Poloniae Pharmaceutica. 2011;68:331–342. [PubMed] [Google Scholar]
- [199].Kandala PK, Srivastava SK. DIMming ovarian cancer growth. Current Drug Targets. 2012;13:1869–1875. doi: 10.2174/138945012804545650. [DOI] [PubMed] [Google Scholar]
- [200].Pappa G, Strathmann J, Lowinger M, Bartsch H, Gerhauser C. Quantitative combination effects between sulforaphane and 3,3'-diindolylmethane on proliferation of human colon cancer cells in vitro. Carcinogenesis. 2007;28:1471–1477. doi: 10.1093/carcin/bgm044. [DOI] [PubMed] [Google Scholar]
- [201].Hutzen B, Willis W, Jones S, Cen L, et al. Dietary agent, benzyl isothiocyanate inhibits signal transducer and activator of transcription 3 phosphorylation and collaborates with sulforaphane in the growth suppression of PANC-1 cancer cells. Cancer Cell International. 2009;9:24. doi: 10.1186/1475-2867-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Letters. 2000;150:49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- [203].Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Current Problems in Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- [204].Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. British Journal of Cancer. 2009;100:1425–1433. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Cheung KL, Khor TO, Kong AN. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharmaceutical Research. 2009;26:224–231. doi: 10.1007/s11095-008-9734-9. [DOI] [PubMed] [Google Scholar]
- [206].Thakkar A, Sutaria D, Grandhi BK, Wang J, Prabhu S. The molecular mechanism of action of aspirin, curcumin and sulforaphane combinations in the chemoprevention of pancreatic cancer. Oncology Reports. 2013;29:1671–1677. doi: 10.3892/or.2013.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Barve A, Khor TO, Hao X, Keum YS, et al. Murine prostate cancer inhibition by dietary phytochemicals--curcumin and phenyethylisothiocyanate. Pharmaceutical Research. 2008;25:2181–2189. doi: 10.1007/s11095-008-9574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Khor TO, Keum YS, Lin W, Kim JH, et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Research. 2006;66:613–621. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- [209].Kim JH, Xu C, Keum YS, Reddy B, et al. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27:475–482. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- [210].Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochemical Pharmacology. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Nair S, Barve A, Khor TO, Shen GX, et al. Regulation of Nrf2- and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta Pharmacologica Sinica. 2010;31:1223–1240. doi: 10.1038/aps.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Chen H, Landen CN, Li Y, Alvarez RD, Tollefsbol TO. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Experimental Cell Research. 2013;319:697–706. doi: 10.1016/j.yexcr.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].De Nicola GR, Montaut S, Rollin P, Nyegue M, et al. Stability of benzylic-type isothiocyanates in hydrodistillation-mimicking conditions. Journal of Agricultural and Food Chemistry. 2013;61:137–142. doi: 10.1021/jf3041534. [DOI] [PubMed] [Google Scholar]
- [214].Franklin SJ, Dickinson SE, Karlage KL, Bowden GT, Myrdal PB. Stability of sulforaphane for topical formulation. Drug Development and Industrial Pharmacy. 2013 doi: 10.3109/03639045.2013.768634. [DOI] [PMC free article] [PubMed] [Google Scholar]