Abstract
Recent data show that people living with HIV/AIDS (PLWHA) are at a greater risk of cardiovascular disease (CVD), which could possibly be explained by an increased prevalence of metabolic syndrome (MetSyn) due to the known toxicities associated with antiretroviral therapy (ART). The purpose of this study is to examine the relationships among physical activity (PA) and components of MetSyn in a sample of PLWHA taking ART. A total of 31 males and 32 females living with HIV and currently taking ART were enrolled in a home-based PA intervention aimed to reduce risk factors for CVD. Clinical assessments included measures of resting blood pressure (BP), waist circumference, height, weight, PA levels via accelerometer, and a fasted blood draw. Components of MetSyn were divided into three clusters (1=0–1; 2 = 2; 3 = 3 or more). A one-way ANOVA was used to determine differences between clusters. Multiple linear regressions were used to identify significant associations between moderate intensity PA (MPA) and sedentary time among components of MetSyn. MPA was significantly lower across MetSyn clusters (p < 0.001), whereas sedentary time was significantly higher (p = 0.01). A multiple linear regression showed MPA to be a significant predictor of waist circumference after controlling for age, race, gender and sedentary time. Routine PA can be beneficial in helping PLWHA reduce waist circumference ultimately leading to metabolic improvements. This in turn would help PLWHA self-manage known components of MetSyn, thus reducing their risk of CVD and mortality.
Keywords: Metabolic syndrome X, aerobic exercise, fitness, lipodystrophy, cardiovascular disease, body composition
Introduction
Now that people living with HIV/AIDS (PLWHA) have lived well into their adult life we are beginning to see the long term consequences of HIV disease and antiretroviral therapy (ART). Data have begun to show increased prevalence rates of metabolic disturbances after initiating treatment (Samaras et al., 2007; Wand et al., 2007). These disturbances, such as lipodystrophy and increased blood lipids, place these individuals at high risk for metabolic syndrome (MetSyn) and other chronic diseases. As a result the primary cause of death among PLWHA are now chronic conditions often seen in elderly populations such as cardiovascular disease (CVD), kidney disease and renal failure, among others (Mayor, Gomez, Rios-Oliveras, & Hunter-Mellado, 2005). Although ART has become a necessity for many people to maintain low viral loads, it also has become a daily burden living with the toxic side effects.
The majority of known side effects associated with ART directly alter metabolic processes, thus increasing the risk of MetSyn. Symptoms of the side effects associated with ART include lipodystrophy (abnormal fat displacement), hypertension, hyperlipidemia (i.e. high triglycerides, cholesterol), decreased HDL-C, and impaired fasting glucose among many others (Montessori, Press, Harris, Akagi, & Montaner, 2004; Crane, Van Rompaey, & Kitahata, 2006). Central fat accumulation is the most common outcome of lipodystrophy resulting in an increased waist circumference, which is also a strong indicator of a person’s risk of developing chronic conditions like hypertension, stroke, or CVD (Janssen, Katzmarzyk, & Ross, 2004; Zhu et al., 2005).
Moderate to high levels of physical activity are known to improve the quality of life in healthy and clinical populations. The importance of physical activity is strongly supported within the literature with studies showing beneficial effects on most of the components associated with MetSyn and positive associations with risk factors for CVD (Andersen et al., 2006; Blair et al., 1996; Warburton, Nicol, & Bredin, 2006). Numerous prospective cohort studies have repeatedly shown that higher levels of physical activity are associated with reduced incidence of CVD, diabetes, certain cancers, all-cause mortality, and many other chronic conditions (Bassuk & Manson, 2005; Blair et al., 1996; Church et al., 2002; Jaggers et al., 2009; Warburton et al., 2006). Emerging data are showing similar benefits among HIV patients (Bopp et al., 2004; Dudgeon et al., 2012; Hand et al., 2008; Hand, Lyerly, Jaggers, & Dudgeon, 2009). However, no one has focused specifically on the components of MetSyn when studying the effects of physical activity among PLWHA. Therefore the purpose of this investigation is to examine the relationships between time spent in MPA and sedentary behavior on components of MetSyn in a sample of HIV+ adults currently taking ART.
Methods
A complete description of the methodology for this home-based physical activity intervention can be found elsewhere (Jaggers et al., 2013). In brief, this was a randomized controlled trial with a standard care control group. The treatment condition consisted of telephone-based coaching sessions with the primary goal to increase daily amounts of moderate-intensity PA. Written informed consent was obtained from all participants prior to any form of data collection. Thorough descriptions of the recruiting and screening processes, as well as the methods are available (Jaggers et al., 2013).
Outcome measures and methods
For this investigation the primary outcome of interest is MPA, as measured by the SenseWear® armband. Secondary outcomes include components of MetSyn, such as blood pressure, waist circumference, fasted glucose, triglycerides, and HDL levels. Blood draws occurred in the early morning following a 12 hour fast. Blood serum was separated via centrifuge and stored in a -80 freezer until ready for analysis.
Physical Activity Assessment
Physical activity was assessed with the SenseWear® armband, a commercially available lightweight physical activity monitor that is worn on the upper left arm halfway between the acromion and olecranon processes. The intra-class correlation has been reported at 0.8 and reports on the validity, reliability and overall accuracy can be found elsewhere (St-Onge, Mignault, Allison, & Rabasa-Lhoret, 2007; Welk, McClain, Eisenmann, & Wickel, 2007). Participants were given the armband for a 7–10 day period and asked to wear the armband at all times. Armband data was considered valid if the participant had a wear time of at least 10 hours a day for a minimum of 3 days. The maximum wear time for some participants consisted of a full 7 days. The daily average of time spent in MPA was used for data analysis.
MetSyn Criteria
Components of MetSyn were identified according to ATPIII criteria and is defined as having three or more of the following disorders: Hypertension (i.e. blood pressure ≥ 130/85) or currently taking hypertension medication; Triglycerides ≥ 150 mg/dL or currently taking lipid lowering medication; Waist circumference > 102 cm for men or > 88 cm for women; HDL cholesterol < 40 mg/dL for men or < 50 mg/dL for women; Fasting glucose ≥ 110 mg/dL (Grundy, Brewer, Jr., Cleeman, Smith, Jr., & Lenfant, 2004). Participants who exhibited any of these components were categorized into one of three groups: 0–1 components of MetSyn; 2 components of MetSyn; 3 or more components of MetSyn.
Statistical Analysis
A one-way ANOVA was used to determine any significant differences between groups. To examine the relationships between MPA and components of MetSyn we ran two separate linear regression analyses with MPA as the dependent variable in one, and sedentary time as the dependent variable in the other. Each model controlled for age, race, gender, and medications. Second regression models were ran that also controlled for the independent variable of the other model (i.e. sedentary time was controlled for in the MPA model). All dependent and independent variables were tested for normality in which it was discovered that the blood lipid measures did not follow a normal distribution. Therefore they were log-transformed prior to running the linear regression.
Results
Table 1 shows the distribution of components for MetSyn and physical activity measures separated by PA tertiles. Levels of PA were determined by splitting the data into three equal groups, which are as followed: Tertile 1: < 42 mins/day of MPA; Tertile 2: 42–90 mins/day of MPA; Tertile III: > 90 mins/day of MPA. Statistically significant differences were observed between groups showing lower values of waist circumference, weight, BMI, and incidence of MetSyn as levels of MPA increased. There was also a significantly higher VO2peak across tertiles indicating higher cardiorespiratory fitness (CRF) levels with increased MPA.
Table 1.
Components of metabolic syndrome and physical activity measures for all participants and separated by physical activity group.
| All | Tertile I | Tertile II | Tertile III | P value | ||
|---|---|---|---|---|---|---|
| Number | 63 | 20 | 23 | 20 | ||
| Age | 48 ± 11 | 49 ±10 | 50 ± 12 | 45 ± 10 | 0.294 | |
| Height | 66.91 ± 3.56 | 65.89 ± 3.51 | 66.85 ± 3.28 | 68.01 ± 3.77 | 0.254 | |
| Weight (lbs) | 193.11 ± 51.97 | 225.43 ± 56.16 | 195.20 ± 45.96 | 160.11 ± 31.79 | < 0.001 | |
| BMI (kg/m2) | 30.40 ± 7.46 | 36.38 ± 6.55 | 30.53 ± 6.28 | 24.28 ± 3.92 | < 0.001 | |
| Waist circumference (cm) | 99.55 ± 18.04 | 112.54 ± 15.9 | 100.38 ± 14.73 | 85.68 ± 12.85 | < 0.001 | |
| Systolic BP | 128 ± 14 | 126 ± 13 | 131 ± 12 | 126 ± 18 | 0.490 | |
| Diastolic BP | 83 ± 9 | 82 ± 9 | 83 ± 9 | 82 ± 11 | 0.885 | |
| Triglycerides | 147.02 ± 122.14 | 162.89 ± 183.01 | 150.24 ± 66.25 | 127.00 ± 82.79 | 0.688 | |
| HDL | 47.88 ± 22.74 | 47.94 ± 19.89 | 46.65 ± 21.85 | 49.06 ± 27.34 | 0.955 | |
| Glucose | 110.64 ± 42.05 | 114.50 ± 40.13 | 122.53 ± 58.10 | 94.64 ± 10.48 | 0.137 | |
| MPA (Avg Min/day) | 88.54 ± 85.66 | 26.00 ± 10.50 | 56.48 ± 13.63 | 187.95 ± 88.61 | < 0.001 | |
| Sedentary Time (Avg Mins/day) | 863.57 ± 209.80 | 880.85 ± 211.48 | 927.96 ± 206.51 | 772.25 ± 188.19 | 0.013 | |
| VO2 peak (ml/kg/min) | 20.13 ± 8.56 | 15.33 ± 3.64 | 17.51 ± 5.19 | 26.84 ± 10.23 | < 0.001 | |
| Number of MetSyn components %, (n) | 0.034 | |||||
| 0–1 | 36.5 (23) | 25 (5) | 30.4 (7) | 55 (11) | ||
| 2 | 27 (17) | 15 (3) | 34.8 (8) | 30 (6) | ||
| 3 or more | 36.5 (23) | 60 (12) | 34.8 (8) | 15 (3) | ||
| Lipid lowering medication %, (n) | 0.182 | |||||
| Yes | 34.9 (22) | 45 (9) | 39.1 (9) | 20 (4) | ||
| No | 65.1 (41) | 55 (11) | 60.9 (14) | 80 (16) | ||
| Blood pressure medication %, (n) | 0.220 | |||||
| Yes | 47.6 (30) | 65 (13) | 39.1 (9) | 40 (8) | ||
| No | 52.4 (33) | 35 (7) | 60.9 (14) | 60 (12) | ||
| Diabetic (Type II) %, (n) | 0.072 | |||||
| Yes | 14.3 (9) | 20 (4) | 21.7 (5) | 0 | ||
| No | 85.7 (54) | 80 (16) | 78.3 (18) | 100 (20) | ||
Tertile 1: < 42 mins/day of MPA; Tertile II: 42–90 mins/day of MPA; Tertile III: > 90 mins/day of PA MPA: Moderate intensity physical activity; BMI: Body mass index
Table 2 shows the results of all regression models. Even when controlling for sedentary time (Model 3) MPA significantly predicted waist circumference while adjusting for the other MetSyn components. No other significant relationships were observed, indicating sedentary time was not a significant predictor for any component of MetSyn. It also did not affect the observed relationship between MPA and waist circumference.
Table 2.
Linear regression of associations between PA and components of metabolic syndrome
| Model 1. Dependent variable: MPA | Model 2. Dependent variable: Sedentary time | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | β | SE B | F | p | β | SE B | F | p |
| Intercept | 5.36 | 2.84 | 5.52 | 1.19 | ||||
| SBP | 0.01 | 0.01 | 1.18 | 0.29 | −0.001 | 0.004 | 0.003 | 0.96 |
| DBP | −0.02 | 0.02 | 0.83 | 0.37 | −0.001 | 0.007 | 0.02 | 0.72 |
| Waist | −0.03 | 0.01 | 12.68 | 0.001 | 0.005 | 0.003 | 3.29 | 0.07 |
| Triglycerides | 0.25 | 0.31 | 0.65 | 0.43 | 0.07 | 0.13 | 0.31 | 0.58 |
| HDL | 0.58 | 0.38 | 2.31 | 0.14 | −0.06 | 0.16 | 0.14 | 0.71 |
| Glucose | −0.27 | 0.39 | 0.48 | 0.49 | 0.19 | 0.17 | 1.27 | 0.27 |
| Note: R2 = 0.63; p < 0.001; N = 65 | Note: R2 = 0.28; p = 0.15; N = 65 | |||||||
| *Both models controlled for sex, age, and race. | ||||||||
| Model 3 | Model 4 | |||||||
| Variable | β | SE B | F | p | β | SE B | F | p |
| Intercept | 5.16 | 2.87 | 6.11 | 1.22 | ||||
| SBP | 0.01 | 0.01 | 1.18 | 0.29 | −0.001 | 0.004 | 0.02 | 0.88 |
| DBP | −0.02 | 0.02 | 0.86 | 0.36 | −0.002 | 0.007 | 0.12 | 0.73 |
| Waist | −0.02 | 0.01 | 11.22 | 0.002 | 0.002 | 0.004 | 0.31 | 0.58 |
| Triglycerides | 0.28 | 0.32 | 0.78 | 0.38 | 0.10 | 0.13 | 0.61 | 0.44 |
| HDL | 0.56 | 0.38 | 2.12 | 0.15 | 0.004 | 0.16 | 0.00 | 0.98 |
| Glucose | −0.18 | 0.41 | 0.18 | 0.67 | 0.19 | 0.17 | 1.22 | 0.27 |
| Note: R2 = 0.64; p = 0.001; N = 65 | Note: R2 = 0.34; p = 0.10; N = 65 | |||||||
| *Same as model 1 but also controlling for sedentary time | *Same as model 2 but also controlling for MPA | |||||||
MPA: Moderate intensity physical activity; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HDL: High density lipoprotein
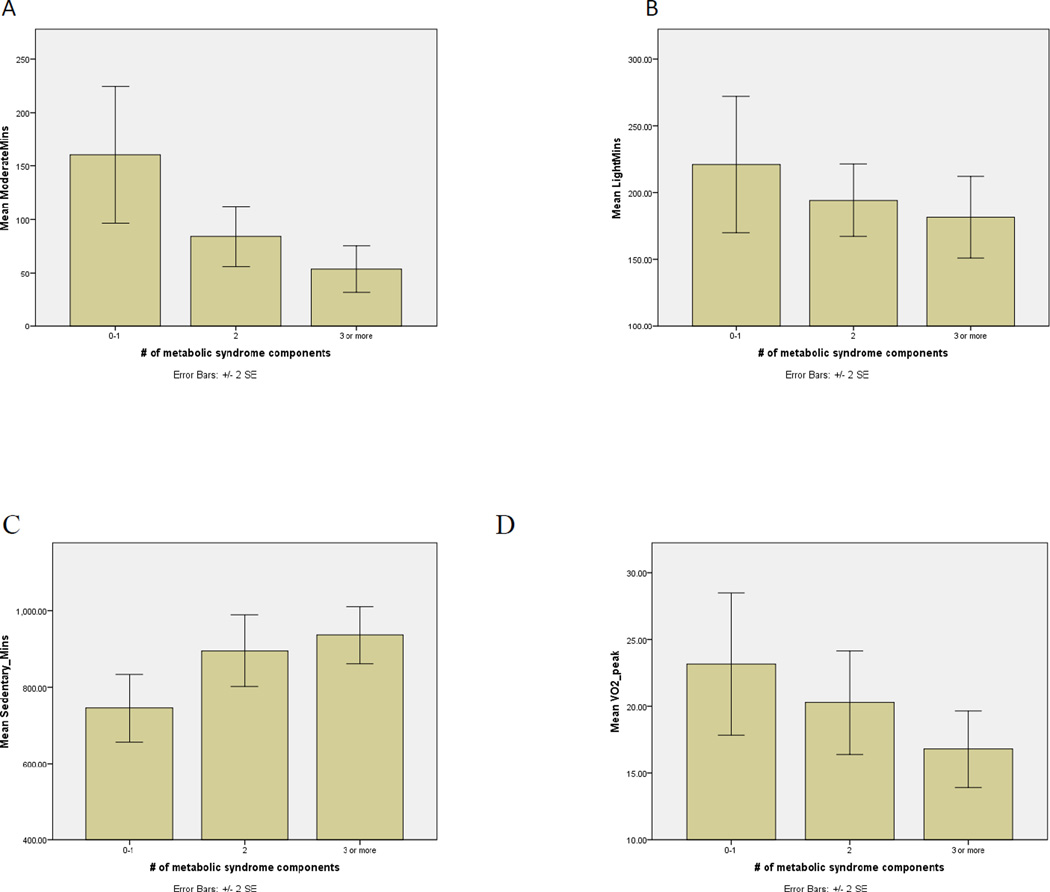
Figure 1 shows differences in MPA, light intensity PA, sedentary time, and CRF across MetSyn clusters. We found significant differences in MPA and sedentary time across all groups. However there was no statistically significant difference in the amount of light intensity PA observed. There was also a trending decrease of CRF levels across MetSyn clusters, which barely reached significance (p = 0.05).
Figure 1.
Differences in physical activity and CRF across metabolic syndrome clusters (A) Moderate
Discussion
The purpose of this investigation was to examine the relationships between MPA and sedentary time among components of MetSyn in a sample of PLWHA currently taking ART. After adjusting for potential confounding variables we observed statistically significant independent associations of MPA, waist circumference, and the number of MetSyn components present. The primary finding of the investigation was the significant association between waist circumference and MPA, even when sedentary time was included in the model. Further there was an inverse relationship between the number of MetSyn components and MPA. There also was a significant difference in the amount of time engaged in sedentary behavior when comparing people with 0–1 MetSyn components to those with 3 or more. Similarly we observed a significant trend among CRF and MetSyn, indicating that those with MetSyn had lower fitness levels.
This study is not without limitations. Even though there was large power detecting the effect size of the relationship between MPA and waist circumference, the small sample size limited the power to detect significant differences in most blood lipids and fasting glucose. These small changes in blood profiles may more accurately be detected with a larger sample. We also acknowledge that since this is a cross-sectional study we cannot suggest causality between the dependent and independent variables.
Our findings are in agreement with the consensus of physical activity investigations in clinical and healthy populations. The significance and novelty behind our findings is that it is the first of its kind to look at extensive physical activity behaviors in a sample of PLWHA currently taking ART. It is well known that ART has toxic side effects often resulting in lipodystrophy, increased abdominal fat, as well as increased blood lipids. What has yet to be addressed is whether or not these toxic effects will inhibit the known benefits of increased physical activity and fitness. In conclusion, our findings show that greater amounts of MPA are significantly associated with a smaller waist circumference for PLWHA currently taking ART. A significant relationship was also observed among sedentary time and waist circumference, however did not retain significance in the linear regression model. Routine MPA can be beneficial in helping PLWHA reduce waist circumference while ultimately leading to metabolic improvements. This in turn would help PLWHA self-manage known components of MetSyn, thus reducing their risk of CVD and mortality.
Acknowledgements
This study was funded by the National Institutes of Health/NINR (R21 Grant 1R21NRO11281). The authors would further like to acknowledge the generosity of Theraband® who provided free elastic resistance bands to all study participants. We thank all participants who volunteered their time for this project.
Footnotes
Conflict of Interest Statement: The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
References
- Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368(9532):299–304. doi: 10.1016/S0140-6736(06)69075-2. doi:S0140-6736(06)69075-2 [pii];10.1016/S0140-6736(06)69075-2 [doi]. Retrieved from PM:16860699. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J.Appl.Physiol. 2005;99(3):1193–1204. doi: 10.1152/japplphysiol.00160.2005. doi:99/3/1193 [pii];10.1152/japplphysiol.00160.2005 [doi]. Retrieved from PM:16103522. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kampert JB, Kohl HW, III, Barlow CE, Macera CA, Paffenbarger RS., Jr Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- Bopp CM, Phillips KD, Fulk LJ, Dudgeon WD, Sowell R, Hand GA. Physical activity and immunity in HIV-infected individuals. AIDS Care. 2004;16(3):387–393. doi: 10.1080/09540120410001665385. doi:10.1080/09540120410001665385 [doi];GH8HKTWQGLQT0NJH [pii]. Retrieved from PM:15203431. [DOI] [PubMed] [Google Scholar]
- Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler.Thromb.Vasc.Biol. 2002;22(11):1869–1876. doi: 10.1161/01.atv.0000036611.77940.f8. Retrieved from PM:12426218. [DOI] [PubMed] [Google Scholar]
- Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20(7):1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. doi:10.1097/01.aids.0000222074.45372.00 [doi];00002030-200604240-00009 [pii]. Retrieved from PM:16603854. [DOI] [PubMed] [Google Scholar]
- Dudgeon WD, Jaggers JR, Phillips KD, Durstine JL, Burgess SE, Lyerly GW, et al. Moderate-Intensity Exercise Improves Body Composition and Improves Physiological Markers of Stress in HIV-Infected Men. ISRN AIDS, 2012. 2012 doi: 10.5402/2012/145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. doi:10.1161/01.CIR.0000111245.75752.C6 [doi];109/3/433 [pii]. Retrieved from PM:14744958. [DOI] [PubMed] [Google Scholar]
- Hand GA, Lyerly GW, Jaggers JR, Dudgeon WD. Impact of Aerobic and Resistance Exercise on the Health of HIV-Infected Persons. Am.J.Lifestyle.Med. 2009;3(6):489–499. doi: 10.1177/1559827609342198. doi:10.1177/1559827609342198 [doi]. Retrieved from PM:20508736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand GA, Phillips KD, Dudgeon WD, William LG, Larry DJ, Burgess SE. Moderate intensity exercise training reverses functional aerobic impairment in HIV-infected individuals. AIDS Care. 2008;20(9):1066–1074. doi: 10.1080/09540120701796900. doi:794184158 [pii];10.1080/09540120701796900 [doi]. Retrieved from PM:18608063. [DOI] [PubMed] [Google Scholar]
- Jaggers JR, Dudgeon W, Blair SN, Sui X, Burgess S, Wilcox S, et al. A home-based exercise intervention to increase physical activity among people living with HIV: study design of a randomized clinical trial. BMC.Public Health. 2013;13:502. doi: 10.1186/1471-2458-13-502. doi:1471-2458-13-502 [pii];10.1186/1471-2458-13-502 [doi]. Retrieved from PM:23706094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggers JR, Sui X, Hooker SP, Lamonte MJ, Matthews CE, Hand GA, et al. Metabolic syndrome and risk of cancer mortality in men. Eur.J.Cancer. 2009;45(10):1831–1838. doi: 10.1016/j.ejca.2009.01.031. doi:S0959-8049(09)00057-4 [pii];10.1016/j.ejca.2009.01.031 [doi]. Retrieved from PM:19250819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am.J.Clin.Nutr. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. Retrieved from PM:14985210. [DOI] [PubMed] [Google Scholar]
- Mayor AM, Gomez MA, Rios-Oliveras E, Hunter-Mellado RF. Mortality trends of HIV-infected patients after the introduction of highly active antiretroviral therapy: analysis of a cohort of 3,322 HIV-infected persons. Ethn.Dis. 2005;15(4 Suppl 5):S5–S62. Retrieved from PM:16312941. [PubMed] [Google Scholar]
- Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 2004;170(2):229–238. Retrieved from PM:14734438. [PMC free article] [PubMed] [Google Scholar]
- Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30(1):113–119. doi: 10.2337/dc06-1075. doi:30/1/113 [pii];10.2337/dc06-1075 [doi]. Retrieved from PM:17192343. [DOI] [PubMed] [Google Scholar]
- St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am.J.Clin.Nutr. 2007;85(3):742–749. doi: 10.1093/ajcn/85.3.742. doi:85/3/742 [pii]. Retrieved from PM:17344495. [DOI] [PubMed] [Google Scholar]
- Wand H, Calmy A, Carey DL, Samaras K, Carr A, Law MG, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21(18):2445–2453. doi: 10.1097/QAD.0b013e3282efad32. doi:10.1097/QAD.0b013e3282efad32 [doi];00002030-200711300-00010 [pii]. Retrieved from PM: 18025881. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. doi:174/6/801 [pii];10.1503/cmaj.051351 [doi]. Retrieved from PM:16534088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welk GJ, McClain JJ, Eisenmann JC, Wickel EE. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity.(Silver.Spring) 2007;15(4):918–928. doi: 10.1038/oby.2007.624. doi:15/4/918 [pii];10.1038/oby.2007.624 [doi]. Retrieved from PM:17426327. [DOI] [PubMed] [Google Scholar]
- Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am.J.Clin.Nutr. 2005;81(2):409–415. doi: 10.1093/ajcn.81.2.409. doi:81/2/409 [pii]. Retrieved from PM:15699228. [DOI] [PubMed] [Google Scholar]