Summary
Wallerian degeneration (WD) occurs after an axon is cut or crushed and entails the disintegration and clearance of the severed axon distal to the injury site. WD was initially thought to result from the passive wasting away of the distal axonal fragment, presumably because it lacked a nutrient supply from the cell body. The discovery of the slow Wallerian degeneration (Wlds) mutant mouse, in which distal severed axons survive intact for weeks rather than only 1–2 days, radically changed our thoughts on the autonomy of axon survival. Wlds taught us that under some conditions the axonal compartment can survive for weeks after axotomy without a cell body. The phenotypic and molecular characterization of Wlds and current models for Wlds molecular function are reviewed herein—the mechanism(s) by which WldS spares severed axons remains unresolved. However, recent studies inspired by Wlds have led to the identification of the first “axon death” signaling molecules whose endogenous activities promote axon destruction during WD.
Introduction
Axons can be enormous structures and constitute the vast majority of the volume of a neuron. Some human sciatic nerve motorneurons are one meter long and attached to a cell body that is only ~50 μm in diameter—meaning the length ratio of cell body to axon is 1:20,000. Maintaining such large and elaborate structures is a major cell biological and bioenergetic challenge for the neuron, but is essential for continued neural circuit function. Axonal injury is quite common in the nervous system, can occur through nerve crush, stretch, or transection, and frequently leads to axonal degeneration. Axon loss is also prominent in neurodegenerative diseases including ALS, Huntington’s, and Parkinson’s disease. Since axonal and synaptic loss are major contributing factors in neural circuit dysfunction, blockade of axon degeneration by any means is of significant clinical interest.
Cutting an axon (axotomy) leads to the granular disintegration of the axon distal to the injury site [1] — a process termed Wallerian degeneration. For ~150 years it was believed that Wallerian degeneration occurred because the portion of the axon distal to the injury site lacked a nutrient supply from the soma. This all changed with the serendipitous discovery of the slow Wallerian degeneration (WldS) mouse, where distal severed axons survived for weeks after axotomy (rather than ~1.5 days) in vivo [2••]. The WldS phenotype was unexpected and remarkable. It demonstrated that—under some conditions—large fragments of severed axons could survive for very long periods of time on their own without a cell body. This observation raised the further intriguing possibility that severed distal axons, rather than waste away, might activate an autonomous “axon death” program akin to apoptotic death [3]. Now 30 years since the discovery of the Wlds mutant mouse the WldS protective mechanism has proven complex and remains controversial [4]. This review summarizes our current understanding of the mechanism by which WldS modulates axon degeneration during Wallerian degeneration, and exciting recent findings that point to the existence of endogenous axon death program(s) required to drive axon death after axotomy.
Dissecting WldS neuroprotective function: what is critical, and where?
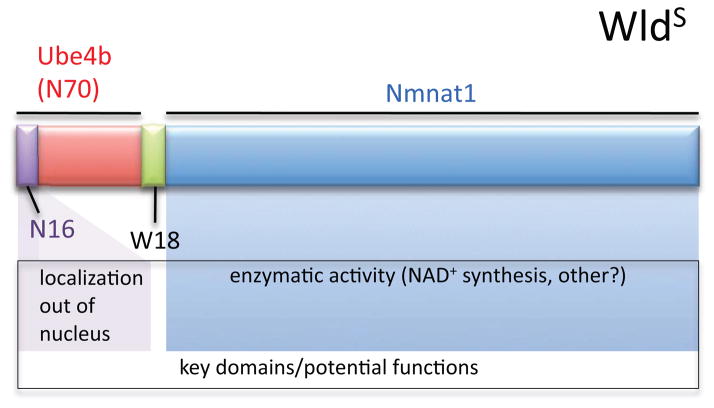
The WldS strain harbors a tandem triplication that results in the fusion of two genes, nmnat1 and ube4b [5]. The WldS protein generated from this locus is composed of 70 amino acids from the N-terminus of the E4 ubiquitin ligase Ube4b (N70), an 18 amino acid linker produced by translation of a short segment of the nmnat1 5′UTR (W18), and full length Nmnat1, a component of the NAD+ scavenging pathway (Figure 1) [6••]. Neuronal expression of WldS is sufficient to suppress the granular disintegration of both motor and sensory axons, and the axons of multiple types of CNS neurons [4]. Somewhat surprisingly, expression of mouse WldS was also shown to robustly suppress Wallerian degeneration in the fruit fly Drosophila [7•], and more recently in zebrafish [8], indicating the mechanistic action of WldS axon protection is evolutionarily conserved.
Figure 1. WldS protein structure.
See text for details.
The mechanism by which overexpression of WldS suppresses axonal degeneration remains incompletely resolved. Studies over the last decade however have clarified the precise domains essential for its axon protective function in vivo. Lentivirus-based overexpression of Nmnat1 was first reported to suppress axon degeneration in cultured mammalian dorsal root ganglion (DRG) neurons. It was therefore proposed that Nmnat1, a nuclear NAD+ biosynthetic enzyme, was the key functional component of WldS responsible for its axon-sparing activity [9••]; and further that Nmnat1 acted in the nucleus prior to injury, by modulating NAD+-dependent activation of the histone deacetylase enzyme Sirtuin1 [9]. A subsequent study also concluded that Nmnat1 was protective in mouse DRG cultures, but argued that the WldS and Nmnat1 acted more locally in axons [10]. NAD+ levels in distal severed axons from wild type animals were found to be maintained for 2–4 hours after axotomy, after which time they dropped precipitously, and this drop in NAD+ along with depletion of ATP immediately preceded axon fragmentation and could be averted by over-expression of WldS or Nmnat1. Moreover, degeneration of axon segments could be suppressed by addition of NAD+ or its precursor nicotinamide even 3–5 hours after axotomy. Despite siRNA-based evidence for a role for Sirt1 in WldS-mediated axon protection [9], neither NAD+-, Nmnat1-, nor WldS-dependent protection of axons was suppressed in DRG cultures from sirt1 knockout animals [10]. WldS-dependent protection of severed Drosophila axons was also not affected in by loss of Sir2, a fly sirtuin [11]. Together these data argued strongly against a pre-injury requirement for WldS in protecting severed axons and the proposed role for Sirt1.
Based on the above in vitro studies the field predicted that Nmnat1 over-expression in mice would provide axonal protection similar to WldS, but this was not the case. Unexpectedly, mice with levels of Nmnat1 expression and NAD+ biosynthetic activity comparable to WldS showed no axonal protective phenotype after axotomy [12]. Nmnat1 enzymatic activity is certainly crucial as enzymatically dead versions of WldS do not suppress Wallerian degeneration strongly in flies [11] or at all in mice [13]. Expression of mouse Nmnat1 in Drosophila provided some protection, but this was clearly diminished compared to expression of WldS and fully eliminated by blocking enzymatic activity [11]. Therefore the mechanism of WldS-dependent axonal preservation is more complex than simply over-expressing Nmnat1. Interestingly, robust axonal protection could be accomplished by including a key portion of the N-terminal Ube4b molecule: the most N-terminal 16 amino acids (N16) that had previously been shown to associate with valosin-containing protein (VCP) [14]. Deletion of N16 from WldS suppressed its neuroprotective function in mouse and flies [11,13]; fusing N16 alone to Nmnat1 resulted in WldS-like protection in flies [11]; replacing N16 in WldS with a VCP-binding motif from Ataxin3 provided WldS-like axon protection in mouse [13]; and knocking down VCP in Drosophila in the presence of WldS reduced its protective capacity to that equivalent to Nmnat1 alone [11]. These observations indicate that both N16 and enzymatically active Nmnat1 are key domains required for maximal protection of axons by WldS in vivo. Thus, both the N-terminus and Nmnat1 are essential components for full WldS-like axon preservation in Wallerian degeneration.
What is N16 doing to promote WldS axon-sparing activity? A likely explanation appears to be that N16 drives the relocalization of a portion of the cellular pool of WldS out of the nucleus to another site of action. Nmnat1 has a strong nuclear localization sequence (NLS) that is included in the WldS fusion protein and results in its primarily nuclear localization [5]. Compelling evidence that extra-nuclear relocalization is a key event in making Nmnat1 neuroprotective came from the observation that deleting the Nmnat1 NLS (to make Nmnat1cyto) results in its cytoplasmic localization, and Nmnat1cyto potently suppresses axon degeneration both in vitro and in vivo [15•]. Deleting the NLS in Nmnat1 from WldS in fact makes it an even more potent protective molecule [16], as does the addition of an axonal targeting domain to a cytoplasmically localized Nmnat1 [17]. WldS itself has been found at low levels in cytoplasmic fractions and axons [16–18] and in synaptosomal preparations [19]. But perhaps the most convincing data that WldS acts in the axon to protect after injury is the observation that lentiviral-based delivery of cytoplasmically targeted Nmnat1 protein directly into axons after axotomy is sufficient to protect them from axonal degeneration [15]. This observation would also seem to eliminate the NAD+/Sirt1-based nuclear model for WldS-mediated axonal protection.
WldS should teach us (again) that localization data must be considered quite carefully, especially in situations where the molecule of interest is being over-expressed. Recently the field appears to have unified behind the idea of a non-nuclear, axonal role for WldS in axon protection, but it was not easy getting to this point—controversy abounded. A number of technical issues might explain some of the controversy and their careful consideration might help moving forward. First, in many cases axon protection was compared across vastly different experimental settings, and this is important for phenotypic characterization of protective function. In cultured DRG neurons Nmnat1 and WldS provide what appears to be similar levels of protection, promoting axon survival at least 72 hours after axotomy. In striking contrast, wild type Nmnat1 provides no protection by 2–3 days after axotomy in vivo [12] while WldS protects the majority of axons for at least two weeks in the mouse [5].
A second point to consider is quantification of both temporal and spatial aspects of axon/synapse preservation. Assays of neuroprotection must be taken to endpoints. Direct comparisons of very late time points in Wallerian degeneration in Drosophila elucidated important differences in neuroprotective effects of WldS versus Nmnat1 and its derivatives [11], as did comparisons of WldS and axonally targeted WldS [17]. The quality of preservation are also important factors to consider—are axons and synapses preserved equally, and are they functionally intact? If protection were weak or specific to a subdomain of a neurite (e.g. dendrite versus axon) for any manipulation, this would impact its consideration as a therapeutic target in important ways. Though not used as a standard, scoring neuroprotection with single axon resolution should be a key goal for the field. In culture preparations of various ganglia, axonal fibers are not reliably identifiable with single axon resolution using light microscopy, so partial protection of axons from disintegration may appear to be complete, or vice versa.
Finally, we need to consider the potential complexity of genetic pathways driving axon degeneration. Although it is the most common positive empirical readout for Wallerian degeneration, what does it mean when an axon undergoes granular disintegration? Is degeneration always the same, or are there multiple Wallerian degeneration-like programs? There appears to be a remarkable disconnect between molecular programs driving developmental neurite pruning and axon degeneration despite the fact these two processes look very similar morphologically [20,21••]. It is therefore reasonable to suspect that a number of pathways exist that can drive granular disintegration of axons. A systematic use of biomarkers for very specific signaling events (e.g. Ca2+ signaling, or reactive oxygen species production) [8,19] or changes in cell biology (e.g. axon trafficking, cytoskeletal breakdown) [22] should go a long way toward determining how distinct genetic pathways might drive the step-wise disassembly of axons in different contexts, and where any genetic or chemical manipulation impinges on these processes.
How does WldS act to suppress axon protection?
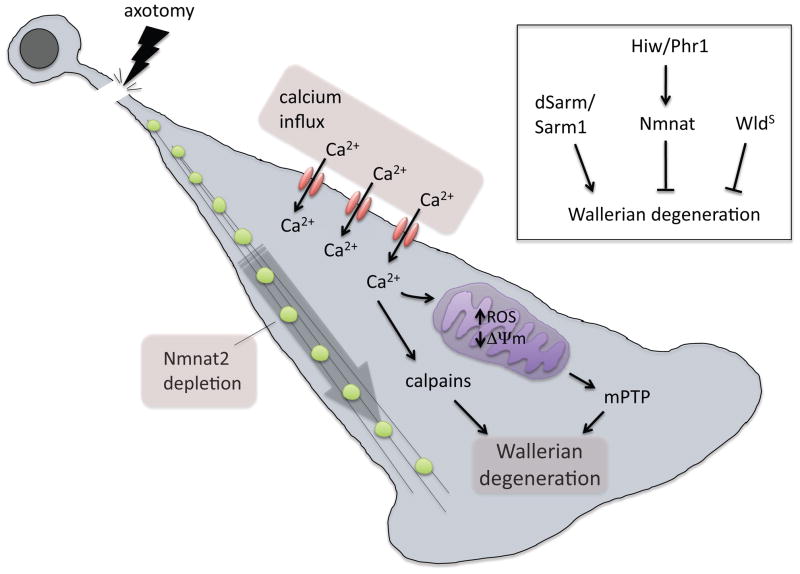
There is a strong correlation between the time of survival of a detached distal axon fragment and its length —longer detached axon fragments degenerated later than shorter fragments. Based on this observation it was proposed that the soma might provide a trophic factor that was continuously transported down the axon [23,24•]. Once severed, the axon would survive until the pool of trophic factor was depleted and degeneration would ensue (Figure 2). Gilley et al. (2010) proposed that this trophic factor was Nmnat2, a cytoplasmic Nmnat molecule that can be found in the axoplasm. Nmnat2 is actively transported down axons, its half-life correlates with the timing of axon degeneration in DRG cultured neuron preparations [24], stabilization of Nmnat2 by mutating is palmitoylation site that tethers it to vesicles stabilizes Nmnat2 and makes it highly protective [25], and, reciprocally, depletion of Nmnat2 from cultured DRG neurons leads to degeneration that can be suppressed by WldS [24]. WldS, they speculated, essentially substitutes for the more labile Nmnat2 activity in the axon. WldS and Nmant1 are indeed more stable than Nmnat2 in vitro [24]. Moreover, while nmnat2 knockout mice are embryonic lethal and grow axons in the PNS which are significantly shorter than control axons both in vivo and DRG cultures [26,27], WldS expression is sufficient to rescue both lethality and short axon phenotypes in the nmnat2 null background [27].
Figure 2. Signaling events initiated by axotomy.
Nmnat2 is continuously delivered on vesicles (green) from the soma to the axon. Axotomy terminates delivery and axons survive until Nmnat2 is depleted. Influx of extracellular Ca2+ is activated by axotomy, which may be amplified by release from internal stores. Axonal Ca2+ activates calpains to drive cytoskeletal degradation, and overwhelms mitochondria, leading to loss of mitochondrial membrane potential and increased ROS production. Eventually mitochondrial undergo PTP formation and granular disintegration of the axon ensues.
Inset box: genetic modulators of Wallerian degeneration, see text for details.
Together these data provide compelling evidence that Nmnat2 is required for maintenance of axonal integrity in cultured neurons and that WldS is capable of substituting for Nmnat2 in multiple contexts. But does this fully explain the WldS phenotype of axon protection? The Nmnat2 depletion model seems quite logical, but a number of observations and experimental caveats argue that the effects of WldS are more complex and not fully explained by Nmnat2 depletion. First, in both mice [18] and Drosophila [19] expression of the mitochondrially-localized Nmnat3 molecule is sufficient to recapitulate the strong protective effects of WldS, and in synaptosome preparations from the WldS mouse brain WldS was found exclusively in mitochondria and not the axoplasm [19]. These data are consistent with a mitochondrial role for WldS. Two groups have purified mitochondria from WldS mouse brain and shown they have an enhanced ability to generate ATP and consume O2 [18,19], and increased capacity to buffer extra-mitochondrial Ca2+ and stave off loss of membrane potential and formation of the permeability transition pore (PTP) [19]. Therefore WldS animals exhibit significant changes in mitochondrial metabolism and handling of Ca2+. This could be very important for induction of axonal degeneration, a Ca2+-stimulated event (below). Axotomy in Drosophila motorneurons leads to a rapid increase in axonal Ca2+ in control animals and this is essentially eliminated in WldS animals, indicating there is a significant in vivo alteration in how severed axons handle Ca2+. Activation of mitochondrial formation of the permeability transition pore (PTP) has long been known to be a key downstream step in the activation of Wallerian degeneration and pathological Ca2+ in the axon, its suppression can strongly delay Wallerian degeneration [28,29], and recent work in zebrafish implicates mitochondrial reactive oxygen species (ROS) production as occurring immediately prior to axon degeneration, and ROS production is strongly reduced in WldS animals [8]. Finally, it seems mitochondrial function is critical to enable WldS to protect severed axons since application of carbonyl cyanide m-chlorophenyl-hydrazone, which uncouples mitochondrial oxidative phosphorylation, eliminates the ability of WldS to protect axons [30].
While the above would appear to support an important role for mitochondria in Wallerian degeneration and WldS function, the story is not entirely clear. Severed Drosophila dendrites also degenerate within a day and this is suppressed by WldS, arguing that dendrites use a similar Wallerian-like degenerative program after cut. However, short portions of dendrites (not expressing WldS) were able to degenerate in the absence of any mitochondria [31•]. Therefore mitochondria are not strictly required for neurite degeneration, but whether the dendrite degenerative program is the same as in axons, and whether the granular disintegration of the mitochondria-free neurite is molecularly similar to Wallerian degeneration needs further exploration. In addition, depletion of mitochondria from Drosophila axons did not fully block the ability of WldS to protect larval axons after cut [32], although this was only examined over the short term (hours rather than days), and so whether this is true of longer time points remains to be determined.
The mechanism of WldS-mediated axon protection it has been difficult to ask key mechanistic questions, often because of experimental limitations. For instance, if the site of action of WldS were mitochondria one would like to transplant mitochondria from donor WldS+ animals into control axons, sever axons and see what happens. But this is not practical experimentally for multiple reasons. Because NAD+ and mitochondria are fundamentally important for axonal and cell survival, determining the precise roles for this molecule and organelle have been challenging—they are needed for cells to simply survive. It is equally important to acknowledge that many experimental manipulations used to dissect WldS function have caveats. Molecules of interest such as Nmnats molecules are over-expressed, hence localization studies could be artifactual and dosages might vary significantly. Manipulations used to alter specific molecular properties might not be as specific as we would hope. For instance, mutation of the palmitoylation site in Nmnat2 that tethers it to axon vesicles indeed led to a much longer protein half-life and significantly increased axon protection, which could be used to argue that increasing the stability of Nmnat2 is a key factor to preserve axons. However this manipulation also results in mislocalization of Nmnat2 [25] which could affect neuroprotection—17 amino acids is all that is need to transform Nmnat1 from a non-protective molecule to one that protects as well as WldS [13]. As such there have been few really definitive experiments explaining how WldS mechanistically blocks Wallerian degeneration. In the future, a greater understanding of the precise cellular and molecular pathways promoting axonal degeneration will be essential to determine precisely where WldS impinges on axonal degeneration after axotomy, and which model best explains its neuroprotective function.
Is there an endogenous axon death pathway?
Perhaps the most important idea inspired by the study of WldS was the notion that severed axons, rather than simply waste away, might activate an active program of axon auto-destruction [3,33]. Wallerian degeneration in Drosophila appears to be genetically distinct from apoptotic cell death and autophagy since elimination of multiple components of these pathways from axons had no effect on Wallerian degeneration [21]. In addition, although caspase activity is required in axons to promote degeneration after trophic factor withdrawal, these same mutants do not suppress Wallerian degeneration [34]. Since WldS is a gain-of-function and likely neomorphic mutation, it does not provide evidence of an axon death pathway per se. A number of genes have been proposed to suppress Wallerian degeneration when mutated including Dual leucine kinase (Dlk) [35], MORN4 [36•], AKT, GSK3 [37], and IκB kinase (IKK) [38], but these all provided quite weak axon protection: while WldS can preserve severed axons for weeks after axotomy, loss of these products only extended survival of severed axons for hours to at most a day.
A major step forward for the field came with the identification of the Drosophila sterile α/Armadillo/Toll-Interleukin receptor homology domain (dsarm) mutants in a forward genetic screen for mutations that suppress Wallerian degeneration [21••]. This was the first mutant that approached levels of axon protection found in WldS: dsarm loss of function mutations resulted in severed axons remaining morphologically intact for several weeks after axotomy. dSarm thus provided direct evidence that genes in fact exist whose endogenous function is to promote Wallerian degeneration. Elimination of the mouse dSarm ortholog Sarm1 suppressed Wallerian degeneration of the majority of axons in sciatic nerve for at least two weeks, protected multiple types of PNS and CNS neurons both in vitro and in vivo, suppressed breakdown of the axonal cytoskeleton (e.g. neurofilament) for at least 6 days after axotomy, and extended the maintenance of neuromuscular synapses of severed axons at levels similar to WldS [21]. Importantly, dsarm null mutations did not suppress apoptotic cell death in Drosophila, nor did it block developmental axon pruning [21••], further supporting the notion that Wallerian degeneration, apoptotic cell death, and developmental neurite pruning are driven by genetically distinct molecular programs.
dSarm/Sarm1 encodes a member of the TIR domain-containing family of proteins, best known for acting downstream of Toll receptors in immune functions. Sarm is unique in its structure within the TIR domain family as it contains Armadillo repeats, relatively rigid structural domains that modulates protein-protein interactions, and a number of SAM domains, which mediate protein-protein interactions [39]. TIR domain containing proteins are generally thought to act as kinase scaffolding molecules that couple Toll-like receptors (TLRs) with essential downstream signaling components. However dSarm/Sarm1 might not necessarily require the presence of a TLR to signal. Tir-1, the C. elegans ortholog of dSarm/Sarm1 acts in a genetic pathway apparently independent of TLRs [40]. In the context of left-right asymmetry of worm olfactory receptor neurons Tir-1 is activated downstream of the voltage gated Ca2+ channel UNC-43 by Ca2+-calmodulin kinase (CaMK), which directly binds to the N-terminal ARM domains in Tir-1. Tir-1 then couples downstream to Apoptosis signaling kinase 1 (Ask1) to execute left-right signaling events [40]. That dSarm/Sarm1 is potentially downstream of a Ca2+ signaling pathway is interesting. Entry of extracellular Ca2+ through L-type Ca2+ channels is essential to activate Wallerian degeneration [41], and a dramatic rise in axoplasmic Ca2+ immediately precedes Wallerian degeneration in zebrafish [42]. An interesting possibility is that dSarm/Sarm1 is activated directly downstream of injury-induced Ca2+ elevation, perhaps via CaMK, and in turn activates axonal degeneration.
How dSarm/Sarm1 activates axonal degeneration is not known. As is the case in C. elegans with Tir-1, the SAM and TIR domains appear to be essential for prodegenerative signaling in cultured DRG neurons [43]. At least two antibodies have been generated to Sarm1: one results in punctate staining in neurites [21,44], the other a more uniform distribution with some enrichment to mitochondria [43]. Epitope-tagged Sarm1 strongly localizes to mitochondria when over-expresed in vitro [45] and mitochondrial localization sequences have been identified [45], but deletion of this motif did not suppress Sarm1-mediated axonal degeneration in vitro after axotomy [43]. Thus, while an exciting new molecule that mediates Wallerian degeneration has been identified, where or even when Sarm1 acts (i.e. before or after injury) remains important questions for the future.
A second exciting recent discovery was that the Drosophila E3 ubiquitin ligase Highwire (Hiw)(Figure 3B), first identified based on its role in synapse formation at the larval neuromuscular junction (NMJ), is required for Wallerian degeneration [46••]. hiw mutant NMJs exhibit robust overgrowth of the presynaptic motorneuron at the larval NMJ [47]. More recently, in a larval nerve crush model of Wallerian degeneration hiw loss of function mutants were shown to suppress axon loss for 2 days longer than wild type, and severed hiw axons appear to remain functionally intact by electrophysiological measures for the duration of this time [46]. Loss of Hiw results in very strong protection (up to 2 weeks) in adult Drosophila, and so hiw mutants also approach levels of protection similar to WldS [46]. As it is an E3 ubiquitin ligase, Hiw might be expected to promote the degradation of specific proteins after axotomy to induce axonal degeneration. Based on the rapid degradation of Nmnat2 in mammalian axons, dNmnat (the sole Drosophila Nmnat ortholog) was proposed as a Hiw target in the distal axon after axotomy [46]. Consistent with the notion that Hiw negatively regulates dNmnat, hiw mutants have elevated dNmnat expression in the nervous system and alterations in the decay of ectopically expressed mouse Nmnat2 [46]. The mouse ortholog of Hiw, called PAM/Highwire/Rpm1 (Phr1), is also essential for Wallerian degeneration. Loss of Phr1 preserves axonal integrity in mouse sciatic nerves for 5–10 days after axotomy in vivo [48•], which is robust, but perhaps not quite as strong as the protection observed in Sarm1−/− animals [21]. Phr1 elimination increases the levels of Nmnat2, consistent with the notion that Phr1 negatively regulates is degradation [48•]. Direct evidence that Nmnat molecules are ubiquitinated prior to degradation and that this is in a Hiw/Phr1-dependent manner is currently lacking, but are important questions for the future. It is also important to note that while dNmnat and Nmnat2 levels are increased in the absence of Hiw/Phr1 [46••] [48•], the specificity of this increase remains unclear. Could stabilization of Nmnats be an indirect effect of broadly increasing protein stability when the Hiw/Phr1 pathway is depleted? At the moment there is a strong correlation, but whether the axon sparing activity of dNmnat or Nmnat2 explains the Hiw/Phr1 protective effect needs further clarification.
Concluding remarks
The phenotype of WldS mutants is fascinating and has inspired intense investigation into the molecular basis of Wallerian degeneration. While excellent progress has been made regarding the essential domains and site of action of WldS over the last decade, its mechanism of protection remains unclear. It seems unlikely that it will be simple, or involve only a single mechanism. WldS has taught us that axons can be remarkably autonomous structures, and based on its partial suppression of some disease models, that the axon is a viable target for therapeutic intervention in disease [4,49]. For instance, the WldS mutation significantly reduced motorneuron loss in the progressive motorneuronopathy (pmn) model of motorneuron disease [50], axon degeneration in paclitaxel (taxol)-based models of progressive peripheral neuropathy [51], dopaminergic fiber loss in a mouse model of Parkinson’s disease [52], and axon degeneration in the DBA/2J glaucoma model [53]. But WldS is not a silver bullet and its protective effect does not likely extend to all diseases where axon loss is observed—WldS has so far failed to affect disease progression in the mouse SOD-G93A model of ALS [54,55], and likely other models of neurodegenerative that likely went unreported. Whether Sarm1 of Phr1 knockouts might meet with greater success is the realm of disease suppression remains an open and exciting question.
The effect of the WldS mutant on the field of axon biology cannot be overstated. However, unraveling its mechanism has been extremely challenging. An important question to now consider is whether to continue to struggle to understand how WldS functions, or to move forward with a deeper analysis of endogenous axon death signaling molecules like dSarm/Sarm1 and Hiw/Phr1? Determining the genetic relationship between these molecules and WldS, and identifying additional conserved axon death molecules seems a priority. Perhaps inhibiting one of these with a small molecule will be a more fruitful approach than trying to mimic WldS activity in patients?
The current state of field of axon degeneration might be likened to the cell death field after the discovery of ced-3 and ced-4 mutants [56]—it is now clear based on genetic evidence that Wallerian degeneration is an active process of auto-destruction driven by an endogenous molecular program. We now need to fully define this genetic program. It is expected that model organisms like Drosophila will lead this effort. Ultimately, central goals for the field should include determining whether such axon death genes are activated in neurological disease, designing therapeutics targeted to suppress axon death, and exploring the possibility that blocking axon death will alleviate suffering in patients with neurological conditions involving axon loss.
Highlights.
The chimeric WldS molecule revealed axons can survive for long periods without a cell body
WldS raised the intriguing possibility that axon degeneration is an active process
WldS functional domains are now defined, but its mechanistic action remains unclear
dSarm/Sarm1 and Hiw/Phr1 are endogenous genes required to drive axon degeneration
“Axon death” pathways therefore exist, and may be relevant to neurological disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waller A. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations of the alternatives produced thereby in the structure of their primitive fibres. Philos Trans R Soc Lond B Biol Sci. 1850;140:423–429. [Google Scholar]
- 2••.Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. This study reported the identification of the WldS mouse, and the observation that severed distal axons survived for remarkably long periods without a cell body. [DOI] [PubMed] [Google Scholar]
- 3.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 4.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. Reported the molecular identification of WldS as a fusion protein composed of N70-18-Nmant1, and demonstrated WldS was a gain of function phenotype due to its overexpression. [DOI] [PubMed] [Google Scholar]
- 7•.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. Demonstrated that Wallerian degeneration occurred in Drosophila which could be suppressed by expression of mouse WldS, indicating evolutionary conservation of the WldS mechanism and opening the door to molecular genetic analysis of WldS and Wallerian degeneration in the fly. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell KC, Vargas ME, Sagasti A. WldS and PGC-1alpha regulate mitochondrial transport and oxidation state after axonal injury. J Neurosci. 2013;33:14778–14790. doi: 10.1523/JNEUROSCI.1331-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. Demonstrated that Nmnat1 was the likely key functional domain required for WldS-mediated protection in DRG cultures. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery MA, Sheehan AE, Kerr KS, Wang J, Freeman MR. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conforti L, Fang G, Beirowski B, Wang MS, Sorci L, Asress S, Adalbert R, Silva A, Bridge K, Huang XP, et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- 13.Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, Mazzola F, Di Stefano M, Hartley R, Babetto E, et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laser H, Conforti L, Morreale G, Mack TG, Heyer M, Haley JE, Wishart TM, Beirowski B, Walker SA, Haase G, et al. The slow Wallerian degeneration protein, WldS, binds directly to VCP/p97 and partially redistributes it within the nucleus. Mol Biol Cell. 2006;17:1075–1084. doi: 10.1091/mbc.E05-04-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Sasaki Y, Milbrandt J. Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J Biol Chem. 2010;285:41211–41215. doi: 10.1074/jbc.C110.193904. Demonstrated that delivery of WldS protein alone into previously severed axons was sufficient for suppression of Wallerian degeneration, clearly demonstrating that WldS neuroprotection can be mediated in axons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beirowski B, Babetto E, Gilley J, Mazzola F, Conforti L, Janeckova L, Magni G, Ribchester RR, Coleman MP. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J Neurosci. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babetto E, Beirowski B, Janeckova L, Brown R, Gilley J, Thomson D, Ribchester RR, Coleman MP. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J Neurosci. 2010;30:13291–13304. doi: 10.1523/JNEUROSCI.1189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avery MA, Rooney TM, Pandya JD, Wishart TM, Gillingwater TH, Geddes JW, Sullivan PG, Freeman MR. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O’Leary DD, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21••.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. dSarm/Sarm1 Is Required for Activation of an Injury-Induced Axon Death Pathway. Science. 2012 doi: 10.1126/science.1223899. Identified in a forward genetic screen in Drosophila the first loss of function mutant that suppressed axon degeneration at levels similar to WldS, and showed that dSarm/Sarm1 prodegenerative function was conserved in Drosophila and mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Stone MC, Tao J, Rolls MM. Axon injury and stress trigger a microtubule-based neuroprotective pathway. Proc Natl Acad Sci U S A. 2012;109:11842–11847. doi: 10.1073/pnas.1121180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubinska L. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977;130:47–63. doi: 10.1016/0006-8993(77)90841-1. [DOI] [PubMed] [Google Scholar]
- 24•.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. Forms the basis for the Nmnat2 depletion model. Nmnat2 was shown to be delivered from the soma to the axon, depleted after axotomy, that Nmnat2 loss induces spontaneous degeneration, and that WldS can substitute for Nmnat2 loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milde S, Gilley J, Coleman MP. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol. 2013;11:e1001539. doi: 10.1371/journal.pbio.1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks AN, Lorenzetti D, Gilley J, Lu B, Andersson KE, Miligan C, Overbeek PA, Oppenheim R, Bishop CE. Nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2) regulates axon integrity in the mouse embryo. PLoS One. 2012;7:e47869. doi: 10.1371/journal.pone.0047869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilley J, Adalbert R, Yu G, Coleman MP. Rescue of peripheral and CNS axon defects in mice lacking NMNAT2. J Neurosci. 2013;33:13410–13424. doi: 10.1523/JNEUROSCI.1534-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunio A, Bittner GD. Cyclosporin A retards the wallerian degeneration of peripheral mammalian axons. Exp Neurol. 1997;146:46–56. doi: 10.1006/exnr.1997.6484. [DOI] [PubMed] [Google Scholar]
- 29.Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, Twiss JL, Alvarez J, Court FA. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci. 2011;31:966–978. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikegami K, Koike T. Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. Neuroscience. 2003;122:617–626. doi: 10.1016/j.neuroscience.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 31•.Tao J, Rolls MM. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J Neurosci. 2011;31:5398–5405. doi: 10.1523/JNEUROSCI.3826-10.2011. Demonstrated that WldS also protects dendrites, and that mitochondria are formally disepensible for neurite fragmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitay BM, McCormack R, Wang Y, Tsoulfas P, Zhai RG. Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLD(S)-mediated axon protection independent of axonal mitochondria. Hum Mol Genet. 2013;22:1601–1614. doi: 10.1093/hmg/ddt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 34.Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O’Leary DD, Hannoush RN, Tessier-Lavigne M. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Bhattacharya MR, Gerdts J, Naylor SA, Royse EX, Ebstein SY, Sasaki Y, Milbrandt J, DiAntonio A. A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J Neurosci. 2012;32:5054–5061. doi: 10.1523/JNEUROSCI.4951-11.2012. Established a Drosophila model for chemical induced neuropathy, reported a screen for RNAi lines suppressing loss of axons after taxol treatment, and described MORN4 as a new gene required for axon degeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakatsuki S, Saitoh F, Araki T. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat Cell Biol. 2011;13:1415–1423. doi: 10.1038/ncb2373. [DOI] [PubMed] [Google Scholar]
- 38.Gerdts J, Sasaki Y, Vohra B, Marasa J, Milbrandt J. Image-based screening identifies novel roles for IkappaB kinase and glycogen synthase kinase 3 in axonal degeneration. J Biol Chem. 2011;286:28011–28018. doi: 10.1074/jbc.M111.250472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 40.Chuang CF, Bargmann CI. A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes Dev. 2005;19:270–281. doi: 10.1101/gad.1276505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M. In vivo nerve-macrophage interactions following peripheral nerve injury. J Neurosci. 2012;32:3898–3909. doi: 10.1523/JNEUROSCI.5225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CY, Lin CW, Chang CY, Jiang ST, Hsueh YP. Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology. J Cell Biol. 2011;193:769–784. doi: 10.1083/jcb.201008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y, Zhou P, Qian L, Chuang JZ, Lee J, Li C, Iadecola C, Nathan C, Ding A. MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med. 2007;204:2063–2074. doi: 10.1084/jem.20070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Xiong X, Hao Y, Sun K, Li J, Li X, Mishra B, Soppina P, Wu C, Hume RI, Collins CA. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 2012;10:e1001440. doi: 10.1371/journal.pbio.1001440. Reported that loss of the Drosophila E3 ubiqutin ligase Hiw potently suppressed axon degeneration (similar to WldS), and proposed Hiw promoted axon degeneration by driving Nmnat degradation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 48•.Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep. 2013;3:1422–1429. doi: 10.1016/j.celrep.2013.04.013. A follow up study to (46), reported that mammalian Hiw, termed Phr1 was also required for Wallerian degeneration and could modulate levels of Nmnat2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 50.Ferri A, Sanes JR, Coleman MP, Cunningham JM, Kato AC. Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr Biol. 2003;13:669–673. doi: 10.1016/s0960-9822(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 51.Wang MS, Davis AA, Culver DG, Glass JD. WldS mice are resistant to paclitaxel (taxol) neuropathy. Ann Neurol. 2002;52:442–447. doi: 10.1002/ana.10300. [DOI] [PubMed] [Google Scholar]
- 52.Sajadi A, Schneider BL, Aebischer P. Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Curr Biol. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 53.Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer LR, Culver DG, Davis AA, Tennant P, Wang M, Coleman M, Asress S, Adalbert R, Alexander GM, Glass JD. The WldS gene modestly prolongs survival in the SOD1G93A fALS mouse. Neurobiol Dis. 2005;19:293–300. doi: 10.1016/j.nbd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Vande Velde C, Garcia ML, Yin X, Trapp BD, Cleveland DW. The neuroprotective factor Wlds does not attenuate mutant SOD1-mediated motor neuron disease. Neuromolecular Med. 2004;5:193–203. doi: 10.1385/NMM:5:3:193. [DOI] [PubMed] [Google Scholar]
- 56.Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]