Abstract
Extensive research has supported the existence of a specialized face-processing network that is distinct from the visual processing areas used for general object recognition. The majority of this work has been aimed at characterizing the response properties of the fusiform face area (FFA) and the occipital face area (OFA), which together are thought to constitute the core network of brain areas responsible for facial identification. Although accruing evidence has shown that face-selective patches in the ventral anterior temporal lobes (vATLs) are interconnected with the FFA and OFA, and that they play a role in facial identification, the relative contribution of these brain areas to the core face-processing network has remained unarticulated. Here we review recent research critically implicating the vATLs in face perception and memory. We propose that current models of face processing should be revised such that the ventral anterior temporal lobes serve a centralized role in the visual face-processing network. We speculate that a hierarchically organized system of face processing areas extends bilaterally from the inferior occipital gyri to the vATLs, with facial representations becoming increasingly complex and abstracted from low-level perceptual features as they move forward along this network. The anterior temporal face areas may serve as the apex of this hierarchy, instantiating the final stages of face recognition. We further argue that the anterior temporal face areas are ideally suited to serve as an interface between face perception and face memory, linking perceptual representations of individual identity with person-specific semantic knowledge.
Keywords: face memory, face perception, social cognition, anterior temporal lobes, perirhinal cortex
1. Introduction
Humans are an intrinsically social species. More sophisticated social-cognitive skills are thought to underlie human's advanced intelligence, our tremendous cultural advances, and the evolution of language (Dunbar & Shultz, 2007; Herrmann, Call, Hernàndez-Lloreda, Hare, & Tomasello, 2007). Superior social skills in humans are driven, in part, by increased proficiency in the identification and discrimination of conspecifics. Humans are remarkably adept at face processing and have even been described as face processing “experts” as shown by extensive clinical and psychophysical research indicating that humans have specialized processes for recognizing faces that are distinct from those used for general object recognition (Farah, Wilson, Drain, & Tanaka, 1998; Gauthier & Tarr, 2002; Moscovitch, Winocur, & Behrmann, 1997). This expertise is supported by increased specialization in a number of cortical regions involved in face processing. Over a decade of neuroimaging work has characterized the neural basis of face perception and identified several nodes or ‘patches’ that preferentially respond to faces and ultimately contribute to humans’ unique face processing proficiencies (Haxby, Hoffman, & Gobbini, 2000; Kanwisher & Yovel, 2006).
Because there are multiple face patches, a natural question is whether there is redundancy in the code or whether any one region is critical for normal face processing abilities. Of the regions that respond to faces more than other objects, the fusiform face area (FFA), occipital face area (OFA), and posterior superior temporal sulcus (pSTS) are proposed to constitute the “core” face recognition system, whereas the ventral anterior temporal lobe (vATL) and the amygdala are part of the “extended network” for face recognition (Haxby, Hoffman, & Gobbini, 2000; Rossion, Schiltz, & Crommelinck, 2003). This proposal has lead to the false presumption that the vATLs play a non-critical role in face processing. However several lines of evidence strongly suggest that the vATLs play a necessary role in face perception and identification. Indeed, face-processing deficits have been more reliably observed following damage to the vATLs than more posterior portions of the face-processing network (Heywood & Cowey, 1992). Furthermore, a small but emerging body of literature has shown that a region in the vATLs is selective for faces and is interconnected with the FFA and the OFA. Early imaging studies of face perception likely missed these face-selective activations in the ATLs because they used a restricted field-of-view that excluded the inferior temporal lobe from image acquisition, or because they suffered from the well known problem of imaging the ATLs: susceptibility artifacts and signal distortion due to the proximity of these regions to the nasal sinuses and ear canals (Devlin et al., 2000; Visser, Jefferies, & Lambon Ralph, 2010). However, recent findings of face-selective cortical areas - “face patches” - in the vATLs of monkeys have spurred fMRI researchers to optimize signal detection in the vATLs, resulting in several recent studies suggesting that functionally homologous face-processing areas may exist in the vATLs of humans (see Figure 1a) (Avidan et al., 2013; Pinsk et al., 2009; Rajimehr, Young, & Tootell, 2009; Tsao, Moeller, & Freiwald, 2008).
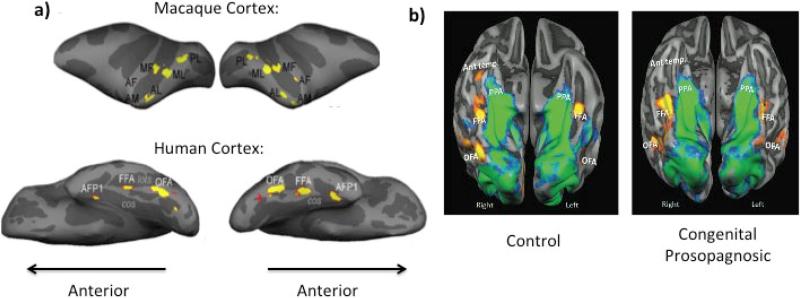
Figure 1.
(a) Face selective regions in a macaque (top) and human (bottom) have been superimposed on a lateral (top) and ventral (bottom) view of the inflated hemispheres with sulci shown in in dark gray. Top: PL, posterior face patch; MF, middle face patch in the STS fundus; ML, middle face patch on the STS lip, AF, anterior face patch in the STS fundus; AL, anterior face patch on the STS lip; AM, anterior face patch on the ventral surface of IT just lateral and anterior to the AMTS. Bottom: OFA, occipital face area; FFA, fusiform face area; AFP1, anterior face patch; cos, collateral; lots, lateral occipitotemporal. Taken with permission from Tsao, Moeller, & Freiwald, (2008) (b) Average activation maps for controls (left) and congenital prosopagnosics (right) for faces presented in a ventral view overlaid on a group-averaged folded cortical mesh of each group. The contrast faces > buildings is presented in red to yellow coloring, whereas the contrast buildings > faces is presented in blue to green coloring. Ant. Temp: anterior temporal cortex; PPA: Parahippocampal place area. Taken with permission from Avidan et al., (2013).
Here, we will review evidence suggesting that that the ventral ATLs play a critical role in face processing. We propose that a hierarchically organized system of face-selective patches extends bilaterally from the inferior occipital gyri to the vATLs and performs the visual computations necessary for accurate facial identification. As feed-forward processing proceeds along this network, facial representations become increasingly complex and abstracted from low-level perceptual features. We further speculate that a face-selective region of the vATLS, which we will refer to as the anterior temporal face area, is the apex of this hierarchy, serving to link viewpoint invariant face representations with person-specific semantic knowledge. In the following sections, we briefly review literature on the posterior face areas (OFA and FFA; see Figure 2) in order to place our proposal in context. The remaining sections will focus on neuroimaging and neuropsychology literature implicating the vATLs in face processing, and will highlight a small but emerging body of work that has examined the response properties of a face-sensitive region within the vATLs, the anterior temporal face area (see Figure 2 for an illustration of the brain regions mentioned in this review). Finally we will suggest additional modifications to current neural models of face processing.
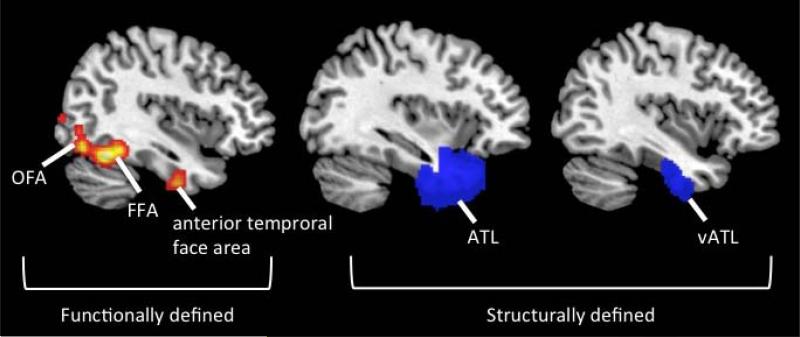
Figure 2.
Location of brain regions mentioned in this review and how they are defined. Functionally defined brain regions are localized using patterns of activation in individual subject space, typically with an independent functional localizer scan. The brain regions in this figure have been localized using the contrast faces > places. The occipital face area (OFA) is a functionally defined face processing area on the inferior occipital gyrus. The fusiform face area (FFA) is a functionally defined face processing area on the lateral middle fusiform gyrus. The anterior temporal face area is a functionally defined face processing area in the ventral ATLs. Structurally defined brain regions are localized using anatomical landmarks. The anterior temporal lobes (ATL) typically include the temporal pole, as well as anterior portions of the inferior, middle, and superior temporal gyri. The ventral anterior temporal lobes (vATL) include the anterior inferior and fusiform temporal gyri.
2. The Fusiform Face Area
A large body research has focused on the fusiform face area (FFA), located in the lateral middle fusiform gyrus (BA 37) which responds more strongly to faces than to other objects (Allison, Puce, Spencer, & McCarthy, 1999; Bentin, Allison, Puce, Perez, & McCarthy, 1996; Halgren, Raij, Marinkovic, Jousmäki, & Hari, 2000; Haxby et al., 1996; Kanwisher, McDermott, & Chun, 1997; Sergent, Ohta, & MacDonald, 1992). Face representations in the FFA are invariant to low-level stimulus manipulations such as position (Kovács, Cziraki, Vidnyánszky, Schweinberger, & Greenlee, 2008), size (Andrews & Ewbank, 2004; Grill-Spector et al., 1999; Kovács et al., 2008), spatial scale (Eger, Schyns, & Kleinschmidt, 2004), and emotional expression (Winston, Henson, Fine-Goulden, & Dolan, 2004); however they are sensitive to changes in the viewing angle of faces (Andrews & Ewbank, 2004; Ewbank & Andrews, 2008; Fang & He, 2005; Fang, Murray, & He, 2007; Pourtois, Schwartz, Seghier, Lazeyras, & Vuilleumier, 2005; Xu, Yue, Lescroart, Biederman, & Kim, 2009) which is consistent with research in macaques (see Figure 2b).
The FFA is primarily implicated in the holistic processing of faces, and responds to the shape of facial features as well as the spacing between them (Liu, Harris, & Kanwisher, 2010; Schiltz, Dricot, Goebel, & Rossion, 2010; Yovel & Kanwisher, 2004). Another study using fMRI adaptation has shown that the FFA contains regions that exhibit both part-based and holistic neural tuning (Harris & Aguirre, 2010). Some (but not all) studies using fMRI repetition suppression have implicated the FFA in processing facial identity. Specifically, it has been shown that the repetition of two face images of the same individual reduces activity in the FFA relative to the repetition of the different individuals (Andrews & Ewbank, 2004; Eger et al., 2004; Gauthier et al., 2000; Gilaie-Dotan & Malach, 2007; Loffler, Yourganov, Wilkinson, & Wilson, 2005; Winston et al., 2004), and that activation in the FFA correlates on a trial-by-trial basis with face identification accuracy (Grill-Spector, Knouf, & Kanwisher, 2004). These findings have been corroborated by studies utilizing multivoxel pattern analysis (MVPA) techniques (Anzellotti, Fairhall, & Caramazza, 2013; Goesaert & Op de Beeck, 2013; Nestor, Plaut, & Behrmann, 2011), but other groups using the same technique have failed to find this effect (Kriegeskorte, Formisano, Sorger, & Goebel, 2007; Natu et al., 2010).
Several neuroimaging studies have examined whether the FFA is sensitive to face familiarity, which is defined in the literature as faces for which there is conceptual or personal familiarity, such as the face of Barack Obama. A few studies reported increased activations in the FFA for faces made familiar through a laboratory training procedure (Lehmann et al., 2004; Verosky, Todorov, & Turk-Browne, 2013) whereas other studies reported no difference in FFA activation for famous as compared to unfamiliar faces (Eger, Schweinberger, Dolan, & Henson, 2005; Gorno-Tempini & Price, 2001; Gorno-Tempini et al., 1998; Pourtois, Schwartz, Seghier, Lazeyras, & Vuilleumier, 2005) and one study showed decreased activation in the FFA for familiar relative to unfamiliar faces (Rossion, Kung, & Tarr, 2004). Thus the jury is still out as to whether the FFA is sensitive to conceptual familiarity.
3. The Occipital Face Area
The OFA is located upstream from the FFA, on the inferior surface of the occipital gyrus (BA 19), and likely contributes to an earlier stage of face analysis than the FFA (Fairhall & Ishai, 2007). This region is primarily sensitive to low-level perceptual attributes of faces, such as spatial frequency (Eger et al., 2004), viewpoint (Ewbank & Andrews, 2008), and location (Kovács et al., 2008; Schwarzlose, Swisher, Dang, & Kanwisher, 2008). The results from several studies have suggested that the OFA is responsible for representing face parts, which are integrated into more complex representations at later processing stages, possibly by the FFA (Arcurio, Gold, & James, 2012; Liu et al., 2010; Pitcher, Charles, Devlin, Walsh, & Duchaine, 2009; Pitcher, Walsh, Yovel, & Duchaine, 2007; Schiltz et al., 2010).
Further supporting the role of the OFA in the low-level analysis of faces, Rothstein and colleagues (2005) showed that the OFA is sensitive to subtle perceptual differences between morphed faces, regardless of whether those faces are perceived as sharing an identity. This finding contrasts with the FFA, which is sensitive to the perceived identity, but not the physical similarity of faces (Rotshtein, Henson, Treves, Driver, & Dolan, 2005). Predictably, the OFA is largely insensitive to cognitive manipulations such as conceptual familiarity (Davies-Thompson, Gouws, & Andrews, 2009; Rotshtein et al., 2005) or task requirements (Nasr & Tootell, 2012).
4. The Ventral Anterior Temporal Lobes
4.1. Evidence from Macaques
Single-unit recording studies have found face sensitive neurons on the inferior bank of the anterior STS, the anterior middle temporal gyrus (MTG), the temporal pole, and the inferior surface of the ATL (De Souza, Eifuku, Tammura, Nishijo, & Ono, 2005; Eifuku, De Souza, Tamura, Nishijo, & Ono, 2004; Hasselmo, Rolls, & Baylis, 1989; Ku, Tolias, Logothetis, & Goense, 2011a; Leopold, Bondar, & Giese, 2006). Using high-resolution fMRI it has been shown that these cells are organized into six face-selective cortical areas (face-patches) on the macaque temporal lobe, each with different functional specializations (see Figure 2a). Three face patches are located on the ATLs: AF, AL, and AM(Bell, Hadj-Bouziane, Frihauf, Tootell, & Ungerleider, 2009; Hadj-Bouziane, Bell, Knusten, Ungerleider, & Tootell, 2008; Ku, Tolias, Logothetis, & Goense, 2011; Moeller, Freiwald, & Tsao, 2008; Pinsk et al., 2012; Rajimehr, Young, & Tootell, 2009; Tsao, Moeller, & Freiwald, 2008b).
The importance of the monkey ATLs in face identification has been supported by several sources of evidence. First, bilateral ablation of the monkey middle face patch does not impair face-identification if the ATLs are intact (Heywood & Cowey, 1992). Second, neuronal sensitivity to face identity is stronger in the anterior temporal regions than in other face sensitive regions (Hasselmo et al., 1989; Perrett, 1992; Rolls, Treves, Tovee, & Panzeri, 1997). Third, the most anterior face patch (AM) demonstrates viewpoint-invariant identity tuning while cells in middle temporal regions are tuned to specific views of a face (Freiwald & Tsao, 2010a). Thus, the anterior-most face patch appears to have the unique ability to represent facial identity in a viewpoint-invariant manner. Although the sensitivity of various face patches is distinct, there is evidence that they interact and modulate one another as a tightly interconnected network, such that electrical stimulation of the middle face patch activates the anterior face patches, and visa-versa (Moeller et al., 2008). It has been shown that the macaque middle face patch initially represents facial category, and after a delay, becomes slightly sensitive to facial-identity information (Tsao, Freiwald, Tootell, & Livingstone, 2006), potentially reflecting feedback from the anterior regions. Thus, the macaque face-processing network appears to be organized in a feed-forward hierarchy, with face representations becoming increasingly viewpoint-invariant and identity specific as they are fed forward in the temporal lobe (see Figure 3b), and activations in the anterior face patches feed back and influence sensitivity in more posterior regions.
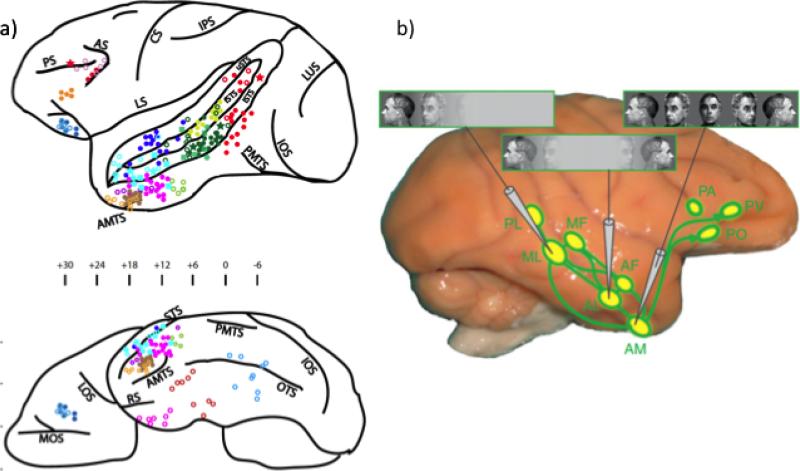
Figure 3.
(a) The locations of face-selective electrode recording cites reported in the macaque literature, superimposed on a side and ventral of the macaque brain. Abbreviation: LOS: lateral orbital sulcus; MOS: medial orbital sulcus; OTS: occipitotemporal sulcus; PMTS: posterior middle temporal sulcus: RS: rhinal sulcus. The vATL face patch would be near the anterior medial temporal sulcus (AMTS). Taken with permission from Ku et al., (2011). (b) The macaque cortical face-processing network, superimposed on a lateral view of the macaque brain with approximate locations of the 6 temporal (PL, ML, MF, AL, AF, and AM) and 3 frontal lobe (PA, PO, and PV) face patches, as well as their known connections. The functional specialization of the temporal face patches is indicated by schematic recording electrodes and sample tuning to head orientation. For instance, face patch ML is tuned to only one head orientation while the most anterior temporal face patch, AM, is sensitive to facial identity regardless of head orientation. Taken with permission from (Brecht & Freiwald, 2012).
The function of the anterior temporal face patches appears to be both mnemonic and perceptual. In regards to memory there is evidence that neurons in the monkey ATLs have response profiles indicative of mnemonic activity: spike rates decrease rapidly with stimulus repetition, firing patterns are maintained over brief delay intervals, and neurons are sensitive to associations between faces and other stimuli (Nakamura & Kubota, 1996; Nakamura, Matsumoto, Mikami, & Kubota, 1994; Sakai & Miyashita, 1991). For instance, recently it was shown that cells in the ATLs of monkeys, but not in more posterior IT regions, can represent a trained associative pairing between faces and abstract patterns (Eifuku, Nakata, Sugimori, Ono, & Tamura, 2010). In regards to perception, neurons in the ATLs are engaged during the passive viewing of faces (Freiwald & Tsao, 2010a; Ku et al., 2011a) and are sensitive to the visual properties of individual faces (Leopold et al., 2006). Specifically, it has been shown that face-sensitive neurons the ATLs responded linearly to the perceptual deviation of morphed faces from an average template faces, thus demonstrating norm-based coding for individual face identities in this brain area (Leopold et al., 2006). Taken together, these findings suggest the intriguing possibility that the face patches may bridge perception and memory, serving to link viewpoint invariant perceptual representations with person (or monkey) -specific identity information.
The relative homologies between the macaque face patches and the face processing regions in humans is not yet clear. Differences in the relative size of the macaque and human cortex, as well as differences in the number of cortical fields between the two species (Krubitzer, 2009) have made it difficult to make a one-to-one mapping across species. Moreover, while the OFA, FFA, and human anterior temporal face area all lay within the ventral cortical areas, the macaque face-processing network is primarily located more superior, near or within the superior temporal sulcus (Tsao et al., 2008). However a functional overlap between the face processing networks of the two species is consistent with a ventral shift in visual areas from the macaque to the human cortex (Orban, Van Essen, & Vanduffel, 2004).
We can gain insight to the possible homologies of the human and macaque face processing network from the functional properties of the respective face-processing regions. The shared mirror symmetric sensitivity of area AL in macaques (Freiwald & Tsao, 2010), and the FFA in humans (Axelrod & Yovel, 2012) suggests that these regions may be functionally homologous, however another study also found mirror-symmetric face representations in the OFA, and distributed throughout higher-visual cortex (Kietzmann, Swisher, König, & Tong, 2012). Notably, when the macaque and human brain are computationally morphed into the same space, the macaque middle face patch (ML) roughly corresponds to the human FFA (Rajimehr et al., 2009; Tsao, Freiwald, Knutsen, Mandeville, & Tootell, 2003) and area AM corresponds to the human anterior temporal face area (Rajimehr et al., 2009). The potential homology between area AM and the human anterior temporal face area is further supported by the a recent study showing that viewpoint invariant face-representations are latent in a more compact neural code in the anterior temporal face processing area than in the OFA or the FFA (Anzellotti et al., 2013). Thus, although future work is needed to establish the functional homologies between the human and macaque face processing network, converging evidence suggests that the human anterior temporal face area may play a similar role in face processing as area AM in the macaque.
4.2 Evidence from Humans
Early PET studies by Justine Sergent and colleagues reported face-sensitive activations in the bilateral ATLs (Sergent et al., 1992). The right ATL is typically activated during the discrimination of familiar and unfamiliar faces (Nakamura et al., 2000) and face naming (Grabowski et al., 2001), and predicts face recognition performance (Kuskowski & Pardo, 1999; Sergent et al., 1992). Additionally, the bilateral ATLs exhibit an adaptation response for repeated presentations of familiar faces (Nakamura et al., 2000; Sugiura et al., 2001) and multivoxel activity patterns in the vATLs discriminate between individual facial identities (Kriegeskorte et al., 2007; Nestor et al., 2011). These findings are consistent with three intracranial electrophysiological recording studies supporting the existence of an “anterior face area” in the human right vATL (Allison et al., 1999; McCarthy, Puce, Belger, & Allison, 1999; Puce, Allison, & McCarthy, 1999). A face-specific late potential termed “AP350” potential originated from this brain area (Allison et al., 1999), following an earlier face-specific N200 originating from posterior ventral temporal cortex (Puce et al., 1999). The AP350 component, but not the N200, is reduced by the repetition of identical faces (Allison et al., 1999), further supporting the role of the vATLs in facial identification. Recent fMRI studies with optimized signal detection in the vATLs, have supported the existence of cortical areas in the human vATLs that respond more to faces than other visual object categories (see Figure 1a)(Avidan et al., 2013; Pinsk et al., 2009; Rajimehr et al., 2009; Tsao et al., 2008).
Although only few studies have investigated the response properties of the functionally localized anterior temporal face area specifically (Anzellotti et al., 2013; Goesaert & Op de Beeck, 2013; Nasr & Tootell, 2012; Pinsk et al., 2009; Tsao et al., 2008), several decades of research have shown that that focal lesions to the ATLs cause face-processing deficits, which have been given the moniker “associative prosopagnosia”. There have been several recent reviews of this literature (Guido Gainotti & Marra, 2011; Olson, Mccoy, Klobusicky, & Ross, 2013) so we will simply summarize the most relevant findings. First, neuropsychological research consistently shows face memory, but not face perception, deficits after ATL resection. In other words, patients with lesions to the ATL, whether from epilepsy resection, head injury, or stroke, tend to have problems identifying individuals, not in differentiating individuals (Damasio, Tranel, & Damasio, 1990; Ellis, Young, & Critchley, 1989; Evans, Heggs, Antoun, & Hodges, 1995; Gainotti, 2003; Tranel, Damasio, & Damasio, 1997), whereas damage to more posterior temporal regions often results in more global face discrimination impairments. Second, there are lateralized deficits with the left ATL being more closely associated with processing verbal information associated with individuals (e.g. proper names and other easily verbalized semantic knowledge), and the right ATL being associated with processing visual and biographical information related to faces, as well as feelings of familiarity (Gainotti, 2007). Finally, ATL lesions can lead to a deficit in forming new person-based associations. Whether these face identification deficits are due to gray matter damage or disconnection of the ATL from other face-processing regions due to destruction of association tracts (e.g. Fox, Iaria, & Barton, 2008) is not known.
The neuropsychological findings strongly suggest that the function of the ATLs in face processing is largely mnemonic, especially in regards to face identification. Indeed, the ATL shows heightened activations to famous and personally familiar faces as compared to unfamiliar faces (Gobbini & Haxby, 2007; Gobbini, Leibenluft, Santiago, & Haxby, 2004; Haxby et al., 2000; Haxby, Hoffman, & Gobbini, 2002; Sugiura et al., 2001), and responses in this area are up regulated by presence of conceptual information about faces signifying semantic uniqueness (Barense, Henson, & Graham, 2011; Eifuku, De Souza, Nakata, Ono, & Tamura, 2011; Ross & Olson, 2012; Tsukiura et al., 2010). Von der Heid and colleagues (Von Der Heide, Skipper, & Olson, 2013) conducted a meta-analysis of existing fMRI studies of famous and personally familiar face processing, as well as an empirical fMRI study using optimized imaging parameters to acquire signal from the ATLs. In both studies, the authors found left-lateralized ATL activations to personally familiar and famous individuals, while novel faces activated the right ATL. Together these findings suggest that face memory-sensitive patches in the human ATL are in the ventral/polar ATL (Von der Heid et al., 2013). These findings are consistent with prior research showing greater fMRI adaptation to famous and personally familiar faces, relative to unfamiliar faces, in the ATL (Motoaki Sugiura, Mano, Sasaki, & Sadato, 2011), and that selectivity for famous faces in the vATLs correlates with pre-experimental familiarity (Rothstein et al., 2005).
However other evidence paints a more nuanced and complex picture, with the vATLs appearing to function at the intersection of high-level visual perception and memory. There is clear evidence that the functionally localized anterior temporal face area is insensitive to many low-level perceptual manipulations that leave facial identity intact such as contrast reversal (Nasr & Tootell, 2012), or viewpoint (Anzellotti et al., 2013), similar to what has been reported in the macaque (Friewald & Tsao, 2010). However, this region is sensitive to the visual features of novel faces, discriminates between individual (unknown) faces (Anzellotti et al., 2013), and unilateral damage to the vATLs impairs the ability to make fine-grained perceptual discriminations between morphed face stimuli, even when there is no time delay (Busigny et al., 2014; Fox, Hanif, Iaria, Duchaine, & Barton, 2011; Olson, Ezzyat, Plotzker, & Chatterjee, accepted pending revisions). Thus, as in monkeys, the evidence from humans indicates that face-sensitive neurons in the human vATL may serve to bridge perception and memory.
In summary, there is suggestive evidence that the anterior temporal face area in humans has both mnemonic and high-level perceptual functions. Yet overall, this region remains relatively unexplored and so we can only speculate on its role in the greater face-processing network, and its potential homology to area AM in monkeys. This is in part to technical difficulties of obtaining sufficient signal in this region. We note that studies that have localized this brain region using traditional imaging techniques have been unable to find it in all participants (Pinsk et al., 2009; Rajimehr et al., 2009; Tsao et al., 2008). Better success is obtained when thin coronal slices are used (Axelrod & Yovel, 2014) and/or a low echo-time (Von Der Heide et al., 2013). It is also possible that investigators failed to appreciate the role of this region in face processing because it is located in perirhinal cortex, a region most commonly associated with episodic memory.
4.3. The Relationship Between the Anterior Temporal Face Area and Perirhinal Cortex
Although traditionally studied in the context two independent bodies of research, the perirhinal cortex (PrC; consisting of BA 35 and 36, see Figure 4) and vATLs are spatially contiguous, highly interconnected, and perform similar computations during visual object processing (see Graham, Barense, & Lee, 2010 for a review of the perirhinal cortex). Several imaging studies have suggested that the human homologue to the monkey anterior face patch is located in the anterior (rostral) collateral sulcus in an area consistent with the PrC (O'Neil, Hutchison, McLean, & Kohler, 2014; Nasr & Tootell, 2012; Rajimehr et al., 2009; Rossion, Hanseeuw, & Dricot, 2012; Tsao et al., 2008). In humans, PrC activations are enhanced for faces relative to other objects (Lee, Bussey, et al., 2005; Lee, Scahill, & Graham, 2008), and face-specific activity in the PrC closely mirrors other face selective areas of the ventral stream (O'Neil, Barkley,& Köhler, 2013). Furthermore, the electrocorticogram of a patient with subdural grid electrodes in the right vATLs revealed a strong face-specific response from an electrode in perirhinal cortex that was reflected in both event-related potentials and broadbannd power changes (Tanji, Iwasaki, Nakasato, & Suzuki, 2012).
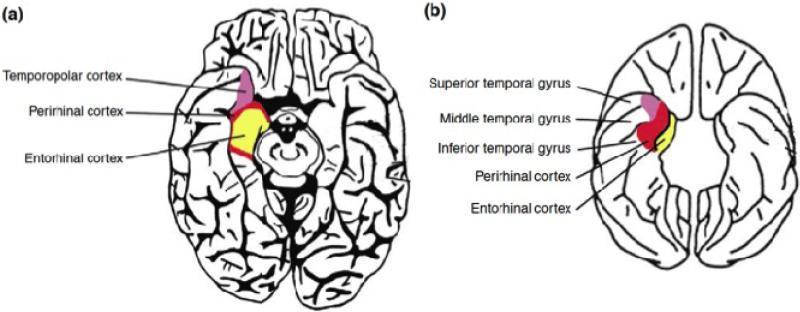
Figure 4.
The location of the primate perirhinal cortex illustrated on the ventral view of a human brain (a) and the macaque brain (b). In humans the perirhinal cortex borders the temporopolar cortex rostrally and the entorhinal cortex caudally. The lateral boundary located in the lateral bank othe collateral sulcus. The macaque perirhinal cortex is made up Brodmann's areas 35 and 36 and is located in the lateral bank of the rhinal cortex and in the laterally adjacent cortex. Taken with permission from Buckley & Gaffan (2006)
The function traditionally ascribed to perirhinal cortex is declarative memory as part of the greater medial temporal lobe memory system (for a review see, Brown, warburton, & Aggleton, 2010), However, teh PrC is highly interconnected with the visual stream, and because of this, it has been argued that PrC may be involved in certain aspects of visual object processing (Bussey & saksida, 2007; Bussey & Saksida, 2005; Saksida & Bussey, 2010). It should be noted that this view is highly controvesial (Buffalo, Reber, & Squire, 1998; Holdstock, Gutnikov, Gaffan, & Mayes, 2000; Levy, Shrager, & Squire, 2005; Stark & Squire, 2000). Nevertheless, data continues to accrue in support of the high-level visual functions of this region. Functional neuroimaging work in humans and lesion studies and non-human primates have supported this suggestion by showing that that the PrC is engaged during the visual discrimination of complex objects (Barense et al., 2011; Barense, Henson, Lee, & Graham, 2010; Baxter, 2009; Lee et al., 2008; Mundy, Downing, Dwyer, Honey, & Graham, 2013; O'Neil et al., 2013; Suzuki & Baxter, 2009), and may represent the conjunction of visual object features (Buckley & Gaffan, 2006; Cate & Köhler, 2006; Fang et al., 2007; Murray & Bussey, 1999; O'Neil, Cate, & Köhler, 2009).
The integration of multiple visual features into a durable representation is critical for face perception (as well as other discrimination tasks involving easily confusable stimuli) and memory. Thus, it is not surprising that damage to the PrC in humans impairs face recognition (Lee, Bussey, et al., 2005; Martin, McLean, O'Neil, & Kohler, 2013). Notably, the PrC is preferentially activated when face discrimination places a higher demand on feature integration (O'neil et al., 2013) due to changes in the viewpoint from which faces are presented (Barense et al., 2010), or the presentation of faces with many, as opposed to few, overlapping features (Mundy, Downing, & Graham, 2012). Thus the PrC appears to be critically involved in feature integration during the visual processing of highly similar faces.
However there is also evidence that the PrC has an important role in person memory. The PrC is preferentially sensitive to famous, as compared to unfamiliar faces (Barbeau et al., 2008; Barense et al., 2011; Martin et al., 2013) and damage to this region impairs one's ability to learn perceptual discriminations between highly similar faces (Mundy et al., 2013). Last, it has been reported that semantic memory deficits for concrete objects, most of which are defined by visual features, are associated with damage to the PrC and surrounding cortex (Mion et al., 2010). One explanation for the perception versus memory findings in this region is that the PrC is functionally coupled with different brain areas depending on task demands (O'Neil et al., 2009).
Together, these findings suggest that representational content, rather than task demands, may drive the functional specialization of the PrC, with the PrC being engaged to represent stimuli that require the integration of multiple visual features for individuation. Furthermore, PrC mechanisms are easily engaged during tasks involving face perception and memory. The representational capacity of the PrC appears well suited to perform the computational metrics attributed to the anterior temporal face area (Anzellotti et al., 2013; Nasr & Tootell, 2012), in which viewpoint-invariant identity representations are utilized for facial individuation, and possibly serve as an interface between perception and memory. It should be noted that we are not suggesting that the PrC as a whole is a face processing area. Rather, we believe that the representational affordances of the PrC are recruited during the discrimination of a variety of stimuli characterized by many overlapping visual features, and that a subpopulation of neurons within the human PrC may be optimally tuned for faces and thus may constitute the human homologue of one of the three macaque ATL face patches.
4.4. Evidence from Congenital Prosopagnosia
Congenital prosopagnosia (CP) is a lifelong inability to recognize people that arises in the absence of any obvious cortical lesions (Jones & Tranel, 2001; Kress & Daum, 2003). The perceptual deficits seen amongst CP patients are often (but not always) selective to faces, and occur despite intact visual, social, and intellectual functions (Behrmann, Avidan, Marotta, & Kimchi, 2005; Behrmann & Avidan, 2005; Bentin, Deouell, & Soroker, 1999; Yovel & Duchaine, 2006). The behavioral profile of face-processing deficits in CPs is heterogeneous in nature, and has been assessed using a variety of tasks (Behrmann et al., 2005; Duchaine & Nakayama, 2005). For example prosopagnosics are impaired at matching sequentially presented facial stimuli across a delay (Yovel & Duchaine, 2006) and at discriminating between simultaneously presented faces (Behrmann et al., 2005; Duchaine & Nakayama, 2005) suggesting that the face-processing impairments experienced by these individuals are both perceptual and mnemonic in nature.
CP's face recognition deficits may be due to the use of a featural processing strategy, which is different than the configural or holistic processing strategy typically adopted in the normal population (Duchaine & Nakayama, 2005). This claim is supported by research showing that CPs do not show an inversion effect for faces, and that they show a bias towards featural processing for non-face objects (Behrmann et al., 2005). Furthermore, it has been shown that CPs are impaired at facial discrimination when the stimuli used differ with respect to the spacing of individual features (i.e. the distance between two eyes), or the shape of individual features (how round the eyes are; Yovel & Duchaine, 2006; see also Garrido et al., 2007).
To understand the neural basis for the face recognition deficits in CP, researchers have used a variety of neuroimaging methods with mixed results. For instance, some fMRI studies of CPs have revealed abnormal response profiles for faces in the FFA (Duchaine, Yovel, Butterworth, & Nakayama, 2006; Hadjikhani & De Gelder, 2002) whereas other studies have not (Avidan, Hasson, Malach, & Behrmann, 2005; Avidan & Behrmann, 2009; Hasson, Avidan, Deouell, Bentin, & Malach, 2003; Von Kriegstein, Kleinschmidt, & Giraud, 2006). More consistent are findings implicating the vATL in the face deficits associated with CP. One study of CPs and matched controls revealed a significant reduction in the size of the anterior fusiform gyrus (Behrmann, Avidan, Gao, & Black, 2007), a region contiguous with the temporal pole, and lying squarely within the vATL face sensitive region. Reduced volume in this vATL region predicted the behavioral face recognition impairment of the patient group, as assessed by a famous face recognition task (Behrmann et al., 2007). This finding is consistent with research on a different group of CPs demonstrating that face selectivity in the anterior temporal lobe was linearly related to behavioral face identification performance on a battery of tasks assessing face discrimination and memory (Furl, Garrido, Dolan, Driver, & Duchaine, 2011). Further supporting the role of the anterior temporal face area in CP a recent functional imaging study demonstrated normal face-related activation patterns in the posterior face processing areas (OFA and FFA) and little or no activation for faces in the vATLs within the patient group (see Figure 1b; Avidan et al., 2013). Using resting state functional connectivity analysis, the authors further demonstrated that functional connectivity between the right vATL (in a region consistent with the anterior temporal face area of the normal participants) and the FFA and OFA was disrupted in congenital prosopagnosics relative to controls, suggesting that connectivity between the anterior temporal face area and the posterior face network is necessary for normal face identification.
5. Connectivity of the Ventral Anterior Temporal Face Area
During the last decade, there has seen a strong emphasis on functional specialization of cortical regions and a relative disregard of structural connectivity as an explanation for various neurological syndromes. However diffusion imaging has reawakened interest in disconnection as a possible explanation for some disorders, including prosopagnosia. As reviewed earlier, there is evidence from macaques that face sensitive patches along the inferior temporal lobe form a tightly interconnected network (Moeller et al., 2008). Consistent with this, a recent study using diffusion imaging has shown that the functionally localized FFA and OFA are strongly interconnected, and that these brain regions are structurally connected with the anterior temporal face area (See Figure 4a; Pyles, Verstynen, Schneider, & Tarr, 2013).
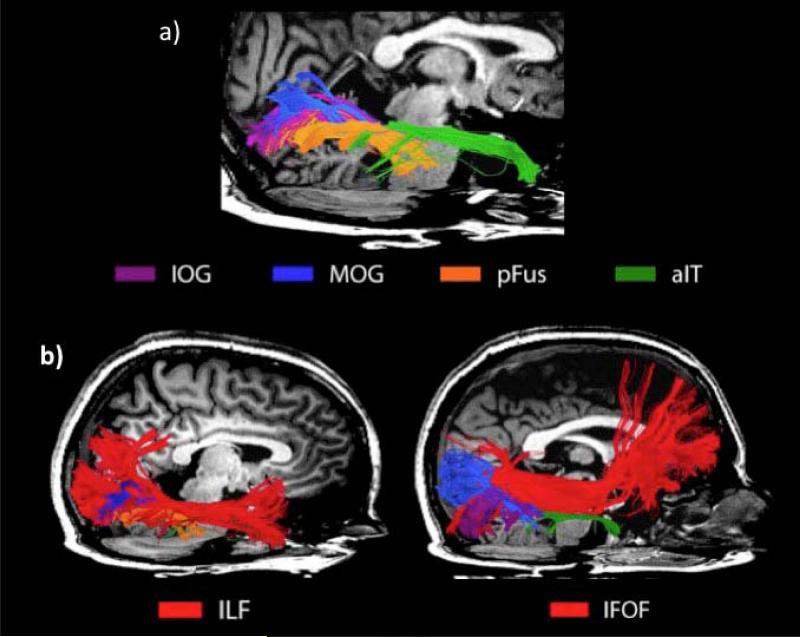
The patterns of white-matter connectivity revealed by this diffusion imaging study overlaps strikingly well with that of two long-range association tracts, the Inferior Longitudinal Fasciculus (ILF), and the Inferior Frontal Occipital Fasciculus (IFOF) (see Figure 5b). The ILF was first discovered over a hundred years ago using gross dissection techniques in monkeys (Polyak, 1957); a homologous white matter tract exists in humans. The ILF is a mono-synaptic pathway connecting ventral extrastriate regions to the ATLs (superior, middle, and inferior gyri, as well as the uncus/parahippocampal gyrus) and the amygdala. It is distinct from the optic radiations and from a series of u-shaped fibers connecting gyri along the interior temporal cortex (Catani, Jones, Donato, & Ffytche, 2003). The IFOF runs superior to the ILF, connecting the ventral occipital lobe to the orbitofrontal cortex (Catani & Thiebaut de Schotten, 2008). The IFOF is a mysterious tract; it has not been identified in monkeys (Schmahmann & Pandya, 2006) but has been identified using diffusion imaging techniques in humans, leading some researchers to suggest that it may be specific to humans (Catani & Thiebaut de Schotten, 2008). Whether or not this is true is still undecided, due in part to the lower precision of diffusion-weighted imaging as compared to gross dissection or autoradiographic tracing methods.
Figure 5.
(a) Top: white-matter pathways connecting the FFA to various face-selective ROIs (as indicated by color key below). (b) The major ILF (left) and the IFOF (right) tracts have been colored red and are overlaid on the pathways linking the face-processing network to highlight their overlap. Taken with permission from Pyles et al., (2013).
An emerging body of literature implicates the ILF, and to a lesser degree the IFOF, in face processing. In one case study, destruction of the ILF caused deficits in identifying facial emotions, presumably due to destruction of the extrastriate-amygdala branch (Philippi, Mehta, Grabowski, Adolphs, & Rudrauf, 2009). In another case, degradation of the right ILF in a variant of frontotemporal dementia called progressive prosopagnosia correlated with face identification deficits (Grossi et al., 2012). These results are compatible with findings showing that white matter scarring of the ILF in multiple sclerosis correlates with prosopagnosia as well as object recognition deficits (Yamasaki et al., 2004). Diffusion imaging studies have also reported associations between the structural integrity of the ILF, and in some cases the IFOF, and face recognition abilities (Tavor et al., 2013; Thomas et al., 2008). In addition, two studies have shown that congenital prosopagnosics, relative to controls, exhibit reduced structural integrity of the right ILF (Thomas et al., 2008; Grossi et al., 2012) and the right IFOF (Thomas et al., 2008) and that this predicts face-recognition impairments (Thomas et al., 2008). In addition to face recognition, the ILF appears to play an important role in object recognition (Coello, Duvaux, De Benedictis, Matsuda, & Duffau, 2013; Gil-Robles et al., 2013; Ortibus et al., 2012; Shinoura et al., 2013) and visual recognition of words (Epelbaum et al., 2008; Gil-Robles et al., 2013; Yeatman, Dougherty, Ben-Shachar, & Wandell, 2012). Like face recognition, these tasks involve integrating perceptual representations with higher-order conceptual knowledge to facilitate rapid recognition. The ILF probably consists of several sub-tracts that serve to link domain-specific visual processing areas performing different computational metrics along the ventral stream, and that the face-network constitutes one of these sub-tracts. Consistent with this possibility, one study has shown that different portions of the ILF are associated with recognition ability for different stimulus classes (Tavor et al., 2013).
The ATLs are monosynaptically connected to specific nuclei in the amygdala involved in learning and memory (Bach, Behrens, Garrido, Weiskopf, & Dolan, 2011; Von der Heide, Skipper, Klobusicky, & Olson, 2013), and unique functional connectivity between the amygdala and the anterior temporal face area has been observed at rest (O'Neil et al., 2014). It has recently been argued that the amygdala should be included as a part of the “core” face-processing network (Mende-Siedlecki, Verosky, Turk-Browne, & Todorov, 2013). This claim was based on findings that the amygdala demonstrates selectivity for neutral facial stimuli, the multivoxel response of this region to faces is reliable across runs, and that this region demonstrates face-specific connectivity with the fusiform gyrus. Earlier studies supported the existence of face-selective amygdala regions in monkeys (Logothetis, Guggenberger, Peled, & Pauls, 1999; Hoffman, Gothard, Schmid, & Logothetis, 2007) and in humans (Engell & McCarthy, 2013; Rossion, Hanseeuw, & Dricot, 2012).
The role of the amygdala in face processing is most closely aligned with facial emotion processing (Fairhall & Ishai, 2007) and the modulation of cortical regions based on the emotional tagging by this region. Monkeys (Hadj-Bouzaine et al., 2013) and humans (Vuilleumier et al., 2004) with amygdala damage fail to show enhanced activity in IT cortex to emotional faces, presumably because these regions are no longer receiving modulatory input (also see Hadj-Bouzaine et al., 2013). Thus, there is converging evidence that the amygdala is sensitive to faces. We speculate that functional interactions between the amygdala and cortical regions may support the prioritization of motivationally relevant individuals.
6. Extending Previous Models of Face Processing
The focus of this review is the contribution of the vATLs to face processing. However we suggest two additional addendums to previous neural models of face perception that we outline below.
The pSTS and the Face Processing Network. Whereas the OFA, FFA, and anterior temporal face area form a coherent network with direct structural and functional connections, two recent studies that used diffusion weighted imaging techniques failed to find white matter pathways between face processing areas in ventral temporal cortex and the pSTS (Gschwind et al., 2012; Pyles et al., 2013). It should be noted that the results from functional connectivity analyses have been more mixed, with functional connectivity between the FFA and pSTS being demonstrated in some studies (Turk-Browne, Norman-Haignere, & McCarthy, 2010) but not others (Davies-Thompson & Andrews, 2012; Fairhall & Ishai, 2007). This lack of structural connectivity between core face processing regions and the pSTS is not surprising given the location of each face processing area. The OFA, FFA, and anterior temporal face area are all located along the ventral visual processing stream and may be connected via the ILF or IFOF (Gschwind et al., 2012; Pyles et al., 2013). In contrast, the pSTS face area is located along the dorsal visual processing stream, and is connected with parietal and frontal regions via the arcuate fasciculus (Gschwind et al., 2012) and the superior longitudinal fasciculus (Ethofer et al., 2013). These findings suggest that the pSTS may interface with face processing regions in ventral-temporal cortex through indirect (i.e. mediated by a separate brain region) connections (O'Neil et al., 2014). We further speculate that these indirect connections are likely up or down regulated by task demands (Baseler, Harris, Young, & Andrews, 2013).
A functional segregation of the pSTS from the OFA, FFA, and anterior temporal face area is further supported by the sensitivity profiles of these brain regions. Whereas the OFA FFA and anterior temporal face area are primarily involved in processing face identity, the pSTS has been implicated in processing facial expression and eye gaze, information that is derived from the moveable or dynamic aspects of a face (Haxby et al., 2000; Nummenmaa, Passamonti, Rowe, Engell, & Calder, 2010; Schultz & Pilz, 2009). Based on these findings we propose that the pSTS is part of a separate but parallel pathway of facial information processing that represents the dynamic aspects of faces important for normal social interactions (for similar arguments see (O'Toole, Roark, & Abdi, 2002; Weiner & Grill-Spector, 2013). The pSTS constitutes a critical node within this pathway, and likely interfaces with several other social-emotional brain areas to support high-level social processes (Allison, Puce, & Mccarthy, 2000; Blakemore, Winston, & Frith, 2004).
A Patchy Functional Architecture Underlies Face Processing. A growing number of face-selective areas or “patches” have been identified in recent fMRI studies that were not present in earlier models of face perception (see Gobbinni & Haxby, 2007; Haxby et al., 2000). For example, two (Avidan et al., 2013; Rajimehr et al., 2009) or three (Pyles et al., 2013) face patches have been identified in occipital cortex near the OFA. Furthermore, multiple face patches have been identified within the fusiform gyrus, posterior or anterior to the FFA (Axelrod & Yovel, 2014.; Engell & McCarthy, 2013; Julian, Fedorenko, Webster, & Kanwisher, 2012; Kietzmann et al., 2012; McGugin et al., 2012; Pinsk et al., 2009; Rossion et al., 2012; Weiner & Grill-Spector, 2010). We speculate that the current characterization of the core face-processing network as consisting of three distinct areas (the OFA, FFA, and pSTS) should be replaced with one in which several ventral face patches extending bilaterally from occipital to anterior temporal cortices contribute to facial identification. Notably, face-selective areas have also been identified in brain regions presumed to play a role in the extended processing of facial identity, such as the amygdala (Avidan et al., 2013; Engell & McCarthy, 2013; Gschwind et al., 2012; Rajimehr et al., 2009; Rossion et al., 2012), orbitofrontal cortex (Gschwind et al., 2012; Julian et al., 2012; Rajimehr et al., 2009; Tsao et al., 2008); and inferior frontal gyrus (Gschwind et al., 2012; Julian et al., 2012; Rajimehr et al., 2009; Rossion et al., 2012; Tsao et al., 2008). The relative contribution of these face patches to the face-processing network remains relatively unexplored and warrants future research.
The variability in the reported face-processing regions across studies raises important methodological questions regarding the optimal way to identify specialized face processing areas in visual cortex and beyond. Moving forward, researchers in the field of face-perception should carefully consider the stimuli, task, and statistical threshold that they use in their functional localizers, as these factors can greatly influence the location and number of face-patches identified (Rossion et al., 2012). Weiner and Grill-Spector (2013) have outlined several recommendations for the acquisition and analysis of fMRI data in high-level visual cortex, including the use of smaller voxels, gray matter segmentation, single subject analyses, and surface based analyses. Furthermore, it is well known that the height and shape of the hemodynamic response function, as well as the signal-to-noise ratio, varies greatly across brain regions and across individual subjects. Thus, the use of varying statistical thresholds for different brains, and different brain regions may be necessary to identify all face processing areas across individuals.
The number and size of reported face-responsive regions also varies across individual subjects (Axelrod & Yovel, 2014; Nasr & Tootell, 2012; Bruno Rossion et al., 2012). Although a portion of this variance may be attributable to a lack of power in single-subject analysis, a more intriguing possibility is that this variability reflects true individual differences in the neural architecture underling face perception and memory. Thus, an important avenue for future research will be to characterize how individual variability in the number, size, and spatial extent of face processing regions relate to individual differences in face processing abilities.
7. Conclusions
Haxby and colleagues (Gobbini & Haxby, 2007; Haxby et al., 2000) distinguish between a core system - the OFA, FFA, and pSTS - that encodes the visual appearance of a face and an extended system -the anterior temporal lobes, as well as several other limbic and non-limbic structures - responsible for processing the meaning of information derived from a face. Although these authors attribute the representation of biographical information to the anterior temporal lobes generally, they made no claims about the presence or absence of a dedicated face processing area in the ATLs. This is not surprising given the fact that the anterior temporal face area has only recently been identified in humans (Tsao et al., 2010; Rajimehr et al., 2009), and the explication of this area requires the use of pulse sequences that are optimized for signal detection in in the ATLs. Based on the findings reviewed here, we believe that current models of face processing should be revised such that the vATLs, and possibly the anterior temporal face area, play a central role in the face processing. We base this proposal on several key points, outlined here, which we elaborate on below: 1) A region of the ventral ATLs is specifically responsive to faces; 2) This region is interconnected with the other regions of the core face network (the OFA and FFA); and 3) The vATLs play a necessary role in the visual encoding of facial identity. We further speculate that the sensitivity of the anterior temporal lobes to familiarity and semantic knowledge makes the anterior temporal face area ideally suited to serve as a nexus between memory and perception, possibly serving to link viewpoint invariant representations of facial identity with person-specific semantic knowledge.
7.1. A Region in the vATLs Demonstrates Selectivity for Faces
Most studies that have functionally localized the anterior face patch in humans have used the contrast faces > scenes (Anzellotti et al., 2013; Avidan et al., 2013; Axelrod & Yovel, 2014.; Nasr & Tootell, 2012; O'Neil, Hutchison, McLean, & Köhler, 2014; Rajimehr et al., 2009) or faces > objects (Axelrod & Yovel, 2014; Pinsk et al., 2009; Rajimehr et al., 2009). Thus, while it is apparent that a discrete region of the vATLs is more active for faces relative to objects or scenes, it is not clear how selective this region is to face stimuli. Two studies have explored the sensitivity of this region to bodies and body parts. Tsao et al., (2010) found that the anterior temporal face area, as well as the FFA and a face-selective region of the inferior frontal sulcus, was more active for headless bodies than for objects, whereas Pinsk et al., (2009) found no differential activity in the anterior temporal face area to body parts and objects. Importantly, Tsao and colleagues found that activity in the anterior temporal face area was greater for faces than for headless bodies, and that activity in the human anterior temporal face area was significantly greater for human faces than for macaque faces. This was not true in the FFA or OFA, which responded similarly to both face types. Thus, converging evidence suggests that activity in the human anterior temporal face area is selective for human faces. Much more work is needed to evaluate the degree of face-selectivity in the human anterior temporal face area. For example, previous work has shown that the FFA responds to non-face objects of expertise (Gauthier et al., 2000; Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999; McGugin, Gatenby, Gore, & Gauthier, 2012) and geometric shapes interacting in a social manner (Schultz et al., 2003), although it should be noted that activations in this region are much more robust to face stimuli than non-face objects (Kanwisher, 2010). Whether the human anterior temporal face area responds similarly to non-face objects of expertise, or social non-face objects, remains an open question.
7.2. The Anterior Temporal Face Area is Interconnected with the Occipital Face Area and the Fusiform Face Area
Single-cell recordings in non-human primates (Moeller et al., 2008), diffusion-weighted imaging in humans (Pyles et al., 2013), and resting state connectivity (O'Neil et al., 2014) have shown that face-sensitive cortical areas in the vATLs are interconnected with the OFA and FFA. The disruption of functional (Avidan et al., 2013) and structural (Thomas et al., 2008) connections between the vATLs and posterior face-processing areas has been implicated in the face-selective visual processing deficits seen in congenital prosopagnosics. Thus the integration of the anterior temporal face area with the OFA and FFA appears to be necessary for normal facial identification (O'Neil et al., 2014).
The interconnectedness of the anterior temporal face area with the OFA and FFA suggest a feed-forward architecture, with facial representations becoming increasingly complex and abstracted from low-level perceptual features as they move forward along this network. Consistent with this possibility, neurons in the macaque vATLs display identity tuning that is more viewpoint invariant than more posterior brain regions (Freiwald & Tsao, 2010); and viewpoint-invariant identity representations are latent in a more compact neural code in the human anterior temporal face area than in more posterior face-processing areas (Anzellotti et al., 2013). One possibility is that the outputs of view-selective cells in the OFA and FFA are combined hierarchically to enable cells “upstream” to generalize across viewpoints, and possibly other image transformations. More work is needed to evaluate this possibility, as the response properties in the human anterior temporal face area remain poorly characterized.
The neural network supporting facial processing is likely also characterized by multiple feed-back interactions between discrete face processing regions, supporting the redundant representation of facial identity in multiple cortical areas (Anzellotti et al., 2013). Consistent with this possibility, electrically stimulating face-processing areas in the inferior occipital gyrus (Jonas et al., 2012), mid fusiform gyrus (Parvizi et al., 2012), or prefrontal cortex (Vignal, Chauvel, & Halgren, 2000), all result in similar face-processing deficits. Furthermore, normal FFA activations for faces have been observed in the absence of an OFA (Rossion, 2008), and it has been shown that identity representations emerge at an earlier latency in the vATLs than the FFA (Tsao et al., 2006), suggesting that feedback may be critical to identity representation in more posterior face areas.
7.3. The vATLs are involved in Normal Face Perception
In this paper we have reviewed evidence from single-unit recordings and fMRI in monkeys, fMRI and lesion studies in humans, as well as findings from developmental prosopagnosia, all pointing towards the conclusion that portions of the vATL play a role in face perception.
The functional role of the vATLs in face perception is still being worked out. We speculate that the ventral anterior temporal lobe's role in identity processing is complex and multifactorial. However, one of these processes appears to be similar to “holistic processing” long noted to be required for normal face processing (Farah et al., 1998). The ability to make certain types of face-based fine-grained discriminations is impaired in patients with damage to the vATL/perirhinal cortex (Barense, Gaffan, & Graham, 2007; Fox et al., 2011; Lee, Buckley, et al., 2005; Olson et al., accepted pending revisions). Such patients are not prosopagnosic in the classic sense (for instance, they perform normally on the Benton Face Inventory; Fox et al., 2011) because they retain the ability to cull information about identity from individual features and non-facial information. For instance, when given enough time, these patients can discern small changes in visual stimuli by using a laborious feature-by-feature comparison strategy. However, when there is a time constraint, their performance on face identity discrimination is poor (Busigny et al., 2014; Olson et al., accepted pending revisions). Paralleling this finding, congenital prosopagnosics, who typically have reduced volume in the vATLs, as well as reduced activation and functional connectivity of the vATLs with the rest of the face processing network (Avidan et al., 2013; Behrmann et al., 2007; Furl et al., 2011), use a similar feature-based strategy during face and object processing (Behrmann et al., 2005; Duchaine & Nakayama, 2005; Yovel & Duchaine, 2006). These findings have recently been corroborated in the normal population; the face inversion effect, a behavioral signature of holistic face processing, is predicted by the strength of resting state connectivity between the anterior temporal face area and the FFA (O'Neil et al., 2014).
Last, there is some degree of mechanistic specificity within the ventral anterior temporal lobes. Perception tasks that do not require within-object feature integration, such as the discrimination of colors or object size, or facial age, gender, or emotional expression, which can be performed by examining discrete features, are performed normally by patients with gross ATL/perirhinal lesions (Barense et al., 2007; Fox et al., 2011; Lee, Buckley, et al., 2005; Olson et al., accepted pending revisions). In both monkeys and humans, the visual perception functions of the vATLs appear to be linked to one task: face identification.
Importantly, the neuropsychological findings discussed earlier suggest that the normal functioning of any one area of the face-processing network is necessary, but not sufficient, for accurate face discrimination. Thus, the ability to accurately discriminate between and identify individual faces relies on the integration of information that is represented within the OFA, FFA, and anterior face patches. Consistent with this suggestion representations of facial identity that are tolerant to image transformation have also been shown in the FFA (Anzellotti et al., 2013; Nestor et al., 2011; Goesaert & Op de Beeck, 2013), and the OFA (Anzellotti et al., 2013; Goesaert & Op de Beeck, 2013). However, the shared sensitivity of the OFA, FFA, and anterior face patches to facial identity does not imply that these brain regions represent identity in the same way. Identity representations in discrete face-processing areas are likely optimized for different functions, and may rely on different information. We argue here that the facial identity information in the vATLs is abstracted from the perceptual features of faces, and is thus more invariant to image transformations relative to the more posterior face-processing regions.
6.4. The vATLs are Involved in Person Memory
A person's identity, and all that goes along with it, can be retrieved from a vast range of cues, both discrete and situational. Simply saying the name “Hitler” evokes a facial image, a voice, various facts about his biography, as well as strong emotional reactions. Several lines of evidence indicate that the vATLs play an important, albeit poorly understood, role in person memory (for reviews see Gainotti & Marra, 2011; Olson et al., 2013). Gross resection of the ATL commonly causes deficits in retrieving information about well-known individuals, such as friends and celebrities. The most common explanation for these deficits is that they reflect a more general problem in accessing unique or specific-level semantic information (Grabowski et al., 2001; Patterson, Nestor, & Rogers, 2007). However the name-retrieval deficits after left ATL resection are only part of the constellation of person-memory deficits observed in these patients: patients with right ATL lesions can have problems retrieving biographical information or in experiencing feelings of familiarity upon re-meeting people. This familiarity deficit corresponds well to findings from neuroimaging and single-unit recordings (Barbeau et al., 2008; Barense et al., 2011; Martin et al., 2013; Rotshtein et al., 2005; Sugiura et al., 2011). It has also been reported that the ability to imagine the faces of famous individuals is completely abolished after damage to the vATLs, but not following damage to other face processing areas (Barton & Cherkasova, 2003). Other studies have linked face-based associative learning to processing in the vATLs (Brambati, Benoit, Monetta, Belleville, & Joubert, 2010; Eifuku et al., 2010; Nieuwenhuis et al., 2012), and activity in the vATL, more so than the FFA or OFA, is up-regulated when faces are paired with semantic content (Ross & Olson, 2012; Von Der Heide et al., 2013).
We speculate that conceptual knowledge of individuals – their names, biographical information, and personal significance – is bound to the view-invariant perceptual representations of faces found in the vATL. This process is accomplished in part by the ability of cells in the vATL to quickly represent associations (Eifuku et al., 2010) as well as mnemonic familiarity in the form of repetition suppression. It is also facilitated by the tight structural interconnectivity of the vATL, amygdala, and hippocampus via short-range fiber pathways (Blaizot et al., 2010; Insausti, Amaral, & Cowan, 1987; Morán, Mufson, & Mesulam, 1987; Suzuki & Amaral, 1994).
7.5. Final Comments
Based on the findings reviewed here we propose that the ventral ATLs are critically involved in face processing. Very few studies have examined the response properties of the functionally localized anterior temporal face area, so we can only speculate on its role in the face-processing network. The connectivity of this region to the OFA and the FFA, and the relevance of this connectivity to face perception are suggestive that this region should be considered as a part of the “core” face-processing network, along with the more posterior face processing areas. We can further speculate based on findings from neuropsychology (which typically involve gross lesions to the ATLs) and fMRI studies involving the vATLs, that this region may serve at the intersection of face-memory and perception, linking viewpoint-invariant facial representations with person-specific semantic knowledge.
Much work is needed to flesh-out the role of the vATLs, and more specifically the anterior temporal face area, in face processing. It is clear that prior models of face processing are incomplete at best, and need to be revised to incorporate brain regions beyond the OFA, FFA as playing centralized roles in facial identification. Importantly, the distinction between “core” and “extended” systems for face processing may obscure important nuances in the functional architecture underling face perception and memory. The face-processing network is highly interconnected, and these connections are variable across different face-processing areas, and under different task demands. A promising avenue for future research will be to explore the relevance of the integrated activity between face-sensitive regions of the vATLs, as well as the OFA, FFA, amygdala, and un-discussed regions of the orbitofrontal cortex to normal face processing, and how individual differences in this connectivity give rise to individual differences in social cognition.
Box 1 Questions for future research.
Several lines of evidence implicate the vATLs in the face processing deficits found in congenital prosopagnosia. Individuals with autism spectrum disorders also have face-processing (usually face memory) deficits, albeit of a less dramatic nature (Weigelt, Koldewyn, & Kanwisher, 2012). Future research should be aimed at understanding the functional profile of the vATLs in autistic individuals, and the connectivity of the vATL face areas with the rest of the face-processing network.
The macaque literature has reported the existence of face patches in the orbitofrontal cortex (Thorpe et al., 1983; Tsao et al., 2008). The functional significance of these patches is poorly understood although it has been suggested that they represent social reward value, such as sexual attractiveness, since the orbitofrontal cortex has been found to be generally sensitive to reward and punishment (Aharon et al., 2001; O'Doherty et al., 2003; Kranz & Ishai, 2006; Ishai, 2007). The ventral anterior temporal lobe is monosynaptically connected with the lateral orbitofrontal cortex via the uncinate fasciculus. It is possible that facial identity information coded by the vATL face patches interact with the OFC face patches to associate reward and punishment history with facial identity information (von der Heide et al., 2013).
Cell loss in perirhinal cortex has been associated with the semantic deficits found in semantic dementia (Mion et al., 2010). The fact that conceptual knowledge, naming, and high-level visual perception would all be associated with the same small region of cortex is rectified by embodied accounts of semantic cognition which claim that our knowledge of various categories is embedded in the same structures that process the sensory or motoric aspects of the stimulus (Barsalou et al., 2008). Thus color concepts should rely on the same cortical processing region (e.g. ventral v4) as color perception. By the same token, person concepts, which necessarily would be at the individual level, and some aspects of person perception, should rely on the same neural processing regions. Whether all the nodes in the face patch network are similarly sensitive to conceptual knowledge is not known.
Single-cell recording studies in the macaque suggest that face representations in the vATL are relatively invariant to low-level perceptual manipulations that leave identity intact. However the dimensions across which the human vATL face patches represent facial identity remain unclear. It has been shown that the vATL face area can represent identity across changes in viewpoint (Friewald & Tsao, 2010; Anzellotti et al., 2013), however in the real world we often need to iad of image changes, including illumination, retinal distance, age, recognize individuals across a myr hair style, etc. A comprehensive understanding of the functional properties of the vATL face areas will require systematic investigation into the perceptual features that the vATL face areas are sensitive, as well as those it is invariant to.
Do individual differences in the size, location, or magnitude of face-selective activations in the vATLs contribute to individual differences in face-processing abilities?
Highlights.
We review research implicating the vATLs in face perception and memory.
We discuss an emerging body of research showing that a portion of the vATLs is selective for faces.
We discuss the human and nonhuman primate face-processing system
The vATLs may be critically involved in congenital prosopagnosia
The human anterior temporal face area may be located in the perirhinal cortex.
Acknowledgments
We would like to thank Vanessa Troiani for assistance with conceptual aspects of this manuscript, and two anonymous reviewers for their very helpful insights on earlier versions of this manuscript. This work was funded by a National Institute of Health grant to I. Olson [RO1 MH091113].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison T, Puce A, Mccarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Science. 2000;4(7):251–291. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cerebral Cortex. 1999;9(5):415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Ewbank MP. Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. NeuroImage. 2004;23(3):905–13. doi: 10.1016/j.neuroimage.2004.07.060. doi:10.1016/j.neuroimage.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Anzellotti S, Fairhall SL, Caramazza A. Decoding representations of face identity that are tolerant to rotation. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht046. Advance online publication. doi:10.1093/cercor/bht046. [DOI] [PubMed] [Google Scholar]
- Arcurio LR, Gold JM, James TW. The response of face-selective cortex with single face parts and part combinations. Neuropsychologia. 2012;50(10):2454–9. doi: 10.1016/j.neuropsychologia.2012.06.016. doi: 10.1016/j.neuropsychologia.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan G, Hasson U, Malach R, Behrmann M. Detailed exploration of face-related processing in congenital prosopagnosia: 2. Functional neuroimaging findings. Journal of Cognitive Neuroscience. 2005;17(7):1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- Avidan G, Tanzer M, Hadj-Bouziane F, Liu N, Ungerleider LG, Behrmann M. Selective dissociation between core and extended regions of the face Ppocessing network in congenital prosopagnosia. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht007. Advance online publication doi:10.1093/cercor/bht007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod V, Yovel G. The challenge of localizing the anterior temporal face area: A possible solution. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2013.05.015. Advance online publication. doi: 10.1016/j.neuroimage.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Axelrod V, Yovel G. Hierarchical processing of face viewpoint in human visual cortex. The Journal of Neuroscience. 2012;32(7):2442–52. doi: 10.1523/JNEUROSCI.4770-11.2012. doi:10.1523/JNEUROSCI.4770-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Behrens TE, Garrido L, Weiskopf N, Dolan RJ. Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. The Journal of Neuroscience. 2011;31(2):618–23. doi: 10.1523/JNEUROSCI.2744-10.2011. doi:10.1523/JNEUROSCI.2744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau EJ, Taylor MJ, Regis J, Marquis P, Chauvel P, Liégeois-Chauvel C. Spatio temporal dynamics of face recognition. Cerebral Cortex. 2008;18(5):997–1009. doi: 10.1093/cercor/bhm140. doi:10.1093/cercor/bhm140. [DOI] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45(13):2963–74. doi: 10.1016/j.neuropsychologia.2007.05.023. doi:10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RNA, Graham KS. Perception and conception: Temporal lobe activity during complex discriminations of familiar and novel faces and objects. Journal of Cognitive Neuroscience. 2011;23(10):3052–67. doi: 10.1162/jocn_a_00010. doi:10.1162/jocn_a_00010. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RNA, Lee ACH, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: Effects of viewpoint. Hippocampus. 2010;20(3):389–401. doi: 10.1002/hipo.20641. doi:10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton JJS, Cherkasova M. Face imagery and its relation to perception and covert recognition in prosopagnosia. Neurology. 2003;61(2):220–5. doi: 10.1212/01.wnl.0000071229.11658.f8. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Harris RJ, Young AW, Andrews TJ. Neural responses to expression and gaze in the posterior superior temporal sulcus interact with facial identity. Cerebral Cortex. 2013;24(3):737–744. doi: 10.1093/cercor/bhs360. doi:10.1093/cercor/bhs360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. 2009;61(5):667–77. doi: 10.1016/j.neuron.2009.02.007. doi:10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G. Congenital prosopagnosia: Face-blind from birth. Trends in Cognitive Sciences. 2005;9(4):180–7. doi: 10.1016/j.tics.2005.02.011. doi:10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cerebral Cortex. 2007;17(10):2354–63. doi: 10.1093/cercor/bhl144. doi:10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Marotta JJ, Kimchi R. Detailed exploration of face-related processing in congenital prosopagnosia: 1. Behavioral findings. Journal of Cognitive Neuroscience. 2005;17(7):1130–1149. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- Bell AH, Hadj-Bouziane F, Frihauf JB, Tootell RBH, Ungerleider LG. Object representations in the temporal cortex of monkeys and humans as revealed by functional magnetic resonance imaging. Journal of Neurophysiology. 2009;101(2):688–700. doi: 10.1152/jn.90657.2008. doi:10.1152/jn.90657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. NeuroReport. 1999;10(40):823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- Blaizot X, Mansilla F, Insausti AM, Constans JM, Salinas-Alamán A, Pró-Sistiaga P, Insausti R. The human parahippocampal region: I. Temporal pole cytoarchitectonic and MRI correlation. Cerebral Cortex. 2010;20(9):2198–212. doi: 10.1093/cercor/bhp289. doi:10.1093/cercor/bhp289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Winston J, Frith U. Social cognitive neuroscience: Where are we heading? Trends in Cognitive Sciences. 2004;8(5):216–22. doi: 10.1016/j.tics.2004.03.012. doi:10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Benoit S, Monetta L, Belleville S, Joubert S. The role of the left anterior temporal lobe in the semantic processing of famous faces. NeuroImage. 2010;53(2):674–81. doi: 10.1016/j.neuroimage.2010.06.045. doi:10.1016/j.neuroimage.2010.06.045. [DOI] [PubMed] [Google Scholar]
- Brecht M, Freiwald WA. The many facets of facial interactions in mammals. Current Opinion in Neurobiology. 2012;22(2):259–66. doi: 10.1016/j.conb.2011.12.003. doi:10.1016/j.conb.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Brown MW, Warburton EC, Aggleton JP. Recognition memory: Material, processes, and substrates. Hippocampus. 2010;20(11):1228–44. doi: 10.1002/hipo.20858. doi:10.1002/hipo.20858. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortical contributions to object perception. Trends in Cognitive Sciences. 2006;10(3):100–7. doi: 10.1016/j.tics.2006.01.008. doi:10.1016/j.tics.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Busigny T, Van Belle G, Jemel B, Hosein A, Joubert S, Rossion B. Face-specific impairment in holistic perception following focal lesion of the right anterior temporal lobe. Neuropsychologia, Advance online publication. 2014 doi: 10.1016/j.neuropsychologia.2014.01.018. doi:10.1016/j.neuropsychologia.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: An alternative approach. Current Opinion in Neurobiology. 2005;15(6):730–7. doi: 10.1016/j.conb.2005.10.014. doi:10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: Thinking outside of the boxes. Hippocampus. 2007;17(9):898–908. doi: 10.1002/hipo.20320. doi:10.1002/hipo. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(9):2093–107. doi: 10.1093/brain/awg203. doi:10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–32. doi: 10.1016/j.cortex.2008.05.004. doi:10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cate AD, Köhler S. The missing whole in perceptual models of perirhinal cortex. Trends in Cognitive Sciences. 2006;10(9):396–7. doi: 10.1016/j.tics.2006.07.004. doi:10.1016/j.tics.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annual Review of Neuroscience. 1990;13(1):89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- Davies-Thompson J, Andrews TJ. Intra- and interhemispheric connectivity between face-selective regions in the human brain. Journal of Neurophysiology. 2012;108(11):3087–95. doi: 10.1152/jn.01171.2011. doi:10.1152/jn.01171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies-Thompson J, Gouws A, Andrews TJ. An image-dependent representation of familiar and unfamiliar faces in the human ventral stream. Neuropsychologia. 2009;47(6):1627–1635. doi: 10.1016/j.neuropsychologia.2009.01.017. doi: 10.1016/j.neuropsychologia.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza WC, Eifuku S, Tamura R, Nishijo H, Ono T. Differential characteristics of face neuron responses within the anterior superior temporal sulcus of macaques. Journal of Neurophysiology. 2005;94(2):1252–1266. doi: 10.1152/jn.00949.2004. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Tyler LK. Susceptibility-induced loss of signal: Comparing PET and fMRI on a semantic task. NeuroImage. 2000;11(6):589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Duchaine BC, Yovel G, Butterworth EJ, Nakayama K. Prosopagnosia as an impairment to face-specific mechanisms: Elimination of the alternative hypotheses in a developmental case. Cognitive Neuropsychology. 2006;23(5):714–747. doi: 10.1080/02643290500441296. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. Dissociations of Face and Object Recognition in Developmental Prosopagnosia. Journal of Cognitive Neuroscience. 2005;17(2):249–261. doi: 10.1162/0898929053124857. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007;317(5843):1344–7. doi: 10.1126/science.1145463. doi:10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. NeuroImage. 2005;26(4):1128–39. doi: 10.1016/j.neuroimage.2005.03.010. doi:10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Eger E, Schyns PG, Kleinschmidt A. Scale invariant adaptation in fusiform face-responsive regions. NeuroImage. 2004;22(1):232–242. doi: 10.1016/j.neuroimage.2003.12.028. Retrieved from http://eprints.gla.ac.uk/24940/ [DOI] [PubMed] [Google Scholar]
- Eifuku S, De Souza WC, Nakata R, Ono T, Tamura R. Neural representations of personally familiar and unfamiliar faces in the anterior inferior temporal cortex of monkeys. PloS One. 2011;6(4):e18913. doi: 10.1371/journal.pone.0018913. doi:10.1371/journal.pone.0018913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifuku S, De Souza WC, Tamura R, Nishijo H, Ono T. Neuronal correlates of face identification in the monkey anterior temporal cortical areas. Journal of Neurophysiology. 2004;91(1):358–371. doi: 10.1152/jn.00198.2003. [DOI] [PubMed] [Google Scholar]
- Eifuku S, Nakata R, Sugimori M, Ono T, Tamura R. Neural correlates of associative face memory in the anterior inferior temporal cortex of monkeys. The Journal of Neuroscience. 2010;30(45):15085–96. doi: 10.1523/JNEUROSCI.0471-10.2010. doi:10.1523/JNEUROSCI.0471-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis AW, Young AW, Critchley EM. Loss of memory for people following temporal lobe damage. Brain. 1989;112(6):1469–83. doi: 10.1093/brain/112.6.1469. [DOI] [PubMed] [Google Scholar]
- Engell AD, McCarthy G. Probabilistic atlases for face and biological motion perception: An analysis of their reliability and overlap. NeuroImage. 2013;74:140–51. doi: 10.1016/j.neuroimage.2013.02.025. doi:10.1016/j.neuroimage.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44(8):962–74. doi: 10.1016/j.cortex.2008.05.003. doi:10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Wiethoff S, Bisch J, Schlipf S, Wildgruber D, Kreifelts B. Functional responses and structural connections of cortical areas for processing faces and voices in the superior temporal sulcus. NeuroImage. 2013;76:45–56. doi: 10.1016/j.neuroimage.2013.02.064. doi:10.1016/j.neuroimage.2013.02.064. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome? Brain. 1995;118(1):1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Andrews TJ. Differential sensitivity for viewpoint between familiar and unfamiliar faces in human visual cortex. NeuroImage. 2008;40(4):1857–70. doi: 10.1016/j.neuroimage.2008.01.049. doi:10.1016/j.neuroimage.2008.01.049. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17(10):2400–6. doi: 10.1093/cercor/bhl148. doi:10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Viewer-centered object representation in the human visual system revealed by viewpoint aftereffects. Neuron. 2005;45(5):793–800. doi: 10.1016/j.neuron.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Fang F, Murray SO, He S. Duration-dependent FMRI adaptation and distributed viewer-centered face representation in human visual cortex. Cerebral Cortex. 2007;17(6):1402–1411. doi: 10.1093/cercor/bhl053. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychological Review. 1998;105(3):482–98. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Fernández Coello A, Duvaux S, De Benedictis A, Matsuda R, Duffau H. Involvement of the right inferior longitudinal fascicle in visual hemiagnosia: A brain stimulation mapping study. Journal of Neurosurgery. 2013;118(1):202–5. doi: 10.3171/2012.10.JNS12527. doi:10.3171/2012.10.JNS12527. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Hanif HM, Iaria G, Duchaine BC, Barton JJS. Perceptual and anatomic patterns of selective deficits in facial identity and expression processing. Neuropsychologia. 2011;49(12):3188–200. doi: 10.1016/j.neuropsychologia.2011.07.018. doi:10.1016/j.neuropsychologia.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Iaria G, Barton JJS. Disconnection in prosopagnosia and face processing. Cortex. 2008;44(8):996–1009. doi: 10.1016/j.cortex.2008.04.003. doi:10.1016/j.cortex.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY. Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science. 2010;330(6005):845–51. doi: 10.1126/science.1194908. doi:10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furl N, Garrido L, Dolan RJ, Driver J, Duchaine B. Fusiform gyrus face selectivity relates to individual differences in facial recognition ability. Journal of Cognitive Neuroscience. 2011;23(7):1723–40. doi: 10.1162/jocn.2010.21545. doi:10.1162/jocn.2010.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain. 2003;126(4):792–803. doi: 10.1093/brain/awg092. doi:10.1093/brain/awg092. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia. 2007;45(8):1591–607. doi: 10.1016/j.neuropsychologia.2006.12.013. doi:10.1016/j.neuropsychologia.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Marra C. Differential contribution of right and left temporo-occipital and anterior temporal lesions to face recognition disorders. Frontiers in Human Neuroscience. 2011:5–55. doi: 10.3389/fnhum.2011.00055. doi:10.3389/fnhum.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Unraveling mechanisms for expert object recognition: Bridging brain activity and behavior. Journal of Experimental Psychology: Human Perception and Performance. 2002;28(2):431–446. doi: 10.1037//0096-1523.28.2.431. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2(2):568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson a W. The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience. 2000;12(3):495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Gil-Robles S, Carvallo A, Jimenez MDM, Gomez Caicoya A, Martinez R, Ruiz-Ocaña C, Duffau H. Double dissociation between visual recognition and picture naming: A study of the visual language connectivity using tractography and brain stimulation. Neurosurgery. 2013;72(4):678–86. doi: 10.1227/NEU.0b013e318282a361. doi:10.1227/NEU.0b013e318282a361. [DOI] [PubMed] [Google Scholar]
- Gilaie-Dotan S, Malach R. Sub-exemplar shape tuning in human face-related areas. Cerebral Cortex. 2007;17(2):325–38. doi: 10.1093/cercor/bhj150. doi:10.1093/cercor/bhj150. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. doi:10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxby JV. Social and emotional attachment in the neural representation of faces. NeuroImage. 2004;22(4):1628–35. doi: 10.1016/j.neuroimage.2004.03.049. doi:10.1016/j.neuroimage.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Goesaert E, Op de Beeck HP. Representations of facial identity information in the ventral visual stream investigated with multivoxel pattern analyses. Journal of Neuroscience. 2013;33(19):8549–8558. doi: 10.1523/JNEUROSCI.1829-12.2013. doi:10.1523/JNEUROSCI.1829-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: A functional neuroimaging study of semantically unique items. Brain. 2001;124(10):2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Tempini ML. The neural systems sustaining face and proper-name processing. Brain. 1998;121(11):2103–18. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Human Brain Mapping. 2001;13(4):199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee ACH. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48(4):831–53. doi: 10.1016/j.neuropsychologia.2010.01.001. doi:10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7(5):555–62. doi: 10.1038/nn1224. doi:10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24(1):187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grossi D, Soricelli A, Ponari M, Salvatore E, Quarantelli M, Prinster A, Trojano L. Structural connectivity in a single case of progressive prosopagnosia: The role of the right inferior longitudinal fasciculus. Cortex. 2012;29:1–10. doi: 10.1016/j.cortex.2012.09.010. doi:10.1016/j.cortex.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P. White-matter connectivity between face-responsive regions in the human brain. Cerebral Cortex. 2012;22(7):1564–76. doi: 10.1093/cercor/bhr226. doi:10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- Hadj-Bouziane F, Bell AH, Knusten TA, Ungerleider LG, Tootell RBH. Perception of emotional expressions is independent of face selectivity in monkey inferior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(14):5591–6. doi: 10.1073/pnas.0800489105. doi:10.1073/pnas.0800489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Bouziane F, Liu N, Bell AH, Gothard KM, Luh W-M, Tootell RBH, Ungerleider LG. Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(52):E3640–8. doi: 10.1073/pnas.1218406109. doi:10.1073/pnas.1218406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, De Gelder B. Neural basis of prosopagnosia: An fMRI study. Human Brain Mapping. 2002;16(3):176–182. doi: 10.1002/hbm.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmäki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex. 2000;10(1):69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Harris A, Aguirre GK. Neural tuning for face wholes and parts in human fusiform gyrus revealed by FMRI adaptation. Journal of Neurophysiology. 2010;104(1):336–45. doi: 10.1152/jn.00626.2009. doi:10.1152/jn.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Rolls ET, Baylis GC. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behavioural Brain Research. 1989;32(3):203–218. doi: 10.1016/s0166-4328(89)80054-3. [DOI] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Deouell LY, Bentin S, Malach R. Face-selective activation in a congenital prosopagnosic subject. Journal of Cognitive Neuroscience. 2003;15(3):419–431. doi: 10.1162/089892903321593135. [DOI] [PubMed] [Google Scholar]
- Haxby JVJ, Hoffman EEA, Gobbini MMI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. doi:10.1016/S1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(2):922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernàndez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317(5843):1360–6. doi: 10.1126/science.1146282. doi:10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Heywood CA, Cowey A. The role of the “face-cell” area in the discrimination and recognition of faces by monkeys. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1992;335(1273):31–7. doi: 10.1098/rstb.1992.0004. discussion 37–8. doi:10.1098/rstb.1992.0004. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Current Biology. 2007;17(9):766–72. doi: 10.1016/j.cub.2007.03.040. doi:10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: III. Subcortical afferents. Journal of Comparative Neurology. 1987;264(3):396–408. doi: 10.1002/cne.902640307. [DOI] [PubMed] [Google Scholar]
- Jonas J, Descoins M, Koessler L, Colnat-Coulbois S, Sauvée M, Guye M, Maillard L. Focal electrical intracerebral stimulation of a face-sensitive area causes transient prosopagnosia. Neuroscience. 2012;222:281–8. doi: 10.1016/j.neuroscience.2012.07.021. doi:10.1016/j.neuroscience.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Jones RD, Tranel D. Severe developmental prosopagnosia in a child with superior intellect. Journal of Clinical and Experimental Neuropsychology. 2001;23(3):263–73. doi: 10.1076/jcen.23.3.265.1183. [DOI] [PubMed] [Google Scholar]
- Julian JB, Fedorenko E, Webster J, Kanwisher N. An algorithmic method for functionally defining regions of interest in the ventral visual pathway. NeuroImage. 2012;60(4):2357–64. doi: 10.1016/j.neuroimage.2012.02.055. doi:10.1016/j.neuroimage.2012.02.055. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(25):11163–70. doi: 10.1073/pnas.1005062107. doi:10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. doi:10.1098/Rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: A cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361(1476):2109–28. doi: 10.1098/rstb.2006.1934. doi:10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann TC, Swisher JD, König P, Tong F. Prevalence of selectivity for mirror-symmetric views of faces in the ventral and dorsal visual pathways. The Journal of Neuroscience. 2012;32(34):11763–72. doi: 10.1523/JNEUROSCI.0126-12.2012. doi:10.1523/JNEUROSCI.0126-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács G, Cziraki C, Vidnyánszky Z, Schweinberger SR, Greenlee MW. Position-specific and position-invariant face aftereffects reflect the adaptation of different cortical areas. NeuroImage. 2008;43(1):156–64. doi: 10.1016/j.neuroimage.2008.06.042. doi:10.1016/j.neuroimage.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Kress T, Daum I. Developmental prosopagnosia: A review. Behavioural Neurology. 2003;14(3-4):109–21. doi: 10.1155/2003/520476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20600–5. doi: 10.1073/pnas.0705654104. doi:10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L. In search of a unifying theory of complex brain evolution. Annals of the New York Academy of Sciences. 2009;1156:44–67. doi: 10.1111/j.1749-6632.2009.04421.x. doi:10.1111/j.1749-6632.2009.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SP, Tolias AS, Logothetis NK, Goense J. fMRI of the face-processing network in the ventral temporal lobe of awake and anesthetized macaques. Neuron. 2011;70(2):352–62. doi: 10.1016/j.neuron.2011.02.048. doi:10.1016/j.neuron.2011.02.048. [DOI] [PubMed] [Google Scholar]
- Kuskowski MA, Pardo JV. The role of the fusiform gyrus in successful encoding of face stimuli. NeuroImage. 1999;9(6):599–610. doi: 10.1006/nimg.1999.0442. doi:10.1006/nimg.1999.0442. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Graham KS. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005;15(6):782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Graham KS. Perceptual deficits in amnesia: Challenging the medial temporal lobe “mnemonic” view. Neuropsychologia. 2005;43(1):1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cerebral Cortex. 2008;18(3):683–96. doi: 10.1093/cercor/bhm104. doi:10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Mueller T, Federspiel A, Hubl D, Schroth G, Huber O, Dierks T. Dissociation between overt and unconscious face processing in fusiform face area. NeuroImage. 2004;21(1):75–83. doi: 10.1016/j.neuroimage.2003.08.038. doi:10.1016/j.neuroimage.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Bondar IV, Giese MA. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature. 2006;442(7102):572–5. doi: 10.1038/nature04951. doi:10.1038/nature04951. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Perception of face parts and face configurations: An fMRI study. Journal of Cognitive Neuroscience. 2010;22(1):203–211. doi: 10.1162/jocn.2009.21203. doi:10.1162/jocn.2009.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler G, Yourganov G, Wilkinson F, Wilson HR. fMRI evidence for the neural representation of faces. Nature Neuroscience. 2005;8(10):1386–90. doi: 10.1038/nn1538. doi:10.1038/nn1538. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nature Neuroscience. 1999;2(6):555–62. doi: 10.1038/9210. doi:10.1038/9210. [DOI] [PubMed] [Google Scholar]
- Martin CB, McLean DA, O'Neil EB, Kohler S. Distinct familiarity-based response patterns for faces and buildings in perirhinal and parahippocampal cortex. Journal of Neuroscience. 2013;33(26):10915–10923. doi: 10.1523/JNEUROSCI.0126-13.2013. doi:10.1523/JNEUROSCI.0126-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Belger A, Allison T. Electrophysiological studies of human face perception. II: Response properties of face-specific potentials generated in occipitotemporal cortex. Cerebral Cortex. 1999;9(5):431–44. doi: 10.1093/cercor/9.5.431. [DOI] [PubMed] [Google Scholar]
- McGugin RW, Gatenby JC, Gore JC, Gauthier I. High-resolution imaging of expertise reveals reliable object selectivity in the fusiform face area related to perceptual performance. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(42):17063–8. doi: 10.1073/pnas.1116333109. doi:10.1073/pnas.1116333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Verosky S, Turk-Browne NB, Todorov A. Robust selectivity for faces in the human amygdala in the absence of expressions. Journal of Cognitive Neuroscience. 2013;25:2086–2106. doi: 10.1162/jocn_a_00469. doi:10.1162/jocn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, Nestor PJ. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133(11):3256–68. doi: 10.1093/brain/awq272. doi:10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Moeller S, Freiwald WA, Tsao DY. Patches with links: A unified system for processing faces in the macaque temporal lobe. Science. 2008;320(5881):1355–9. doi: 10.1126/science.1157436. doi:10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. The Journal of Comparative Neurology. 1987;256(1):88–103. doi: 10.1002/cne.902560108. doi:10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G, Behrmann M. What is special about face recognition? Ninteen experiments on a person with visual object agnosia and dyslexia but normal face recognition. Journal of Cognitive Neuroscience. 1997;9(5):555–604. doi: 10.1162/jocn.1997.9.5.555. [DOI] [PubMed] [Google Scholar]
- Mundy ME, Downing PE, Dwyer DM, Honey RC, Graham KS. A critical role for the hippocampus and perirhinal cortex in perceptual learning of scenes and faces: Complementary findings from amnesia and fMRI. Journal of Neuroscience. 2013;33(25):10490–10502. doi: 10.1523/JNEUROSCI.2958-12.2013. doi:10.1523/JNEUROSCI.2958-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy ME, Downing PE, Graham KS. Extrastriate cortex and medial temporal lobe regions respond differentially to visual feature overlap within preferred stimulus category. Neuropsychologia. 2012;50(13):3053–61. doi: 10.1016/j.neuropsychologia.2012.07.006. doi:10.1016/j.neuropsychologia.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Murray E, Bussey T. Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Sciences. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Zilles K. Functional delineation of the human occipito-temporal areas related to face and scene processing. A PET study. Brain. 2000;123(9):1903–12. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. The primate temporal pole: Its putative role in object recognition and memory. Behavioural Brain Research. 1996;77(1-2):53–77. doi: 10.1016/0166-4328(95)00227-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto K, Mikami A, Kubota K. Visual response properties of single neurons in the temporal pole of behaving monkeys. Journal of Neurophysiology. 1994;71(3):1206–21. doi: 10.1152/jn.1994.71.3.1206. [DOI] [PubMed] [Google Scholar]
- Nasr S, Tootell RBH. Role of fusiform and anterior temporal cortical areas in facial recognition. NeuroImage. 2012;63(3):1743–53. doi: 10.1016/j.neuroimage.2012.08.031. doi:10.1016/j.neuroimage.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu VS, Jiang F, Narvekar A, Keshvari S, Blanz V, O'Toole AJ. Dissociable neural patterns of facial identity across changes in viewpoint. Journal of Cognitive Neuroscience. 2010;22(7):1570–82. doi: 10.1162/jocn.2009.21312. doi:10.1162/jocn.2009.21312. [DOI] [PubMed] [Google Scholar]
- Nestor A, Plaut DC, Behrmann M. Unraveling the distributed neural code of facial identity through spatiotemporal pattern analysis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(24):9998–10003. doi: 10.1073/pnas.1102433108. doi:10.1073/pnas.1102433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis ILC, Takashima A, Oostenveld R, McNaughton BL, Fernández G, Jensen O. The neocortical network representing associative memory reorganizes with time in a process engaging the anterior temporal lobe. Cerebral Cortex. 2012;22(11):2622–33. doi: 10.1093/cercor/bhr338. doi:10.1093/cercor/bhr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Passamonti L, Rowe J, Engell AD, Calder AJ. Connectivity analysis reveals a cortical network for eye gaze perception. Cerebral Cortex. 2010;20(8):1780–7. doi: 10.1093/cercor/bhp244. doi:10.1093/cercor/bhp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil EB, Barkley VA, Köhler S. Representational demands modulate involvement of perirhinal cortex in face processing. Hippocampus. 2013;23(7):592–605. doi: 10.1002/hipo.22117. doi:10.1002/hipo.22117. [DOI] [PubMed] [Google Scholar]
- O'Neil EB, Cate AD, Köhler S. Perirhinal cortex contributes to accuracy in recognition memory and perceptual discriminations. The Journal of Neuroscience. 2009;29(26):8329–34. doi: 10.1523/JNEUROSCI.0374-09.2009. doi:10.1523/JNEUROSCI.0374-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil EB, Hutchison RM, McLean DA, Köhler S. Resting-state fMRI reveals functional connectivity between face-selective perirhinal cortex and the fusiform face area related to face inversion. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2014.02.005. Advanced online publication. doi:10.1016/j.neuroimage.2014.02.005. [DOI] [PubMed] [Google Scholar]
- O'Toole AJ, Roark DA, Abdi H. Recognizing moving faces: A psychological and neural synthesis. Trends in Cognitive Sciences. 2002;6(6):261–266. doi: 10.1016/s1364-6613(02)01908-3. [DOI] [PubMed] [Google Scholar]
- Olson IR, Ezzyat Y, Plotzker A, Chatterjee A. Perceptual Deficits in Face and Non-Face Performance Following Unilateral Anterior Temporal Lobe Damage. Neurocase. doi: 10.1080/13554794.2014.959025. accepted pending revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Mccoy D, Klobusicky E, Ross LA. Social Cognition and the Anterior Temporal Lobes: A Review and Theoretical Framework. Social Cognitive and Affective Neuroscience. 2013;8:123–133. doi: 10.1093/scan/nss119. doi:10.1093/scan/nss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban G. a, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences. 2004;8(7):315–24. doi: 10.1016/j.tics.2004.05.009. doi:10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L. Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: A diffusion tensor imaging study. Developmental Medicine and Child Neurology. 2012;54(1):38–43. doi: 10.1111/j.1469-8749.2011.04147.x. doi:10.1111/j.1469-8749.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Jacques C, Foster BL, Withoft N, Rangarajan V, Weiner KS. Electrical stimulation of human fusiform face-selective regions distorts face perception. Journal of Neuroscience. 2012;32(43):14915–14920. doi: 10.1523/JNEUROSCI.2609-12.2012. doi:10.1523/JNEUROSCI.2609-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Perrett D. Organization and functions of cells responsive to faces in the temporal cortex. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience. 2009;29(48):15089–99. doi: 10.1523/JNEUROSCI.0796-09.2009. doi:10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S. Neural representations of faces and body parts in macaque and human cortex: A comparative FMRI study. Journal of Neurophysiology. 2009;101(5):2581–600. doi: 10.1152/jn.91198.2008. doi:10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Charles L, Devlin JT, Walsh V, Duchaine B. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Current Biology. 2009;19(4):319–324. doi: 10.1016/j.cub.2009.01.007. doi:10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Current Biology. 2007;17(18):1568–73. doi: 10.1016/j.cub.2007.07.063. doi:10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Polyak S. The vertebrate visual system. University of Chicago Press; 1957. [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: An event-related fMRI study. NeuroImage. 2005;24(4):1214–24. doi: 10.1016/j.neuroimage.2004.10.038. doi:10.1016/j.neuroimage.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cerebral Cortex. 1999;9(5):445–58. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- Pyles JA, Verstynen TD, Schneider W, Tarr MJ. Explicating the face perception network with white matter connectivity. PLoS ONE. 2013;8(4):e61611. doi: 10.1371/journal.pone.0061611. doi:10.1371/journal.pone.0061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajimehr R, Young JC, Tootell RBH. An anterior temporal face patch in human cortex, predicted by macaque maps. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):1995–2000. doi: 10.1073/pnas.0807304106. doi:10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Treves A, Tovee MJ, Panzeri S. Information in the neuronal representation of individual stimuli in the primate temporal visual cortex. Journal of Computational Neuroscience. 1997;4(4):309–33. doi: 10.1023/a:1008899916425. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. What's unique about unique entities? An fMRI investigation of the semantics of famous faces and landmarks. Cerebral Cortex. 2012;22(9):2005–2015. doi: 10.1093/cercor/bhr274. doi:10.1093/cercor/bhr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B. Constraining the cortical face network by neuroimaging studies of acquired prosopagnosia. NeuroImage. 2008;40(2):423–6. doi: 10.1016/j.neuroimage.2007.10.047. doi:10.1016/j.neuroimage.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Rossion B, Hanseeuw B, Dricot L. Defining face perception areas in the human brain: A large-scale factorial fMRI face localizer analysis. Brain and Cognition. 2012;79(2):138–57. doi: 10.1016/j.bandc.2012.01.001. doi:10.1016/j.bandc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Rossion B, Kung CC, Tarr MJ. Visual expertise with nonface objects leads to competition with the early perceptual processing of faces in the human occipitotemporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(40):14521–14526. doi: 10.1073/pnas.0405613101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M. The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. NeuroImage. 2003;19(3):877–883. doi: 10.1016/s1053-8119(03)00105-8. doi:10.1016/S1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing marilyn into maggie dissociates physical and identity face representations in the brain. Nature Neuroscience. 2005;8(1):107–13. doi: 10.1038/nn1370. doi:10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Russell R, Duchaine B, Nakayama K. Super-recognizers: People with extraordinary face recognition ability. Psychonomic Bulletin & Review. 2009;16(2):252–7. doi: 10.3758/PBR.16.2.252. doi:10.3758/PBR.16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354(6349):152–5. doi: 10.1038/354152a0. doi:10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ. The representational – hierarchical view of amnesia: Translation from animal to human. Neuropsychologia. 2010;48(8):2370–2384. doi: 10.1016/j.neuropsychologia.2010.02.026. doi:10.1016/j.neuropsychologia.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Schiltz C, Dricot L, Goebel R, Rossion B. Holistic perception of individual faces in the right middle fusiform gyrus as evidenced by the composite face illusion. Journal of Vision. 2010;10:1–16. doi: 10.1167/10.2.25. doi:10.1167/10.2.25. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Schultz J, Pilz KS. Natural facial motion enhances cortical responses to faces. Experimental Brain Research. 2009;194(3):465–75. doi: 10.1007/s00221-009-1721-9. doi:10.1007/s00221-009-1721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Shultz RT. The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2003;358(1430):415–27. doi: 10.1098/rstb.2002.1208. doi:10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzlose RF, Swisher JD, Dang S, Kanwisher N. The distribution of category and location information across object-selective regions in human visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4447–4452. doi: 10.1073/pnas.0800431105. doi:10.1073/pnas.0800431105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115(1):15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Midorikawa A, Onodera T, Tsukada M, Yamada R, Tabei Y, Yagi K. Damage to the left ventral, arcuate fasciculus and superior longitudinal fasciculus-related pathways induces deficits in object naming, phonological language function and writing, respectively. The International Journal of Neuroscience. 2013 doi: 10.3109/00207454.2013.765420. doi:10.3109/00207454.2013.765420. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, Sato N, Nakamura A, Kato T, Fukuda H. Activation reduction in anterior temporal cortices during repeated recognition of faces of personal acquaintances. NeuroImage. 2001;13(5):877–90. doi: 10.1006/nimg.2001.0747. doi:10.1006/nimg.2001.0747. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Mano Y, Sasaki A, Sadato N. Beyond the memory mechanism: Person-selective and nonselective processes in recognition of personally familiar faces. Journal of Cognitive Neuroscience. 2011;23(3):699–715. doi: 10.1162/jocn.2010.21469. doi:10.1162/jocn.2010.21469. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. The Journal of Comparative Neurology. 1994;350(4):497–533. doi: 10.1002/cne.903500402. doi:10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Baxter MG. Memory, perception, and the medial temporal lobe: A synthesis of opinions. Neuron. 2009;61(5):678–9. doi: 10.1016/j.neuron.2009.02.009. doi:10.1016/j.neuron.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Tanji K, Iwasaki M, Nakasato N, Suzuki K. Face specific broadband electrocorticographic spectral power change in the rhinal cortex. Neuroscience Letters. 2012;515(1):66–70. doi: 10.1016/j.neulet.2012.03.020. doi:10.1016/j.neulet.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Tavor I, Yablonski M, Mezer A, Rom S, Assaf Y, Yovel G. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. NeuroImage. 2013;86:123–30. doi: 10.1016/j.neuroimage.2013.07.085. doi:10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, Behrmann M. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. Journal of Cognitive Neuroscience. 2008;20(2):268–84. doi: 10.1162/jocn.2008.20025. doi:10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35(10):1319–27. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tsao D, Freiwald W, Tootell R, Livingstone M. A cortical region consisting entirely of face-selective cells. Science. 2006;311(5761):670–674. doi: 10.1126/science.1119983. doi:10.1126/science.1119983.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RBH. Faces and objects in macaque cerebral cortex. Nature Neuroscience. 2003;6(9):989–95. doi: 10.1038/nn1111. doi:10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19514–9. doi: 10.1073/pnas.0809662105. doi:10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nature Neuroscience. 2008;11(8):877–9. doi: 10.1038/nn.2158. doi:10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Mano Y, Sekiguchi A, Yomogida Y, Hoshi K, Kambara T, Kawashima R. Dissociable roles of the anterior temporal regions in successful encoding of memory for person identity information. Journal of Cognitive Neuroscience. 2010;22(10):2226–2237. doi: 10.1162/jocn.2009.21349. doi:10.1162/jocn.2009.21349. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Norman-Haignere SV, McCarthy G. Face-specific resting functional connectivity between the fusiform gyrus and posterior superior temporal sulcus. Frontiers in Human Neuroscience. 2010;4:176. doi: 10.3389/fnhum.2010.00176. doi:10.3389/fnhum.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verosky SC, Todorov A, Turk-Browne NB. Representations of individuals in ventral temporal cortex defined by faces and biographies. Neuropsychologia. 2013;51(11):1–9. doi: 10.1016/j.neuropsychologia.2013.07.006. doi:10.1016/j.neuropsychologia.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal JP, Chauvel P, Halgren E. Localised face processing by the human prefrontal cortex: Stimulation-evoked hallucinations of faces. Cognitive Neuropsychology. 2000;17(1):281–91. doi: 10.1080/026432900380616. doi:10.1080/026432900380616. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience. 2010;22(6):1083–1094. doi: 10.1162/jocn.2009.21309. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. 2013;136(6):1692–707. doi: 10.1093/brain/awt094. doi:10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Olson IR. Anterior temporal face patches: A meta-analysis and empirical study. Frontiers in Human Neuroscience. 2013;7(17) doi: 10.3389/fnhum.2013.00017. doi:10.3389/fnhum.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kriegstein K, Kleinschmidt A, Giraud AL. Voice recognition and cross-modal responses to familiar speakers’ voices in prosopagnosia. Cerebral Cortex. 2006;16(9):1314–1322. doi: 10.1093/cercor/bhj073. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–8. doi: 10.1038/nn1341. doi:10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. NeuroImage. 2010;52(4):1559–73. doi: 10.1016/j.neuroimage.2010.04.262. doi:10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Neural representations of faces and limbs neighbor in human high-level visual cortex: evidence for a new organization principle. Psychological Research. 2013;77(1):74–97. doi: 10.1007/s00426-011-0392-x. doi:10.1007/s00426-011-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Henson RNA, Fine-Goulden MR, Dolan RJ. fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. Journal of Neurophysiology. 2004;92(3):1830–9. doi: 10.1152/jn.00155.2004. doi:10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- Xu X, Yue X, Lescroart MD, Biederman I, Kim JG. Adaptation in the fusiform face area (FFA): Image or person? Vision Research. 2009;49(23):2800–7. doi: 10.1016/j.visres.2009.08.021. doi:10.1016/j.visres.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Taniwaki T, Tobimatsu S, Arakawa K, Kuba H, Maeda Y, Kira J. Electrophysiological correlates of associative visual agnosia lesioned in the ventral pathway. Journal of the Neurological Sciences. 2004;221(1-2):53–60. doi: 10.1016/j.jns.2004.03.024. doi:10.1016/j.jns.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(44):E3045–53. doi: 10.1073/pnas.1206792109. doi:10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, Duchaine B. Specialized face perception mechanisms extract both part and spacing information: Evidence from developmental prosopagnosia. Journal of Cognitive Neuroscience. 2006;18(4):580–593. doi: 10.1162/jocn.2006.18.4.580. [DOI] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. Face perception: domain specific, not process specific. Neuron. 2004;44(5):889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]