Abstract
The longevity of an organism is directly related to its ability to effectively cope with cellular stress. Heat shock response (HSR) protects the cells against accumulation of damaged proteins after exposure to elevated temperatures and also in ageing cells. To understand the role of Hsp70 in regulating life span of Daphnia, we examined the expression of Hsp70 in two ecotypes that exhibit strikingly different life spans. D. pulicaria, the long lived ecotype, showed a robust Hsp70 induction as compared to the shorter lived D. pulex. Interestingly, the short-lived D. pulex isolates showed no induction of Hsp70 at the mid point in their life span. In contrast to this, the long-lived D. pulicaria continued to induce Hsp70 expression at an equivalent age. We further show that the Hsp70 expression was induced at transcriptional level in response to heat shock. The transcription factor responsible for Hsp70 induction, heat shock factor-1 (HSF-1), although present in aged organisms did not exhibit DNA-binding capability. Thus, the decline of Hsp70 induction in old organisms could be attributed to a decline in HSF-1’s DNA-binding activity. These results for the first time, present a molecular analysis of the relationship between HSR and life span in Daphnia.
Keywords: Daphnia, heat shock, Hsp70, HSF-1, longevity, ageing
1. Introduction
Ageing is a universal property of multicellular organisms that causes functional decline of all biological systems. Proteotoxicity due to misfolded proteins is a central component of ageing and the ability of organisms to deal with misfolded proteins is crucial for longevity (Taylor and Dillin 2011). As an organism ages, the free radicals increase within cells due to mitochondrial malfunctions and inefficiency, which in turn leads to an increase in altered proteins (Cui et al. 2012). The pathologies and phenotypes of ageing are caused primarily by the inability to deal with proteotoxic stress and an accumulation of altered proteins (Clancy and Birdsall 2013). In order to appropriately respond to proteotoxic stress, a physiological response termed the heat shock response (HSR) is induced, which involves a rapid and transient increase in expression of molecular chaperones such as heat shock proteins (Hsps) following a proteotoxic stimulus (Calderwood et al. 2009). HSR enables an organism to handle proteotoxic conditions and survive without an extended, damaging imbalance in protein homeostasis. Molecular chaperones, such as Hsp70, act to renature the denatured or misfolded proteins or trigger their degradation if renaturation is not possible (Bukau et al. 2006). The Hsp gene expression is induced at transcriptional level and is mainly regulated by the transcription factor Heat Shock Factor-1 (HSF-1) (Anckar and Sistonen 2011).
Among molecular chaperones, Hsp70 plays a regulatory role in the ageing process since it mitigates the effects of proteotoxic stress (Calderwood et al. 2009; Kim et al. 2013). Overexpression of Hsp70 by knocking in more copies of the gene in C. elegans led to an overall increase in lifespan (Yokoyama et al. 2002). Consistent with this, knockdown of Hsp70 led to accelerated aging including premature death (Kimura et al. 2007). Hsp70 and its transcriptional regulator HSF-1 are both important in coping with proteotoxic stress as well as the general increase of altered and misfolded proteins in aged organisms (Morley and Morimoto 2004; Morimoto and Cuervo 2009; Anckar and Sistonen 2011). Hsp70 protein levels can be used as a predictor of total life span in C. elegans and D. melanogaster, because the organisms that display a more robust HSR and a higher expression of Hsp70 also have longer lifespans (Rea et al. 2005; Tower 2011). In the present study we have investigated the HSR of two different ecotypes of Daphnia that have very different life spans by examining the expression of Hsp70.
Daphnia are small freshwater crustaceans that are an important model system in ecology, evolutionary biology, and ecotoxicology (Benzie 2005). These organisms are easy to maintain, reproduce via cyclic parthenogenesis, have transparent carapaces, and have relatively short life-span, thus making them a good experimental model. In cyclic parthenogenesis, wild populations reproduce mainly via ameiotic cloning (parthenogenesis), but periodic environmental stress induces sexual reproduction. Parthenogenetic reproduction can be enforced in the lab, making it possible to maintain isogenic individuals from one generation to the next that reflect naturally occurring genomes. These unique properties have made them a useful model for research on naturally occurring patterns of variation in ageing (Dudycha 2001; Dudycha and Hassel 2013) and on the effect of environmental variation on ageing (Dudycha 2003; Steinberg et al. 2010). We focus on two ecotypes of the D. pulex species that are adapted to distinct habitats, and thus have evolved sharply divergent lifespans (Dudycha and Tessier 1999). D. pulex inhabits temporary ponds, where it faces high extrinsic mortality risk and has a short lifespan with an average being about 25-30 days (Dudycha 2001; Dudycha 2004). In contrast, D. pulicaria inhabits large stratified lakes, encounters low extrinsic mortality, and has a long lifespan with an average being about 60-65 days (Dudycha 2001, 2004). Though the ecotypes have different names, they are not fully distinct species, and abundant evidence supports ongoing genetic exchange (Dudycha 2004; Cristescu et al. 2012). Estimated divergence time is only ~82,000 years (Omilian and Lynch 2009), a relatively short evolutionary span that is lower than the divergence among some human populations. The complete genome sequence of D. pulex was recently published (Colbourne et al. 2011). For simplicity, throughout the manuscript, we use the specific terminology D. pulex and D. pulicaria to distinguish the pond- and lake- ecotypes with short and long life spans respectively. All of the clones used in this study have been acclimated to the lab for >3 years (i.e., >75 asexual generations).
It is clear that these named taxa, though ecologically separate, are not evolutionarily distinct species, and gene flow continues to occur between the habitats at a local scale. Heier & Dudycha (2009) showed that experimental crosses between the ecotypes were fully fertile, and recent population genetic and genome-scale analyses confirm that gene flow and introgression is widespread (Daniel et al. 1995; Cowan et al. 1996; Vergilino et al. 2011; Cristescu et al. 2012; Tucker et al. 2013; Xu et al. 2013). The two ecotypes face different patterns of temperature variation in nature, but the ranges of temperatures they experience are similar. In a study of field demography (Dudycha 2004) populations in southwest Michigan, including the sources of our study clones, were monitored for a year, confirming that these populations experience similar temperature ranges. In both habitats, Daphnia will experience temperatures ranging from 4°C to the >28°C, but the physics of water bodies dictates that they experience them differently (Wetzel 2001). In temporary ponds, the largest change is seasonal, with cold temperatures as snow melts shortly before the population emerges from overwintering dormant eggs, and warmer temperatures as the population produces dormant eggs in summer. There is some daily variation with changes in air temperature, but small ponds are primarily insulated by and reflect ground temperatures. Lakes also have seasonal dynamics, but the populations are active year round, and in winter experience a continuous temperature of 4°C. By summer, lakes stratify into a warm upper layer (typically ranging from 24° - 28°) and a cool bottom layer (~10°C), with Daphnia migrating between the two temperature zones on a daily basis.
Previous reports have shown that despite the limited genetic divergence between the ecotypes that there is substantial differentiation of life history and related traits. They differ in lifespan, age-specific mortality rates, the timing and rate of age-dependent increases in mortality rates, maximum reproductive rate, the rate of reproductive decline with aging, and juvenile growth rate (Dudycha and Tessier 1999). This study included naturally occurring hybrids between the ecotypes which showed intermediate characteristics, and the results were confirmed in later studies (Dudycha 2001, 2003). Other direct comparisons have indicated there are differences in investment in dormancy and sexual reproduction (Caceres and Tessier 2011). Physiological studies on Daphnia are very few in number; we are unaware of any comparisons of respiration or metabolic rate, though there is limited evidence that D. pulicaria has superior photoenzymatic repair of DNA damage (Connelly et al. 2009).
In our present work we establish a foundation for the use of Daphnia as a model system in molecular biology of ageing. We evaluate age-dependence of the HSR in D. pulex and D. pulicaria, testing its relationship to their life span. Our results show that the two ecotypes respond differently to heat stress, as measured by analysis of Hsp70 protein expression. D. pulicaria, the long lived ecotype, responds better than the shorter lived D. pulex, supporting a role for HSR in differential ageing of the ecotypes. For the first time, these results present a molecular analysis of the relationship between stress response and regulation of life span in Daphnia. In addition, our findings offer a mechanistic insight for the lack of HSR in older organisms by demonstrating that the transcription factor HSF-1 responsible for transcriptional induction of Hsp70 loses its ability to bind DNA as Daphnia age.
2. Material and Methods
2.1. Daphnia Cultures
D. pulex and D. pulicaria ecotypes used in this study were isolated from ponds in southwest Michigan, USA in 2008 (except for clone “TCO” which was isolated from Oregon, USA) and have since been cultured in the lab. Daphnia are maintained at a temperature of 20° C with a photoperiod of 12:12 L:D (12 hours of light followed by 12 hours of dark) within a Percival growth chamber. Daphnia were maintained at a concentration of 3 to 5 animals per 250 ml beaker in 200 ml of filtered lake water. Daphnia were cleared of young and transferred to a new beaker with fresh water on alternate days. They were fed every day with vitamin-supplemented algae Ankistrodesmus falcatus at a concentration of 20,000 cells/ml. To generate experimental animals, even-aged cohorts were begun by placing neonates individually in 100 ml of filtered lake water. Experimental animals were otherwise maintained as in the source cultures.
2.2. Heat Shock Treatment
Daphnia of specific ages were placed into groups of 25-30 organisms in 1 ml of lake water in a microcentrifuge tube. The tubes were placed in a heating block at 32°C for 30 minutes. Daphnia were allowed to recover at room temperature (22° C) for 4 or 6 hr. The control samples were also separated into groups of 25-30 but were kept at room temperature for the duration of the experiment and then harvested.
2.3. Western Blot Analysis
D. pulex and D. pulicaria total protein extracts were prepared from either treated or untreated organisms. All water was removed from the tubes containing Daphnia, the organisms were washed once with 1 ml cold PBS. The PBS was removed and the organisms were homogenized using a tight-fitting pestle in RIPA buffer (150 mM NaCl, 1.0% IGEPAL, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) containing protease inhibitor cocktails (Sigma and Calbiochem). The samples were chilled on ice for 5 minutes, centrifuged at 13,000 × g for 4 minutes and supernatant was saved as total protein extract. The protein concentration was determined immediately using the BCA kit (Pierce), Laemmli’s sample loading buffer (4×) was immediately added to the extract following protein concentration determination and samples were heated at 95° C for 5 minutes to further inactivate any residual proteases from the Daphnia. This protocol ensures efficient inactivation of gut proteases. 50 μg of total protein were separated by SDS-PAGE and a western blot analysis was performed using ECL plus (Pierce) chemifluorescence detection. The primary antibodies used are as follows: Hsp70 (Fisher MAB3-007, 1:1000), β-actin (Sigma A5441, clone AC-15, 1:5000), α-tubulin (Sigma, T5168, 1:5000). Chemifluorescent signal was detected using a Molecular Dynamics Storm 860 Phosphor Imager.
2.4. Statistics
For the analysis of fold-inductions in western blot and Real Time-PCR analyses a two-tailed Student’s t test was performed with equal variance comparing values as indicated by brackets. A P value of 0.01 or lower was considered statistically significant.
2.5. Reverse Transcriptase PCR
RNA was isolated from Daphnia using RNAzol B reagent (TelTest). Between 25 and 30 individuals were separated into a microcentrifuge tube, subjected to heat shock or left untreated, all water was removed after heat shock, 1 wash with 1 ml cold PBS was given, and 0.8 ml of RNAzol B was added. The samples were homogenized and total RNA was isolated as per the manufacturer’s instructions. cDNA was synthesized at 42°C for 1 h using random hexamer primers, 1 μg total RNA, M-MuLV reverse transcriptase, 500 μM dNTPs, and RNase Inhibitor RNasin (Promega). For each PCR reaction, 2 μl of total cDNA and 50 pmoles each of the forward and reverse primers designed to amplify a 418 bp region of Hsp70 transcript or a 500 bp region of β-actin transcript were used with the Promega GoTaq PCR kit. The following conditions were used for PCR: 95° C for 5 minutes (initial denaturation), denaturation at 95° C for 30 seconds, annealing at 52° C for 30 seconds, extension at 72° C for 30 seconds for 20 cycles in order to stay within linear range of amplification. The linear range was determined by varying cycle numbers and performing a densitometric analysis of the amplified product.
2.6. Real Time PCR
RNA was isolated from Daphnia after heat shock after indicated recovery times (as well as a control, non-heat shocked sample). cDNA was synethsized as described before using random hexamers. We first standardized our cDNA by performing real time PCRs with serial dilutions of cDNA to ensure appropriate efficiency and correlation. Every reaction was performed in triplicate in a total volume of 20 μl. This included 4 μl cDNA, 250nM Hsp70 or β-actin primers, and SsoFast EvaGreen Supermix (BioRad). β-actin was used for normalization. All reactions were run on a BioRad CFX96 Real Time System C1000 Thermal cycler machine with the following conditions: 95° C for 30 seconds, 95° C for 5 seconds, 52° C for 5 seconds (the last three steps repeated for 40 cycles), 65° C for 5 seconds, and then 95° C for 5 seconds. We analyzed our data using the Bio-Rad CFX Manager Software and used the 2−ΔΔCt method to compare Hsp70 expression in heat shocked versus the non-heat shocked samples. Note that 3 separate RNA isolations were utilized from 3 separate groups of Daphnia to serve as biological replicates for each clone for each treatment.
2.7. Electrophoretic Mobility Shift Assay (EMSA)
The probe used was heat shock element (HSE) as previously described previously (Akerfelt et al. 2010), sequence of the upper strand: GGGCAGAAATTTCTAGAATCAGC. The double-stranded synthetic oligonucleotide corresponding to HSE sequence was end labeled with [γ-32P] ATP using T4 polynucleotide kinase. To determine specificity of interaction, HSE oligonucleotide and a consensus Oct1 oligonucleotide (upper strand sequence: TGTCGAATGCAAATCACTAGAA) were used as unlabeled competitors in 50 molar fold excess. HSE oligonucleotide serves as the specific competitor and Oct-1 oligonucleotide, which is the specific binding sequence for Oct-1 (octamer-binding transcription factor -1) transcription factor serves as the non-specific competitor for HSF-1. Whole cell extracts were prepared as described previously with some modifications (Murata et al. 1999). For analysis of HSF-1 DNA-binding activity after heat shock, whole cell extracts have been used widely as HSF-1 undergoes post-translational modification and trimerization leading to its activation in response to heat shock. Prior to heat shock activation, HSF-1 is non active and exhibits no DNA-binding activity. Daphnia were heat shocked as described before and homogenized in 3 volumes of extraction buffer (20mM HEPES pH 7.9, 0.4 M KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol (DTT), 25% glycerol and 0.2 mM phenylmethylsulfonyl fluoride), extracts were centrifuged at 13,000 xg for 5 minutes. The supernatants were diluted with an equal volume of the extraction buffer lacking KCl and were stored at −80 C. Binding reaction mixture included 18 mM HEPES pH 7.9, 80 mM KCl, 2 mM MgCl2, 10 mM DTT, 10% glycerol, 0.2 mg/ml poly (dI-dC), 15 fmol of 32P-labeled probe, and 5 μg of whole cell extract in 12.5 μl. The reaction mixtures were incubated at 25° C for 20 minutes and analyzed on a 5% native polyacrylamide gel via PAGE. The gel was dried and radioactivity was visualized via Phosphor Imager analysis (Molecular Dynamics Storm 860).
3. Results
3.1. Induction of Hsp70 in Daphnia
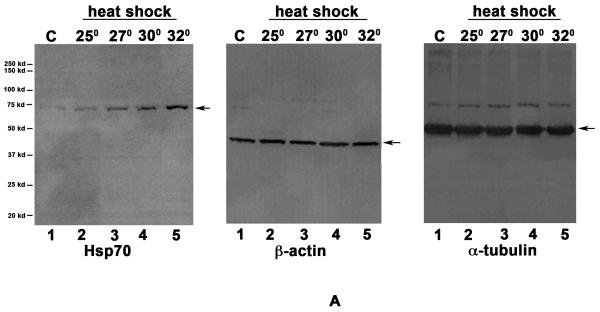
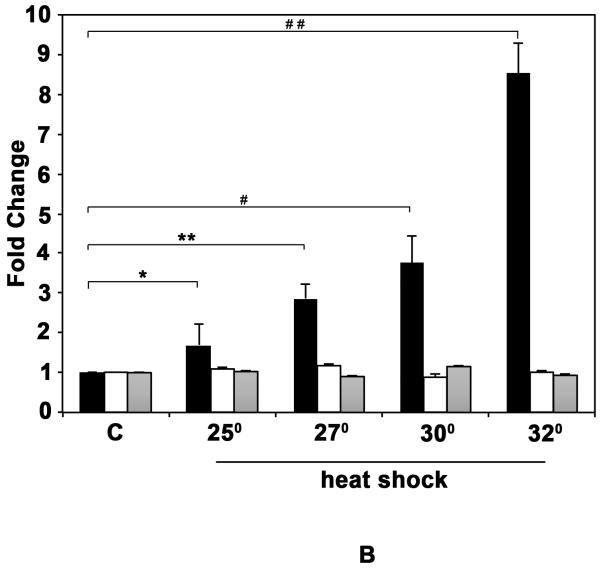
In order to characterize the Hsp70 induction in response to heat shock, we analyzed the induction of Hsp70 at different temperatures in D. pulicaria clone Lake XVI-11 (Fig. 1 A). D. pulicaria were normally grown and maintained at 20° C and in order to determine the temperature at which Hsp70 would be induced optimally we performed a western blot analysis on protein extracts prepared from Daphnia heat shocked for 30 minutes at each of the indicated temperatures and allowed to recover for 4h. As seen in Fig. 1 A, there was a basal level of Hsp70 expression in control sample without heat shock (lane 1), which increased in a temperature dependent manner (lanes 2-5). Heat shock at 34° C resulted in Hsp70 induction at the same level as at 32° C (data not shown), but exhibited some lethality in the organisms and thus, we decided on using 32° C as the temperature for all future experiments. We tested several anti-Hsp70 antibodies, of which only one detected a heat-shock inducible band at the appropriate molecular weight in Daphnia extracts. As seen in Fig. 1 A, the antibody detected a single band on western blots and was used for all further analyses. Both anti-β-actin and anti-α-tubulin antibodies were used as loading controls because at present there is limited information on which antibodies recognize corresponding Daphnia proteins. Daphnia β-actin protein has a predicted molecular weight of 41.8 kd and exhibits a high degree of homology to human β-actin with 367 amino acids being identical out of the total 376 amino acids (97 % sequence conservation). Daphnia α-tubulin protein has a predicted molecular weight of 49.9 kd and shows a high degree of homology to human α-tubulin with 419 amino acids being identical out of the total 450 amino acids (93% sequence conservation). Based on the percentage homologies between human and Daphnia proteins, both antibodies that were raised against the human homologs were able to detect bands at expected molecular weights in Daphnia extracts as seen in Fig. 1 A. Our results establish that either one of these proteins could be used as a loading control to normalize western blot analysis since both antibodies detect corresponding Daphnia proteins. The western blot signals were quantified using STORM phosphorimager and Imagequant software and the data is represented as bar graphs in Fig. 1 B. The levels of β-actin and α-tubulin did not vary after heat shock, whereas the Hsp70 protein was significantly induced in response to heat shock. The P values are indicated in the figure legend and were obtained by a statistical analysis of data obtained from 4 independent biological replicates.
Fig. 1. Induction of Hsp70 in D. pulicaria in response to heat shock.
A. Western blot: D. pulicaria (lake XVI-clone11) were subjected to heat shock at indicated temperatures for 30 minutes and allowed to recover for 4h. Western Blot analysis was performed using 50 μg of total protein using anti-Hsp70 antibody. C lanes indicate control samples with no heat shock treatment. The blot was re-probed with anti-β actin and anti-α tubulin antibodies to ensure equal amounts of protein were loaded in each lane. The positions of molecular weight markers are as indicated on the left. The arrows indicate bands corresponding to Hsp70, β-actin, and α-tubulin. The antibody used in each panel is indicated at the bottom of the blot. B: Quantification of western blot data: The chemifluorescent band intensities were quantified using STORM phosphorimager and the averages from 4 independent biological replicates is represented as a bar graph and the error bars represent standard deviations. Fold-changes are calculated with respect to signals in control lanes. The black bars represent Hsp70, the white bars represent β-actin, and the grey bars represent α-tubulin bands. As shown above bars, *, * *, #, and # # symbols indicate P values that indicate a significant difference (0.0019, 0.0009, 0.0008, and 0.0007 resp.). There was no significant difference observed in signals from β-actin or α-tubulin bands in various lanes with significant values being < 0.01.
3.2. Long-lived D. pulicaria displays a more robust HSR than short-lived D. pulex
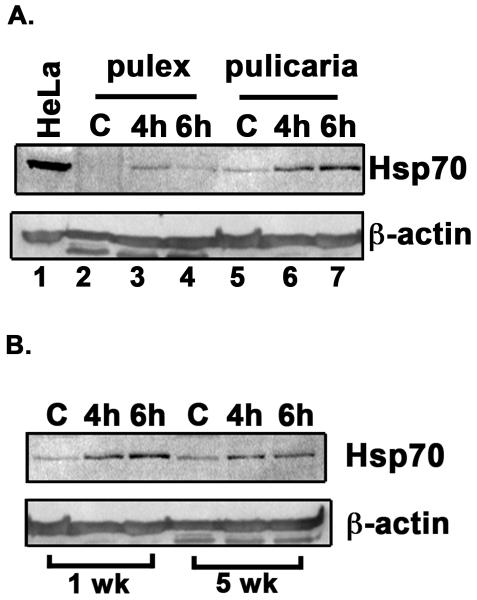
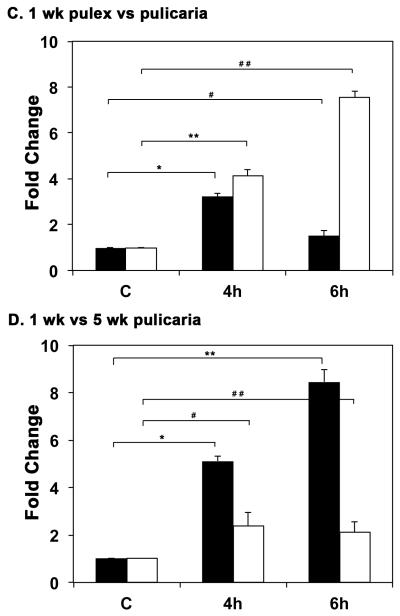
Since the two ecotypes differ substantially in lifespan we examined the induction of Hsp70 in response to heat shock in D. pulex and D. pulicaria. As seen in Fig. 2 A, D. pulicaria, the long-lived ecotype, displayed a more robust induction of Hsp70 (4.1-fold and 7.5-fold induction of Hsp70 in lanes 6 and 7) in response to heat shock than D. pulex at the age of 1 week at both 4h and 6h recovery time points (3.2-fold and 1.5-fold induction of Hsp70 in lanes 3 and 4). In addition, the basal level of Hsp70 expression was also higher in D. pulicaria than in D. pulex (lanes 5 and 2 respectively). The band intensities were quantified using STORM phosphorimager and Imagequant software and the data is represented as a bar graph in Fig. 2 C. The levels of β-actin and α-tubulin did not vary after heat shock, whereas the Hsp70 protein was significantly induced in response to heat shock. The P values are indicated in the figure legend and were obtained by a statistical analysis of data obtained from 5 independent biological replicates.
Fig. 2.
A. Comparison of HSR in D. pulex and D. pulicaria. One week old D. pulex (TCO) and D. pulicaria (lake XVI-clone11) were subjected to heat shock at 32° C for 30 minutes, extracts were prepared after 4h or 6h recovery period. Western Blot analysis was performed using 50 μg of total protein with anti-Hsp70 antibody. The source of the extract and recovery periods after heat shock are as indicated above each lane. C indicates control extract from organisms that were not subjected to heat shock. HeLa extract from heat-shocked cells was used as a positive control for Hsp70. The blot was re-probed with anti-β actin antibody to ensure equal loading. B. Comparison of HSR in young versus old D. pulicaria. One and five week old D. pulicaria (lake XVI-clone11) were subjected to heat shock at 32° C for 30 minutes, allowed to recover for indicated time periods, and protein was extracted. Western Blot analysis was performed using 50 μg of total protein with anti-Hsp70 antibody. Lanes C: non-heat shocked control samples, 4h: after 4h recovery period, and 6h: after 6h recovery period. The age of Daphnia is indicated below the lanes. Blot was re-probed with anti-β actin antibody to ensure even loading. C. Quantification of western blot data in Fig. 2 A: The chemifluorescent band intensities were quantified using STORM phosphorimager and the averages from 5 independent biological replicates is represented as a bar graph and the error bars represent standard deviations. Fold-changes are calculated with respect to signals in control lanes and all Hsp70 signals were normalized to band intensities of β-actin in each lane. The black bars represent D. pulex, and the white bars represent D. pulicaria. As shown above bars, *, * *, #, and # # symbols indicate P values that indicate a significant difference (0.0009, 0.0007, 0.0018, and 0.0008 resp.). There was no significant difference observed in signals for β-actin in various lanes with significant values being < 0.01. D. Quantification of western blot data in Fig. 2 B: The chemifluorescent band intensities were quantified using STORM phosphorimager and the averages from 3 independent biological replicates is represented as a bar graph and the error bars represent standard deviations. Fold-changes are calculated with respect to signals in control lanes and all Hsp70 signals were normalized to band intensities of β-actin in each lane. The black bars represent samples from 1 wk old D. pulicaria, and the white bars represent samples from 5 wk old D. pulicaria. As shown above bars, *, #, * *, and # # symbols indicate P values that indicate a significant difference (0.0013, 0.0027, 0.0018, and 0.0038 resp.). There was no significant difference observed in signals for β-actin in various lanes with significant values being < 0.01.
3.3. Young Daphnia exhibit a more robust HSR than old Daphnia
Next, we wanted to examine the ability to respond to heat stress in relation to age. We thus characterized the induction of Hsp70 in response to heat shock at two different ages of D. pulicaria. As seen in Fig. 2 B, the 1 week-old D. pulicaria exhibited a more robust HSR (5.1-fold and 8.1-fold induction of Hsp70 in lanes 2 and 3) than the 5 week-old organisms (2.3-fold and 2.1-fold induction of Hsp70 in lanes 5 and 6). The band intensities were quantified using STORM phosphorimager and Imagequant software and the data is represented as a bar graph in Fig. 2 D. The levels of β-actin and α-tubulin did not vary after heat shock, whereas the Hsp70 protein was significantly induced in response to heat shock. The P values are indicated in the figure legend and were obtained by a statistical analysis of data obtained from 3 independent biological replicates.
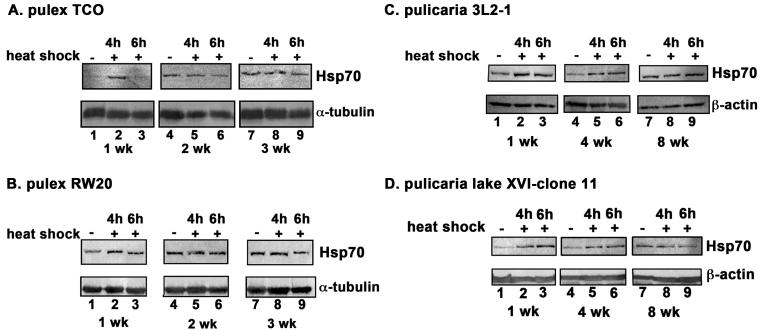
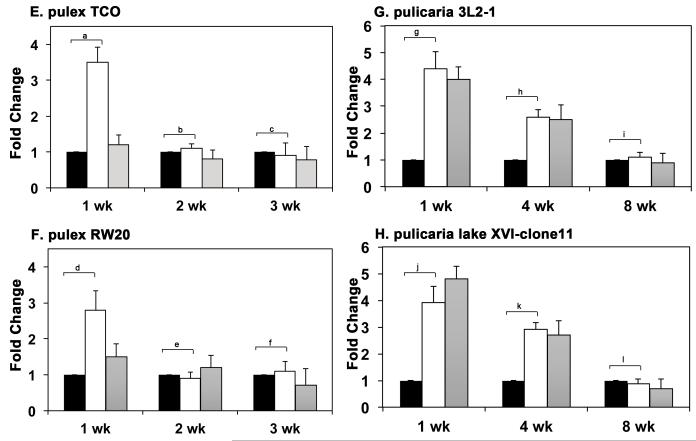
3.4. HSR decreases with age in multiple clones of D. pulex and D. pulicaria
Two of the isolated clones within the D. pulex ecotype were chosen for further analysis, TCO (The Chosen One, Fig. 3 A) and RW20 (Roughwood 20, Fig. 3 B) and two of the isolated clones of D. pulicaria were investigated further, 3L2-1 (Three Lakes Two-1, Fig. 3 C) and XVI-11 (Lake Sixteen-11, Fig. 3 D). Both D. pulex clones exhibit short lifespans (~ 4 weeks) as compared to D. pulicaria clones, which display longer lifespans (~ 9 weeks). The difference in their lifespans is more than two-fold. We examined the induction of Hsp70, in response to heat shock at different ages in two clones for each ecotype. In all clones, the HSR as indicated by induction of Hsp70 expression, decreases with age (Fig. 3 A-D) while the basal level of Hsp70 protein increases with age (lanes 1, 4, 7). It is interesting to note that in RW20, the Hsp70 induction persisted for a longer duration compared to TCO, since at 6h recovery, there was significant amount of Hsp70 expression in RW20 (Fig. 3 B lane 3), whereas for TCO Hsp70 expression returned to basal levels by that time (Fig. 3 A, lane 3). Any significance of this observation to ageing related physiology remains to be explored in future. In addition to a more robust HSR in D. pulicaria (Fig. 3 C and D, lanes 1-3) as compared to D. pulex at 1 week (Fig. 3 A and B, lanes 1-3), there were other significant differences observed in HSR of short-lived ecotypes as compared to long-lived ecotypes. Both TCO and RW20 ecotypes of D. pulex stopped responding to heat shock at the age of 2 weeks and showed no induction of Hsp70 (Fig. 3 A and B, lanes 4-6). Both these ecotypes also showed increased basal expression of Hsp70 at 2 weeks of age as compared to 1 week (Fig. 3 A and B, lanes 1,4, and 7). In contrast to this, both 3L2-1 and lake XVI-11 ecotypes of D. pulicaria showed an induction of Hsp70 levels in response to heat shock at the age of 4 weeks (Fig. 3 C and D, lanes 4-6). Since the average life spans of pulex and pulicaria are 4 weeks and 9 weeks, these ages are midpoints of their average life span and thus are equivalent time points in their respective life spans. Thus, it can be concluded that the long-lived pulicaria ecotypes continue to respond to heat shock by inducing Hsp70 expression until their middle age but the short-lived pulex ecotypes cease to respond to heat shock by their corresponding middle age. These results are highly significant since it is known that induction of Hsp70 in response to heat shock protects against proteotoxicity and limits damage to the cellular function.
Fig. 3. Comparison of HSR at different ages in two isolates each of D. pulex and D. pulicaria.
A. HSR in D. pulex isolate TCO. B: HSR in D. pulex isolate RW20. C. HSR in D. pulicaria isolate 3L2-1. D. HSR in D. pulicaria isolate lake XVI-clone 11. Daphnia were subjected to heat shock at 32° C for 30 minutes, allowed to recover for indicated time periods, and protein was extracted. Western Blot analysis was performed using 50 μg of total protein with anti-Hsp70 antibody. The recovery periods after heat shock are as indicated above the lanes and the age of the Daphnia are indicated in weeks below the panels. Blots were re-probed with anti-α tubulin antibody to ensure even loading. E-F. Quantification of western blot data in Figs. 3 A-D: The chemifluorescent band intensities were quantified using STORM phosphorimager and the averages from several independent biological replicates (3 replicates for pulex ecotypes and 4 replicates for pulicaria ecotypes) is represented as bar graphs and the error bars represent standard deviations. Fold-changes are calculated with respect to signals in control lanes and all Hsp70 signals were normalized to band intensities of β-actin for each lane. The black bars represent control samples, the white bars represent samples after 4 h recovery, and grey bars represent samples after 6 h recovery. As shown above bars, a, d, g, h, j and k labels indicate P values that show a significant difference (0.0010, 0.0018, 0.0014, 0.0008, 0.0021, and 0.0038 resp.) compared to controls. The P values indicated by labels b, c, e, f, i, and l exhibited no significant difference (0.034, 0.045, 0.086, 0.052, 0.11, and 0.21) compared to controls.There was no significant difference observed in signals for β-actin in various lanes with significant values being < 0.01.
The data in western blot analyses (Fig. 3 A-D) was quantified using STORM phosphorimager and Imagequant software and is represented as bar graphs in Fig. 3 E-H. The P values are indicated in the figure legend and were obtained by a statistical analysis of data obtained from 3 independent biological replicates for D.pulex ecotypes and 4 independent biological replicates for D. pulicaria.
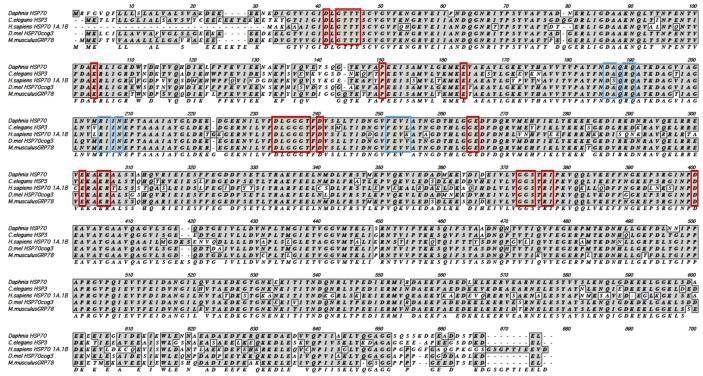
3.5. The Daphnia Hsp70 protein is highly homologous to known Hsp70 sequences
There are five different Hsp70 isoforms that can be identified in D. pulex genome based on sequence homology with mammalian Hsp70. In order to determine which of these genes represents the isoform primarily induced by heat shock, we performed RT-PCR using primers corresponding to the conserved regions of the five Hsp70 nucleotide sequences so that they could amplify all possible Hsp70 sequences if they were expressed in response to heat shock. RT-PCR was performed on RNA isolated from control and heat shocked organisms. Sequence analysis of these products revealed that the control as well as heat shocked samples contained only the sequence from genomic scaffold 3. An alignment of the predicted Hsp70 protein sequence from this chromosomal location is represented in Fig. 4. The protein sequence is highly homologous to Hsp70 sequence from C. elegans, D. melanogaster, as well as human and murine sequences. Based on this information, we designed specific primers for Daphnia Hsp70 sequence from scaffold 3 to determine if the Hsp70 induction in response to heat shock resulted from an increase in Hsp70 mRNA levels.
Fig. 4. Sequence alignment of Daphnia Hsp70.
D. pulex Hsp70 protein sequence is aligned with Hsp70 sequence from other organisms. Gray shaded boxes indicate identity or similarity. Red boxes indicate residues that are involved in nucleotide binding while blue boxes indicate residues that are involved in substrate binding. Gaps (designated as dashes) were entered for the best alignment. C. elegans: Caenorhabditis elegans. D. mel: Drosophila melanogaster. H. sapiens: Homo sapiens. M. musculus: Mus musculus.
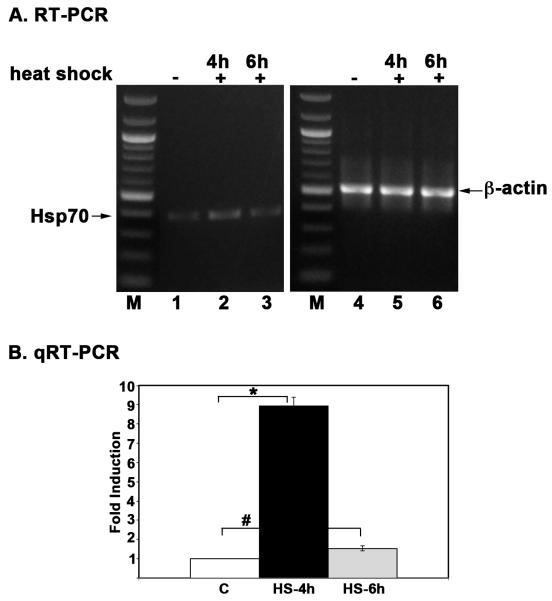
3.6. Hsp70 transcript levels increase after heat shock
Hsp70 induction after heat shock is regulated primarily at the level of transcription by the transcription factor HSF-1 (Morimoto et al. 1992). Various proteotoxic stimuli lead to trimerization of HSF, which translocates to the nucleus to induce the transcription of Hsp70 and consequently leads to an increase in Hsp70 mRNA and protein levels (Akerfelt et al. 2010; Anckar and Sistonen 2011). We next asked if heat shock in Daphnia led to an increase in mRNA levels for Hsp70. We performed RT-PCR and qRT-PCR analysis to quantify Hsp70 mRNA levels in total RNA isolated from control and heat shocked D. pulicaria (lakeXVI-clone 11) at age of 1 week. Using RT-PCR, we found that the Hsp70 mRNA levels were induced after heat shock at a 4 hours recovery time point (Fig. 5 A lane 2) and returned to basal levels at 6 hour recovery time point (Fig. 5 A lane 3) although the Hsp70 protein levels remained high at 6 hours (Figs. 2, and 3 D). The qRT-PCR analysis also showed a 8.5-fold elevation of Hsp70 mRNA levels at 4 hours recovery and return to basal levels at 6 hours recovery (Fig. 5 B). Thus, similar to other organisms, in Daphnia the Hsp70 mRNA levels are elevated in response to heat shock.
Fig. 5. Heat shock leads to an increase in Hsp70 mRNA levels in D. pulicaria.
A. RT-PCR analysis. Total RNA was isolated from 1 week-old D. pulicaria (lake XVI-clone 11) either after heat shock or non-heat shock (control) conditions. Hsp70 mRNA levels were analyzed via reverse-transcriptase PCR and visualized on a 1% agarose gel. β-actin mRNA levels were utilized as a control for normalization. The labels above the lanes indicate control or heat-shocked samples and the length of recovery periods after heat shock. B: qRT-PCR analysis. qRT-PCR analysis was performed to examine the fold-change in expression between non-heat shocked and heat shocked samples from one week old D. pulicaria (lake XVI-clone 11). Data represents the average from nine replicate experiments from three different RNA isolations. C: without heat shock, HS-4h: 4h recovery after heat shock, and HS-6h: 6h recovery after heat shock. All results are normalized to β-actin. Error bars indicate standard deviation calculated from the nine replicates. Statistical analysis was performed to calculate P values and the symbol * represents P value (0.0012) that indicated significant difference compared to control and the symbol # represents P value (0.032) that indicates no significant difference compared to control values.
3.7. The ability of HSF-1 to bind DNA decreases with age in Daphnia
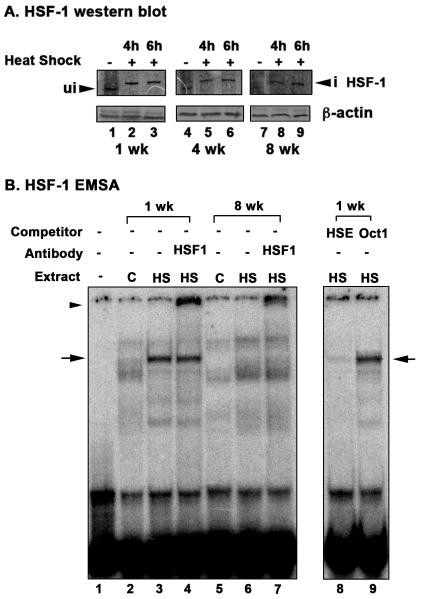
Transcriptional induction of Hsp70 is primarily regulated by transcription factor HSF in all organisms studied for HSR (Akerfelt et al. 2010). In order to gain an insight about why the Hsp70 induction declines with age, we investigated if the HSF levels or activity also shows similar decline with age. In response to heat shock, HSF is phosphorylated and trimerizes to form a functional complex that translocates to nucleus (Akerfelt et al. 2010). We first examined the expression of HSF in Daphnia by western blot analysis. Since there is a single HSF gene in Daphnia and it is most homologous to human HSF-1, we used anti-human HSF-1 antibody for our analysis. As seen in Fig. 6 A, HSF-1 is activated in response to heat shock at 1 week age as indicated by the characteristic upward mobility shift (lanes 2 and 3), that is a likely consequence of HSF-1 phosphorylation. HSF-1 activation is not compromised with increasing age as indicated by the same characteristic upward mobility shift in response to heat shock at ages 4 weeks (lanes 5 and 6) and 8 weeks (lanes 8 and 9). HSF-1 phosphorylation in response to heat shock leads to an increase in the apparent molecular weight of the monomer on SDS-PAGE, by as much as 12 kDa, which has been described previously in literature (Westwood and Wu 1993). We also observe an increased apparent molecular weight of HSF-1 after heat shock on SDS-PAGE, which corresponds to ~10 kDa increase in its apparent molecular weight (Fig. 6 A, lanes 2, 3, 5, 6, 8, and 9). Thus, it can be concluded that activation of HSF-1 is not compromised with age in D. pulicaria. It is worth noting that there is a slight reduction in the abundance of HSF-1 protein at 4 and 8 weeks of age as seen in lanes 4-9 in comparison to age of 1 week (lanes 1-3).
Fig. 6. Activation of HSF-1 in response to heat shock at different ages in D. pulicaria.
A. Western blot analysis. D. pulicaria (lake XVI-clone 11) were subjected to heat shock at 32° C for 30 minutes, allowed to recover for indicated time periods, and protein was extracted. Western Blot analysis was performed using 50 μg of total protein with anti-HSF-1 antibody. The recovery periods after heat shock are as indicated above the lanes and the age of the Daphnia are indicated in weeks below the panels. Blots were re-probed with anti-β actin antibody to ensure even loading. Arrowhead marked “ui” indicates HSF-1 position from non-heat shocked samples, and an arrowhead marked “i” indicates upward mobility shift of HSF-1 in heat shocked samples. B. Electrophoretic mobility shift analysis. EMSA was performed using nuclear extracts prepared from D. pulicaria (lake XVI-clone 11) with (HS) or without heat shock (C) at different ages as indicated. 5 μg of nuclear extracts were incubated with P32-labeled HSE probe. Above each lane, the age of the Daphnia in weeks, addition of a competitor (HSE: specific, Oct1: nonspecific), or addition of an antibody (HSF-1) is as indicated. The ‘-’ lane indicates probe alone lane without any added nuclear extract. Arrows indicate the position of HSF-1 containing complex, and arrowhead indicates position of complex after antibody super-shift. Specific and non-specific non-radioactive competitor oligonucleotides were used in 50-fold molar excess to confirm the specificity of the bound complex.
We next examined if HSF-1 DNA binding activity is reduced with age and can explain the decline in Hsp70 expression in 4 and 8 week old organisms. We analyzed DNA-binding activity of HSF-1 by performing EMSAs with heat shock element (HSE) oligonucleotide using nuclear extracts from 1 week and 8 week-old D. pulicaria (Lake XVI-clone 11). As seen in Fig. 6 B, extract from the 1 week old individuals showed a strong mobility shifted band (indicated by an arrow) with HSE after heat shock (lane 3). This complex was missing with the extract from control (non-heat shocked) individuals (lane 2) thereby indicating that that this is the complex that is strongly induced in response to heat shock. Part of this complex could also be super-shifted (lane 4, indicated by an arrowhead) with HSF-1 antibody thereby demonstrating the presence of HSF-1 transcription factor in the complex. Competition with unlabeled specific (HSE oligo, lane 8) and the lack of competition with unlabeled non-specific oligonucleotides (Oct1 oligo, lane 9) confirmed that the complex indicated by an arrow resulted from specific recognition of the HSE probe. 8 week old individuals showed no complex at the equivalent position of HSF-1 complex (lane 6), thereby indicating that HSF-1 is not competent to bind DNA in aged organisms. It is interesting to note the presence of two much weaker complexes in lane 6. These complexes did not exhibit any significant super shift with HSF-1 antibody (lane 7), thereby indicating lack of HSF-1 in these complexes. The composition and significance of these complexes for transcriptional regulation of Hsp70 expression in older organisms remains to be explored in future studies. Our results establish that the DNA-binding ability of HSF-1 is diminished in older animals.
In summary, we have presented results which establish that the freshwater crustacean Daphnia responds to heat shock by inducing the expression of Hsp70 and the ability to induce Hsp70 in response to heat shock decreases with age in both short-lived and long-lived ecotypes. We also show that the long lived ecotype, D. pulicaria, exhibits a better induction of Hsp70 in response to heat shock than the short lived D. pulex at a young age. In both short- and long-lived species the ability to respond to heat shock by inducing Hsp70 decreases with age. Furthermore, the short-lived pulex ecotypes stop responding at about midpoint of their average life span, but the long-lived pulicaria ecotypes show HSR at an age that is equivalent of the midpoint in their longer life span. In addition, we demonstrate that the lack of HSR in older organisms is not due to lack of HSF-1 activation, but rather can be attributed to a lack of DNA-binding by HSF-1 in older organisms. These results for the first time characterize HSR and Hsp70 induction in Daphnia and this is first report to study ageing mechanisms at molecular level using Daphnia as a model organism.
4. Discussion
4.1. Daphnia as a new model organism for research on ageing
Organisms sense and respond to physiological and environmental stress by inducing specific stress response pathways that function to protect the core biological processes by promoting protein folding, and triggering degradation of damaged proteins (Kim et al. 2013). The HSR pathway protects the proteome of all cells against acute damage resulting from exposure to elevated temperatures, oxidative damage, heavy metals, and ageing related accumulation of misfolded and aggregated proteins (Calderwood et al. 2009). Induction of HSR leads to a rapid and robust expression of Hsps that function as molecular chaperones, which prevent protein misfolding and aggregation to promote recovery from stress. In this report, we investigated the regulation of Hsp70 expression in relation to ageing in D. pulex and D. pulicaria. These two Daphnia ecotypes vary about 2-fold in their relative life spans, thereby making it possible to identify and study molecular pathways that regulate ageing and longevity (Dudycha and Tessier 1999). Daphnia have numerous characteristics that make them a novel and versatile model for ageing research. The parthenogenetic mode of reproduction in Daphnia is particularly useful because large numbers of genetically identical individuals can be easily produced without creating artificially homozygous genetic backgrounds. They have short lifespans, tractable genetics, an available completed D. pulex genome sequence with additional genome sequences in progress, and adult tissue regeneration. They have sexual and asexual phases to their life cycle, permitting crosses as well as straightforward generation of many genetically identical individuals. Furthermore, Daphnia have significantly more genes than Drosophila or C. elegans, raising the possibility that additional genes with relevance to human ageing could be identified in them (Colbourne et al. 2011). Currently the biomedical research community uses Daphnia primarily in the contexts of ecotoxicology. In order to establish Daphnia as a model system for ageing, we have analyzed the well-established HSR pathway to determine if the longer lived ecotype, D. pulicaria displayed a better HSR in comparison to the short lived D. pulex. There are relatively few molecular studies involving Daphnia, none published on the relationship between HSR and molecular biology of ageing. Our results presented here for the first time present an analysis of regulation of Hsp70 expression during Daphnia life span.
4.2. HSR in short-lived ecotype versus the long-lived ecotype
Our results establish that the long-lived D. pulicaria displays a more robust induction of Hsp70 in response to elevated temperatures than the short-lived D. pulex. Within each ecotype, the young organisms display a more robust HSR than the old organisms, which is in accordance with data from studies in mammals (Gagliano et al. 2007; Kayani et al. 2008). In addition, the short lived D. pulex show no induction of Hsp70 by the average midpoint in their life span (2 weeks), but the long-lived D. pulicaria continues to exhibit good induction of Hsp70 at the average midpoint in their life span (4 weeks). Our results indicate that the lack of Hsp70 induction in older organisms results from a lack of DNA-binding by HSF-1. These results are similar to data from other model organisms that the ability of HSF-1 to bind to DNA decreases with age (Heydari et al. 2000; Westerheide et al. 2009). Thus, Daphnia have shown similar characteristics about HSR in relation to ageing as other model organisms, while offering a direct comparison between HSR in ecotypes that have evolved short and long life spans, which is unique to our system.
4.3. Basal versus heat-induced Hsp70 expression during ageing
Both D. pulicaria and D. pulex display an increase in basal Hsp70 expression as they age, with the oldest organisms displaying the highest basal expression of Hsp70. The basal expression level of Hsp70 in the absence of any proteotoxic signal such as heat shock is known to increase as the organisms age in D. melanogaster (Wheeler et al. 1995). Tissue-specific induction of stress proteins in mammals is also observed during normal ageing (Schultz et al. 2001) indicating that induction of Hsp expression during ageing is evolutionarily conserved across a broad range of taxa. Ageing-associated up-regulation of Hsps has also been documented including some recent genome-wide studies of gene expression changes (Wheeler et al. 1995; King and Tower 1999; Wheeler et al. 1999; Landis et al. 2004). Although some studies indicate that a functional HSE is required in the Hsp promoters for this age-dependent induction of basal transcription, posttranscriptional regulation at the level of Hsp translation or stability has also been documented (Wheeler et al. 1995). This could explain the age-dependent upregulation of Hsp70 protein levels in Daphnia while HSF-1 is not competent for DNA-binding in old organisms. In contrast to this, induction of Hsp70 expression in response to proteotoxic stimuli (HSR) has been shown to decrease with age in other model organisms, which is also the case in two isolates of each of the two ecotypes of Daphnia that we investigated (Morley and Morimoto 2004; Rea et al. 2005; Tower 2011).
4.4. Mechanism of reduced HSR in older Daphnia
The functional role of HSF-1 as a transcription factor that regulates stress-induced expression of Hsps including Hsp70 has been unambiguously established (Akerfelt et al. 2010). Given the central role played by HSF-1 in inducing Hsp expression in response to proteotoxic stimuli and the need for proteome maintenance for increased organismal life span, it is not surprising that HSF-1 is required for longevity. In C. elegans a reduction in HSF-1 levels by RNAi reduces life span by 30-40% (Morley and Morimoto 2004) and worms carrying additional copies of HSF-1 gene are resistant to elevated temperatures and live about 40% longer than their wild type counterparts (Hsu et al. 2003). Extensive studies done in C. elegans also indicate HSF-1 as a longevity factor affected by insulin-regulated signaling pathways (Barna et al. 2012). Previous studies have found that HSF-1’s ability to bind to DNA decreases with age; therefore, causing an age-dependent decrease in HSR (Fawcett et al. 1994; Jurivich et al. 1997). In our present study, we find that the Daphnia HSF-1 also shows a decrease in its DNA-binding activity with age. Many post translational modifications such as phosphorylation, sumoylation, and acetylation occurring at several sites are known to affect HSF-1 DNA binding (Anckar and Sistonen 2011). The molecular mechanism of the observed age dependent decrease in DNA-binding of HSF-1 in Daphnia remains to be determined in future studies.
Traditionally, Daphnia have been studied as an ecological model. The ability of Daphnia to respond to environmental stressors and toxins is crucial for the survival and propagation of the species, especially the short lived D. pulex, which lives in temporary ponds that may only exist for a few weeks. In addition, predation is one of the major components of a changing, unstable environment (Lampert 1987; Pijanowska and Kloc 2004). Some of the antipredator defenses in Daphnia involve a variety of morphological, behavioral, and life-history adaptations that may not be life-saving, but may enable organisms to complete reproduction before predation. Most of these phenotypic changes are induced by chemical compounds (kairomones) released by a predator into water. Exposure of Daphnia to kairomones results in a change in motion, behavior, and life history (Miyakawa et al. 2010). At the molecular level, the response also involves induction of Hsps. Since some of the kairomone-induced effects are transgenerational, i.e., they are passed on from the mother to her offspring, it may be interesting to test if the short lived D. pulex exposed to kairomones at a young age may show extension of life span under laboratory setting which may be attributed to induction of Hsp70 expression.
Conclusions
We examined the HSR, and more specifically the expression of Hsp70 in two Daphnia ecotypes that exhibit strikingly different life spans. D. pulicaria, the long lived ecotype, showed a robust Hsp70 induction as compared to the shorter lived D. pulex. The short-lived D. pulex isolates showed no induction of Hsp70 at the mid point in their life span. In contrast to this, the long-lived D. pulicaria continued to induce Hsp70 expression at an equivalent age. The Hsp70 expression was induced at transcriptional level in response to heat shock and the decline of Hsp70 induction in old organisms could be attributed to a decline in the transcription factor HSF-1’s DNA-binding activity. This is a first report of the molecular analysis of relationship between HSR and life span in Daphnia.
Highlights.
We present the freshwater microcrustacean Daphnia as a new model system for aging research
HSR is more robust in younger compared to older Daphnia
HSR is more robust in long-lived compared to short-lived ecotypes of Daphnia
Old organisms show a reduction in HSF-1’s DNA-binding ability as compared to young organisms
Acknowledgements
The authors would like to thank the technical support and help with Daphnia maintenance provided by Christine M. Ansell.
Funding:
This work was supported by National Institutes of Health grant 1R01AG037969-01 awarded to JLD and RCP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
CS, and IH performed the experiments, and analyzed the data. JLD helped with designing the experiments and gave conceptual advice. RCP conceived, designed and supervised all the experiments. CS and RCP wrote the manuscript.
References
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar J, Sistonen L. Regulation of HSF-1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- Barna J, Princz A, Kosztelnik M, Hargitai B, Takacs-Vellai K, Vellai T. Heat shock factor-1 intertwines insulin/IGF-1, TGF-beta and cGMP signaling to control development and aging. BMC Dev Biol. 2012;12:32. doi: 10.1186/1471-213X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie J. The Genus Daphnia (Including Daphniopsis) (Anomopoda: Daphniidae). Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. Ghent and Backhuys Publishers; Leiden: 2005. [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125(3):443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Caceres CE, Tessier AJ. To sink or swim: variable diapause strategies among Daphnia species. Limnology and Oceanography. 2011;49:1333–1340. [Google Scholar]
- Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging--a mini-review. Gerontology. 2009;55(5):550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy D, Birdsall J. Flies, worms and the Free Radical Theory of ageing. Ageing Res Rev. 2013;12(1):404–412. doi: 10.1016/j.arr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, Frohlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak JR, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Koonin EV, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL. The ecoresponsive genome of Daphnia pulex. Science. 2011;331(6017):555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly SJ, Moeller RE, Sanchez G, Mitchell DL. Temperature effects on survival and DNA repair in four freshwater cladoceran Daphnia species exposed to UV radiation. Photochem Photobiol. 2009;85(1):144–152. doi: 10.1111/j.1751-1097.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- Cowan PJ, Shinkel TA, Witort EJ, Barlow H, Pearse MJ, d’Apice AJ. Targeting gene expression to endothelial cells in transgenic mice using the human intercellular adhesion molecule 2 promoter. Transplantation. 1996;62(2):155–160. doi: 10.1097/00007890-199607270-00002. [DOI] [PubMed] [Google Scholar]
- Cristescu ME, Constantin A, Bock DG, Caceres CE, Crease TJ. Speciation with gene flow and the genetics of habitat transitions. Mol Ecol. 2012;21(6):1411–1422. doi: 10.1111/j.1365-294X.2011.05465.x. [DOI] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:646354. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel EE, van Breemen C, Schilling WP, Kwan CY. Regulation of vascular tone: cross-talk between sarcoplasmic reticulum and plasmalemma. Can J Physiol Pharmacol. 1995;73(5):551–557. doi: 10.1139/y95-070. [DOI] [PubMed] [Google Scholar]
- Dudycha JL. The senescence of Daphnia from risky and safe habitats. Ecology Letters. 2001;4:102–105. [Google Scholar]
- Dudycha JL. A multi-environment comparison of senescence between sister species of Daphnia. Oecologia. 2003;135(4):555–563. doi: 10.1007/s00442-003-1230-7. [DOI] [PubMed] [Google Scholar]
- Dudycha JL. Mortality dynamics of Daphnia in contrasting habitats and their role in ecological divergence. Freshwater Biology. 2004;49(5):505–514. [Google Scholar]
- Dudycha JL, Hassel C. Aging in sexual and obligately asexual clones of from temporary ponds. J Plankton Res. 2013;35(2):253–259. doi: 10.1093/plankt/fbt008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudycha JL, Tessier AJ. Natural Genetic Variation of Life Span, Reproduction, and Juvenile Growth in Daphnia. Evolution. 1999;53(6):1744–1756. doi: 10.1111/j.1558-5646.1999.tb04559.x. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Sylvester SL, Sarge KD, Morimoto RI, Holbrook NJ. Effects of neurohormonal stress and aging on the activation of mammalian heat shock factor 1. J Biol Chem. 1994;269(51):32272–32278. [PubMed] [Google Scholar]
- Gagliano N, Grizzi F, Annoni G. Mechanisms of aging and liver functions. Dig Dis. 2007;25(2):118–123. doi: 10.1159/000099475. [DOI] [PubMed] [Google Scholar]
- Heydari AR, You S, Takahashi R, Gutsmann-Conrad A, Sarge KD, Richardson A. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res. 2000;256(1):83–93. doi: 10.1006/excr.2000.4808. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Jurivich DA, Qiu L, Welk JF. Attenuated stress responses in young and old human lymphocytes. Mech Ageing Dev. 1997;94(1-3):233–249. doi: 10.1016/s0047-6374(96)01856-8. [DOI] [PubMed] [Google Scholar]
- Kayani AC, Morton JP, McArdle A. The exercise-induced stress response in skeletal muscle: failure during aging. Appl Physiol Nutr Metab. 2008;33(5):1033–1041. doi: 10.1139/H08-089. [DOI] [PubMed] [Google Scholar]
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Ulrich Hartl F. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- Kimura K, Tanaka N, Nakamura N, Takano S, Ohkuma S. Knockdown of mitochondrial heat shock protein 70 promotes progeria-like phenotypes in caenorhabditis elegans. J Biol Chem. 2007;282(8):5910–5918. doi: 10.1074/jbc.M609025200. [DOI] [PubMed] [Google Scholar]
- King V, Tower J. Aging-specific expression of Drosophila hsp22. Dev Biol. 1999;207(1):107–118. doi: 10.1006/dbio.1998.9147. [DOI] [PubMed] [Google Scholar]
- Lampert W. Predictability in lake ecosystems: the role of biotic interactions. Ecological Studies. 1987;61:333–346. [Google Scholar]
- Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, Tavare S, Tower J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101(20):7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa H, Imai M, Sugimoto N, Ishikawa Y, Ishikawa A, Ishigaki H, Okada Y, Miyazaki S, Koshikawa S, Cornette R, Miura T. Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev Biol. 2010;10:45. doi: 10.1186/1471-213X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64(2):167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992;267(31):21987–21990. [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15(2):657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Gong P, Suzuki K, Koizumi S. Differential metal response and regulation of human heavy metal-inducible genes. J Cell Physiol. 1999;180(1):105–113. doi: 10.1002/(SICI)1097-4652(199907)180:1<105::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Omilian AR, Lynch M. Patterns of intraspecific DNA variation in the Daphnia nuclear genome. Genetics. 2009;182(1):325–336. doi: 10.1534/genetics.108.099549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijanowska J, Kloc M. Daphnia response to predation threat involves heat-shock proteins and the actin and tubulin cytoskeleton. Genesis. 2004;38(2):81–86. doi: 10.1002/gene.20000. [DOI] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37(8):894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C, Dick EJ, Cox AB, Hubbard GB, Braak E, Braak H. Expression of stress proteins alpha B-crystallin, ubiquitin, and hsp27 in pallido-nigral spheroids of aged rhesus monkeys. Neurobiol Aging. 2001;22(4):677–682. doi: 10.1016/s0197-4580(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Steinberg CEW, Ouerghemmi N, Herrmann S, Bouchnak R, Timofeyev MA, Menzel R. Stress by poor food quality and exposure to humic substances:Daphnia magna responds with oxidative stress, lifespan extension, but reduced offspring numbers. Hydrobiologia. 2010;652:223–236. [Google Scholar]
- Taylor RC, Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol. 2011;3(5) doi: 10.1101/cshperspect.a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. Heat shock proteins and Drosophila aging. Exp Gerontol. 2011;46(5):355–362. doi: 10.1016/j.exger.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AE, Ackerman MS, Eads BD, Xu S, Lynch M. Population-genomic insights into the evolutionary origin and fate of obligately asexual Daphnia pulex. Proc Natl Acad Sci U S A. 2013;110(39):15740–15745. doi: 10.1073/pnas.1313388110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergilino R, Markova S, Ventura M, Manca M, Dufresne F. Reticulate evolution of the Daphnia pulex complex as revealed by nuclear markers. Mol Ecol. 2011;20:1191–1207. doi: 10.1111/j.1365-294X.2011.05004.x. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Jr., Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JT, Wu C. Activation of Drosophila heat shock factor: conformational change associated with a monomer-to-trimer transition. Mol Cell Biol. 1993;13(6):3481–3486. doi: 10.1128/mcb.13.6.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel RG. Limnology: Lake and River Ecosystems. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Wheeler JC, Bieschke ET, Tower J. Muscle-specific expression of Drosophila hsp70 in response to aging and oxidative stress. Proc Natl Acad Sci U S A. 1995;92(22):10408–10412. doi: 10.1073/pnas.92.22.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JC, King V, Tower J. Sequence requirements for upregulated expression of Drosophila hsp70 transgenes during aging. Neurobiol Aging. 1999;20(5):545–553. doi: 10.1016/s0197-4580(99)00088-3. [DOI] [PubMed] [Google Scholar]
- Xu S, Innes DJ, Lynch M, Cristescu ME. The role of hybridization in the origin and spread of asexuality in Daphnia. Mol Ecol. 2013;22(17):4549–4561. doi: 10.1111/mec.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516(1-3):53–57. doi: 10.1016/s0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]