Abstract
Neuronal competence to re-extend axons and a permissive environment that allows growth cone navigation are two major determinants for successful axon regeneration. Here, we review the roles of bone morphogenetic protein (BMP) signaling in mediating both neuronal and glial injury responses after CNS injury. BMPs can activate a proregenerative transcription program in neurons through Smad-mediated canonical pathway, or act locally on cytoskeleton assembly at distal axons via non-canonical pathways. Emerging evidence implicates retrograde BMP signalosomes in connecting the cytoskeletal and nuclear responses. In addition, BMP/Smad signaling modulates neurotrophin-mediated axonal outgrowth, and interacts with the epigenetic machinery to initiate epigenetic reprogramming for axon regeneration. Besides their influences on neurons, BMPs also regulate astrogliosis, inflammatory processes, and neural progenitor cell differentiation at the injury site, all of which can either positively or negatively modify the injury microenvironment. Lastly, an increasing number of BMP signaling partners, sensitizers, and downstream effectors collectively fine-tune the signaling intensity and spatiotemporal dynamics of BMP activity in an integrated signaling network during axon regeneration.
Introduction
Mature neurons in the CNS regenerate their injured axons minimally, owing to an age-dependent decline of axon growth potential [1] and an inhibitory environment [2]. Inactivation of the external inhibitory molecules is insufficient for long-distance axon regeneration, as demonstrated in studies on functional interference with myelin-based inhibitors [3] or chondroitin sulfate proteoglycans (CSPGs) [4]. On the other hand, enhancing the intrinsic axon growth potential of adult neurons by itself also results in modest regeneration [1]. Hence, successful regenerative strategies need to address both intrinsic and extrinsic hurdles. Bone morphogenetic proteins (BMPs) have emerged as an important class of signaling molecules that mediate both neuronal axon growth potential and glial injury responses.
BMPs are members of the TGFβ superfamily that signal through serine/threonine kinase receptors to activate Smad family transcription factors through C-terminal phosphorylation (pSmadC) [5]. Smad1, -5, and -8 isoforms mediate canonical signaling of the BMP family, while Smad2 and -3 mediate signaling of the TGFβ family. In addition to the pSmadC-mediated transcriptional program, BMPs can also activate non-canonical, Smad-independent pathways that include LIM kinase (LIMK), p38/MAPK, phosphatidylinositol 3-kinase (PI3K), and Rho-like small GTPases. Some of the known functions of these pathways involve local cytoskeletal dynamics by way of modulating the actin depolymerization factor cofilin. Additionally, BMP co-receptors and signaling sensitizers further increase the functional complexity of BMPs in the CNS (for an in-depth review on BMP pathways in the nervous system, please refer to [6]). Here, we discuss the involvement of both the canonical and non-canonical BMP signaling in coordinating neuronal and glial injury responses, focusing on those that influence the outcome of axon regeneration after CNS injury.
BMP signaling mediates axon growth potential
To date, BMPs have not been directly linked to developmental axon elongation in vivo. This could be due to their functional redundancy (the BMP family consists of 20 members) [7], or to the early embryonic lethality of currently available mouse knockout lines. The development of more sophisticated conditional mouse models may yield conclusive evidence regarding the role of BMP signaling in axon development. In primary neuron cultures, BMPs function as growth factors to stimulate axon growth in developing striatal GABAergic neurons [8], raphe serotonergic neurons [9], cerebellar granule neurons [10], retinal ganglion cells (RGCs) [11], and spinal motor neurons (SMNs) [12]. BMP also interacts with neurotrophin signaling as part of a tightly regulated signaling network to ensure proper axon development. For instance, in developing DRG sensory neurons, neurotrophins robustly promote axonal outgrowth through the RAF-MEK kinase cascade [13]. Conditional activation of B-RAF kinase promotes both developmental and regenerative axon growth [14]. BMP signaling is required for neurotrophin-mediated axonal outgrowth in DRG neurons, by way of transcriptional regulation of two effectors of RAF-MEK kinase signaling, Erk1 and Erk2, thereby sensitizing neurotrophin responsiveness [15]. In vivo, abrogation of NGF or RAF-MAP kinase signaling results in axonal terminal branching defect [13], which is phenocopied by conditional knockout of Smad1 in neural-crest derived cells, including DRG neurons [15]. In addition, BMP signaling potentiates NGF induction of the transcription factor Egr [16]. BMPs also potentiate neurotrophin 3-induced neurite outgrowth of peripheral neurons [17]. On the other hand, BMP4 has been shown to function as a target-derived cue that limits the number of sensory neurons and the extent of terminal peripheral nerve innervation [18]. In developing trigeminal ganglia (TG) sensory neurons, a forward genetic screen has identified Megf8, a large putative transmembrane protein, as a modifier of BMP4 signaling that inhibits axon growth [19]. Interestingly, in TG sensory neurons, retrograde BMP signaling regulates gene expression and patterning along the dorsoventral axis of the TG [20]. Furthermore, BMP7 in the roof plate of spinal cord functions to repel axons of developing commissural neurons [21]. Taken together, BMPs mediate axon development in a cell-type specific and context dependent manner. For a full understanding, one must consider which signaling cascade is activated by BMPs, in what neuronal subtypes, in which subcellular compartment, and at what developmental stages.
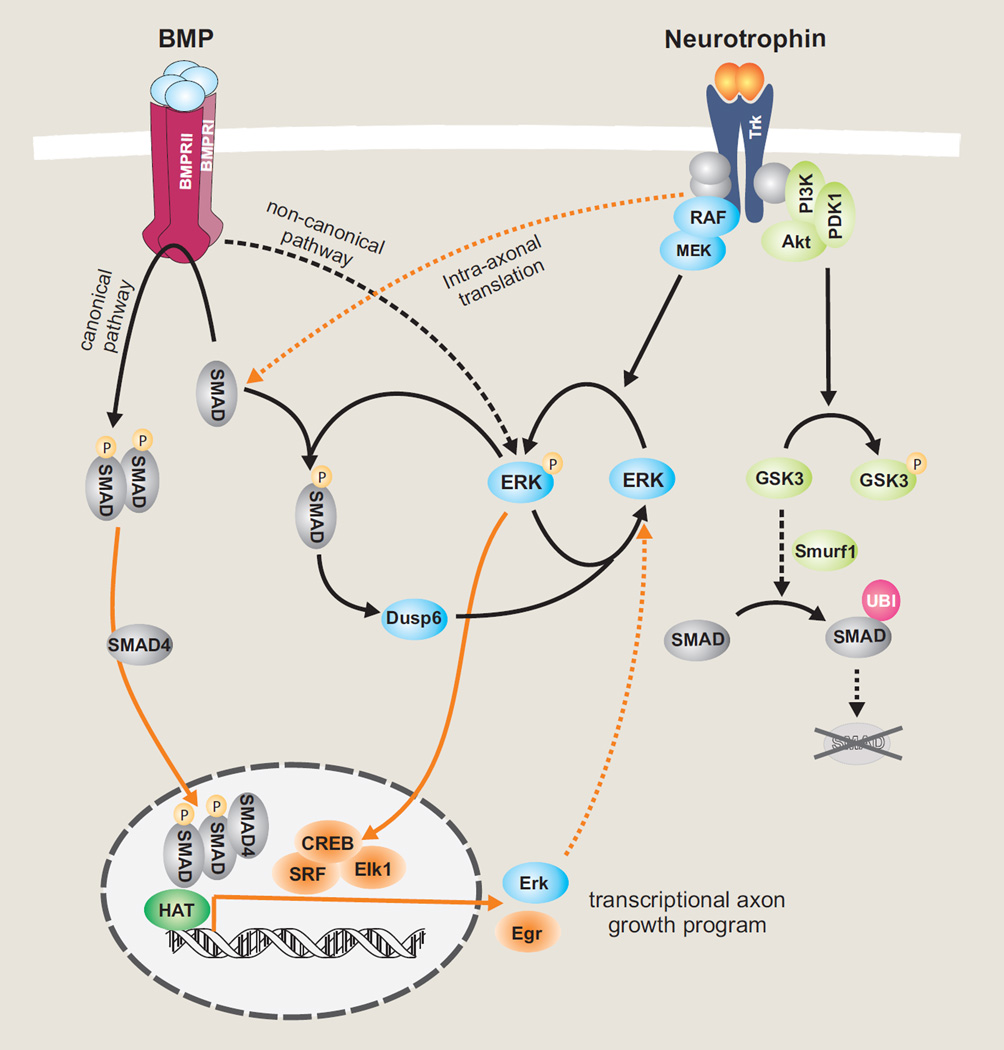
Initial evidence for a pro-regenerative role of BMP-Smad signaling came from studies of the conditioning lesion paradigm, where a prior peripheral axotomy of DRG neurons drastically increases axon growth potential of the centrally projecting axons that are usually refractory to regeneration [22]. Smad1 is induced after a conditioning lesion, and its activation is required for enhanced axon growth potential. In contrast, a central axotomy fails to induce or activate Smad1 [23]. In a followup study, we designed an intrathecal injection of an adeno-associated virus that encodes BMP4 (AAV-BMP4) to specifically target DRG neurons. This led to nuclear accumulation of activated Smad1, promotion of axon growth potential and sensory axon regeneration in a mouse model of SCI [24]. Notably, Smad1 is developmentally regulated, with robust expression in developing DRG neurons. Aside from regulating Erk1 and Erk2 transcription by pSmad1C, Smad1 can also respond to neurotrophin stimulation via linker phosphorylation (pSmad1L). pSmad1L takes part in a negative feedback mechanism to prevent axonal overgrowth in the target field by upregulating DUSP6, an Erk-specific dual-specificity phosphatase, leading to reduced pErk1/2. Hence, through differential phosphorylation, Smad1 helps to shape sensory axon development by converging neurotrophin and BMP signaling to maintain a balanced ratio of Erk1/2 and pErk1/2 [15]. In further support for a positive role of BMP-Smad signaling in axon regeneration, acute knockdown of Smad1 hinders axonal regeneration in a sciatic crush model in vivo [25]. Moreover, Noggin, an endogenous BMP signaling antagonist, inhibits neurite outgrowth in DRG explants and retards early axonal regeneration in injured sciatic nerve. It is worth mentioning that Noggin is downregulated in DRGs after a conditioning lesion [26].
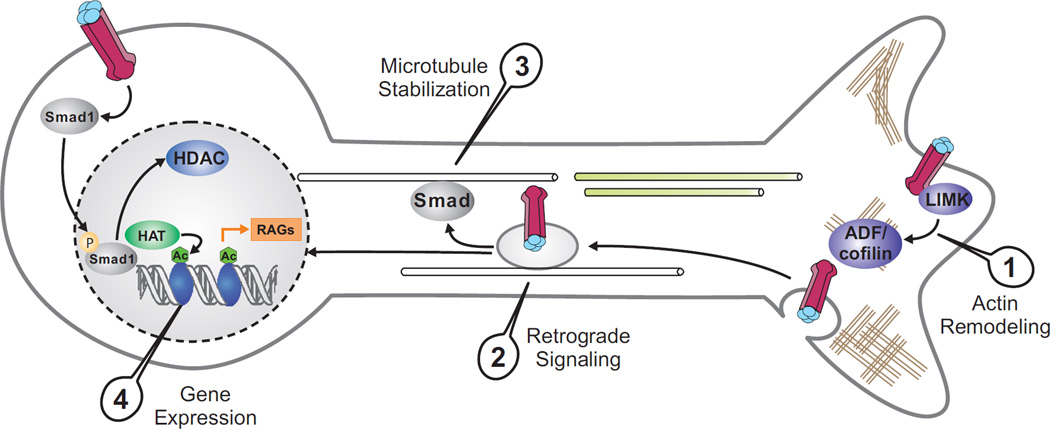
Expression profiling of regeneration-associated genes (RAGs) showed that Sprr1a, Npy, Galanin and to a lesser extent, Vip, are downregulated in DRGs with Smad1 ablation. Chromatin immunoprecipation (ChIP) studies confirmed specific binding of Smad1 at the promoters of target genes. Our recent studies also demonstrate that Smad1 helps to recruit histone-modifying enzymes such as histone acetylases (HATs) and dissipate histone deacetylases (HDACs), leading to increased histone H4 acetylation (AcH4); AcH4 enrichment in turn facilitates Smad1 promoter occupancy. It turns out that diminished axon growth potential is associated with histone hypoacetylation, whereas a conditioning lesion can restore histone acetylation levels [27, 28]. Importantly, pharmacological inhibition of HDACs is capable of initiating epigenetic reprogramming, leading to induction of multiple RAGs and promotion of sensory axon regeneration after SCI. Together, these studies identify a pro-regenerative transcriptional module, consisting of Smad1 and histone enzymes for RAG regulation [28]. Consistently, Smad1 is among the differentially upregulated genes in axon-sprouting vs. non-sprouting neurons after cerebral ischemia [29]. Moreover, in injured hypoglossal motor neurons, Smad1, 2, and 4 are upregulated [30], and in RGC, BMP-Smad signaling promotes survival after NMDA induced retina damage [31]. However, BMPs' role in axon regeneration in other classes of neurons awaits future studies.
BMP signaling regulates cytoskeletal dynamics during axon development
BMP signaling can act locally to mediate cytosketetal assembly in distal axonal or dendritic compartments, through Smad-independent, non-canonical pathways. For instance, LIMK can be activated by binding to type II BMPR and regulates BMP-dependent dendritogenesis [32]. On the other hand, in cerebellar granule neurons, BMP2 inhibits neurite outgrowth by a LIMK-dependent mechanism [33]. BMP7 has been shown to induce growth cone turning via local actin dynamics through ADF/cofilin activity controlled by LIMK and slingshot phosphatase [34]. In Drosophila motor neurons, both local LIMK-mediated and retrograde BMP signaling are involved in synaptic formation and stability at the neuromuscular junction (NMJ) [35, 36]. Whether modulation of the BMP-LIMK-ADF/Cofilin pathway could enhance axon regeneration needs to be tested.
Aside from the LIMK/cofilin pathway, the glycosylphosphatidylinositol (GPI)-anchored repulsive guidance molecules (RGMs) have been identified as BMP co-receptors as they can form a complex with BMP receptors [37, 38]. RGMb (Dragon) is highly expressed in adult DRG sensory neurons and promotes neurite outgrowth by sensitizing BMP signaling [26]. RGMb deletion decreases BMP signaling and inhibits early axonal regeneration in the sciatic nerve crush model [26]. In contrast, RGMa causes, by a BMPR-independent mechanism, growth cone collapse and neurite outgrowth inhibition by engaging the transmembrane receptor neogenin to activate the small GTPase RhoA and its downstream effectors Rho kinase and PKC [39]. Intrathecal administration of a blocking antibody against RGMa enhances CST regeneration and improves functional recovery [40]. It is also noteworthy that BMP can directly bind to neogenin, leading to RhoA activation and a dampening effect on Smad in non-neuronal cell culture studies [41]. The relevance of the BMP-RGM or BMP-neogenin signaling in axon regeneration in vivo has not been determined.
Intra-axonal BMP signalosome trafficking
Long-range, retrograde signaling of target-derived BMPs helps to shape neural circuit assembly [42]. The effects of target-derived BMP4 and BDNF are interconnected: BDNF induces translation of axonally localized Smad1/5/8 transcripts, and the axon-derived Smad1/5/8 is then translocated to the soma, where it is activated by BMP-induced signaling endosomes [43].
BMP signaling is also involved in axonal transport and organization of the microtubule network in developing motor neurons in Drosophila [44]. Endocytosis of BMP receptors and dynein-dependent retrograde transport of BMP signalosome along the axon have been demonstrated [45]. In zebrafish SMN axons, type I BMPR is observed in punctate vesicular structures distributed along the neurite, and BMP signalosome trafficking, mediated by atlastin, a membrane-bound GTPase linked to cases of hereditary spastic paraglegia (HSP), has been implicated in microtubule dynamics [46]. Knockdown of atlastin leads to enhanced BMP signaling and aberrant axonal branching due to lack of stable microtubules. It is conceivable that intra-axonal BMP receptor trafficking may affect axon regeneration by regulating microtubule stability and by coupling the cytoskeletal dynamics at the distal axon with the nuclear transcriptional response.
Studies using microfluidic chambers to separately manipulate BMP signaling in the soma vs. axon terminal may shed light on this hypothesis.
BMP signaling regulates glial scar formation
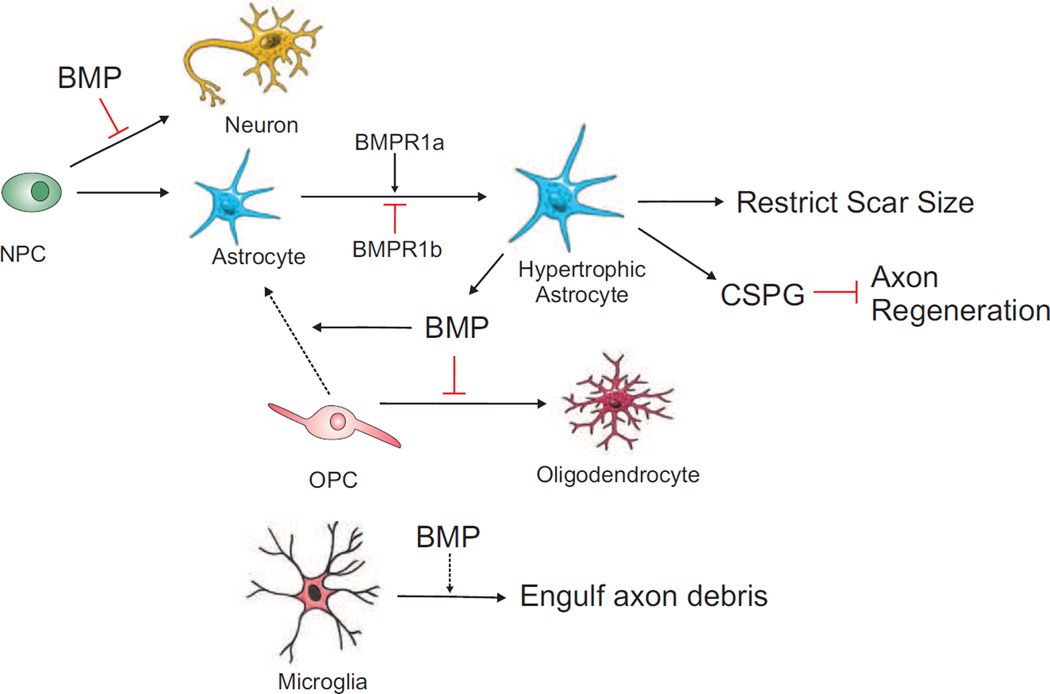
One of the major differences between axon regeneration after injury and developmental axon growth is the interference from glial scarring after CNS injury. While the dense glial scar that builds up over time is detrimental to axon regeneration due to increased deposition of CSPGs [47], the early hypertrophic astroglial response is thought to be beneficial for neural repair by restricting scar size [48]. Engraftment of astrocytes derived from glial-restricted embryonic precursors treated with BMP4 after SCI promotes axon regeneration and functional recovery [49].
The local injury microenvironment favors astrocyte differentiation as illustrated by the preferential differentiation of engrafted pluripotent embryonic neural precursor cells (NPCs) into astrocytes in the injured spinal cord [50]. After SCI, a number of BMPs are upregulated in local neurons, astrocytes, oligodendrocytes, microglia/macrophages, and endogenous NPCs in the spinal cord, with different temporal patterns [51, 52]. BMPs are thought to restrict NPCs to the astroglial lineage while suppressing the neuronal and oligodendroglial differentiation, similar to their effect in neurosphere cultures derived from adult mouse spinal cord [53]. In cultured astrocytes, BMPs increase CSPG expression, in line with the notion that BMPs drives gliogenesis and glial scar formation, which has also been demonstrated in a demyelinating spinal cord lesion model [54]. However, global inhibition of BMP signaling in vivo at the SCI site yields conflicting results. In one study, transplantation of fetal NPCs engineered to express Noggin promoted neuronal and oligodendrocyte differentiation while suppressing generation of astrocytes, along with functional recovery after SCI [55]; but in another study, astroglial differentiation was unaffected, but instead, it led to an increase in both lesion volume and the number of infiltrating macrophages [56]. Intrathecal delivery of Noggin also gave rise to conflicting results: it enhanced locomotor activity and CST axons regeneration in one study [57], but failed to attenuate GFAP expression in another [53]. These discrepancies are attributed to different grafted cells, various injury models (compression vs. contusion and ischemia), and use of cyclosporine A as immunosuppressor in some studies, which may mask the effect of Noggin. Another contributing factor may be the varying ratio of BMPR isoform expression in different glial cells or NPCs. BMPR1a and -1b exert opposing effects, with BMPR1a positively, and BMPR1b negatively regulating astrogliosis and wound closure after SCI [58]. Conditional knockout of BMPR1a in GFAP-expressing cells results in defective astrocytic hypertrophy, increased inflammatory cell infiltration, in conjunction with reduced axonal density and deficits in locomotor recovery after severe SCI. Notably, these effects of BMPR1a are independent of nuclear Smad signaling. In contrast, BMPR1b deletion leads to hyperactive reactive astrocytes and reduced lesion volume. Loss of both BMPR isotypes resulted in normal-appearing reactive astrocytes [58].
BMP also promotes astrocyte differentiation from oligodendrocyte precursor cells (OPCs) at the expense of oligodendrocytes [59]. This occurs through upregulation of Id2 and Id4, which in turn interact with Olig1 and Olig2 [60]. Furthermore, reactive astrocytes in the contused spinal cord themselves secrete BMP, which further limits oligodendrocyte differentiation from OPCs [61]. BMPs also have immunomodulatory roles. In an in vitro co-culture of cortical explants with primary microglia, p38 MAPK is activated in primary microglia after axonal transection and is required for phagocytic activity of microglia to engulf axon debris [62].
TGFβ is another important activator of astrogliosis through the Smad pathway [63]. Upon CNS injury, fibrinogen leaks out of injured vasculature and functions as a carrier of a latent form of TGFβ, which is then activated by astrocytes, leading to Smad2 phosphorylation and glial scarring [64]. On the other hand, microtubule stabilization by Taxol treatment reduces scar formation and promotes axonal regeneration after SCI [65]. The decreased CSPG in Taxol-treated animals is ascribed to decreased nuclear translocation of Smads, leading to dampening of the TGFβ signaling. These results raise the possibility of an involvement of fibrinogen or microtubule-Smad interaction in BMP-mediated astrogliosis.
Conclusion
As in developmental processes, BMPs exert a wide range of influences over both neurons and glia after CNS injury. BMPs can activate a pro-regenerative transcription program in neurons through Smad-mediated canonical pathway, or act locally on cytoskeletal dynamics at the growth cone via non-canonical pathways. The responses of the two subcellular compartments to BMPs may be connected via intra-axonal BMP signalosome trafficking. BMPs also function as an injury signal to coordinate glial responses and neural progenitor cell differentiation at the injury site. Establishing a comprehensive view of the multifaceted roles of BMP signaling after CNS injury is a prerequisite to design BMP-based therapeutic strategies to promote axonal repair and functional recovery.
Figure 1.
Crosstalk of BMP and neurotrophin signaling in neurons. Through differential phosphorylation, Smad1 serves as a converging node to maintain a balanced ratio of ERK/pERK. BMPs signal through C-terminal phosphorylation of Smad1, which then makes nuclear entry and regulates transcription of downstream targets, such as Erk1 and Erk2. Neurotrophin stimulation leads to ERK/MAPK-mediated linker phosphorylation of Smad1, which in turn upregulates DUSP6, an ERK-specific phosphatase, thereby reducing pErk levels and constituting a negative feedback loop. PI3K signaling can induce Smad1, while GSK-mediated linker phosphorylation of Smad1 marks it for proteasome degradation via Smurf1-dependent polyubiquitination pathway. BDNF can also stimulate intra-axonal translation of Smads, which are then translocated to soma to be activated by BMP signalosomes, thereby connecting retrograde signaling of BDNF and BMP.
Figure 2.
Schematic of BMP signaling in promoting axon regeneration. BMP can activate a pro-regenerative transcriptional program through the Smad-mediated canonical pathway. Smad1 works together with histone-modifying enzymes to increase histone acetylation levels, which in turn facilitates Smad1 promoter binding and transcription of regeneration-associated genes (RAGs) by way of local chromatin relaxation. BMP also regulates local cytoskeletal assembly through non-canonical pathways, such as LIMK-cofilin signaling. Retrogradely transported signaling endosomes may control microtubule stability and link cytoskeletal and nuclear responses to BMPs.
Figure 3.
Schematic of BMP signaling in neural progenitor cell differentiation, gliogenesis and scar formation after CNS injury. Astroglial activation plays a dual role: restricting scar size, but also hindering axon regeneration via CSPG deposition. BMPR1a positively and BMPR1b negatively regulates astrocytosis. BMP also drives astroglial differentiation of OPCs, and BMP secreted by reactive astrocytes further limits oligodendrocyte differentiation.
Highlights.
BMP regulates axon growth potential via Smad1-mediated canonical signaling.
BMP mediates cytoskeletal assembly in distal axon via non-canonical pathways.
Retrograde signalosomes couple cytoskeletal and nuclear effects of BMP.
BMP coordinates neuronal and glial injury responses after CNS injury.
Acknowledgements
We apologize to colleagues whose work could not be cited owing to space limitations. J. Z. is supported by NIH (R01EY022409, R01EY022409-01S1) and H. Z. by NIH (NS073596) and the IrmaT. Hirschl / Monique Weill-Caulier Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
• • of outstanding interest
- 1.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 2.Fawcett JW, Schwab ME, Montani L, Brazda N, Muller HW. Defeating inhibition of regeneration by scar and myelin components. Handb Clin Neurol. 2012;109:503–522. doi: 10.1016/B978-0-444-52137-8.00031-0. [DOI] [PubMed] [Google Scholar]
- 3.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 6.Gamez B, Rodriguez-Carballo E, Ventura F. BMP signaling in telencephalic neural cell specification and maturation. Front Cell Neurosci. 2013;7:87. doi: 10.3389/fncel.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins. a critical review. Cell Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Gratacos E, Gavalda N, Alberch J. Bone morphogenetic protein-6 is a neurotrophic factor for calbindin-positive striatal neurons. J Neurosci Res. 2002;70:638–644. doi: 10.1002/jnr.10438. [DOI] [PubMed] [Google Scholar]
- 9.Galter D, Bottner M, Krieglstein K, Schomig E, Unsicker K. Differential regulation of distinct phenotypic features of serotonergic neurons by bone morphogenetic proteins. Eur J Neurosci. 1999;11:2444–2452. doi: 10.1046/j.1460-9568.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 10.Yabe T, Samuels I, Schwartz JP. Bone morphogenetic proteins BMP-6 and BMP-7 have differential effects on survival and neurite outgrowth of cerebellar granule cell neurons. J Neurosci Res. 2002;68:161–168. doi: 10.1002/jnr.10210. [DOI] [PubMed] [Google Scholar]
- 11.Kerrison JB, Lewis RN, Otteson DC, Zack DJ. Bone morphogenetic proteins promote neurite outgrowth in retinal ganglion cells. Mol Vis. 2005;11:208–215. [PubMed] [Google Scholar]
- 12.Kelly CE, Thymiakou E, Dixon JE, Tanaka S, Godwin J, Episkopou V. Rnf165/Ark2C enhances BMP-Smad signaling to mediate motor axon extension. PLoS Biol. 2013;11:e1001538. doi: 10.1371/journal.pbio.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nature neuroscience. 2007;10:598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
- 14. O’Donovan KJ, Ma K, Guo H, Wang C, Sun F, Han SB, Kim H, Wong KJ, Charron J, Zou H, et al. B-RAF kinase drives developmental axon growth and promotes axon regeneration in the injured mature CNS. Journal of Experimental Medicine. 2014 doi: 10.1084/jem.20131780. in press. This study demonstrated that activation of the RAF/MAPK signaling is sufficient to promote long-distance peripheral axon projection of sensonry neurons during development, as well as axon regeneration of both DRG neurons and retinal ganglinotic neurons after injury.
- 15. Finelli MJ, Murphy KJ, Chen L, Zou H. Differential phosphorylation of Smad1 integrates BMP and neurotrophin pathways through Erk/Dusp in axon development. Cell Rep. 2013;3:1592–1606. doi: 10.1016/j.celrep.2013.04.011. This study elucidates molecular mechanisms by which BMP-Smad1 facilitates neurotrophin signaling during sensory axon development. It depicts an integrated BMP and neurotrophin signaling network with multiple converging nodes, and highlights the importance of a balanced ratio of ERK/pERK to ensure the precise connection between sensory neurons and peripheral targets.
- 16.Lonn P, Zaia K, Israelsson C, Althini S, Usoskin D, Kylberg A, Ebendal T. BMP enhances transcriptional responses to NGF during PC12 cell differentiation. Neurochem Res. 2005;30:753–765. doi: 10.1007/s11064-005-6868-6. [DOI] [PubMed] [Google Scholar]
- 17.Althini S, Usoskin D, Kylberg A, Kaplan PL, Ebendal T. Blocked MAP kinase activity selectively enhances neurotrophic growth responses. Mol Cell Neurosci. 2004;25:345–354. doi: 10.1016/j.mcn.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Guha U, Gomes WA, Samanta J, Gupta M, Rice FL, Kessler JA. Target-derived BMP signaling limits sensory neuron number and the extent of peripheral innervation in vivo. Development. 2004;131:1175–1186. doi: 10.1242/dev.01013. [DOI] [PubMed] [Google Scholar]
- 19.Engelhard C, Sarsfield S, Merte J, Wang Q, Li P, Beppu H, Kolodkin AL, Sucov HM, Ginty DD. MEGF8 is a modifier of BMP signaling in trigeminal sensory neurons. Elife. 2013;2:e01160. doi: 10.7554/eLife.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodge LK, Klassen MP, Han BX, Yiu G, Hurrell J, Howell A, Rousseau G, Lemaigre F, Tessier-Lavigne M, Wang F. Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron. 2007;55:572–586. doi: 10.1016/j.neuron.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- 22.McQuarrie IG, Grafstein B. Axon outgrowth enhanced by a previous nerve injury. Arch Neurol. 1973;29:53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- 23.Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acad Sci U S A. 2011;108:E99–E107. doi: 10.1073/pnas.1100426108. This study employed an AAV-based in vivo gene transfer method to specifically target adult DRG neurons. The authors demonstrated that Smad1 activation by AAV-BMP4 promotes ascending sensory axon regeneration following spinal cord injury.
- 25. Saijilafu Hur EM, Liu CM, Jiao Z, Xu WL, Zhou FQ. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun. 2013;4:2690. doi: 10.1038/ncomms3690. The authors demonstrated that PI3K is activated after a peripheral axotomy and that it is required for the conditioning effect in DRG neurons. They also placed Smad1 downstream of the PI3K-GSK3 signaling. Interfering with either PI3K or Smad1 signaling resulted in inhibition of axon regeneration in a peripheral nerve injury model in vivo.
- 26.Ma CH, Brenner GJ, Omura T, Samad OA, Costigan M, Inquimbert P, Niederkofler V, Salie R, Sun CC, Lin HY, et al. The BMP coreceptor RGMb promotes while the endogenous BMP antagonist noggin reduces neurite outgrowth and peripheral nerve regeneration by modulating BMP signaling. J Neurosci. 2011;31:18391–18400. doi: 10.1523/JNEUROSCI.4550-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-Induced HDAC5 Nuclear Export Is Essential for Axon Regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. The authors described an injury-induced HDAC5 nuclear export through a retrograde propagating calcium wave that reaches the soma. This leads to histone acetylation enrichment and actviation of a proregenerative gene-expression program. HDAC5 therefore serves as a transcriptional switch that controls axon regeneration.
- 28. Finelli MJ, Wong JK, Zou H. Epigenetic regulation of sensory axon regeneration after spinal cord injury. J Neurosci. 2013;33:19664–19676. doi: 10.1523/JNEUROSCI.0589-13.2013. The authors established a novel link between axon growth potential and histone acetylation and identified a pro-regenerative transcription module – consisting of Smad1 and histone-modifying enzymes – that regulates induction of regeneration-associated genes (RAGs). Pharmacological manipulation that increases histone acetylation can induce multiple RAGs and promote axon regeneration.
- 29.Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH, Carmichael ST. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okuyama N, Kiryu-Seo S, Kiyama H. Altered expression of Smad family members in injured motor neurons of rat. Brain Res. 2007;1132:36–41. doi: 10.1016/j.brainres.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Ueki Y, Reh TA. Activation of BMP-Smad1/5/8 signaling promotes survival of retinal ganglion cells after damage in vivo. PLoS One. 2012;7:e38690. doi: 10.1371/journal.pone.0038690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. Embo J. 2004;23:4792–4801. doi: 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura I, Endo M, Hata K, Kubo T, Yamaguchi A, Saeki N, Yamashita T. BMP inhibits neurite growth by a mechanism dependent on LIM-kinase. Biochem Biophys Res Commun. 2007;360:868–873. doi: 10.1016/j.bbrc.2007.06.157. [DOI] [PubMed] [Google Scholar]
- 34.Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66:536–549. doi: 10.1016/j.neuron.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Eaton BA, Davis GW. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Babitt JL, Zhang Y, Samad TA, Xia Y, Tang J, Campagna JA, Schneyer AL, Woolf CJ, Lin HY. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:29820–29827. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 38.Samad TA, Rebbapragada A, Bell E, Zhang Y, Sidis Y, Jeong SJ, Campagna JA, Perusini S, Fabrizio DA, Schneyer AL, et al. DRAGON, a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:14122–14129. doi: 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- 39.Conrad S, Genth H, Hofmann F, Just I, Skutella T. Neogenin-RGMa signaling at the growth cone is bone morphogenetic protein-independent and involves RhoA, ROCK, and PKC. J Biol Chem. 2007;282:16423–16433. doi: 10.1074/jbc.M610901200. [DOI] [PubMed] [Google Scholar]
- 40.Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, Mueller BK, Yamashita T. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagihara M, Endo M, Hata K, Higuchi C, Takaoka K, Yoshikawa H, Yamashita T. Neogenin, a receptor for bone morphogenetic proteins. J Biol Chem. 2011;286:5157–5165. doi: 10.1074/jbc.M110.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.da Silva S, Wang F. Retrograde neural circuit specification by target-derived neurotrophins and growth factors. Curr Opin Neurobiol. 2011;21:61–67. doi: 10.1016/j.conb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ji SJ, Jaffrey SR. Intra-axonal Translation of SMAD1/5/8 Mediates Retrograde Regulation of Trigeminal Ganglia Subtype Specification. Neuron. 2012;74:95–107. doi: 10.1016/j.neuron.2012.02.022. This is the first study to link BDNF signaling and intra-axonal translation of Smads. The authors further show that locally translated Smads are then translocated to the soma and activated by BMP signaling endosomes.
- 44.Wang X, Shaw WR, Tsang HT, Reid E, O'Kane CJ. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat Neurosci. 2007;10:177–185. doi: 10.1038/nn1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith RB, Machamer JB, Kim NC, Hays TS, Marques G. Relay of retrograde synaptogenic signals through axonal transport of BMP receptors. J Cell Sci. 2012;125:3752–3764. doi: 10.1242/jcs.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fassier C, Hutt JA, Scholpp S, Lumsden A, Giros B, Nothias F, Schneider-Maunoury S, Houart C, Hazan J. Zebrafish atlastin controls motility and spinal motor axon architecture via inhibition of the BMP pathway. Nat Neurosci. 2010;13:1380–1387. doi: 10.1038/nn.2662. [DOI] [PubMed] [Google Scholar]
- 47.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 48.Barnabe CC, LeClercq SA, Fitzgerald AA. The development of inflammatory arthritis and other rheumatic diseases following stem cell transplantation. Semin Arthritis Rheum. 2009;39:55–60. doi: 10.1016/j.semarthrit.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- 51.Setoguchi T, Yone K, Matsuoka E, Takenouchi H, Nakashima K, Sakou T, Komiya S, Izumo S. Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–225. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Leong SY, Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur J Neurosci. 2005;22:1895–1906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- 53.Xiao Q, Du Y, Wu W, Yip HK. Bone morphogenetic proteins mediate cellular response and, together with Noggin, regulate astrocyte differentiation after spinal cord injury. Exp Neurol. 2010;221:353–366. doi: 10.1016/j.expneurol.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Fuller ML, DeChant AK, Rothstein B, Caprariello A, Wang R, Hall AK, Miller RH. Bone morphogenetic proteins promote gliosis in demyelinating spinal cord lesions. Ann Neurol. 2007;62:288–300. doi: 10.1002/ana.21179. [DOI] [PubMed] [Google Scholar]
- 55.Setoguchi T, Nakashima K, Takizawa T, Yanagisawa M, Ochiai W, Okabe M, Yone K, Komiya S, Taga T. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Enzmann GU, Benton RL, Woock JP, Howard RM, Tsoulfas P, Whittemore SR. Consequences of noggin expression by neural stem, glial, and neuronal precursor cells engrafted into the injured spinal cord. Exp Neurol. 2005;195:293–304. doi: 10.1016/j.expneurol.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 57.Matsuura I, Taniguchi J, Hata K, Saeki N, Yamashita T. BMP inhibition enhances axonal growth and functional recovery after spinal cord injury. J Neurochem. 2008;105:1471–1479. doi: 10.1111/j.1471-4159.2008.05251.x. [DOI] [PubMed] [Google Scholar]
- 58.Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, McGuire TL, Stupp SI, Kessler JA. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30:1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka T, Ueno M, Yamashita T. Engulfment of axon debris by microglia requires p38 MAPK activity. J Biol Chem. 2009;284:21626–21636. doi: 10.1074/jbc.M109.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Susarla BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-beta-mediated induction of chondroitin sulfate proteoglycans. J Neurochem. 2011;119:868–878. doi: 10.1111/j.1471-4159.2011.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]