Abstract
The axon initial segment (AIS) is a structurally and molecularly unique neuronal compartment of the proximal axon that functions as both a physiological and physical bridge between the somatodendritic and axonal domains. The AIS has two main functions: to initiate action potentials and to maintain neuronal polarity. The cytoskeletal scaffold ankyrinG is responsible for these functions and clusters ion channels at the AIS. Recent studies reveal how the AIS forms and remarkable diversity in its structure, function, and composition that may be modulated by neuronal activity and post-translational modifications of AIS proteins. Furthermore, AIS proteins have been implicated in a variety of human diseases. Here, we discuss these findings and what they teach us about the dynamic AIS.
Introduction
A mature neuron is structurally and functionally polarized, typically with a single long axon and several dendrites. Neuronal polarity is essential for the directional flow of action potentials from dendrites to axons. The axon initial segment (AIS) is a highly specialized neuronal compartment in the proximal axon (Figure 1) [1–3] that has key roles as both a ‘gatekeeper’ separating the somatodendritic and axonal domains, and a spike initiation zone where action potentials (APs) are generated. The AIS restricts membrane proteins and even lipids to axons or dendrites [4,5], while simultaneously regulating vesicular trafficking by excluding dendritic cargoes from axons [6–8]. Although all AIS have ion channels, cell adhesion molecules, extracellular matrix molecules and cytoskeletal scaffolds, the defining molecular component of the AIS is the cytoskeletal protein ankyrinG (AnkG) since it is responsible for clustering Na+ channels and maintaining neuronal polarity; loss of AnkG blocks Na+ channel clustering and causes axons to acquire the characteristics of dendrites [9,10]. While AnkG’s role as an ‘ion channel accumulator’ is fairly well understood, how AnkG regulates neuronal polarity remains unknown. Recently, the first human mutations in AnkG were reported and shown to be associated with severe intellectual disability, attention deficit hyperactivity disorder, and autism[•11].
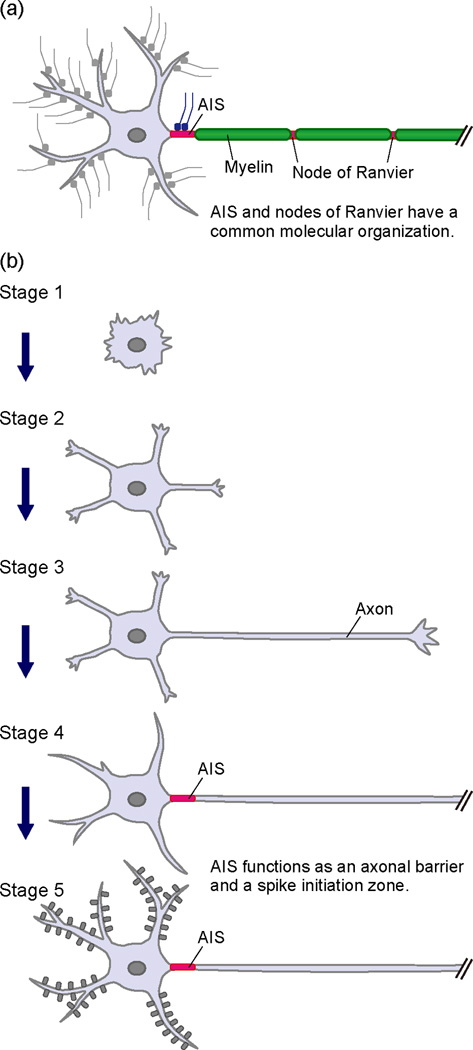
Figure 1.
The axon initial segment (AIS) in the most proximal part of the axon. (a) Schema of a neuron with a myelinated axon. Specific types of ion channels, receptors, adhesion molecules and molecular scaffolds are enriched in both the AIS and nodes of Ranvier. (b) Establishment of neuronal polarity in cultured hippocampal neurons. Cultured pyramidal neurons from a rodent hippocampus acquire their characteristic polarized morphology in five successive stages. The first step in neuronal polarization is initial axon formation in the transition from stage 2 to stage 3. The AIS is formed from stage 3 to stage 4. The AIS maintains neuronal polarity and regulates action potential initiation.
Studies of AIS physiology (reviewed in [12] and [13]) and advances in the characterization and description of AIS protein components and their interactions (reviewed in [1] and [3]) reveal remarkable diversity in AIS structure and function. For example, AIS may have unique compositions of ion channels, accessory proteins, and even modulatory synaptic inputs, each reflecting the unique physiologies of different neuronal cell types and the circuits to which they belong. Furthermore, although the AIS was once thought to be uniform and static in nature, recent evidence shows that it may be dynamically modified to control excitability [14,15] thereby expanding diversity even more and in response to different states of excitability or disease. Here, we discuss recent advances in our understanding of AIS diversity and how the AIS is assembled. We consider mechanisms, both intrinsic and extrinsic, diseases, and injuries that can modulate AIS structure and function.
AIS diversity
The AIS integrates somatodendritic inputs and generates the AP [12]. Different kinds of voltage-gated channels and modulatory proteins found at the AIS underlie unique neuronal and axonal physiologies. For example, different classes of neurons have distinct types, distributions, and/or combinations of AIS Na+, K+, and Ca2+ channels. While most excitatory neurons have Nav1.2 and Nav1.6 [16,17], some neurons, including cortical inhibitory neurons, motor neurons, retinal ganglion cells, and some retinal bipolar cells, have Nav1.1 [18–21]; loss-of-function mutations in Nav1.1 cause Dravet syndrome, characterized by decreased excitability of cortical interneurons leading to seizures, epilepsy, and cognitive dysfunction [22].
Different Na+ and K+ channels can occupy distinct subdomains of the AIS. In cortical excitatory neurons, Nav1.2 and Nav1.6 are found in the proximal and distal regions of the AIS, respectively [16]; Kv1.1, Kv1.2, and Kv1.4 K+ channels are enriched in the distal segment of cortical neuron AIS and colocalize with Nav1.6 [19,23]. The Na+ channels Nav1.1 and Nav1.6 occupy proximal and distal AIS domains, respectively, in retinal ganglion cells and motor neurons [19,21]. Since neuronal Na+ channels all share a common ankyrin-binding motif, it is unclear what molecular mechanisms regulate the differential distribution of AIS Na+ channels. Furthermore, the genetic mechanisms controlling expression of AIS ion channels are unknown.
The synaptic scaffolding protein PSD-93 is enriched at the AIS and clusters Kv1 channels [24]. However, the mechanisms responsible for AIS recruitment of PSD-93 remain unknown. It is interesting that the Kv1 channel and PSD-93 associated cell adhesion molecule Caspr2 (CNTNAP2) is also enriched at the AIS [24] and is a major susceptibility gene for autism spectrum disorder, schizophrenia, major depression, epilepsy, and impaired language development [25,26].
Some interneurons have AIS T-type Ca2+ channels. These channels are modulated by dopamine, and control spontaneous burst generation [27]. Although the physiological evidence for these channels at the AIS of cartwheel neurons is very strong, direct immunostaining has not been observed. It is not known if Ca2+ channels are found at all AIS, and the molecular mechanisms regulating their AIS clustering are unknown.
Nav channel function is modulated by accessory Na+ channel β-subunits. For example, Navβ4, thought to underlie resurgent current and open channel block, is found at the AIS of fast-spiking interneurons, cerebellar Purkinje neurons, dorsal root ganglia, and motor neurons [28]; one common feature of these cell types is their ability to sustain high AP firing rates. Interestingly, AIS clustering of Navβ4 occurs through a covalent disulfide bond with the Nav1α subunit rather than binding AnkG like most other AIS proteins. We speculate that other modulatory AIS proteins will be discovered, and their AIS clustering will occur through interactions with ‘core’ AIS proteins whose activity they modulate.
Besides differences in ion channel composition, many cortical pyramidal neuron AIS receive GABAergic synaptic input by chandelier neurons [29]. Chandelier neurons are fast-spiking cortical interneurons that modulate AIS excitability. Some studies suggest they play an excitatory role [30], and others suggest that altered AIS synaptic input may contribute to schizophrenia [31]. Recent advances in genetic methods to manipulate chandelier neurons provide much needed tools to elucidate the functions of these enigmatic cells and the molecular mechanisms underlying their AIS interactions [•32].
Neuronal polarity and axon initial segment assembly
The AIS is first detected around three to four days in cultured hippocampal neurons, a well-established model used to study neuronal polarity (Figure 1b) [33]. As they mature, these neurons undergo dramatic changes in morphology. Banker and colleagues described this process and divided the morphological events into five stages [34]. Shortly after attachment to the substratum, a neuron displays intense lamellipodial and filopodial protrusive activity (stage 1). These protrusions then develop into several short immature neurites (stage 2). At this stage, neurons still appear unpolarized. All neurites alternate phases of elongation and retraction and are approximately equal in length. Next, one of the immature neurites rapidly grows into a long neurite, which soon acquires axonal characteristics (stage 3). A few days after the formation of the axon, the remaining neurites slowly elongate to become dendrites (stage 4). The AIS, defined by AnkG clustering, is first observed between stages 3 and 4, indicating that axon specification occurs independently of AnkG clustering. This is consistent with in vivo studies demonstrating axon specification by cortical neurons in the absence of AnkG [••35]. Axons and dendrites continue to mature until neurons harboring dendritic spines form synaptic contacts and establish a neuronal network (stage 5). The AIS distinguishes the axon from the rest of the neuron and functions to maintain neuronal polarity.
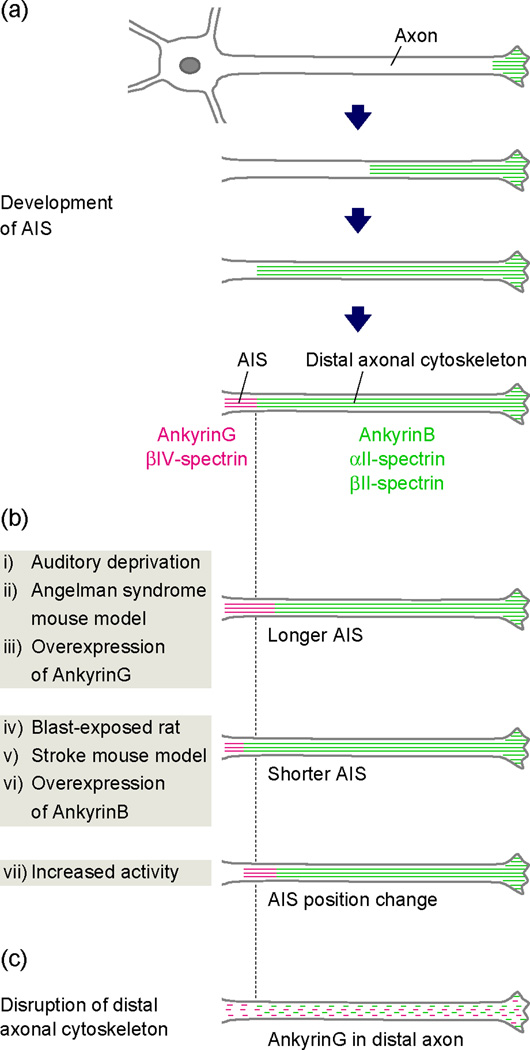
While many of the molecular events regulating axon specification and these early morphological changes have been described [33], the mechanisms regulating AnkG clustering at the AIS have remained elusive. The earliest efforts to identify AnkG’s AIS clustering mechanism assumed that some motif, or protein module, in AnkG was responsible for its active recruitment to the proximal axon. However, deletion studies failed to reveal any single critical motif [36]. Instead, it was concluded that AnkG’s AIS localization depends on the cooperative action of multiple protein modules that work together for AnkG clustering. More recently, we showed the mechanism for AnkG clustering is a surprising example of domain assembly by exclusion rather than recruitment (Figure 2a) [••35]. After neurons break symmetry (stage 2/3, Figure 1b), neurons assemble an axonal cytoskeleton, comprised of ankyrinB (AnkB), αII-spectrin, and βII-spectrin. This cytoskeleton progressively fills the axon from its distal growing end back towards the cell body, and defines a boundary limiting AnkG to the remaining proximal axon that will become the AIS. Suppression of AnkB, αII-spectrin, or βII-spectrin expression in cultured neurons disrupts the intra-axonal boundary and permits AnkG to be found in the distal axon (Figure 2c). Furthermore, AnkG clustering is impaired and the AIS is disrupted in the cortex and hippocampus of αII-spectrin- and βII-spectrin-deficient mice [••35]. In further support of the concept of intra-axonal boundaries, we recently showed that βII-spectrin-deficient myelinated dorsal root ganglion axons fail to establish the paranodal, lateral diffusion barriers that normally restrict Kv1 channels to the juxtaparanodes flanking nodes of Ranvier [•37]. In control axons, βII-spectrin is highly enriched at paranodes [38]. Together, these results suggest a model where intra-axonal boundaries are established by submembranous spectrin-based cytoskeletons, and these boundaries function to establish and maintain specialized membrane domains like AIS, juxtaparanodes, and nodes of Ranvier.
Figure 2.
The formation and plasticity of the AIS. (a) After the axon specification, an AnkB/αII-spectrin/βII-spectrin-based distal cytoskeleton is assembled in the distal axon and progressively fills the axon toward the cell body. Later expression of AnkG and exclusion from the distal axonal cytoskeleton results in the accumulation of AnkG in the remaining proximal axon that becomes the AIS. (b) The AIS is a new target of intrinsic plasticity. In neurons from the nucleus magnocellularis of chicks, auditory deprivation leads to an increase in AIS length and increased neuronal excitability (i). The AIS is longer in a mouse model of Angelman syndrome (ii). Overexpression of AnkG induces a longer AIS in cultured neurons (iii). Exposure to a single blast wave decreases AIS length in rats (iv). AIS length is decreased within the peri-infarct cortex after white matter stroke in mice (v). Overexpression of AnkB induces a shorter AIS in cultured neurons (vi). Increased neuronal activity shifts AIS location and leads to reduced neuronal excitability (vii). (c) Loss of the intra-axonal boundary by disruption of the distal axonal cytoskeleton blocks AIS assembly and permits AnkG to be found in the distal axon.
Why is AnkG excluded from the αII-spectrin/βII-spectrin/AnkB- based cytoskeleton? Recently, Xu et al. [•39] described the structure and organization of the distal cytoskeleton using super-resolution microscopy. They described a cytoskeleton consisting of actin, spectrin, and AnkB that forms a periodic network of ring-like structures that wrap around the circumference of the axon and are evenly spaced along axonal shafts. We speculate that this remarkably regular and periodic structure may exclude AnkG/βIV-spectrin complexes from integrating into the network. Indeed, it is not clear that βIV-spectrin functions as a canonical tetrameric spectrin comprised of two α- and two β-spectrins, since no α-spectrin has been identified at the AIS, and the major splice variant of βIV-spectrin in mature neurons lacks the actin-binding domain [40]. These observations argue that the intra-axonal boundary is not a strict boundary in the sense that AnkG is excluded from entering the axon (after all, AnkG is a major component of nodes), but rather the boundary reflects a submembranous cytoskeleton from which AnkG is excluded. The only location where AnkG participates in a submembranous cytoskeleton is where AnkB, αII-spectrin, and βII-spectrin are not found.
AIS plasticity
The brain constantly changes by experience, age, and disease. Although plasticity of synaptic connections between neurons has received the most attention, recent studies demonstrate the AIS is also plastic and may change in response to activity and disease (Figure 2b). For example, in avian nucleus magnocellularis neurons, auditory deprivation caused by removal of the cochlea increases the length of the AIS, thereby augmenting neuronal excitability [15]. Similar morphological changes have been reported in rodent disease and injury models, and in manipulated cultured neurons. In a mouse model of Angelman syndrome where the resting membrane potential is more hyperpolarized, the AIS is longer [41], and these length changes can be reversed by restoring the membrane potential [42]. In contrast, rats exposed to a single blast wave, designed to mimic blast-induced mild traumatic brain injury, experienced memory impairment and had significant reductions in AIS length in the cortex and hippocampus [43]. A computational model suggested that such a reduction in length could result in increased interspike interval, reflecting decreased neuronal excitability. Reductions in AIS length were also reported within peri-infarct cortex after white matter stroke [44]. Alternatively, increasing the activity of cultured excitatory (but not inhibitory) hippocampal neurons shifts the entire AIS to a more distal position in the axon, resulting in decreased neuronal excitability [14]. These observations suggest increased neuronal excitability (due to increased glutamate release after traumatic brain injury or stroke) or decreased neuronal excitability (due to synaptic deprivation or hyperpolarizing shifts in membrane potential) result in chronic homeostatic changes reflected by decreases or increases in AIS length, respectively. It is unknown whether AIS length changes are accompanied by changes in AIS channel density or composition.
What molecular mechanisms could account for a change in AIS length? In cultured hippocampal neurons, overexpression of AnkG shifts the intra-axonal boundary away from the cell body, resulting in a longer AIS [••35]. Conversely, overexpression of the distal axonal cytoskeletal protein AnkB causes a proximal shift of the intra-axonal boundary and a shorter AIS [••35]. Thus, the simplest means to change AIS length is to modify the position of the intra-axonal boundary. This could happen by changes in expression level of AIS or distal cytoskeletal proteins, or by dynamic modification of individual elements of the cytoskeletal network such that a very small length change in a given protein can result in large changes when summed throughout the axon.
Post-translational modifications of AIS proteins
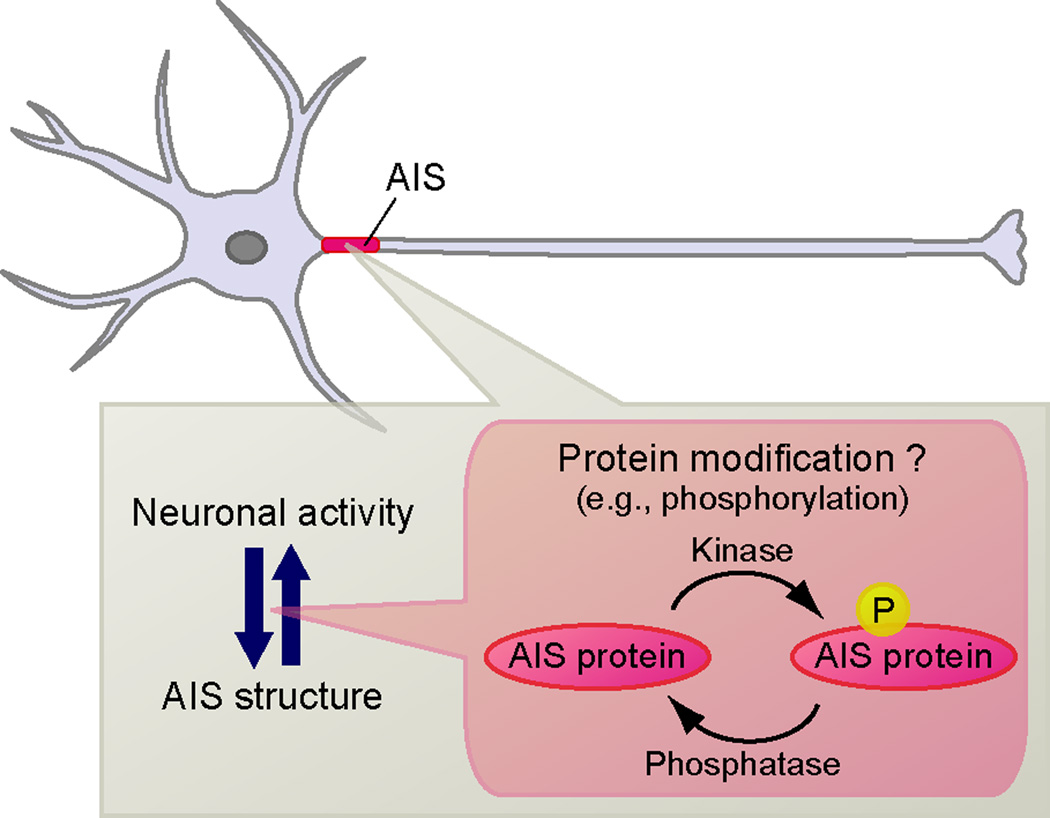
Although no signaling pathways or posttranslational modifications have been described that change the amount or length of AnkG or AnkB, the Ca2+- and calmodulin-dependent protein phosphatase calcineurin was recently shown to mediate activity-dependent AIS relocation [•45]. Interestingly, in mushroom body neurons of the Drosophila central brain, cyclin dependent kinase 5 (Cdk5) regulates the length of an AIS-like compartment [46]. How calcineurin and Cdk5 regulate these changes is unknown.
Besides regulation of AIS position and size, post-translational modifications of AIS proteins have been reported to modulate AIS structure and function (Figure 3). For example, AnkG and Neurofascin-186 are palmitoylated (palmitoylation is a reversible modification that promotes membrane association) and palmitoylation-deficient AnkG is unable to cluster at the AIS [47,48]. Casein kinase 2 (CK2) is highly enriched at the AIS and phosphorylates Na+ channels, increasing their affinity for AnkG [49]. Furthermore, CK2 inhibition impairs the tethering of Na+ channels and AnkG in the AIS [50].
Figure 3.
Dynamic regulation of AIS proteins. Neuronal activity can ‘tune’ AIS structure, and vice versa. Post-translational modifications (e.g. phosphorylation) of AIS proteins may play key roles to modulate AIS assembly, structure and function.
Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been reported at the AIS of cerebellar Purkinje neurons, binds to βIV-spectrin, and regulates Na+ channel function by direct phosphorylation of the channel itself [51]. The phosphorylation of the Kvβ2 subunit of Kv1 channels by Cdk2 and Cdk5 negatively regulates Kvβ2–EB1 interaction, and Kvβ2 phosphorylation is critical for the AIS membrane insertion of Kv1 K+ channels [52]. However, not all modifications promote interactions among AIS proteins. For example, the tyrosine phosphorylation of neurofascin and NrCAM abolishes their ankyrin-binding activity [53]. Together, these observations suggest the AIS may be a ‘hotspot’ for protein phosphorylation. Indeed, many phospho-antibodies have been shown to bind non-specifically to the AIS [54].
Glycogen synthase kinase-3 (GSK-3) is also found at the AIS, and although it is not exclusive or even enriched here, it is emerging as a key regulator of AIS structure and function. GSK-3 phosphorylates AIS β-catenin, which in turn functions to maintain high levels of Na+ channels at the AIS [•55]. Furthermore, GSK-3 also regulates the interaction between AIS Na+ channels and fibroblast growth factor 14 (FGF14) [56] and the histone deacetylase 6 (HDAC6) which can function as a tubulin deacetylase [57]. Remarkably, inhibition of HDAC6 interferes with AIS development [58]. These results suggest that GSK-3 may regulate AIS development through modification of microtubules by HDAC6. Microtubule-AnkG interactions have been reported through the microtubule end-binding proteins EB1 and EB3 [•59]. Interestingly, GSK-3β is also critical for axon formation through the phosphorylation of collapsin response mediator protein-2 (CRMP-2) [60]. In Caenorhabditis elegans, UNC-44 (ankyrin) was reported to direct UNC-33 (CRMP) to axons, and these proteins organize microtubules and localize kinesins to regulate polarized axon-dendrite sorting [61]. Thus, the molecular mechanisms involved in axon formation may also be used at later stages in AIS development and function.
Conclusions
Although the AIS’s importance for action potential initiation has long been appreciated, AIS diversity, its role in polarity and disease, and its plasticity have only recently been recognized. AIS size, location, and molecular composition can change in response to developmental programs and injury. It remains to be seen if similar dynamic modifications to the AIS occur in the course of normal learning and behavior.
Highlights.
The axon initial segment (AIS) maintains neuronal polarity and regulates action potential initiation.
AIS have diverse compositions.
The distal axonal cytoskeleton is crucial for AIS formation.
Neuronal activity affects AIS structure and function.
Post-translational modification of AIS proteins may modulate AIS structure and function.
Acknowledgements
We thank Dr. Keiichiro Susuki, Kae-Jiun Chang and Tammy Szu-Yu Ho (Baylor College of Medicine, TX) for suggestions. Work in the authors’ laboratory was supported by NIH grants NS044916 and NS069688, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. We apologize for the omission of citations or discussion of relevant papers due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- 2.Grubb MS, Burrone J. Building and maintaining the axon initial segment. Curr Opin Neurobiol. 2010;20:481–488. doi: 10.1016/j.conb.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leterrier C, Dargent B. No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin Cell Dev Biol. 2013 doi: 10.1016/j.semcdb.2013.11.001. in press. [DOI] [PubMed] [Google Scholar]
- 4.Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol. 2003;5:626–632. doi: 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- 5.Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep. 2012;2:1546–1553. doi: 10.1016/j.celrep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, Schultz C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci U S A. 2009;106:17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iqbal Z, Vandeweyer G, van der Voet M, Waryah AM, Zahoor MY, Besseling JA, Roca LT, Vulto-van Silfhout AT, Nijhof B, Kramer JM, et al. Homozygous and heterozygous disruptions of ANK3: at the crossroads of neurodevelopmental and psychiatric disorders. Hum Mol Genet. 2013;22:1960–1970. doi: 10.1093/hmg/ddt043. This is the first report of human mutations in AnkG and the diseases that result from these mutations.
- 12.Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Bender KJ, Trussell LO. The Physiology of the Axon Initial Segment. Annu Rev Neurosci. 2012;35:249–265. doi: 10.1146/annurev-neuro-062111-150339. [DOI] [PubMed] [Google Scholar]
- 14.Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;65:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 17.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puthussery T, Venkataramani S, Gayet-Primo J, Smith RG, Taylor WR. Nav1.1 channels in axon initial segments of bipolar cells augment input to magnocellular visual pathways in the primate retina. J Neurosci. 2013;33:16045–16059. doi: 10.1523/JNEUROSCI.1249-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- 20.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duflocq A, Le Bras B, Bullier E, Couraud F, Davenne M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol Cell Neurosci. 2008;39:180–192. doi: 10.1016/j.mcn.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol. 2008;18:307–313. doi: 10.1016/j.conb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci. 2008;28:5731–5739. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji W, Li T, Pan Y, Tao H, Ju K, Wen Z, Fu Y, An Z, Zhao Q, Wang T, et al. CNTNAP2 is significantly associated with schizophrenia and major depression in the Han Chinese population. Psychiatry Res. 2013;207:225–228. doi: 10.1016/j.psychres.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender KJ, Uebele VN, Renger JJ, Trussell LO. Control of firing patterns through modulation of axon initial segment T-type calcium channels. J Physiol. 2012;590:109–118. doi: 10.1113/jphysiol.2011.218768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buffington SA, Rasband MN. Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier. J Neurosci. 2013;33:6191–6202. doi: 10.1523/JNEUROSCI.4051-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inan M, Blazquez-Llorca L, Merchan-Perez A, Anderson SA, DeFelipe J, Yuste R. Dense and overlapping innervation of pyramidal neurons by chandelier cells. J Neurosci. 2013;33:1907–1914. doi: 10.1523/JNEUROSCI.4049-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 31.Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118–127. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. This paper describes chandelier neurons, their origin, and how to genetically label them for future studies of these cells.
- 33.Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 35. Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. This study demonstrates that a distal axonal cytoskeleton, comprised of ankyrin B, αII-spectrin, and βII-spectrin, is crucial for the AIS formation. This study is first evidence to reveal the molecular mechanism by which AnkG is accumulated to the segment of proximal axons.
- 36.Zhang X, Bennett V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J Cell Biol. 1998;142:1571–1581. doi: 10.1083/jcb.142.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang C, Susuki K, Zollinger DR, Dupree JL, Rasband MN. Membrane domain organization of myelinated axons requires betaII spectrin. J Cell Biol. 2013;203:437–443. doi: 10.1083/jcb.201308116. This paper provides additional evidence that as for the AIS, submembranous spectrin-based cytoskeletons function as intra-axonal boundaries in myelinated axons and contribute to the assembly of axonal subdomains.
- 38.Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu K, Zhong G, Zhuang X. Actin, Spectrin, and Associated Proteins Form a Periodic Cytoskeletal Structure in Axons. Science. 2013;339:452–456. doi: 10.1126/science.1232251. This study shows the distal axonal cytoskeleton and AIS cytoskeleton precisely by using super-resolution microscopy. Actin, spectrin, and adducin form a coordinated ladder-like lattice submembrane structure in axons.
- 40.Komada M, Soriano P. βIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaphzan H, Buffington SA, Jung JI, Rasband MN, Klann E. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of angelman syndrome. J Neurosci. 2011;31:17637–17648. doi: 10.1523/JNEUROSCI.4162-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaphzan H, Buffington SA, Ramaraj AB, Lingrel JB, Rasband MN, Santini E, Klann E. Genetic reduction of the alpha1 subunit of Na/K-ATPase corrects multiple hippocampal phenotypes in Angelman syndrome. Cell Rep. 2013;4:405–412. doi: 10.1016/j.celrep.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baalman KL, Cotton RJ, Rasband SN, Rasband MN. Blast wave exposure impairs memory and decreases axon initial segment length. J Neurotrauma. 2013;30:741–751. doi: 10.1089/neu.2012.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinman JD, Rasband MN, Carmichael ST. Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke. 2013;44:182–189. doi: 10.1161/STROKEAHA.112.668749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Evans MD, Sammons RP, Lebron S, Dumitrescu AS, Watkins TB, Uebele VN, Renger JJ, Grubb MS. Calcineurin signaling mediates activity-dependent relocation of the axon initial segment. J Neurosci. 2013;33:6950–6963. doi: 10.1523/JNEUROSCI.0277-13.2013. The authors show that Ca2+ flux through L-type voltage-gated Ca2+ channels activate the downstream phosphatase calcineurin to regulate AIS location in the axon. This is the first evidence of a phosphatase regulating AIS properties.
- 46.Trunova S, Baek B, Giniger E. Cdk5 regulates the size of an axon initial segment-like compartment in mushroom body neurons of the Drosophila central brain. J Neurosci. 2011;31:10451–10462. doi: 10.1523/JNEUROSCI.0117-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren Q, Bennett V. Palmitoylation of neurofascin at a site in the membrane-spanning domain highly conserved among the L1 family of cell adhesion molecules. J Neurochem. 1998;70:1839–1849. doi: 10.1046/j.1471-4159.1998.70051839.x. [DOI] [PubMed] [Google Scholar]
- 48.He M, Jenkins P, Bennett V. Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly. J Biol Chem. 2012;287:43995–44005. doi: 10.1074/jbc.M112.417501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bréchet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sánchez-Ponce D, Muñoz A, Garrido JJ. Casein kinase 2 and microtubules control axon initial segment formation. Mol Cell Neurosci. 2011;46:222–234. doi: 10.1016/j.mcn.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, et al. A βIV-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vacher H, Yang JW, Cerda O, Autillo-Touati A, Dargent B, Trimmer JS. Cdk-mediated phosphorylation of the Kvβ2 auxiliary subunit regulates Kv1 channel axonal targeting. J Cell Biol. 2011;192:813–824. doi: 10.1083/jcb.201007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buffington SA, Sobotzik JM, Schultz C, Rasband MN. IκBα is not required for axon initial segment assembly. Mol Cell Neurosci. 2012;50:1–9. doi: 10.1016/j.mcn.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tapia M, Del Puerto A, Puime A, Sanchez-Ponce D, Fronzaroli-Molinieres L, Pallas-Bazarra N, Carlier E, Giraud P, Debanne D, Wandosell F, et al. GSK3 and β-catenin determines functional expression of sodium channels at the axon initial segment. Cell Mol Life Sci. 2013;70:105–120. doi: 10.1007/s00018-012-1059-5. This paper provides compelling evidence that phosphorylation of β-catenin by GSK-3 regulates AIS structure and function.
- 56.Shavkunov AS, Wildburger NC, Nenov MN, James TF, Buzhdygan TP, Panova-Elektronova NI, Green TA, Veselenak RL, Bourne N, Laezza F. The fibroblast growth factor 14•voltage-gated sodium channel complex is a new target of glycogen synthase kinase 3 (GSK3) J Biol Chem. 2013;288:19370–19385. doi: 10.1074/jbc.M112.445924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S, Owens GC, Makarenkova H, Edelman DB. HDAC6 regulates mitochondrial transport in hippocampal neurons. PLoS One. 2010;5:e10848. doi: 10.1371/journal.pone.0010848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tapia M, Wandosell F, Garrido JJ. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS One. 2010;5:e12908. doi: 10.1371/journal.pone.0012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leterrier C, Vacher H, Fache MP, d'Ortoli SA, Castets F, Autillo-Touati A, Dargent B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc Natl Acad Sci U S A. 2011;108:8826–8831. doi: 10.1073/pnas.1018671108. This paper reveals the end binding proteins EB1 and EB3 may play key roles in linking AnkG to microtubules. This is important because the results link AnkG more directly to regulation of axonal trafficking.
- 60.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat Neurosci. 2012;15:48–56. doi: 10.1038/nn.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]