Abstract
Background
Despite their high rates of depression, homebound older adults have limited access to evidence-based psychotherapy. The purpose of this paper was to report both depression and disability outcomes of telehealth problem-solving therapy (tele-PST via Skype video call) for low-income homebound older adults over 6 months postintervention.
Methods
A 3-arm randomized controlled trial compared the efficacy of tele-PST to in-person PST and telephone care calls with 158 homebound individuals who were aged 50+ and scored 15+ on the 24-item Hamilton Rating Scale for Depression (HAMD). Treatment effects on depression severity (HAMD score) and disability (score on the WHO Disability Assessment Schedule [WHODAS]) were analyzed using mixed-effects regression with random intercept models. Possible reciprocal relationships between depression and disability were examined with a parallel-process latent growth curve model.
Results
Both tele-PST and in-person PST were efficacious treatments for low-income homebound older adults; however the effects of tele-PST on both depression and disability outcomes were sustained significantly longer than those of in-person PST. Effect sizes (dGMA-raw) for HAMD score changes at 36 weeks were 0.68 for tele-PST and 0.20 for in-person PST. Effect sizes for WHODAS score changes at 36 weeks were 0.47 for tele-PST and 0.25 for in-person PST. The results also supported reciprocal and indirect effects between depression and disability outcomes.
Conclusions
The efficacy and potential low cost of tele-delivered psychotherapy show its potential for easy replication and sustainability to reach a large number of underserved older adults and improve their access to mental health services.
Keywords: depression, disability, tele-psychotherapy, homebound older adults
INTRODUCTION
Because of their chronic medical conditions, mobility impairment, and social isolation, homebound older adults are more vulnerable to depression and suicidal ideation than their mobile peers. Previous studies found that 13.5% of homecare patients (65+ years) had major depressive disorder and 13% reported suicidal ideation.[1, 2] Other studies also found the rates of clinically significant depression (PHQ-9 ≥ 10[3, 4]) to be 11–12% among homebound older adults.[3, 4] When younger homebound persons (50–65 years) were included, the rate was 17%.[5] Along with individual suffering, healthcare spending often doubles or triples for people with chronic conditions as well as disability and depression, compared to those with just chronic conditions.[6–8]
Pharmacotherapy is a common treatment for depression in homebound older adults.[9] However, many older adults on antidepressants have limited response to medication alone and remain symptomatic, due in part to subtherapeutic dosing, inadequate monitoring by the clinicians, and poor patient adherence.[10, 11] More important, pharmacotherapy does not provide coping skills training for low-income homebound older adults with multiple life stressors that are associated with social isolation, managing chronic medical conditions and formal and informal care arrangements, and financial difficulties.[11, 12] Thus, depression remains insufficiently treated and persistent in the majority of these seniors.
Most older adults prefer psychotherapy to pharmacotherapy for their depression, especially given that they already take multiple medications for their chronic illnesses.[13, 14] However, referring homebound older adults to specialty mental health services for psychotherapy seldom succeeds due to inaccessibility, shortage of geriatric mental health providers, and cost.[15] Providing in-person psychotherapy is especially expensive for homebound patients, given the costs associated with travel—of clinicians to homes or disabled patients to offices. Although a handful of successful, longstanding home-based mental health services for older adults are available in select localities,[16] in-home psychotherapy services are not widely available in most communities.
Synchronous telemental health services (i.e., live, interactive videoconferenced assessment and treatment) have become important parts of mental health service delivery in recent years, and systematic reviews found no significant differences in treatment efficacy for psychotherapy administered via telehealth as compared to standard nontelehealth approaches.[17, 18] Especially for homebound persons who have limited access to in-person psychotherapy, videoconferenced sessions in which they can interact with a therapist can offer benefits comparable to those of in-person sessions and improve their access to evidence-based psychotherapy. However, home-based telemental health service delivery is still an emerging area, and there have been relatively few controlled studies of outcomes in the geriatric population.[19, 20]
The authors recently completed a randomized controlled trial (RCT) to test the efficacy of in-home, telehealth problem-solving therapy (tele-PST via Skype video call) compared to in-person PST and telephone support call (care call) for depressed, low-income home-bound older adults. Our previous papers[21, 22] reported tele-PST’s feasibility, acceptability, and short-term efficacy for treating depression. The purpose of this paper was to report longer term (i.e., 24 and 36 weeks after baseline assessment) outcomes of tele-PST for both depression and disability. The study hypotheses were tele-PST and in-person PST will be more efficacious than care call in reducing (H1) depressive symptoms and (H2) disability over 6 months after intervention. Given the previous research findings of significant mutual links between the onset and deterioration of disability and the onset and deterioration of depression over time,[23, 24] we also examined possible reciprocal and indirect (i.e., mediation) effects of depression and disability outcomes.
MATERIALS AND METHODS
PARTICIPANTS AND SETTINGS
The participants were English-speaking, non-Hispanic White, Black/African American, and Hispanic homebound (as defined in Medicare[25]) adults aged 50 or older, who were served by a large home-delivered meal (HDM) program and four other aging-service agencies in Central Texas. The inclusion criterion for depression was HAMD ≥ 15 (24-item Hamilton Rating Scale for Depression[26, 27]). The exclusion criteria, including high suicide risk, possible dementia (assessed with the Mini-Cog[28]), bipolar disorder, and psychotic disorder, are described in our earlier paper.[21] Written informed consent, approved by the first author’s university institutional review board, was obtained from each participant after the study procedures had been fully explained.
Of the 238 persons referred by their case managers during the 30-month recruitment period, 74 were found ineligible because of low HAMD scores or other mental health problems (e.g., bipolar or psychotic disorder); seven had possible dementia; and four had alcohol/drug problems. Of the 164 eligible individuals, six declined participation and 158 were enrolled based on simple random assignment by the project manager using a random number sequence generated by the investigators: 56 in tele-PST, 63 in in-person PST, and 39 in care call. Of the enrollees, 139 (49 tele-PST; 54 in-person PST; and 36 care call) completed all six intervention sessions and provided 12-week follow-up data; 125 (41 tele-PST; 52 in-person PST; and 32 care call) provided 24-week follow-up data; and 116 (40 tele-PST; 45 in-person PST; and 31 care call) provided 36-week follow-up data. Attrition during intervention and follow-up was due mostly to deteriorating health problems that resulted in hospitalization, nursing home placement, and death; however, the baseline demographic and clinical characteristics of the dropouts did not differ significantly from those who continued in the study. All the assessments were conducted in person, in home by four master’s-level social workers who were specifically trained in all the study instruments by a PhD-level clinical psychologist. The assessors were not blinded to the participants’ group assignments as some questions were specific to the group (e.g., technology assessment for the tele-PST group), but they did not know the study hypotheses.
INTERVENTION
Problem-solving therapy in this trial followed the PST-PC (primary care; PST hereafter) protocol, which was developed in England in the 1980s[29, 30] and adapted for delivery in fast-paced primary care settings in the United States during the 1990s.[31, 32] PST is grounded in the cognitive-behavioral theory of mental health and posits that people with deficits in problem-solving skills become vulnerable to depression because such deficits lead to ineffective coping attempts under high levels of stress.[33, 34] The evidence base of PST for late-life depression has been established in multiple RCTs in both primary care and in-home settings.[35–38]
The fourth author (M.T.H.) trained two master’s-level social workers to deliver both tele-PST and in-person PST, monitored fidelity, and provided clinical supervision. The tele-PST participants received in-person PST for the first session; at the end of which the therapist provided each of them a laptop computer with Skype video call installed and demonstrated its use for the next five tele-sessions. In-person PST participants engaged in six face-to-face PST sessions in their own homes. In each, 60-min PST session, the therapist and participant used a worksheet to progress through the seven steps of PST-PC, focusing on participants’ appraisal and evaluation of specific problems, their identification of the best possible solutions, and the practical implementation of those solutions, as well as on addressing anhedonia and psychomotor retardation through behavioral activation and increased exposure to pleasant events. The participants in the care call group received six weekly, 30-min telephone support calls from two research associates. The purpose of the calls was to provide nonspecific support and to monitor the participants’ depressive symptoms to ensure their safety. The detailed intervention and fidelity monitoring procedures are described elsewhere.[21, 22]
MEASURES
Depressive Symptoms
The 24-item HAMD consists of the GRID-HAMD-21 structured interview guide[26] augmented with three additional items assessing feelings of hopelessness, helplessness, and worthlessness, with specific probes and follow-up questions developed by Moberg et al.[27]
Disability Status
The 12-item WHODAS II[39] (World Health Organization Disability Assessment Schedule) assesses the degree of difficulties (1 = none; 5 = extreme/cannot do) that people have had in the preceding 30 days due to “health conditions,” without asking respondents to identify whether their health conditions are medical or mental. The maximum possible score is 60.
Other Participant Characteristics
Demographic variables included age; gender; race/ethnicity; living arrangement; education; and family income. Health-related variables included the number of diagnosed chronic illnesses and the activities and instrumental activities of daily living (ADL/IADL) impairments.
STATISTICAL ANALYSIS
Between-group one-way ANOVA (with Bonferroni-corrected post hoc tests), χ2 tests, and t tests were used to assess group differences in participant characteristics. All tests of significance were two tailed with α set at .05. Treatment effects on depression severity (HAMD score) and disability (WHODAS score) were analyzed from an intent-to-treat approach using piecewise mixed-effects regression with random intercept models[40] using SPSS v.21 (IBM Corp., Armonk, NY). Treatment group, time, and the interaction terms between treatment group and time were included in the models, with group as a between-subject effect and piecewise time as a continuous within-subject variable coded as two distinct time periods (T1: baseline to 12 weeks; T2: 12–36 weeks). The four time points were coded as [−12, 0, 0, 0] and [0, 0, 12, 24] for T1 and T2, respectively. Because the 12-week time point was zero for both time variables, the models’ intercepts represent the 12-week assessment scores. Following recommendations from Feingold,[41] effect sizes at 12 and 36 weeks were estimated by dividing the difference between the estimated means of treatment groups by the baseline standard deviation. The formula generates an effect size (dGMA-raw) in a growth model context that is equivalent to traditional effect sizes (e.g., Cohen’s d).
To examine the reciprocal relationship between depression and disability, we fit a parallel-process latent growth curve (LGC) model in a structural equation modeling framework (SEM)[42, 43] using Mplus 7.11.[44] The piecewise mixed-effects regression models were replicated in the LGC framework by fixing latent variable parameters,[45] resulting in three growth parameters representing the three sequential stages of change: (1) T1 (baseline to 12 weeks) change, (2) at 12 weeks (the model intercept), and (3) T2 (12–36 weeks) change. The combination of the mixed-effects models and the parallel-process LGC tested the following four criteria for mediation described by Baron and Kenny[46]: (1) the initial variable (i.e., treatment group) predicts the outcome (i.e., HAMD and WHODAS scores at follow-ups; path c); (2) the initial variable predicts change in the mediator (T1 change in HAMD and WHODAS scores; path a); (3) the mediator predicts the outcome (path b); and (4) the relationship between the initial variable and the outcome is reduced to zero if the mediator is included in the model (path c′). Paths a, b, and c′ were simultaneously fit in a parallel-process LGC model. We used bias-corrected bootstrap estimates[43] to test the indirect effect from the initial variable to the outcome variable through the mediator. We examined modification indices for theoretically viable parameters to improve model fit and added one parameter, the covariance between HAMD and WHODAS score errors at 36 weeks. We treated all latent variables as random effects with the exception of T2 WHODAS score change, which did not significantly differ from zero. We assessed univariate normality using the criteria of skewness < 2 and kurtosis < 7, following SEM guidelines by Curran et al.[47] All variables included in the model met these criteria (maximum skew = 0.83 and the maximum kurtosis = 1.91). Model fit was evaluated using root mean square error of approximation (RMSEA) < 0.05,[48] the comparative fit index (CFI) > 0.95,[49] and the standardized root mean square residual (SRMR) < 0.08.[49]
RESULTS
Participants’ demographic and clinical characteristics are presented in Table 1. At baseline, 45% were in the age 60–69 category and the rest were evenly divided between the age 50–59 and 70+ categories. The distributions of gender (78.5% female), racial/ethnic group (58% Black or Hispanic), and low-income status of the sample (84% with ≤$25,000) reflected those of the clientele of the HDM program from which 85% of the referrals came. No significant difference was found in any baseline demographic and clinical characteristics (medical morbidity, ADL/IADL limitations, HAMD and WHODAS scores, and DSM-IV-TR diagnosis) among the three treatment groups and in age, gender, and other baseline characteristics by race/ethnicity.
TABLE 1.
Baseline characteristics of study participants (n = 158)
| Age | |
| Mean (SD) | 64.80 (9.18) |
| Age group (n, %) | |
| 50–59 | 44 (27.8) |
| 60–69 | 71 (44.9) |
| 70–79 | 29 (18.4) |
| 80+ | 14 (8.9) |
| Gender | |
| Male | 34 (21.5) |
| Female | 124 (78.5) |
| Race/ethnicity | |
| Non-Hispanic White | 67 (42.4) |
| African American/Black | 52 (32.9) |
| Hispanic | 39 (24.7) |
| Education | |
| <High school diploma | 34 (7.6) |
| High school diploma/GED | 31 (19.6) |
| Some college (no degree) | 53 (33.5) |
| 2- or 4-year college degree | 22 (13.9) |
| Graduate school | 15 (9.5) |
| Family income | |
| Up to $15,000 | 98 (62.0) |
| $15,001–$25,000 | 35 (22.2) |
| $25,001–$35,000 | 11 (7.0) |
| $35,001+ | 7 (4.4) |
| Refused | 7 (4.4) |
| Self-reported financial situation | |
| Can’t make ends meet | 40 (25.3) |
| Just about manage to get by | 90 (57.0) |
| Have enough to get along | 23 (14.6) |
| Money is not a problem | 4 (2.5) |
| Refused | 1 (0.6) |
| Living arrangement | |
| Living alone | 101 (64.3) |
| Living with spouse and/or other | 57 (35.7) |
| Number of diagnosed chronic illness | |
| Mean (SD) | 3.06 (1.54) |
| Number of activities of daily living impairment | |
| Mean (SD) | 1.43 (1.31) |
| Number of instrumental activities of daily living impairment | |
| Mean (SD) | 2.69 (1.51) |
| DSM-IV-TR diagnosis of depression | |
| Major depressive disorder | 103 (65.2) |
| Depressive disorder, NOS | 47 (29.7) |
| Dysthymia | 8 (5.1) |
| HAMD score | |
| Mean (SD) | 23.89 (6.52) |
| Range | 15–42 |
| WHODAS II score | |
| Mean (SD) | 35.97 (9.06) |
| Range | 13–56 |
GROUP AND TIME EFFECTS ON DEPRESSION SEVERITY AND DISABILITY
Table 2 shows the results of the mixed-effects regression analysis for depression severity and disability. The main effects of group on HAMD scores were significant for both tele-PST and in-person PST, as compared to care call. The main effects of T1 and T2 were also significant. Group by T1 interaction effects were significant for both tele-PST and in-person PST. However, group by T2 interaction effect was significant for in-person PST group only, showing that the HAMD scores of in-person PST participants have increased between T1 and T2, while there was no change in the HAMD scores of tele-PST participants since T1, as compared to the HAMD scores of call participants.
TABLE 2.
Treatment effects on depression and disability by group, time, and group by time: mixed-effects regression results
| Variable | Depression (HAMD scores)
|
Disability (WHODAS II scores)
|
||||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | 95% CI | t | P | B (SE) | 95% CI | t | P | |
| Intercept | 18.93 (1.17) | (16.64, 21.23) | 16.25 | <.000 | 34.97 (1.47) | (32.07, 37.87) | 23.77 | <.001 |
| Treatment group | ||||||||
| Tele-PST | −5.25 (1.53) | (−8.26, −2.23) | −3.42 | .001 | −5.25 (1.93) | (−9.06, −1.45) | −2.72 | .007 |
| In-person PST (care call) | −4.85 (1.50) | (−7.80, −1.90) | −3.24 | .001 | −4.84 (1.90) | (−8.56, −1.12) | −2.56 | .011 |
| Time 1: baseline to 12 weeks | −0.48 (0.10) | (−0.67, −0.28) | −4.72 | <.001 | −0.25 (0.11) | (−0.47, 0.04) | −2.32 | .021 |
| Time 2: 12–36 weeks | −0.14 (0.06) | (−0.25, −0.03) | −2.56 | .011 | −0.07 (0.06) | (−0.19, 0.05) | −1.12 | .265 |
| Group by time | ||||||||
| Tele-PST × Time 1 | −0.35 (0.13) | (−0.61, −0.08) | −2.61 | .010 | −0.31 (0.14) | (−0.60, −0.03) | −2.16 | .032 |
| In-person PST × Time 1 (care call × Time 1) | −0.33 (0.13) | (−0.58, −0.08) | −2.55 | .011 | −0.09 (0.14) | (−0.36, 0.19) | −0.60 | .546 |
| Tele-PST × Time 2 | 0.04 (0.07) | (−0.11, 0.18) | 0.47 | .636 | 0.04 (0.08) | (−0.12, 0.20) | 0.49 | .623 |
| In-person PST × Time 2 (care call × Time 2) | 0.15 (0.07) | (0.00, 0.29) | 2.03 | .043 | 0.11 (0.08) | (−0.05, 0.26) | 1.36 | .175 |
Parentheses denote reference category.
The main effects of group on WHODAS scores were significant for both tele-PST and in-person PST, as compared to care call. The main effects of T1 were also significant; however, they were nonsignificant for T2. Group by T1 interaction effects were significant only for tele-PST. Group by T2 interaction effects were nonsignificant for both groups.
Table 3 shows the baseline HAMD and WHODAS scores by group and the group differences in predicted mean HAMD and WHODAS scores at follow-ups. At both 12 and 24 weeks, both HAMD and WHODAS scores were significantly lower in both tele-PST and in-person PST participants than in care call participants, with no significant difference between tele-PST and in-person PST. However, at 36 weeks, the group differences in HAMD and WHODAS scores remained significant between tele-PST and care call, but they were nonsignificant between in-person PST and care call. For HAMD scores, the difference between tele-PST and in-person PST also became significant, with tele-PST participants’ HAMD scores being the lowest among the three groups. For WHODAS scores, no difference was found between tele-PST and in-person PST. Further analysis showed that at 36 weeks, 42.5% of tele-PST participants, compared to 26.7% of in-person PST and 25.8% of care call participants, achieved remission (HAMD < 10), although these differences were not statistically significant (χ2(2) = 3.66, P = .21).
TABLE 3.
Pairwise comparisons of baseline and follow-up predicted mean HAMD and WHODAS scores
| Baseline | F | df | P | |
|---|---|---|---|---|
| HAMD | 0.35 | 2 | .703 | |
| Tele-PST | 23.54 (0.86) | |||
| In-person PST | 27.75 (0.83) | |||
| Care call | 24.64 (1.07) | |||
| WHODAS | 2.33 | 2 | .101 | |
| Tele-PST | 36.52 (1.16) | |||
| In-person PST | 34.21 (1.23) | |||
| Care call | 38.03 (1.28) | |||
| HAMD | 12-week follow-up | t | df | P |
|
|
||||
| Tele-PST vs. care call | 13.68 (1.00) vs. 18.93 (1.17) | −3.42 | 327.24 | .001 |
| In-person PST vs. care call | 14.08 (0.94) vs. 18.93 (1.17) | −3.24 | 327.05 | .001 |
| Tele-PST vs. in-person PST | 13.68 (1.00) vs. 14.08 (0.94) | −0.06 | 334.68 | .772 |
| WHODAS | ||||
| Tele-PST vs. care call | 29.72 (1.26) vs. 34.97 (1.48) | −2.72 | 270.07 | .007 |
| In-person PST vs. care call | 30.13 (1.19) vs. 34.97 (1.48) | −2.56 | 270.40 | .011 |
| Tele-PST vs. in-person PST | 29.72 (1.25) vs. 30.13 (1.19) | 0.24 | 278.41 | .809 |
| 24-week follow-up | t | df | P | |
| HAMD | ||||
|
|
||||
| Tele-PST vs. care call | 12.38 (0.85) vs. 17.21 (0.99) | −3.70 | 189.13 | <.001 |
| In-person PST vs. care call | 14.12 (0.80) vs. 17.21 (0.99) | −2.43 | 186.77 | .016 |
| Tele-PST vs. in-person PST | 12.38 (0.85) vs. 14.12 (0.80) | −1.49 | 194.20 | .138 |
| WHODAS | ||||
| Tele-PST vs. care call | 29.38 (1.12) vs. 34.15 (1.32) | −2.77 | 178.24 | .006 |
| In-person PST vs. care call | 30.60 (1.05) vs. 34.15 (1.32) | −2.11 | 176.72 | .036 |
| Tele-PST vs. in-person PST | 29.38 (1.12) vs. 30.60 (1.05) | −0.80 | 186.62 | .426 |
| 36-week follow-up | t | df | P | |
| HAMD | ||||
|
|
||||
| Tele-PST vs. care call | 11.08 (1.07) vs. 15.49 (1.23) | −2.72 | 363.21 | .007 |
| In-person PST vs. care call | 14.16 (0.99) vs. 15.49 (1.23) | −0.84 | 360.40 | .400 |
| Tele-PST vs. in-person PST | 11.08 (1.07) vs. 14.16 (0.99) | −2.11 | 374.10 | .035 |
| WHODAS | ||||
| Tele-PST vs. care call | 29.04 (1.32) vs. 33.33 (1.54) | −2.12 | 303.52 | .034 |
| In-person PST vs. care call | 31.07 (1.24) vs. 33.33 (1.54) | −1.15 | 300.37 | .251 |
| Tele-PST vs. in-person PST | 29.04 (1.32) vs. 31.07 (1.24) | −1.12 | 314.10 | .261 |
Parentheses denote standard error.
Note. 95% CI of the predicted HAMD means at 12-week follow-up: 11.72–15.65 for tele-PST group, 12.23–15.94 for in-person PST group, and 16.64–21.23 for care call group; at 24-week follow-up: 10.70–14.06 for tele-PST group, 12.55–15.69 for in-person PST group, and 15.26–19.16 for care call group; and at 36-week follow-up: 8.99–13.18 for tele-PST group, 12.21–16.11 for in-person PST group, and 13.08–17.90 for care call group.
95% CI of the predicted WHODAS means at 12-week follow-up: 27.25–32.19 for tele-PST group, 27.80–32.47 for in-person PST group, and 32.07–37.87 for care call group; at 24-week follow-up: 27.17–31.59 for tele-PST group, 28.53–32.68 for in-person PST group, and 31.57–36.74 for care call group; and at 36-week follow-up: 26.44–31.64 for tele-PST group, 28.64–33.50 for in-person PST group, and 30.32–36.35 for care call group.
The predicted mean HAMD and WHODAS scores by group and time are plotted in Figs. 1 and 2. Effect sizes for HAMD score changes were 0.81 for tele-PST and 0.74 for in-person PST at 12 weeks and 0.68 for tele-PST and 0.20 for in-person PST at 36 weeks. Effect sizes for WHODAS score changes were 0.58 for tele-PST and 0.53 for in-person PST at 12 weeks and 0.47 for tele-PST and 0.25 for in-person PST at 36 weeks.
Figure 1.
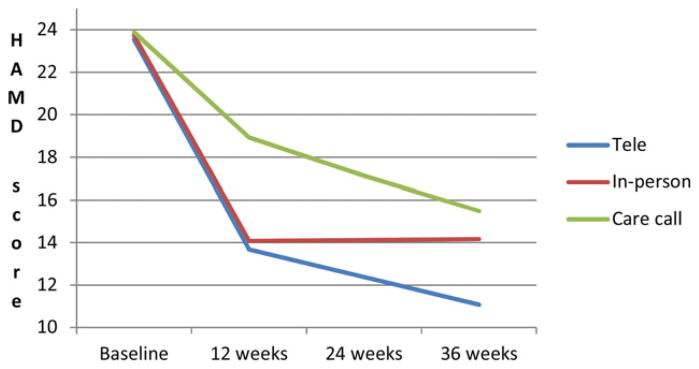
Changes in predicted mean HAMD score.
Figure 2.
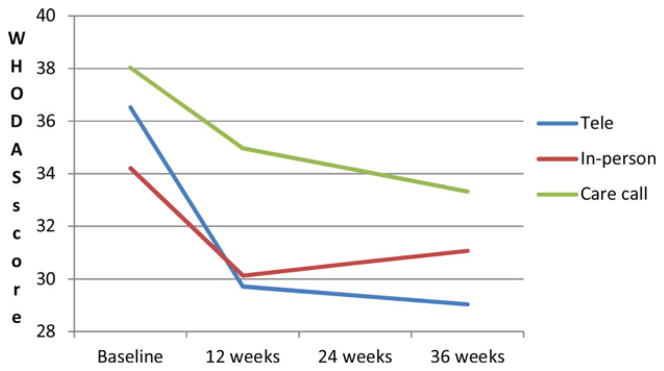
Changes in predicted mean WHODAS score.
RECIPROCAL EFFECTS OF DEPRESSION AND DISABILITY
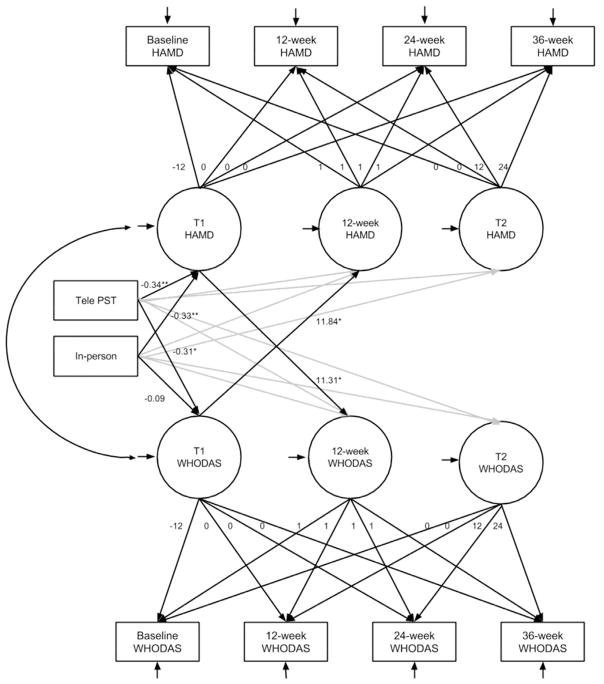
Figure 3 presents the path coefficients from the parallel-process LGC model. The model fit indices (RMSEA = 0.04, CFI = 0.94, SRMR = 0.05, χ2(22) = 27.53, P = .192) met all cutoff criteria. Both tele-PST and in-person PST, contrasted with care call, were significant predictors of the T1 HAMD (B = −0.34, SE = 0.12, P = .004; B = −0.033, SE = 0.12, P = .003); however, only tele-PST was a significant predictor of the T1 WHODAS (B = −0.31, SE = 0.14, P = .027 vs. B = −0.09, SE = 0.14, P = .516 for in-person PST). The results, replicating those reported in Tables 2 and 3, indicate that compared with care calls, tele-PST was associated with larger decreases in both HAMD and WHODAS scores, whereas in-person PST was associated with larger decreases in HAMD only. The T1 HAMD was a significant predictor of the WHO-DAS at 12 weeks (B = 11.31, SE = 0.14, P = .027), and T1 WHODAS was a significant predictor of the HAMD at 12 weeks (B = 11.84, SE = 4.66, P = .015). The indirect effect of tele-PST on T2 HAMD was significant (CI −11.63, −0.67), as were the indirect effects of tele-PST and in-person PST on T2 WHO-DAS (CIs −10.46, −1.05 and −9.81, −0.96). Furthermore, the direct effect of tele-PST and in-person PST on T2 HAMD score became nonsignificant in this model (B = −1.43, SE = 2.55, P = .576; B = −3.77, SE = 2.05, P = .066) as did the direct effect of tele-PST on T2 WHODAS score (B = −1.46, SE = 2.60, P = .573), whereas in-person PST remained nonsignificant (B = −1.18, SE = 2.74, P = .667), providing support for complete mediation for the three observed significant indirect effects.
Figure 3.
Parallel-process latent growth curve mediation model.
Note. Gray paths indicate paths included in the model; coefficients are not displayed in order to simplify the figure.
Notations within circles: T1 HAMD and T1 WHODAS: score changes from baseline to 12 weeks; 12-week HAMD and 12-week WHODAS: intercept at 12 weeks; and T2 HAMD and T2 WHODAS: score changes from 12 to 36 weeks.
Although the model supports a reciprocal mediation process, because changes in HAMD and WHODAS scores appear to operate in parallel, the temporal precedence of the change in HAMD scores or the change in WHODAS scores cannot be established. It is possible that (1) a change in depression severity produced a subsequent change in WHODAS at 12 weeks (or vice versa), but it could have created the impression that depression and disability independently influence each other because changes occurred in both outcomes; or (2) changes could have occurred more incrementally whereby changes in depression led to changes in disability and the latter in turn led to changes in depression in a process that continued over time.
DISCUSSION
The major findings of this study were (1) both in-person PST and tele-PST, compared with care call, were efficacious treatments for depression in low-income homebound older adults; (2) the independent effects of tele-PST on both depression and disability outcomes were sustained significantly longer than those of in-person PST; and (3) there was a support for reciprocal and indirect effects between depression and disability outcomes. Use of a freely available and widely used video call function to tele-deliver short-term evidence-based psychotherapy to low-income homebound older adults in their own homes can alleviate access barriers to depression treatment among this underserved population group. Since the videoconferencing equipment in this study consisted of a laptop and a wireless card, the equipment cost was relatively low. In the near future, as more older adults will be Internet-connected, the cost of delivery will go down further. Low-cost laptop computers (e.g., Chromebooks) with wireless card or tablets can be loaned to those without their own equipment for tele-sessions and then the devices can be moved on to other people. Our earlier study[38] found that although many tele-PST participants who had not been exposed to computer and Internet use initially felt uncomfortable with tele-delivery, the discomfort disappeared as soon as they started engaging in tele-sessions and recognized the convenience and the in-person-like qualities of this type of interaction with the therapist. Also because they could engage in therapy in the privacy of their own homes, they felt more comfortable with tele-delivery than with going to a clinic for therapy and gave it high approval ratings.[50]
The long-term efficacy of tele-PST may have resulted, in part, from the participants’ positive expectancies because of the use of technology itself and their perception of it being new and innovative compared to usual in-person psychotherapy. Although the therapists’ treatment fidelity ratings did not vary between tele-PST and in-person PST, the two therapists observed that the patients tended to be more focused during tele-PST than during in-person PST sessions. This was supported by the fact that even when the usual 10 min of greeting and settling-in common to in-person sessions were not counted, in-person PST sessions (65.8 ± 0.17 min) were longer than tele-PST sessions (54.3 ± 0.27 min). The added length of in-person sessions was often due to household or routine distractions, including getting up to fetch a glass of water, answering the phone, and/or using the restroom during the session. Such a distraction was rare during tele-PST sessions. Compared to in-person treatment in one’s own home, tele-delivered treatment may have contributed to more professional ambience for the sessions and more investment in the therapy on the part of the participants.
The study had a couple of limitations. (1) The sample was small and limited to those who were willing to be randomized to tele-psychotherapy and to those without cognitive deficits and other mental health conditions. Further research is needed to test the effectiveness of tele-PST when it is offered on a routine basis and for those with other comorbid conditions. (2) It is not surprising that telephone support call participants experienced some symptom reductions between baseline and 12 weeks, owing to caring social interactions because these were socially isolated older adults. However, the continued decline in their depressive symptoms between 12 and 36 weeks is surprising. The present study could not identify the factors that may have contributed to improved mood among this group.
A recent report of the Institute of Medicine (IOM)[51] pointed out the serious problem of unmet mental health needs of older adults in a rapidly aging society. The unmet mental health needs are the most pronounced in low-income homebound older adults who simply do not have adequate access to psychotherapy. The results of this RCT suggest that technology offers a potentially low-cost solution for the problem of access that is as good as, if not better than, in-person psychotherapy for depressed, low-income homebound older adults. Late-life depression is increasingly seen as a chronic condition, and prevention is more cost-effective than treatment. Given the current and projected geriatric mental health workforce shortage identified in the IOM report, strategies to tele-deliver depression prevention and treatment may be more economical and sustainable over time than in-person strategies because they enable more low-income homebound older adults to be served by fewer therapists.
CONCLUSIONS
Problem-solving therapy, with its focus on “here-and-now” issues and a short-term, structured problem-solving skills training approach, was proven to be an acceptable and effective psychotherapy for depressed older adults in primary care and in-home settings. The present study found that tele-delivered PST for low-income homebound older adults is an efficacious treatment that maintains its effects long term. The economical nature of tele-delivery also shows its potential for easy replication and sustainability to reach a large number of underserved older adults and improve their access to evidence-based psychotherapy.
Acknowledgments
This research was supported by the National Institute of Mental Health (R34 MH083872; PI: N.C.); St. David’s Foundation (PI: N.C.); and with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX (M.K. and N.W.).
References
- 1.Bruce ML, McVay J, Raue PJ, et al. Major depression in elderly home health care patients. Am J Psychiatry. 2002;159:1367–1374. doi: 10.1176/appi.ajp.159.8.1367. [DOI] [PubMed] [Google Scholar]
- 2.Raue PJ, Meyers BS, Rowe JL, et al. Suicidal ideation among elderly homecare patients. Int J Geriatr Psychiatry. 2007;22:32–37. doi: 10.1002/gps.1649. [DOI] [PubMed] [Google Scholar]
- 3.Ell K, Unützer J, Aranda M, et al. Routine PHQ-9 depression screening in home health care: depression prevalence, clinical and treatment characteristics, and screening implementation. Home Health Care Serv Q. 2005;24:1–19. doi: 10.1300/J027v24n04_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirey JA, Bruce ML, Carpenter M, et al. Depressive symptoms and suicidal ideation among older adults receiving home-delivered meals. Int J Geriatr Psychiatry. 2008;23:1306–1311. doi: 10.1002/gps.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi NG, Teeters M, Perez L, et al. Severity and correlates of depressive symptoms among recipients of meals in wheels: age, gender, and racial/ethnic difference. Aging Ment Health. 2010;14:145–154. doi: 10.1080/13607860903421078. [DOI] [PubMed] [Google Scholar]
- 6.Anderson G. Chronic Care: Making the Case for Ongoing Care. Princeton, NJ: Robert Wood Johnson Foundation; 2010. Available at: http://www.rwjf.org/content/dam/farm/reports/reports/2010/rwjf54583. [Google Scholar]
- 7.Katon WJ, Lin E, Russo J, et al. Increased medical care costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 8.Unutzer J, Schoenbaum M, Katon WJ, et al. Healthcare costs associated with depression in medically ill fee-for-service medicare participants. J Am Geriatr Soc. 2009;57:506–510. doi: 10.1111/j.1532-5415.2008.02134.x. [DOI] [PubMed] [Google Scholar]
- 9.Weissman J, Meyers BS, Ghosh S, Bruce ML. Demographic, clinical and functional factors associated with antidepressant use in the home healthcare elderly. Am J Geriatr Psychiatry. 2011;19:1042–1045. doi: 10.1097/JGP.0b013e318235b743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21:973–986. doi: 10.1097/JGP.0b013e31826576cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen A, Houck PR, Szanto K, et al. Social inequalities in response to antidepressant treatment in older adults. Arch Gen Psychiatry. 2006;63:50–56. doi: 10.1001/archpsyc.63.1.50. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CM, Sharkey JR, Dean WR. Indicators of material hardship and depressive symptoms among homebound older adults living in North Carolina. J Nutr Gerontol Geriatr. 2011;30:154–168. doi: 10.1080/21551197.2011.566527. [DOI] [PubMed] [Google Scholar]
- 13.Gum AM, Areán PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist. 2006;46:14–22. doi: 10.1093/geront/46.1.14. [DOI] [PubMed] [Google Scholar]
- 14.Choi N, Morrow-Howell N. Older adults’ attitudes toward depression treatment modalities: within-group differences and comparison with their caregivers. Aging Ment Health. 2007;11:422–433. doi: 10.1080/13607860600963802. [DOI] [PubMed] [Google Scholar]
- 15.Choi NG, Lee A, Goldstein M. Meals on wheels: exploring potential for and barriers to integrating depression intervention for homebound older adults. Home Health Care Serv Q. 2011;30:214–230. doi: 10.1080/01621424.2011.622251. [DOI] [PubMed] [Google Scholar]
- 16.Reifler BV, Bruce ML. Home-based mental health services for older adults: a review of ten model programs. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2012.12.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osenbach JE, O’Brien KM, Mishkind M, Smolenski DJ. Synchronous telehealth technology in psychotherapy for depression: a meta-analysis. Depress Anxiety. 2013 doi: 10.1002/da.22165. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Hilty DM, Ferrer DC, Parish MB, et al. The effectiveness of telemental health: a 2013 review. Telemed J E Health. 2013;19:444–454. doi: 10.1089/tmj.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luxton DD, O’Brien K, McCann RA, Mishkind MC. Home-based telemental healthcare safety planning: what you need to know. Telemed J E Health. 2012;18:629–633. doi: 10.1089/tmj.2012.0004. [DOI] [PubMed] [Google Scholar]
- 20.Grady B, Myers KM, Nelson EL, et al. Evidence-based practice for telemental health. Telemed J E Health. 2011;17:131–48. doi: 10.1089/tmj.2010.0158. [DOI] [PubMed] [Google Scholar]
- 21.Choi NG, Hegel MT, Sirrianni L, et al. Passive coping response to depressive symptoms among low-income homebound older adults: does it affect depression severity and treatment outcome? Behav Res Ther. 2012;50:668–674. doi: 10.1016/j.brat.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi NG, Hegel MT, Marti CM, et al. Telehealth problem-solving therapy for depressed low-income homebound older adults: acceptance and preliminary efficacy. Am J Geriatr Psychiatry. 2014 doi: 10.1097/JGP.0b013e318266b356. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruce ML. Depression and disability in late life: directions for future research. Am J Geriatr Psychiatry. 2001;9:102–112. [PubMed] [Google Scholar]
- 24.Ormel J, Rijsdijk FV, Sullivan M, et al. Temporal and reciprocal relationship between IADL/ADL disability and depressive symptoms in late life. J Gerontol B Psychol Sci Soc Sci. 2002;57:P338–P347. doi: 10.1093/geronb/57.4.p338. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Medicare and Medicaid Services. Medicare benefit policy manual. Chapter 7. Home health services; 2011. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/bp102c07.pdf. [Google Scholar]
- 26.Depression Rating Scale Standardization Team. GRID-HAMD-17, GRID-HAMD-21 Structured Interview Guide. San Diego: International Society for CNS Drug Development; 2003. [Google Scholar]
- 27.Moberg PJ, Lazarus LW, Mesholam RI, et al. Comparison of the standard and structured interview guide for the Hamilton Depression Rating Scale in depressed geriatric inpatients. Am J Geriatr Psychiatry. 2001;9:35–40. [PubMed] [Google Scholar]
- 28.Borson S, Scanlan J, Brush M, et al. The mini-cog. A cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;25:1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Catalan J, Gath DH, Anastasiades P, et al. Evaluation of a brief psychological treatment for emotional disorders in primary care. Psychol Med. 1991;21:1013–1018. doi: 10.1017/s0033291700030002. [DOI] [PubMed] [Google Scholar]
- 30.Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, et al. Randomized controlled trial comparing problem-solving treatment with amitriptyline and placebo for major depression in primary care. BMJ. 1995;310:441–445. doi: 10.1136/bmj.310.6977.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegel MT, Barrett JE, Cornell JE, et al. Predictors of response to problem-solving treatment of depression in primary care. Behav Ther. 2002;33:511–527. [Google Scholar]
- 32.Hegel MT, Barrett JE, Oxman TE. Training therapists in problem-solving treatment of depressive disorders in primary care. Lessons learned from the “Treatment Effectiveness Project. Fam Syst Health. 2000;18:423–435. [Google Scholar]
- 33.D’Zurilla TJ, Nezu AM. Problem-Solving Therapy: A Positive Approach to Clinical Intervention. New York: Springer; 2007. [Google Scholar]
- 34.Nezu AM, Nezu CM, Perri MG. Problem-Solving Therapy for Depression: Theory, Research, and Clinical Guidelines. New York: Wiley; 1989. [Google Scholar]
- 35.Areán PA, Hegel MT, Vannoy S, et al. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT study. Gerontologist. 2008;48:311–324. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 36.Areán PA, Raue PJ, Mackin S, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167:1391–1398. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciechanowski P, Wagner E, Schmaling K, et al. Community-integrated home-based depression treatment in older adults. JAMA. 2004;29:1569–1577. doi: 10.1001/jama.291.13.1569. [DOI] [PubMed] [Google Scholar]
- 38.Gellis Z, McGinty J, Horowitz A, et al. Problem-solving therapy for late-life depression in home care: a randomized field trial. Am J Geriatr Psychiatry. 2007;15:968–978. doi: 10.1097/JGP.0b013e3180cc2bd7. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. World Health Organization Disability Assessment Schedule II. Available at: http://www.who.int/classifications/icf/12int.pdf.
- 40.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 41.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheong J, MacKinnon DP, Khoo ST. Investigation of mediational processes using parallel-process latent growth curve modeling. Struct Equ Modeling. 2003;10:238–262. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacKinnon DP. Multivariate Applications: Introduction to Statistical Mediation Analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- 44.Muthén LK, Muthén BO. Mplus 7.11. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- 45.Flora D. Specifying piecewise latent trajectory models for longitudinal data. Struct Equ Modeling. 2008;15:513–533. [Google Scholar]
- 46.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.Curran PJ, West SG, Finch JF. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol Methods. 1996;1:16–29. [Google Scholar]
- 48.MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1:130–149. [Google Scholar]
- 49.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 50.Choi NG, Wilson N, Sirrianni L, et al. Acceptance of home-based telehealth problem-solving therapy for depressed, low-income homebound older adults: qualitative interviews with the participants and aging service case managers. Gerontologist. doi: 10.1093/geront/gnt083. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Institute of Medicine (IOM) The Mental Health and Substance Use Workforce for Older Adults: In Whose Hands? Washington, DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- 52.Licht-Strunk E, Van Marwijk HWJ, Hoekstra T, et al. Outcome of depression in later life in primary care: longitudinal cohort study with three years’ follow-up. BMJ. 2009;338:a3079. doi: 10.1136/bmj.a3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van’t Veer-Tazelaar P, Smit F, van Hout H, et al. Cost-effectiveness of a stepped care intervention to prevent depression and anxiety in late life: randomised trial. Br J Psychiatry. 2010;196:319–325. doi: 10.1192/bjp.bp.109.069617. [DOI] [PubMed] [Google Scholar]