Abstract
Chondroitin sulfate proteoglycans (CSPGs) are a diverse family of extracellular matrix (ECM) molecules that make significant contributions to the patterning and routing of migrating neural cells and extending axons. Three distinct modes of migration mediation have been observed, which result from the relative abundance and positioning of expressed CSPGs, the profile of CSPG receptors expressed by the motile cell types, and the overall way in which the CSPGs integrate into and stabilize the neural ECM. Here we discuss recent findings that help to clarify the molecular mechanisms that underlie these distinct migration-regulating properties as they pertain to neural development, CNS injury, and gliomagenesis.
Keywords: CSPG, Chondroitin Sulfate Proteoglycan, Cell Migration, Axon Guidance, Glial Scar, Brain Tumor, Glioma, Glioblastoma, Invasion, CSPG Receptor, LAR
Introduction
Chondroitin sulfate proteoglycans (CSPGs) are a large and diverse family of extracellular matrix (ECM) molecules. The central nervous system (CNS) enriched CSPGs, referred to collectively as the lectican subfamily, share three key features. The core protein structure of each lectican, which ranges from 97 to >262kD, begins and ends with a conserved N-terminal (G1) and C-terminal (G3) globular domain linked by a central, CS-GAG anchoring backbone, of variable length, bound to at least one, long-chain chondroitin sulfate glycosaminoglycan (CS-GAG) polysaccharide [1]. By binding hyaluronan and tenascin-R through their G1 and G3 domains, respectively, CSPGs provide one of three major components of the tripartite lattice that forms the CNS ECM [1,2]. While the contribution of CSPGs to the inhibitory nature of certain restricted territories within the embryo as well as the glial scar in the adult has been known for many years (see review by [3]), until recently, a mechanistic explanation of how CSPGs might redirect the advancement of cells or growth cones was lacking. This brief review will describe work that begins to elucidate how CSPGs provide varied biological effects during normal development and after trauma in the adult and, in addition, present some new ideas on how CSPGs orchestrate glioma invasion or the lack thereof.
Expression and the role of CSPGs During Development
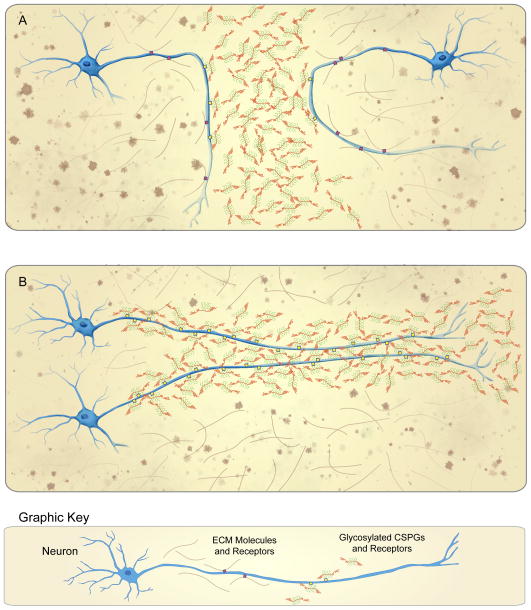
During CNS development, glycosylated CSPGs are highly expressed in specific locations and were first thought to serve only as molecular barriers, blocking cells or axons from moving across the boundary between two adjacent, emerging structures (Fig. 1A). For example, CSPGs repel axons from extending across the roof plate of the spinal cord [4,5], the midline rhombencephalon and mesencephalon [6,7], the periphery of the developing retina [8,9], certain portions of the optic chiasm and distal optic tract [10,11] and the posterior somite [12]. A second mode of CSPG-mediated migration was then discovered within the sub-ventricular germinal centers of the embryonic and adult brain. This “addictive” growth constrained movement to pathways that were spatially defined by the uniform and robust expression of glycosylated CSPGs [13–16]. Unlike classic, chemo-attractive guidance, which involves forward movement up an increasing gradient of attractant, the addictive growth observed within the sub-plate of the developing forebrain [17] and the raphe [18], restricted cellular movement and process extension within specific, spatially-defined highways of CSPGs (Fig. 1B). Thus, two distinct cell migratory behaviors are associated with CSPGs during development: (1) turning away from a zone of CSPG production, and also (2) a type of “addictive” growth constrained within CSPG containing territories (Figure 1). What is the mechanism that allows for such diverse motile behaviors during interactions with the same family of molecules?
Figure 1.
CSPGs mediate the distinct migratory behaviors of turning and addiction. (A) Extending axons and migrating neuroblasts are re-directed, or “turned” away from developmentally-regulated proteoglycan boundaries when their receptor profiles favor adhesion to the non-addictive ECM molecules (i.e. laminin and fibronectin) adjacent to these CSPG-rich barriers. (B) In contrast, migrating neuroblasts and extending axons may become “addicted” to moving within CSPG-rich tracts through preferential expression of CSPG-receptors.
For years it was posited that CSPGs exert their effects through relatively nonspecific mechanisms such as substrate occlusion [19], or presentation of negative charge [20]. This view has evolved considerably with the discovery of several receptors that directly bind sulfated glycosaminoglycan moieties [21–23]. The receptor protein tyrosine phosphatase sigma (PTPRs, or PTPσ) was the first receptor identified with the ability to both bind CS-GAGs and convey a signal for growth inhibition [23]. PTPσ, together with PTPδ and LAR, are members of the class IIa/Leukocyte common antigen-related (LAR) family of receptor tyrosine phosphatases. Since all three LAR family receptors exhibit high sequence homology and contain a cluster of lysine residues that comprise a canonical glycosaminoglycan-binding domain [24,25], it was not unexpected when LAR was later shown to be another CS-GAG binding, CSPG receptor [22]. In addition to LAR family phosphatases, the NOGO receptors NgR1, NgR2, and NgR3 have been recently identified as receptors for CSPGs [21,26]. This discovery established a link between myelin derived inhibitors and proteoglycans, the two major classes of molecules that inhibit axon regeneration and sprouting. Whether signaling by NgR1-3 and LAR family receptors converges on a common effector is an important and unanswered question (see review by [27]). Here, we will focus on LAR family receptors and discuss the implications of their differential expression mediating disparate CSPG-mediated migratory behaviors.
Turning versus addictive growth patterns may depend on a receptor competition mechanism
In vitro, neurons exhibit different growth patterns depending upon which substrate (CSPG containing or CSPG lacking) the neuron cell body first resides and the length of time upon which their axons remain on this surface [28,29]. For instance, neurons co-cultured with CSPG and laminin-expressing Schwann cells tend to preferentially extend their processes along the Schwann cell membranes over the laminin only coated background. Interestingly, chondroitinase ABC mediated CS-GAG removal mitigates this phenomenon and allows the axons to abandon the Schwann cell. [The bacterial enzyme chondroitinase ABC (Ch’ase ABC) catalyzes the degradation of CS-GAGs.] In vitro stripe assays have revealed similar addiction-like behaviors. Specifically, Ch’ase ABC digestion frees dorsal root ganglion (DRG) neurons from exclusive growth within tracts of purified CSPGs (combined with other ECM molecules, such as laminin or fibronectin). As mentioned above, during development it has been shown that in certain regions, migrating neuronal precursors or growing axons actually prefer the CSPG containing SVZ or sub-plate (Fig. 1B). Alternatively, the turning behaviors, observed at the roof plate [5], the developing mid and hindbrain [6,7], the retina [8], etc., occur when neurons arrive from a journey within a CSPG-free ECM and are suddenly presented with abundant CSPGs at a sharp interface (Fig. 1A). Thus, the abundance of CSPGs, especially the concentration of CS-GAGs, relative to other adhesive matrix molecules appears to predict turning versus additive migration behavior.
There is increasing evidence that neurons express different receptor profiles based upon which substrate molecules they encounter first and for how long. Further, cross talk between CSPGs and CS-GAG receptors and other ECM molecules and their receptors, such as laminin, fibronectin, and certain integrins is conceivable [30–33]. Thus, we hypothesize that neurons beginning on non-addictive, non-CSPG substrates, express high amounts of ECM adhesion receptors (i.e. integrins) and minute quantities of LAR family receptors. Cells or axons are therefore able to enter territories containing permissive molecules even when they contain relatively low amounts of CSPGs. Up-regulation of LAR family receptors may take some time, so short encounters with CSPGs are not enough to cause permanent addiction. However, if the neurite enters and grows within a CSPG and laminin or CSPG and fibronectin-containing ECM for extended periods, it will eventually also increase both integrin as well as LAR family receptors and will no longer leave without removing or interfering with the LAR/CS-GAG interaction [34].
A Novel Mechanism for Regeneration Failure: CSPG-Mediated Entrapment
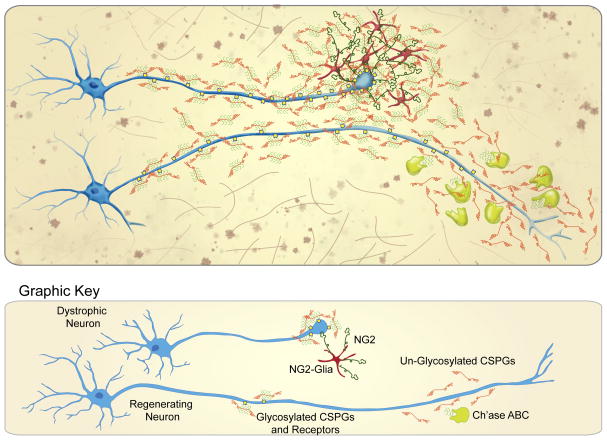
While the contribution of CSPGs to the inhibitory nature of the glial scar has been known for many years (see review by [3]), a mechanistic explanation as to how CSPGs permanently thwart advancing growth cones was lacking – until recently. Our laboratory has developed a simplified version of the injury site ECM, in which adult sensory neurons develop classic dystrophic endballs as they attempt to traverse an increasing gradient of CSPGs. Herein, adult DRG axons initially encounter a non-addictive ECM component, such as laminin or fibronectin, where they display dynamic filopodia with a highly motile growth cone that rarely collapses. However, when growth cones are exposed to a gradient of CSPGs they enter within the low end of the gradient and ascend until their initially elaborate growth cones convert into an immobilized state with slender tips and one or several extremely strong focal adhesions. The morphology of these dystrophic growth cones in vitro is remarkably similar to those described after spinal cord injury (SCI) by Ramon y Cajal in the early 20th century [35]. The molecular mechanism that leads to such strong bonds between neurons and CSPG-containing substrates is dependent upon the pro-synaptic LAR family receptors, which become highly concentrated in dystrophic growth cones. Using cell-permeable peptides that regulate the receptor and markedly reduce adhesion, we found that PTPσ in particular plays a critical role in converting growth cones into a dystrophic state by first mediating addiction, and eventually binding them so tightly to CSPG containing substrates that they can no longer advance [36] (Fig. 2).
Figure 2.
Extreme “addiction,” or entrapment occurs within the CSPG-rich glial scar that results from injury to the CNS. Overabundant expression of CSPG-receptors results in tight adhesion to injury-associated CSPGs, especially to the NG2 expressed by polydendrocytes within the lesion. Entrapped axons can escape and regenerate beyond the glial scar through treatments (i.e. Ch’ase ABC) that sever the CS-GAG/CSPG receptor connection.
Interestingly, in vivo, this injury-associated “extreme” addiction or entrapment within CSPGs may depend less on secreted lecticans and more on the membrane-anchored CSPG, NG2. NG2 is purportedly one of the more inhibitory CSPGs produced after SCI [37–39]. Upon SCI, severed axons dieback from the lesion core into the injury penumbra, where they closely associate with NG2+ glial cells [40,41]. These polydendrocytes (for review see [42]) produce a complex ECM containing the membrane-bound CSPG as well as laminin and fibronectin. We investigated the role that membrane-bound CSPGs played in this tight cell-axon interaction and whether over-adhesion to these NG2-expressing cells might play a role in regeneration failure. Studies using CSPGs in combination with other adhesion molecules as well as adult cord-derived NG2+ glia suggest that CSPGs are, indeed, involved in entrapping neurons. Once dystrophic axons become stabilized on polydendrocytes, they form synaptic-like connections in vitro and in vivo, which could potentially trap axons within the peri-lesional white matter for decades (Fig. 2). PTPσ knockouts exhibit increased axonal regeneration after optic nerve crush [43], dorsal column lesion [23], and corticospinal tract injury [44].
Expression of CSPGs within Diffusely Invasive Gliomas
There is potentially a third CSPG-mediated migration behavior that occurs in infiltrative glioma. High-grade astrocytoma is characterized by the diffuse infiltration of tumor cells throughout the brain. CSPGs have long been implicated in this diffusely invasive pathology [45,46], however the aforementioned turning and addiction mechanisms likely do not apply. Recall that both turning and addiction depend heavily on the relative abundance and positioning of CSPGs as well as the expression profile of CSPG receptors. On the receptor side, recent data suggests that LAR, NgR1, and NgR3-expression in invasive glioma are only minimally expressed and indistinguishable from the adult, non-tumor bearing brain [47]. Further, CSPG core protein levels [48] as well as glycosylated CSPGs [47] are also in low abundance and not significantly different between the glioma microenvironment and healthy, non-malignant brain tissue. Thus, whereas the turning and addictive behaviors represent restrictions and constraints on the movement of cells, the widespread infiltration of glioma likely represents a separate biology.
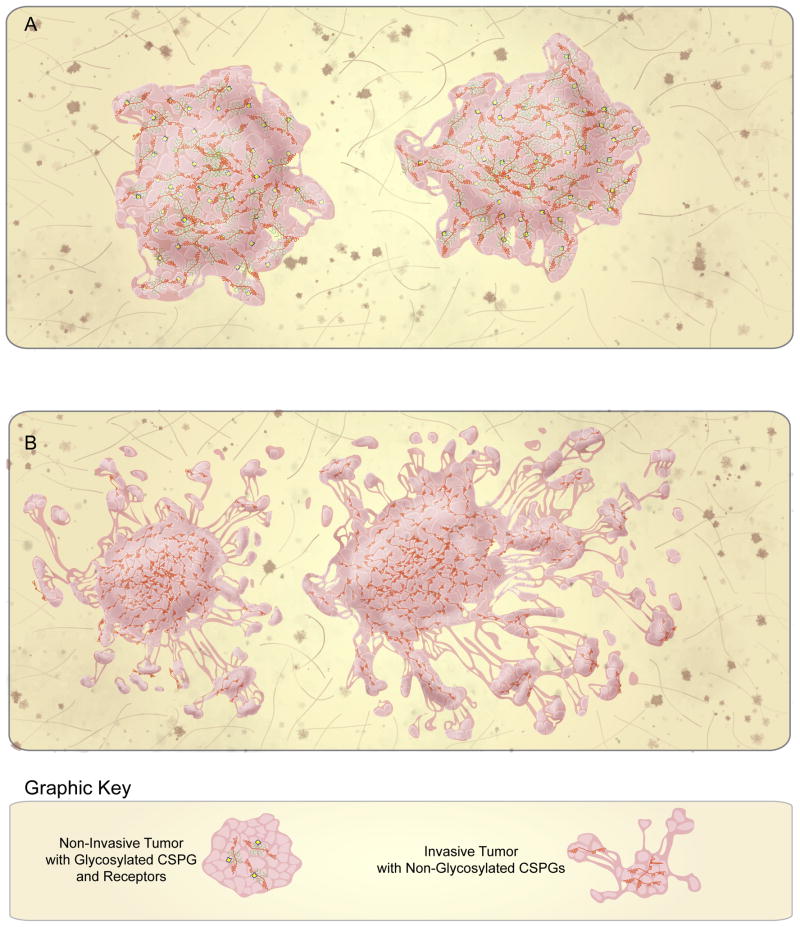
If not turning or addiction, how can we reconcile the diffuse infiltration of tumor cells within a CSPG-containing ECM? Because CSPG-mediated migration is dependent on CS-GAGs, it is striking that more and more evidence suggests that the invasive glioma ECM is comprised of CSPGs that almost entirely lack CS-GAG side chains [46,47,49,50] (Fig. 3B). In this regard, it is worth exploring the possibility that the long-standing association between CSPGs and tumor infiltration might more accurately represent a lack of inhibition rather than the promotion of invasion. Multiple reports corroborate the scarcity of CS-GAGs from the glioma microenvironment and multiple splice variations and proteolytic cleavage reactions have been discovered in glioma cells that lead to the generation of non-glycosylated CSPGs [49–52].
Figure 3.
CSPG addiction distinguishes non-invasive from invasive brain tumors. (A) Non-invasive brain tumors, such as non-neural brain metastases and certain low-grade gliomas express abundant CSPG receptors and glycosylated CSPGs, which mediate the self-containment of these tumors. (B) In contrast, invasive brain tumors, such as GBM, express various CSPG core proteins, but lack CS-glycosylation and CSPG receptors. The absence of addiction and the “loosening” of the brain ECM facilitates the diffuse infiltration of these tumors throughout the brain.
The idea that CSPGs enable invasion extends from a series of reports published between the late 1990s and the early 2000s, in which a connection between brevican (BCAN) and invasive glioma was first described and then elaborated. Interestingly, while the structure and nomenclature of this BCAN molecule has been revised, investigators have held to the conclusion that glioma associated BCAN is an under-glycosylated or non-glycosylated species [45,46,50]. Inspired by these studies, investigators have expanded the scope of the analysis to additional lecticans as well as PCAN. For instance, Muller and colleagues demonstrated that the short (6.4kb), non-glycosylated, soluble splice variant of PCAN predominates the glioma ECM [49] and we have very recently demonstrated abundant, non-glycosylated BCAN, NCAN, PCAN, and VCAN species within patient-derived, infiltrative astrocytomas [47].
Functionally, multiple studies have demonstrated that CSPGs make important regulatory contributions to tumor cell invasion, however the molecular/mechanistic details of these regulations remain largely uncertain. CSPGs are known to bind adhesion [53] and motility [51] factors, which in turn, may act directly on invasive tumor cells. However, in terms of the CSPGs specifically, there is simply the indication, from multiple in vitro invasion assays as well as in vivo xenograft and allograft tumor models that CS-GAG side chain depletion is important in promoting glioma invasion. For instance, over-expression of the non-glycosylated hyaluronan-binding domain of BCAN [46] or the full-length molecule [52] from which this domain is readily generated, results in measurably increased tumor volumes.
Interestingly, in contrast it is clear that CS-GAG-rich, LAR-expressing tumors derived from the human glioma cell line U-87MG and the human grade II glioma cell line L7, grow expansively without microscopic infiltration and essentially mirror the CSPG-mediated addictive growth pattern displayed by developing and and regenerating neurons [47] (Fig. 3A). We have also observed an addictive form of growth from brain metastases generated by the CS-GAG-expressing human breast carcinoma line M4A4 (D. J. Silver, unpublished data). Importantly, we have demonstrated that enzymatic digestion of CS-GAGs away from glioma-associated CSPGs can convert these non-invasive, U-87MG-derived tumors into diffusely infiltrating lesions [47].
One potential explanation for the role of CSPGs in diffuse glioma invasion was provided by the hyaluronan-lectican-tenascin-R (HLT) matrix model of the brain ECM [2]. The HLT model explains that the “tightness” of the brain ECM, or the degree to which the ECM resists the movement of cells, is dependent on abundant CS-GAGs. Thus, an invasion-permissive environment is established in glioma by destabilizing the otherwise inhibitory brain ECM with less inhibitory, CS-GAG deplete CSPGs. In total, these reports present CSPGs as important, albeit indirect facilitators or enablers of invasion. Further, these results hint that CS-GAGs could potentially oppose tumor cell infiltration (in accordance with their turning and addictive functions) but their absence or removal from the invasive glioma microenvironment instead serves to facilitate tumor cell infiltration.
Conclusion
This synthesis of classic and more recent studies presents a novel perspective into the biology of a family of molecules that has been studied in the nervous system for nearly 3 decades. It is clear that CSPGs, which were initially characterized as strictly inhibitory, actually mediate a diverse array of migratory behaviors that can both promote and inhibit the movement of cells and extending processes. Clinically, uncoupling entrapped axons from their constraints within the glial scar or peri-neuronal net has long been an attractive therapeutic possibility. The recent discoveries of the CS-GAG-binding, CSPG receptors LAR, PTPσ, NgR1, 2, and 3 offers the promise of currently unexplored therapeutic targets to leverage against this goal. On the tumor side, the picture may be more complicated. On one hand, the absence of CS-GAGs from the glioma microenvironment suggests that activation of one (or more) critical members of the CS-GAG post-translational modification machinery might be effective in a novel anti-invasive strategy. However, based on this new understanding of CSPGs, CS-GAGs, and CS-GAG receptors, could anti-invasive tumor therapies compromise the intrinsic plasticity of the CNS? What of the rare CNS injured patients with co-morbid CNS tumors or brain metastases? Could attempted regeneration through CSPG modification (especially if applied for long time periods) inadvertently exacerbate tumor infiltration? Seeking the foundational knowledge that clarifies this spectrum of cellular/environmental interactions has untold value. We offer this, more holistic view of matrix biology of the developing, injured, and tumor-bearing CNS as one attempt that may prove provocative and insightful.
Highlights.
CSPGs mediate three distinct modes of cellular migration.
CSPGs can redirect, confine, or amplify cell migration.
CSPG structure, time, location, and receptor profile determine migratory mode.
Acknowledgments
We would like to thank Mesa Schumacher for her assistance with the graphical illustration of these concepts. These efforts were supported by NINDS R01 NS025713 (JS).
Footnotes
Conflict of Interest Statement:
The authors have no financial or personal conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 4.Katoh-Semba R, Matsuda M, Kato K, Oohira A. Chondroitin sulphate proteoglycans in the rat brain: candidates for axon barriers of sensory neurons and the possible modification by laminin of their actions. Eur J Neurosci. 1995;7:613–621. doi: 10.1111/j.1460-9568.1995.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 5(•).Snow DM, Steindler DA, Silver J. Molecular and cellular characterization of the glial roof plate of the spinal cord and optic tectum: a possible role for a proteoglycan in the development of an axon barrier. Dev Biol. 1990;138:359–376. doi: 10.1016/0012-1606(90)90203-u. This is the first paper that described the presence of sulfated proteoglycans within a prospective astrogial structure that formed a normal boundary region to the passage of axons within the developing spinal cord. [DOI] [PubMed] [Google Scholar]
- 6.Cole GJ, McCabe CF. Identification of a developmentally regulated keratan sulfate proteoglycan that inhibits cell adhesion and neurite outgrowth. Neuron. 1991;7:1007–1018. doi: 10.1016/0896-6273(91)90345-z. [DOI] [PubMed] [Google Scholar]
- 7.Wu DY, Schneider GE, Silver J, Poston M, Jhaveri S. A role for tectal midline glia in the unilateral containment of retinocollicular axons. J Neurosci. 1998;18:8344–8355. doi: 10.1523/JNEUROSCI.18-20-08344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- 9.Jhaveri S. Midline glia of the tectum: a barrier for developing retinal axons. Perspect Dev Neurobiol. 1993;1:237–243. [PubMed] [Google Scholar]
- 10.Becker CG, Becker T. Repellent guidance of regenerating optic axons by chondroitin sulfate glycosaminoglycans in zebrafish. J Neurosci. 2002;22:842–853. doi: 10.1523/JNEUROSCI.22-03-00842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung KY, Taylor JS, Shum DK, Chan SO. Axon routing at the optic chiasm after enzymatic removal of chondroitin sulfate in mouse embryos. Development. 2000;127:2673–2683. doi: 10.1242/dev.127.12.2673. [DOI] [PubMed] [Google Scholar]
- 12.Oakley RA, Tosney KW. Peanut agglutinin and chondroitin-6-sulfate are molecular markers for tissues that act as barriers to axon advance in the avian embryo. Dev Biol. 1991;147:187–206. doi: 10.1016/s0012-1606(05)80017-x. [DOI] [PubMed] [Google Scholar]
- 13.Akita K, von Holst A, Furukawa Y, Mikami T, Sugahara K, Faissner A. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem Cells. 2008;26:798–809. doi: 10.1634/stemcells.2007-0448. [DOI] [PubMed] [Google Scholar]
- 14.Gates MA, Thomas LB, Howard EM, Laywell ED, Sajin B, Faissner A, Gotz B, Silver J, Steindler DA. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 15.Silver DJ, Steindler DA. Common astrocytic programs during brain development, injury and cancer. Trends Neurosci. 2009;32:303–311. doi: 10.1016/j.tins.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas LB, Gates MA, Steindler DA. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17(•).Bicknese AR, Sheppard AM, O’Leary DD, Pearlman AL. Thalamocortical axons extend along a chondroitin sulfate proteoglycan-enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci. 1994;14:3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. This is an interesting paper that first questioned the role of CSPGs as absolute barriers to axon growth since it described a cohort of axons that preferred to grow within a CSPG laden territory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawthorne AL, Wylie CJ, Landmesser LT, Deneris ES, Silver J. Serotonergic neurons migrate radially through the neuroepithelium by dynamin-mediated somal translocation. J Neurosci. 2010;30:420–430. doi: 10.1523/JNEUROSCI.2333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 20.Dillon GP, Yu X, Bellamkonda RV. The polarity and magnitude of ambient charge influences three-dimensional neurite extension from DRGs. J Biomed Mater Res. 2000;51:510–519. doi: 10.1002/1097-4636(20000905)51:3<510::aid-jbm28>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21(•).Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. An interesting study that extends the capacity of CS-GAG binding beyond the LAR family of receptors to classic myelin associated inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22(•).Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. The second in a series of papers that identify novel CS-GAG binding, CSPG-receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23(••).Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. This critical paper is the first to have identified a receptor that mediates the inhibitory actions of CSPGs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan Y, Giger RJ. A new role for RPTPsigma in spinal cord injury: signaling chondroitin sulfate proteoglycan inhibition. Sci Signal. 2010;3:pe6. doi: 10.1126/scisignal.3110pe6. [DOI] [PubMed] [Google Scholar]
- 25.Hileman RE, Fromm JR, Weiler JM, Linhardt RJ. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays. 1998;20:156–167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Bäumer BE, Kurz A, Borrie SC, Sickinger S, Dours-Zimmermann MT, Zimmermann DR, Bandtlow CE. Nogo Receptor Homolog NgR2 Expressed in Sensory DRG Neurons Controls Epidermal Innervation by Interaction with Versican. J Neurosci. 2014;34:1633–1646. doi: 10.1523/JNEUROSCI.3094-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimpe B, Pressman Y, Lupa MD, Horn KP, Bunge MB, Silver J. The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol Cell Neurosci. 2005;28:18–29. doi: 10.1016/j.mcn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Kuffler DP, Sosa IJ, Reyes O. Schwann cell chondroitin sulfate proteoglycan inhibits dorsal root ganglion neuron neurite outgrowth and substrate specificity via a soma and not a growth cone mechanism. J Neurosci Res. 2009;87:2863–2871. doi: 10.1002/jnr.22132. [DOI] [PubMed] [Google Scholar]
- 30.Condic ML. Adult neuronal regeneration induced by transgenic integrin expression. J Neurosci. 2001;21:4782–4788. doi: 10.1523/JNEUROSCI.21-13-04782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condic ML, Snow DM, Letourneau PC. Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression. J Neurosci. 1999;19:10036–10043. doi: 10.1523/JNEUROSCI.19-22-10036.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu WL, Fu SL, Wang YX, Li Y, Lu HZ, Xu XM, Lu PH. Chondroitin sulfate proteoglycans regulate the growth, differentiation and migration of multipotent neural precursor cells through the integrin signaling pathway. BMC Neurosci. 2009;10:128. doi: 10.1186/1471-2202-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31:6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filous AR, Evans TA, Lang BT, Silver J. Synaptic-like connections between dystrophic axons and NG2+ cells: Another reason for regeneration failure after spinal cord injury. Society for Neuroscience Annual Meeting; November 9–13; San Diego, California. 2013. [Google Scholar]
- 35.Ramon y Cajal S. Degeneration and regeneration of the nervous system. Vol. 2. New York, New York, USA: Haffner Publishing Co; 1928. [Google Scholar]
- 36.Lang BT, Cregg JM, DePaul MA, Filous AR, Tran AP, Li S, Evans TA, Busch SA, Silver J. Non-invasive systemic modulation of the CSPG receptor PTPσ promotes locomotor and urinary recovery following severe contusive spinal cord injury. Society for Neuroscience Annual Meeting; November 9–13; San Diego, California. 2013. [Google Scholar]
- 37.Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. J Anat. 2005;207:717–725. doi: 10.1111/j.1469-7580.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40(••).Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci. 2010;30:255–265. doi: 10.1523/JNEUROSCI.3705-09.2010. Here, we demonstrate a complex cellular interplay between injured axons, NG2-glia and infiltrating macrophages. This study was foundational for understanding how CSPGs might entrap frustrated axons within the penumbral zone of a CNS injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busch SA, Horn KP, Silver DJ, Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci. 2009;29:9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 43.Sapieha PS, Duplan L, Uetani N, Joly S, Tremblay ML, Kennedy TE, Di Polo A. Receptor protein tyrosine phosphatase sigma inhibits axon regrowth in the adult injured CNS. Mol Cell Neurosci. 2005;28:625–635. doi: 10.1016/j.mcn.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58:423–433. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- 45(•).Jaworski DM, Kelly GM, Piepmeier JM, Hockfield S. BEHAB (brain enriched hyaluronan binding) is expressed in surgical samples of glioma and in intracranial grafts of invasive glioma cell lines. Cancer Res. 1996;56:2293–2298. Seminal paper, which first described the link between CSPG-expression and infiltrative glioma. [PubMed] [Google Scholar]
- 46.Zhang H, Kelly G, Zerillo C, Jaworski DM, Hockfield S. Expression of a cleaved brain-specific extracellular matrix protein mediates glioma cell invasion In vivo. J Neurosci. 1998;18:2370–2376. doi: 10.1523/JNEUROSCI.18-07-02370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47(••).Silver DJ, Siebzehnrubl FA, Schildts MJ, Yachnis AT, Smith GM, Smith AA, Scheffler B, Reynolds BA, Silver J, Steindler DA. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J Neurosci. 2013;33:15603–15617. doi: 10.1523/JNEUROSCI.3004-12.2013. With this study, we demonstrate that invasion of high-grade glioma occurs in the absence of a CS-GAG-rich, inhibitory matrix in contrast to non-invasive, CS-GAG and LAR-expressing non-invasive tumors. Further, we suggest that the absence of glycosylated CSPGs provides favorable conditions for the diffuse infiltration that typifies high-grade glioma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varga I, Hutoczki G, Szemcsak CD, Zahuczky G, Toth J, Adamecz Z, Kenyeres A, Bognar L, Hanzely Z, Klekner A. Brevican, neurocan, tenascin-C and versican are mainly responsible for the invasiveness of low-grade astrocytoma. Pathol Oncol Res. 2012;18:413–420. doi: 10.1007/s12253-011-9461-0. [DOI] [PubMed] [Google Scholar]
- 49.Muller S, Kunkel P, Lamszus K, Ulbricht U, Lorente GA, Nelson AM, von Schack D, Chin DJ, Lohr SC, Westphal M, et al. A role for receptor tyrosine phosphatase zeta in glioma cell migration. Oncogene. 2003;22:6661–6668. doi: 10.1038/sj.onc.1206763. [DOI] [PubMed] [Google Scholar]
- 50.Viapiano MS, Bi WL, Piepmeier J, Hockfield S, Matthews RT. Novel tumor-specific isoforms of BEHAB/brevican identified in human malignant gliomas. Cancer Res. 2005;65:6726–6733. doi: 10.1158/0008-5472.CAN-05-0585. [DOI] [PubMed] [Google Scholar]
- 51.Arslan F, Bosserhoff AK, Nickl-Jockschat T, Doerfelt A, Bogdahn U, Hau P. The role of versican isoforms V0/V1 in glioma migration mediated by transforming growth factor-beta2. Br J Cancer. 2007;96:1560–1568. doi: 10.1038/sj.bjc.6603766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viapiano MS, Hockfield S, Matthews RT. BEHAB/brevican requires ADAMTS-mediated proteolytic cleavage to promote glioma invasion. J Neurooncol. 2008;88:261–272. doi: 10.1007/s11060-008-9575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu B, Kong LL, Matthews RT, Viapiano MS. The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. J Biol Chem. 2008;283:24848–24859. doi: 10.1074/jbc.M801433200. [DOI] [PMC free article] [PubMed] [Google Scholar]