Abstract
Signaling pathways and cellular processes that regulate neural development are used post-developmentally for proper function and maintenance of the nervous system. Genes that have been studied in the context of the development of Drosophila peripheral nervous system (PNS) and neuromuscular junction (NMJ) have been identified as players in the pathogenesis of human neurodegenerative diseases, including spinocerebellar ataxia, amyotrophic lateral sclerosis, and spinal muscular atrophy. Hence, by unraveling the molecular mechanisms that underlie proneural induction, cell fate determination, axonal targeting, dendritic branching, and synapse formation in Drosophila, novel features related to these disorders have been revealed. In this review, we summarize and discuss how studies of Drosophila PNS and NMJ development have provided guidance in experimental approaches for these diseases.
Keywords: spinocerebellar ataxia, SCA, amyotrophic lateral sclerosis, ALS, spinal muscular atrophy, SMA, neurodegenerative diseases, peripheral nervous system, PNS development, neuromuscular junction, NMJ
Introduction
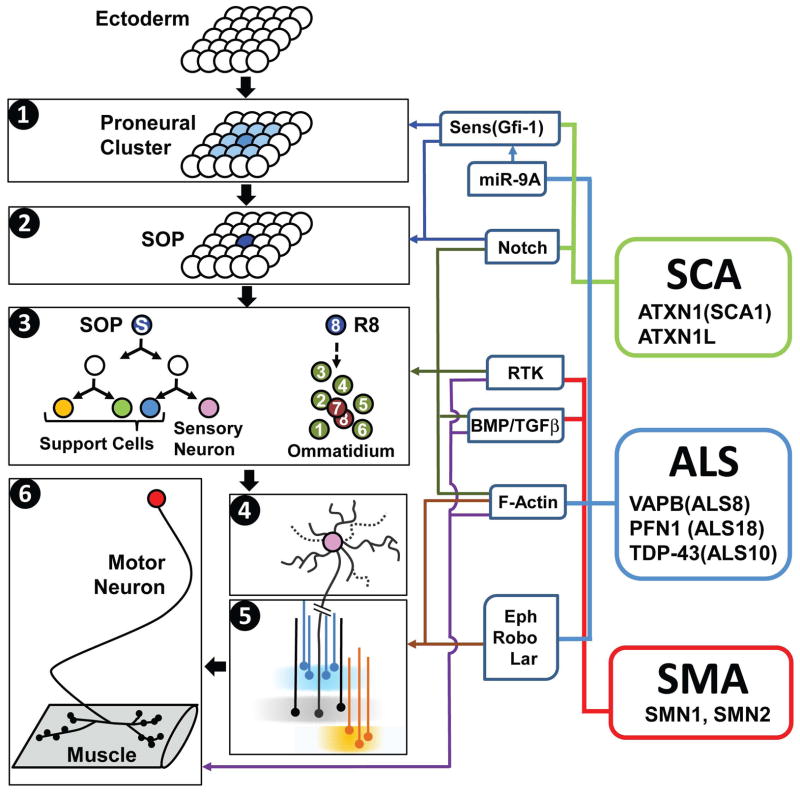
Drosophila is a powerful model organism to study neural development and neuronal maintenance [1]. The development of a neuron typically includes proneural induction, cell fate determination, axonal targeting, dendritic branching, and synapse formation (Figure 1). Based on their anatomical features and accessibility, different types of neurons are better suited to study different steps. For example, proneural induction and cell fate specification is more easily studied in external sensory organs (ESOs) [2], axonal targeting in photoreceptor cells [3], dendrite branching and pruning in multidendritic neurons [4], and synapse formation and function in the neuromuscular junction (NMJ) [5,6]. Although invertebrate biologists typically consider NMJs as part of the CNS, considering their peripheral localization and significant contribution to our understanding of synaptogenesis [5], we also included NMJ studies in this review. By making use of the unique features of different cell types of the fly PNS, and by applying sophisticated genetic manipulations [7], many evolutionarily conserved genes and pathways that regulate these processes have been discovered [1,8].
Figure 1. Molecular links between Drosophila PNS/NMJ development and human neurodegenerative diseases.
Left: Schematic diagrams of different steps of fly PNS/NMJ development. (1) Proneural induction, (2) lateral inhibition, (3) cell fate specification in the ESO (left) and in the eye (right), (4) dendrite development, (5) axon growth and targeting, (6) synapse development and maintenance. Middle: Selected signaling pathways and components that are commonly involved in different steps of fly PNS/NMJ development and human neurodegenerative diseases. Right: Selected human neurological diseases and selected causative genes. See main text for abbreviations.
Studies of genes involved in the development of Drosophila PNS and NMJ have provided important insights in the pathogenesis of several neurodegenerative diseases, including spinocerebellar ataxias (SCA), amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA). For example, the proneural gene senseless (sens) was shown to regulate PNS development and later was implicated in the pathogenesis of SCA1 [9]. The Eph receptor was found to bind to major sperm protein (MSP) at NMJs and was later shown to be a key modifier of ALS in human [10–12]. Moreover, Fibroblast Growth Factor (FGF) signaling at the NMJ provided a link between this pathway and SMA pathogenesis [13,14]. This review will focus on the contribution and the interplay between basic research in the Drosophila PNS and NMJ fields and neurodegenerative diseases.
Links between molecular mechanisms of neurodegenerative diseases and PNS/NMJ development
Spinocerebellar ataxia (SCA)
SCAs are neurodegenerative diseases that primarily affect the spinal cord and the cerebellum [15]. A subset of SCA types (SCA1–3, 6–7, and 17) are caused by CAG trinucleotide repeat expansion which encode polyglutamine (polyQ) tracts in a variety of proteins. While long polyQ tracts by themselves can be cell toxic, recent studies indicate that modulations of the normal functions of the disease-causing proteins are also important in the pathogenesis [15].
SCA1 is caused by a polyQ expansion in ATAXIN-1 (ATXN1) [16]. The first link between ATXN1 and Drosophila PNS development came from a yeast two-hybrid screen which used Sens as bait to identify ATXN1 as an interactor [9]. Sens, a zinc finger transcription factor, regulates the early development of PNS organs [17]. Absence of sens leads to embryonic PNS cell death in Drosophila [17] and loss of the mouse homolog, Growth Factor Independent 1 (Gfi1), causes inner ear hair cell death [18]. During proneural induction in PNS development, the expression of proneural transcription factors is initiated in a group of ectodermal cells (proneural cluster) [19]. Subsequently, proneural proteins and Sens form a positive feedback loop to designate a single sensory organ precursor cell [20] through lateral inhibition, a process mediated by Notch signaling [17,21]. The SOP then undergoes several rounds of asymmetric cell division to generate a PNS organ. Differential Notch signaling activation and a combinatorial transcription factor code determines the fate of each cell type [2,22,23]. Overexpression of human ATXN1 can impact fly PNS development by promoting degradation of Sens and inhibiting SOP formation [9].
Similar to the fly, human ATXN1 interacts with Gfi1 [9]. Gfi1 is expressed in vertebrate PNS neurons [18] and cerebellar Purkinje cells (PC) [9], the most affected neurons in SCA1. Interestingly, overexpression of the polyQ-containing ATXN1 in mice (SCA1 mice) decreases the levels of Gfi1 prior to PC death, a phenotype that is further enhanced by loss of one copy of endogenous Gfi1. In summary, these data indicate that loss of Gfi1 and the consequent transcriptional dysregulation contributes to PC death in SCA1 pathology, and hence striking parallels can be drawn between PNS development in Drosophila and PC maintenance in vertebrates.
Another study links SCA1 and fly PNS development through Notch signaling [24]. In vertebrates, ATXN1 has another homolog, named ATAXIN-1 like (ATXN1L). To probe its function, Tong et al. (2011) overexpressed ATXN1L in Drosophila and observed a Notch signaling defect [24]. This phenotype is suppressed by mutations in Suppressor of Hairless [Su(H)], a transcriptional effector of Notch signaling [25]. Similarly, ATXN1L and ATXN1 exhibit repressive effects on Notch signaling through binding to the mammalian homolog of Su(H), CBF1/RBP-J_in cultured cells. Hence, ATXN1 and ATXN1L may form a repressor complex with Su(H) to inhibit Notch signaling, an idea that should be tested in vivo in mice. Interestingly, the protein level of ATXN1L is reduced prior to PC death in SCA1 mice [24], although Notch signaling activity in these dying cells has not been documented. Nevertheless, given that Sens/Gfi1 and Notch signaling form a feedback loop in the fly PNS [21] as well as in mammalian hematopoietic stem cell lineages [26], it is tempting to speculate that the disruption of this network in adult PC contributes to SCA1 pathogenesis.
The study of signaling pathways in Drosophila PNS development can also drive the discovery of potential therapeutic treatments. Phosphorylation of ATXN1 at Serine 776 (S776) is critical for its stability, and the polyQ-containing ATXN1 with S776A mutation exhibits reduced toxicity [27]. Therefore, suppressing the kinase activity for S776 phosphorylation is likely to reduce ATXN1 toxicity. A recent RNAi-based screen for such kinases in mammalian cells and fly retina identified the Ras-MAPK pathway as a regulator of ATXN1 phosphorylation [28]. Inhibition of numerous components in this pathway suppresses the neurodegenerative phenotype in SCA1 flies and mice, providing a potential therapeutic target. Ras-MAPK pathway is the downstream effector of receptor tyrosine kinase (RTK) signaling and has been extensively studied in Drosophila photoreceptor cell fate determination [8]. Many important components in this pathway, including downstream of receptor kinases (Drk) and Son of Sevenless (SOS), were identified in Drosophila forward genetic screens [20]. Therefore, identification and characterization of signaling pathway components in the Drosophila PNS facilitates in vivo studies in flies and mice.
Amyotrophic lateral sclerosis (ALS)
ALS is characterized by progressive loss of both upper and lower motor neurons, leading to muscle atrophy [29]. About 15% of all ALS are familial and are inherited dominantly. ALS causing genes are functionally diverse and mutations in these genes often interfere with normal protein functions [29,30]. Most of the genes that cause ALS are evolutionarily conserved and numerous Drosophila ALS related studies have revealed interesting features of pathogenesis [31]. Here, we focus on the studies of VAMP-associated membrane protein B (VAPB), Profilin1 (PFN1), and TAR DNA-binding protein 43 (TDP-43).
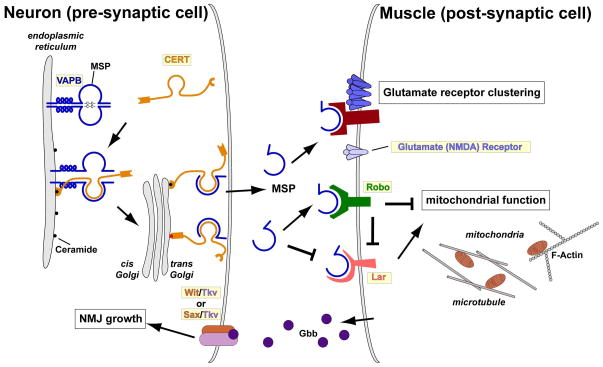
VAPB is the causative gene of ALS8 and SMA [32]. Its function was studied at the Drosophila NMJ prior to its identification as an ALS causing gene [33]. Drosophila VAPB (dVAP) mutants exhibit a severe reduction in bouton number and an increase in bouton size, while motor neuron specific dVAP overexpression causes the opposite NMJ phenotype. Based on experiments at the NMJ in flies and worms, the following model has emerged (Figure 2): VAPB is associated with the Endoplasmic Reticulum (ER) [9,34] where it is implicated in protein folding and ceramide transport together with ceramide transfer protein (CERT) to the Golgi complex [35,36]. Loss of VAPB in vertebrate cells leads to defects in ceramide transport from the ER to the Golgi [35]. Consistently, ceramide is enriched in the spinal cord in ALS patients [37]. In addition to its role in lipid homeostasis, VAPB also serves as a signaling molecule. Its MSP domain is cleaved and secreted from motor neurons into circulation, and the MSP moiety is also present in mammalian blood [11,38]. The disease causing mutation (P56S) in VAPB disrupts this cleavage and secretion, leading to accumulation of ubiquitinated aggregates in the ER. This induces an ER-associated unfolded protein response (UPR) [10,11,39], which is also observed in ALS model mice and ALS patients [30]. Secreted VAPB binds to Eph receptor (an RTK) on muscles and leads to un-clustering of glutamate receptors, whereas loss of VAPB causes hyper-clustering [10,11]. Glutamate receptor hyper-clustering promotes Ca2+ influx, which may mediate excitotoxicity. Interestingly, EPHA4, one of the human Eph homologs, has recently been identified as a modifier of ALS related phenotypes in mice and human [12]. Secreted VAPB also binds to Robo (a growth cone guidance molecule associated with kinases) and Lar (a receptor tyrosine phosphatase) to control mitochondria structure/function in muscles through Arp2/3-dependent actin cytoskeleton remodeling [38]. Loss of VAPB, Lar, or Robo impairs muscle function, resulting in severe mitochondrial defects and subsequent neurodegeneration. Similar muscular defects have also been reported in ALS model mice [40]. These data suggest that loss of VAPB is an initiator of disease progression, and that reintroduction of exogenous secreted VAPB may be a beneficial therapeutic intervention.
Figure 2. A schematic model of the VAPB signaling pathway.
VAPB (blue) is associated with the ER and regulates protein folding and ceramide transport from the ER to the Golgi together with CERT. The full length VAP protein is cleaved, releasing the MSP domain from the neuron. MSP binds the growth cone guidance receptors in the muscle, including Eph, Lar, and Robo. MSP binding to Eph leads to unclustering of Glutamate receptors, and binding to Lar and Robo is required for proper mitochondrial morphology, localization, and function through regulation of the actin and tubulin cytoskeleton. See main text for abbreviations.
Profilin is a downstream effector of Robo and Lar that regulates the formation of filamentous-actin. Drosophila Profilin is required for axon outgrowth during motor neuron development [41,42]. Recently, PFN1 was found to cause ALS18 and be responsible for 1–2% of familial ALS [43]. Overexpression of mutant PFN1 leads to formation of ubiquitinated protein aggregates and causes cytoskeletal defects including axonal outgrowth defects in mammalian cells, consistent with earlier fly studies. The studies on Profilin and VAPB indicate that the dynamics of actin polymerization in muscles is important in ALS pathogenesis. Actin and microtubules are key coordinators of many cellular processes [44] and in many cases, alterations in actin dynamics affect microtubule structure and vice versa. Moreover, Drosophila dVAP mutants exhibit disorganized microtubular structures [33], and mutations in Dynactin 1 (DCTN1; a microtubule and dynein binding protein) are also associated with ALS [45]. These studies indicate that cytoskeleton alterations play key roles in the pathogenesis of ALS. The main question is to determine if they all impinge on similar or different cellular processes.
TDP-43 is the causative gene of ALS10 [46] and encodes a DNA/RNA-binding protein that regulates transcription, alternative splicing, and stability of hundreds of RNAs [29]. Among these RNA targets, mutations in TDP-43 may affect certain RNAs which play a predominant role in the pathogenesis of ALS. A recent Drosophila study revealed one such potential RNA, microRNA-9a (miR-9a) [47]. miR-9a, a conserved miRNA that regulates SOP development by fine-tuning the expression of Sens [48,49]. Loss of miR-9a leads to Sens upregulation, causing formation of additional sensory organs. In Drosophila TDP-43 mutants, the level of miR-9a is reduced and hence the number of SOPs is increased. While it remains to be established whether miR-9a is reduced and whether Gfi1 is upregulated in TDP-43 mutant mice, fly data provide an interesting inroad to investigate this avenue in vertebrates. Moreover, miR-9a minimizes the phenotypic variation resulting from individual genetic variants in Drosophila: i.e. miR-9a sets a threshold for the amount of Sens, and the consequences of genomic variations that affect the levels of Sens are filtered, providing phenotypic stability in SOP formation [50]. When the level of miR-9a decreases, SOP formation becomes sensitive to small genetic variances. In ALS10, if the level of miR-9a or other TDP-43 target miRNAs are affected, loss of buffering in genetic variation may promote the pathogenesis caused by minor mutations in the patient’s genome.
Spinal muscular atrophy (SMA)
SMA is an autosomal recessive disorder with progressive loss of lower motor neurons and muscle atrophy [51]. Mutations in Survival of Motor Neuron 1 (SMN1) contribute to the majority of SMA [52]. SMN1 is ubiquitously expressed and regulates pre-mRNA splicing. Drosophila smn mutants exhibit alterations in NMJ morphology, neurotransmission, and locomotion, similar to SMA patients [51,53–56]. Novel insights into the molecular mechanisms of SMA originated from a modifier screen.
Components of the BMP/TGFβ and FGF (an RTK) signaling pathways were identified as enhancers of the smn mutant phenotypes [14,54]. BMP/TGFβ signaling is an important retrograde signal for synapse development at the NMJ, while the role of FGF signaling in this context is not well established [6,57]. Loss of BMP/TGFβ signaling at the NMJ leads to defects in pre-synaptic structure and neurotransmitter release [6,57]. When the level of SMN decreases, the level of phosphorylated Mothers Against Decapentaplegic (pMAD), an effector of BMP/TGFβ signaling, is reduced [54]. In addition, elevated BMP/TGFβ signaling can suppress the NMJ morphology defect in smn mutant, indicating that defective BMP/TGFβ signaling contributes to mutant phenotypes [54]. The same modifier screen also isolated FGF signaling components as modifiers, and activation of FGF signaling specifically in the muscle is sufficient to reverse the NMJ morphology defects in smn mutants [14]. Subsequent studies have documented that FGF signaling is also misregulated in an SMA mouse model [13]. Therefore, pharmacological manipulation of BMP/TGFβ and FGF signaling can be considered as possible therapeutic strategies against SMA.
Studies of the PNS of smn mutant flies also shed light on the neuronal circuitry critical for SMA. Reintroduction of SMN protein in a subpopulation of cholinergic proprioceptive and interneurons of the motor circuit, but not muscles or motor neurons, can rescue NMJ phenotypes in smn mutants [55]. Conversely, specific knockdown of smn in these cholinergic neurons causes SMA-related phenotypes. Therefore, the defects in the sensory-motor circuit may be the primary cause for the motor system defects in smn mutant flies. Interestingly, SMN1 overexpression in cholinergic motor neurons largely rescues motor symptoms in SMA model mice [58,59]. These data indicate that cholinergic neurons of motor circuits are the major site of action for SMN in flies (peripheral/interneurons) and in mammals (lower motor neurons) [60].
Since SMN is ubiquitously expressed, the question remains as to which neurons are selectively vulnerable in SMA patients. One recent study found that splicing of several U12 intron-containing pre-mRNAs are impaired in smn mutant flies [56]. Among these, selective knockdown of stasimon (Stas, a putative vesicular trafficking protein) in cholinergic neurons exhibited SMA-related defects, while many of the SMA-related defects in smn mutant flies can be repaired by overexpression of stas in the motor circuit. These data suggest that mRNA splicing defects in the motor circuit, rather than in motor neurons per se, are responsible for SMA-related motor defects in smn mutant flies. The same splicing defect in Tmem41b (mouse homolog of stas) is also observed in SMA mice, indicating that this is an evolutionarily conserved target [56]. It will be interesting to investigate whether overexpression of Tmem41b can correct the SMA-related defects in SMA mice.
Conclusions
Many evolutionarily conserved genes involved in Drosophila PNS and NMJ development have not yet been linked to neurological disorders in humans. On the other hand, sequencing of human exomes is revealing numerous variants in patients with rare genetic diseases, and many homologs of these genes have been or can be studied in flies. Although mutant flies often do not display the exact phenotypes as humans, the information obtained from studying the endogenous function of a conserved gene is very valuable to identify and characterize the molecular mechanisms underlying diverse human diseases. For example, mutations in flies that cause PNS organ defects may affect Notch signaling and help identify genes that cause neurological or other diseases related to Notch signaling in human [61]. Another examples are Atonal and Sens, fly proneural transcription factors which are required for PNS and eye development [62,63]. In vertebrate, loss of Atoh1/Math1, a homolog of Atonal, leads to cerebellar defects [64], and loss of Gfi1 (Sens homolog) leads to PC loss [9]. Hence, similar molecular mechanisms are probably used in both development and maintenance of the nervous system. Moreover, a method to identify non-obvious equivalences between mutant phenotypes in various species has been proposed. Such “phenologs” can now be used to predict genes associated with human diseases [65]. By systematically identifying and characterizing additional genes involved in PNS and NMJ development, we foresee that one will be able to predict and discover novel human disease genes using a similar approach. In sum, the Drosophila PNS and NMJ have been and will continue to be valuable model systems to reveal the molecular mechanisms underlying neurodegenerative disorders.
Highlights.
VAPB signaling at the NMJ is involved in the pathogenesis of ALS
PNS studies implicate a link between microRNA mediated genomic stability and ALS
A major mRNA splicing target of SMN in cholinergic neurons contributes to SMA
Acknowledgments
We would like to apologize to those whose work has not been cited because of topic and space constraints. We would like to thank Drs. Karen L. Schulze and Hsiang-Chih Lu for useful suggestions and critical comments. W.-L.C. was supported by Taiwan Merit Scholarships Program sponsored by the National Science Council (NSC-095-SAF-I-564-015-TMS). S.Y. is a Fellow of the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital. H.J.B. is a Howard Hughes Medical Institute Investigator. We acknowledge support from the NIH grant 1RC4GM096355-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandachar V, Roegiers F. Endocytosis and control of Notch signaling. Curr Opin Cell Biol. 2012;24:534–540. doi: 10.1016/j.ceb.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjieconomou D, Timofeev K, Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol. 2011;21:76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda H, Jafar-Nejad H, Patel AJ, Sun Y, Chen HK, Rose MF, Venken KJ, Botas J, Orr HT, Bellen HJ, et al. The AXH domain of Ataxin-1 mediates neurodegeneration through its interaction with Gfi-1/Senseless proteins. Cell. 2005;122:633–644. doi: 10.1016/j.cell.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Chai A, Withers J, Koh YH, Parry K, Bao H, Zhang B, Budnik V, Pennetta G. hVAPB, the causative gene of a heterogeneous group of motor neuron diseases in humans, is functionally interchangeable with its Drosophila homologue DVAP-33A at the neuromuscular junction. Hum Mol Genet. 2008;17:266–280. doi: 10.1093/hmg/ddm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, et al. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. This paper provides evidence that VAPB can be cleaved and release the MSP moiety that binds to Eph receptors. The authors also document that the ALS8 mutant form of VAPB cannot be cleaved and secreted, leading to protein aggregates in the ER that triggers an UPR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Van Hoecke A, Schoonaert L, Lemmens R, Timmers M, Staats KA, Laird AS, Peeters E, Philips T, Goris A, Dubois B, et al. EPHA4 is a disease modifier of amyotrophic lateral sclerosis in animal models and in humans. Nat Med. 2012;18:1418–1422. doi: 10.1038/nm.2901. In this paper, the authors performed a genetic modifier screen in zebrafish and identified Epha4 as a modifier for the ALS phenotype. Manipulation of Epha4 genetically or pharmacologically can suppress the ALS-related phenotypes in SOD1 and TDP-43 mice. More importantly, Epha4 expression level and mutations are associated with survival and disease onset in humans. [DOI] [PubMed] [Google Scholar]
- 13.Hensel N, Ratzka A, Brinkmann H, Klimaschewski L, Grothe C, Claus P. Analysis of the fibroblast growth factor system reveals alterations in a mouse model of spinal muscular atrophy. PLoS One. 2012;7:e31202. doi: 10.1371/journal.pone.0031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Sen A, Yokokura T, Kankel MW, Dimlich DN, Manent J, Sanyal S, Artavanis-Tsakonas S. Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling. J Cell Biol. 2011;192:481–495. doi: 10.1083/jcb.201004016. This paper continued the work from a previous modifier screen in smn mutants and found that FGF signaling activation in the post-synaptic muscle is able to restore NMJ abnormality in smn mutants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr HT. Cell biology of spinocerebellar ataxia. J Cell Biol. 2012;197:167–177. doi: 10.1083/jcb.201105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr HT, Chung MY, Banfi S, Kwiatkowski TJ, Jr, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LP, Zoghbi HY. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4:221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- 17.Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 18.Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, Bellen HJ. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 20.Sopko R, Perrimon N. Receptor tyrosine kinases in Drosophila development. Cold Spring Harb Perspect Biol. 2013:5. doi: 10.1101/cshperspect.a009050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jafar-Nejad H, Acar M, Nolo R, Lacin H, Pan H, Parkhurst SM, Bellen HJ. Senseless acts as a binary switch during sensory organ precursor selection. Genes Dev. 2003;17:2966–2978. doi: 10.1101/gad.1122403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews HK, Giagtzoglou N, Yamamoto S, Schulze KL, Bellen HJ. Sequoia regulates cell fate decisions in the external sensory organs of adult Drosophila. EMBO Rep. 2009;10:636–641. doi: 10.1038/embor.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore AW, Jan LY, Jan YN. hamlet, a binary genetic switch between single- and multiple- dendrite neuron morphology. Science. 2002;297:1355–1358. doi: 10.1126/science.1072387. [DOI] [PubMed] [Google Scholar]
- 24.Mizutani A, Wang L, Rajan H, Vig PJ, Alaynick WA, Thaler JP, Tsai CC. Boat, an AXH domain protein, suppresses the cytotoxicity of mutant ataxin-1. Embo J. 2005;24:3339–3351. doi: 10.1038/sj.emboj.7600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 26.Phelan JD, Saba I, Zeng H, Kosan C, Messer MS, Olsson HA, Fraszczak J, Hildeman DA, Aronow BJ, Moroy T, et al. Growth factor independent-1 maintains Notch1-dependent transcriptional programming of lymphoid precursors. PLoS Genet. 2013;9:e1003713. doi: 10.1371/journal.pgen.1003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emamian ES, Kaytor MD, Duvick LA, Zu T, Tousey SK, Zoghbi HY, Clark HB, Orr HT. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 2003;38:375–387. doi: 10.1016/s0896-6273(03)00258-7. [DOI] [PubMed] [Google Scholar]
- 28**.Park J, Al-Ramahi I, Tan Q, Mollema N, Diaz-Garcia JR, Gallego-Flores T, Lu HC, Lagalwar S, Duvick L, Kang H, et al. RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature. 2013;498:325–331. doi: 10.1038/nature12204. This paper combined Drosophila genetics with mammalian cell culture studies to screen for suppressors of neurodegeneration caused by overexpression of the pathogenic ATXN1. They identified several components of the RAS–MAPK–MSK1 pathway and further showed that inhibition of this pathway can suppress neurodegeneration in Drosophila and mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 31.Jaiswal M, Sandoval H, Zhang K, Bayat V, Bellen HJ. Probing mechanisms that underlie human neurodegenerative diseases in Drosophila. Annu Rev Genet. 2012;46:371–396. doi: 10.1146/annurev-genet-110711-155456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002;35:291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 34.Wyles JP, McMaster CR, Ridgway ND. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J Biol Chem. 2002;277:29908–29918. doi: 10.1074/jbc.M201191200. [DOI] [PubMed] [Google Scholar]
- 35.Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry RJ, Ridgway ND. Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol Biol Cell. 2006;17:2604–2616. doi: 10.1091/mbc.E06-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- 38**.Han SM, Tsuda H, Yang Y, Vibbert J, Cottee P, Lee SJ, Winek J, Haueter C, Bellen HJ, Miller MA. Secreted VAPB/ALS8 major sperm protein domains modulate mitochondrial localization and morphology via growth cone guidance receptors. Dev Cell. 2012;22:348–362. doi: 10.1016/j.devcel.2011.12.009. This paper identified that secreted MSP from neurons regulates the actin structure and mitochondria arrangement/function in the muscle through binding to Lar and Robo receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moustaqim-Barrette A, Lin YQ, Pradhan S, Neely GG, Bellen HJ, Tsuda H. The Amyotrophic Lateral Sclerosis 8 protein, VAP, is required for ER protein quality control. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Yi J, Fu R, Liu E, Siddique T, Rios E, Deng HX. Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J Biol Chem. 2010;285:705–712. doi: 10.1074/jbc.M109.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YS, Furman S, Sink H, VanBerkum MF. Calmodulin and profilin coregulate axon outgrowth in Drosophila. J Neurobiol. 2001;47:26–38. doi: 10.1002/neu.1013. [DOI] [PubMed] [Google Scholar]
- 42.Wills Z, Marr L, Zinn K, Goodman CS, Van Vactor D. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron. 1999;22:291–299. doi: 10.1016/s0896-6273(00)81090-9. [DOI] [PubMed] [Google Scholar]
- 43*.Wu CH, Fallini C, Ticozzi N, Keagle PJ, Sapp PC, Piotrowska K, Lowe P, Koppers M, McKenna-Yasek D, Baron DM, et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature. 2012;488:499–503. doi: 10.1038/nature11280. The authors identify profilin, an actin binding protein, in ALS. Actin polymerization may play a critical role in ALS given the phenotypes described in worms and flies in ref. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 45*.Munch C, Sedlmeier R, Meyer T, Homberg V, Sperfeld AD, Kurt A, Prudlo J, Peraus G, Hanemann CO, Stumm G, et al. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology. 2004;63:724–726. doi: 10.1212/01.wnl.0000134608.83927.b1. This paper shows that VAPB is required for ER protein quality control and this function is mediated by an ER lipid-binding protein, Oxysterol binding protein (Osbp). dVAP mutant flies exhibit protein accumulation, ER expansion and ER stress and restoration of Osbp in the ER can suppress these phenotypes. The authors also show that VAPB with P56S mutation has impaired interaction with Osbp, which may contribute to ALS pathogenesis. [DOI] [PubMed] [Google Scholar]
- 46.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 47*.Li Z, Lu Y, Xu XL, Gao FB. The FTD/ALS-associated RNA-binding protein TDP-43 regulates the robustness of neuronal specification through microRNA-9a in Drosophila. Hum Mol Genet. 2013;22:218–225. doi: 10.1093/hmg/dds420. The authors identify a target for TDP-43 in flies: miR-9a. This miRNA regulates the level of the Senseless protein in fruit flies, which in turn minimizes genomic variation in ref 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christodoulou F, Raible F, Tomer R, Simakov O, Trachana K, Klaus S, Snyman H, Hannon GJ, Bork P, Arendt D. Ancient animal microRNAs and the evolution of tissue identity. Nature. 2010;463:1084–1088. doi: 10.1038/nature08744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Wang F, Lee JA, Gao FB. MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes Dev. 2006;20:2793–2805. doi: 10.1101/gad.1466306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Cassidy JJ, Jha AR, Posadas DM, Giri R, Venken KJ, Ji J, Jiang H, Bellen HJ, White KP, Carthew RW. miR-9a minimizes the phenotypic impact of genomic diversity by buffering a transcription factor. Cell. 2013;155:1556–1567. doi: 10.1016/j.cell.2013.10.057. This paper provides an example of how miR-9a sets a threshold for phenotypic output and stabilizes the variability in individual genomes, a mechanism that may also underlie TDP-43 mediated ALS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markowitz JA, Singh P, Darras BT. Spinal muscular atrophy: a clinical and research update. Pediatr Neurol. 2012;46:1–12. doi: 10.1016/j.pediatrneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 53.Chan YB, Miguel-Aliaga I, Franks C, Thomas N, Trulzsch B, Sattelle DB, Davies KE, van den Heuvel M. Neuromuscular defects in a Drosophila survival motor neuron gene mutant. Hum Mol Genet. 2003;12:1367–1376. doi: 10.1093/hmg/ddg157. [DOI] [PubMed] [Google Scholar]
- 54.Chang HC, Dimlich DN, Yokokura T, Mukherjee A, Kankel MW, Sen A, Sridhar V, Fulga TA, Hart AC, Van Vactor D, et al. Modeling spinal muscular atrophy in Drosophila. PLoS One. 2008;3:e3209. doi: 10.1371/journal.pone.0003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Imlach WL, Beck ES, Choi BJ, Lotti F, Pellizzoni L, McCabe BD. SMN is required for sensory-motor circuit function in Drosophila. Cell. 2012;151:427–439. doi: 10.1016/j.cell.2012.09.011. This paper identified cholinergic neurons as the primary affected neuronal population in smn mutant flies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Lotti F, Imlach WL, Saieva L, Beck ES, Hao le T, Li DK, Jiao W, Mentis GZ, Beattie CE, McCabe BD, et al. An SMN-dependent U12 splicing event essential for motor circuit function. Cell. 2012;151:440–454. doi: 10.1016/j.cell.2012.09.012. The authors identified stasimon (a putative vesicular trafficking protein) as a major mRNA target of SMN in cholinergic neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayat V, Jaiswal M, Bellen HJ. The BMP signaling pathway at the Drosophila neuromuscular junction and its links to neurodegenerative diseases. Curr Opin Neurobiol. 2011;21:182–188. doi: 10.1016/j.conb.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gogliotti RG, Quinlan KA, Barlow CB, Heier CR, Heckman CJ, Didonato CJ. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J Neurosci. 2012;32:3818–3829. doi: 10.1523/JNEUROSCI.5775-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez TL, Kong L, Wang X, Osborne MA, Crowder ME, Van Meerbeke JP, Xu X, Davis C, Wooley J, Goldhamer DJ, et al. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J Neurosci. 2012;32:8703–8715. doi: 10.1523/JNEUROSCI.0204-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roselli F, Caroni P. A circuit mechanism for neurodegeneration. Cell. 2012;151:250–252. doi: 10.1016/j.cell.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Louvi A, Artavanis-Tsakonas S. Notch and disease: a growing field. Semin Cell Dev Biol. 2012;23:473–480. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frankfort BJ, Nolo R, Zhang Z, Bellen H, Mardon G. senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron. 2001;32:403–414. doi: 10.1016/s0896-6273(01)00480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- 64.Gazit R, Krizhanovsky V, Ben-Arie N. Math1 controls cerebellar granule cell differentiation by regulating multiple components of the Notch signaling pathway. Development. 2004;131:903–913. doi: 10.1242/dev.00982. [DOI] [PubMed] [Google Scholar]
- 65*.McGary KL, Park TJ, Woods JO, Cha HJ, Wallingford JB, Marcotte EM. Systematic discovery of nonobvious human disease models through orthologous phenotypes. Proc Natl Acad Sci U S A. 2010;107:6544–6549. doi: 10.1073/pnas.0910200107. This paper introduces a concept called phenologs or orthologous phenotypes. The authors describe a method to identify candidate disease genes using the orthologous gene network in model organisms despite the phenotypic discrepancy between human and model organisms. [DOI] [PMC free article] [PubMed] [Google Scholar]