Abstract
Background
Treatment with intravenous enzyme replacement therapy and hematopoietic stem cell transplantation for mucopolysaccharidosis (MPS) type I does not address joint disease, resulting in persistent orthopedic complications and impaired quality of life. A proof-of-concept study was conducted to determine the safety, tolerability, and efficacy of intra-articular recombinant human iduronidase (IA-rhIDUA) enzyme replacement therapy in the canine MPS I model.
Methods
Four MPS I dogs underwent monthly rhIDUA injections (0.58 mg/joint) into the right elbow and knee for six months. Contralateral elbows and knees concurrently received normal saline. No intravenous rhIDUA therapy was administered. Monthly blood counts, chemistries, anti-rhIDUA antibody titers, and synovial fluid cell counts were measured. Lysosomal storage of synoviocytes and chondrocytes, synovial macrophages and plasma cells were scored at baseline and one month following the final injection.
Results
All injections were well-tolerated without adverse reactions. One animal required prednisone for spinal cord compression. There were no clinically significant abnormalities in blood counts or chemistries. Circulating anti-rhIDUA antibody titers gradually increased in all dogs except the prednisone-treated dog; plasma cells, which were absent in all baseline synovial specimens, were predominantly found in synovium of rhIDUA-treated joints at study-end.
Lysosomal storage in synoviocytes and chondrocytes following 6 months of IA-rhIDUA demonstrated significant reduction compared to tissues at baseline, and saline-treated tissues at study-end. Mean joint synovial GAG levels in IA-rhIDUA joints was 8.62±5.86 μg/mg dry weight and 21.6±10.4 μg/mg dry weight in control joints (60% reduction). Cartilage heparan sulfate was also reduced in the IA-rhIDUA joints (113±39.5 ng/g wet weight) compared to saline-treated joints (142±56.4 ng/g wet weight). Synovial macrophage infiltration, which was present in all joints at baseline, was abolished in rhIDUA-treated joints only.
Conclusions
Intra-articular rhIDUA is well-tolerated and safe in the canine MPS I animal model. Qualitative and quantitative assessments indicate that IA-rhIDUA successfully reduces tissue and cellular GAG storage in synovium and articular cartilage, including cartilage deep to the articular surface, and eliminates inflammatory macrophages from synovial tissue.
Keywords: mucopolysaccharidosis, lysosomal storage disorder, orthopedic, therapy, canine, model, joint, chondrocyte, synovium, enzyme replacement, articular, treatment
1. INTRODUCTION
1.1
The mucopolysaccharidoses (MPSs) are a group of inborn errors of metabolism linked by deficiencies in lysosomal hydrolases that catalyze the stepwise degradation of glycosaminoglycans (GAGs), modified saccharide polymers found throughout the body. As a result of the enzyme deficiency, GAGs that are normally recycled in a healthy individual cannot be degraded in the MPS patient. MPS type I is caused by a deficiency of the lysosomal enzyme α-L-iduronidase (IDUA) and results in progressive hepatosplenomegaly, airway compromise, corneal clouding, cardiovascular disease, degenerative joint disease with reduced mobility, and varying degrees of cognitive impairment. Prior to the development of treatments, MPS I patients suffered tremendous morbidity and childhood mortality arising from storage of the heparan and dermatan sulfate GAG species, such as neurodegeneration, cor pulmonale, aspiration pneumonia, and myocardial infarction. However, hematopoietic stem cell transplantation (HSCT) to introduce the missing lysosomal hydrolase systemically and into the central nervous system via donor neuroglia, and intravenous enzyme replacement therapy (ERT) with recombinant human IDUA (rhIDUA) to introduce the deficient enzyme peripherally have successfully reduced bodily GAG storage and emerged as life-saving treatments for MPS I. Now, MPS I patients are surviving through childhood and beyond, with rescue of cognitive outcomes and partial amelioration of some somatic symptoms [1,2].
1.2
Despite these advances in therapies for MPS I, significant limitations in efficacy still exist. Orthopedic complications are especially problematic for long-term survivors. Post-HSCT or ERT delivery of IDUA into cartilage and joints is limited by their avascular nature and disruption of normal synovial trophic function by GAG storage [3,4]. Consequently, GAG storage in cartilage and bone continues to occur, resulting in progressive cervical spinal cord stenosis, spinal kyphosis, restriction of joint mobility, hip dysplasia, and osteoarthritis and significant impairment of quality of life. Patients with MPS I must endure continued orthopedic surgeries to palliate or correct these [5,6]. We hypothesize that direct, intra-articular administration of rhIDUA (IA-ERT) can safely circumvent the impediments presented by HSCT and ERT, reduce synovial and cartilage GAG storage, and reduce markers of joint inflammation. The purpose of this study was to determine the safety, tolerability, and efficacy of rhIDUA IA-ERT in the canine model of MPS I. Concerning sanctuary tissues unreachable by conventional treatment, there is precedent for multimodal approach to therapy for MPS. Prior IA-ERT studies on the feline model of MPS type VI have shown clearance of GAG and reduced lysosomal storage in treated joint tissues [4,7]. Intra-thecal ERT has been studied for treatment of central nervous system disease in canine MPS I, a well-characterized animal model extensively utilized for ERT trials [8–10]. We report results of a safety, tolerability, and efficacy study of IA-ERT with rhIDUA in the canine model of MPS I.
2. MATERIALS AND METHODS
2.1 Test animals and husbandry
2.1.1
Four MPS I canines were bred by artificial insemination, diagnosed via α-iduronidase enzyme assay and PCR, and maintained at Iowa State University until 1 year of age, after which they were transported to the Los Angeles Biomedical (LA BioMed) Research Institute at Harbor-UCLA, an AAALAC accredited facility under the care of a veterinarian. The dog colony has a null mutation in intron 1 of the canine α-L-iduronidase gene that results in abnormal mRNA splicing, introduces a premature termination codon, and completely eliminates IDUA protein expression [11]. The four dogs were neither tolerized nor treated with IV rhIDUA [12]. This study was reviewed and approved by the LA BioMed Institutional Animal Care and Use Committee (IACUC #20013-01).
2.2 Intra-articular rhIDUA injections and dosage rationale
2.2.1
Test canines cleared the standard two week quarantine period before initiation of the study protocol. They were fasted for 12 – 16 hours prior to each procedure and received pre-procedural medication with intramuscular diphenhydramine 2.2 mg/kg for prophylaxis against reaction to enzyme injection, atropine 0.04 mg/kg to reduce salivation, and propofol 6 mg/kg for anesthesia. General anesthesia as described previously was administered for all IA injections and biopsies [13]. Using sterile technique, monthly joint aspirations were performed in bilateral elbows and knees, for a total of six months. Immediately following joint aspiration, 0.58 mg of rhIDUA (formulated as Laronidase, BioMarin Pharmaceutical, Novato CA, lot V011004, 0.58 mg/mL in 150 mM NaCl, 100 mM sodium phosphate, 0.001% polysorbate 80, pH 5.8) dissolved in 1 mL of sterile normal saline were administered to the right elbow and knee (“rhIDUA-treated joints”). This is equivalent to a dose of 100 rhIDUA units per joint per injection, with one enzyme unit catalyzing the release of 1 micromole 4-methylumbelliferyl-α-iduronate substrate per mg of protein per hour at 37 C. The contralateral elbows and knees received 1 mL sterile normal saline (“saline-treated joints”). The rationale for the 100 unit / joint rhIDUA IT-ERT dose and monthly frequency was to reduce the likelihood of storage reaccumulation, which was observed by Auclair and associates utilizing a maximum IA-ERT recombinant human arylsulfatase B dose of 35 units (500 micrograms) per joint per month in the MPS VI feline model [4,7]
2.3 Safety and tolerability monitoring
2.3.1
At each injection, heart rate, body temperature, and respiratory rate were monitored. In addition, complete blood count with differential, electrolytes, creatine phosphokinase, and other chemistry values were obtained and submitted to a commercial veterinary lab (Antech, Irvine, CA). Joint fluid obtained from each aspiration was submitted for analysis of protein (reference range ≤ 2.5 mg/dL) and cell count (reference range ≤ 3000 cells/mm3) [14]. Monthly serum anti-rhIDUA antibody titers, and synovial anti-rhIDUA antibody titers at baseline and study-end, were measured as previously described [15].
2.3.2
All dogs were recovered after general anesthesia and monitored for pain, distress, difficult ambulation, joint effusions, fever, or infection. A general physical exam noting posture, activity, demeanor and appearance was conducted 1 day after each intra-articular injection and periodically thereafter. Because MPS I canines manifest joint hyperlaxity, as opposed to the progressive joint restriction seen in humans with MPS I, assessments of joint mobility were not performed. Body weight measurements were taken every 2 weeks.
2.4 Tissue collection and euthanasia
2.4.1
Four weeks prior to the first IA injection, biopsy samples of articular cartilage and synovium were obtained from the elbow and knee joints of each dog. One month following the sixth IA injection, all dogs were euthanized with a 1 cc / 10 lb dose of euthasol and identical samples of articular cartilage and synovium were obtained post-mortem. Biopsy and post-mortem samples were immediately placed in fixative after removal including: 1) 10% neutral buffered formalin (NBF), or 2) 3% glutaraldehyde in 0.1M cacodylate buffer. After 12–24 hours in fixative, samples in NBF were rinsed and stored in phosphate-buffered saline at 4° C until processing; glutaradehyde fixed samples were washed and stored at 4° in cacodylate buffer.
2.5 Light Microscopy
2.5.1
Tissue samples fixed in NBF were routinely processed into paraffin for histologic sectioning. Deparaffinized 4 micron tissue sections were stained with hematoxylin and eosin (H&E) for routine light microscopic evaluation.
2.6 Transmission Electron Microscopy
2.6.1
Following an initial fixation in glutaraldehyde fixative, samples were washed in cacodylate buffer then post-fixed in cacodylate buffered (0.1M) 2% osmium tetroxide for 60 minutes. Samples were then washed in water and dehydrated through a graded ethanol series with final dehydration in 2 changes of propylene oxide. Following dehydration, samples were embedded in Spurr epoxy over a 24 hour period then placed in a 68° C oven for 24 hours. Semi-thin (1micron) and thin (100nm) sections were cut on a Leica EMUC7 ultramicrotome. Sections for electron microscopy were post stained with 50% methanolic uranyl acetate washed and then stained with lead citrate before examination in a Zeiss Libra 200MC.
2.7 Scoring of GAG and inflammatory infiltrates in articular tissues
2.7.1
Tissues removed at biopsy and necropsies were assessed via light and electron microscopy by a veterinary pathologist blinded to intervention. The amount of GAG storage in the synovium and entire articular cartilage segments were scored on a scale of 0–3 in the following manner: 0, little or no storage (0–1 vesicle per cell); 1, mild storage (2–4 vesicles); 2, moderate storage (5–10 vesicles); 3, severe (as seen in untreated MPS I dog tissues). An identical 0–3 scale was used to score the amount of tissue macrophages and plasma cells: 0, no cellular infiltrates; 1, mild inflammatory cell infiltrate; 2, moderate inflammatory cell infiltrate; 3, severe (macrophages as seen in untreated MPS I dog tissues; heaviest tissue plasma cell infiltration). Plasma cells did not require special staining as they were readily recognizable with their distinct nuclear chromatin pattern and cytoplasmic characteristics, including pallor of the perinuclear Golgi apparatus.
2.8 Heparan sulfate quantification in articular cartilage
2.8.1
At the end of the study, tissue samples were collected at necropsy and snap-frozen with dry ice. Cartilage samples were treated with protease and the GAGs were purified by anion exchange chromatography as described [16]. GAGs were enzymatically depolymerized with lyases and the liberated disaccharides were mass labeled by reductive amination with [12C6]aniline as previously described [16]. Standards representing the various internal disaccharides and the non-reducing end carbohydrate biomarker for MPS I (Iduronic acid-N-sulfoglucosamine) were tagged with [13C6]aniline and mixed with the samples. The mixed samples were then analyzed by LC-MS using an LTQ Orbitrap Discovery electrospray ionization mass spectrometer (Thermo Scientific) equipped with quaternary HPLC pump (Finnigan Surveyor MS pump) and a reverse-phase capillary column as previously described [17].
2.9 Total GAG quantification in synovium
2.9.1
For tissue GAG analysis, sulfated GAG was measured using a modification of the Alcian Blue method of Björnsson [8]. The GAG quantities were determined by comparison to standards of dermatan sulfate.
2.10 Articular fluid ceramidase and acid sphingomyelinase activity
2.10.1
We chose to measure acid ceramidase and sphingomyelinase enzyme activities in articular fluid because we previously identified elevations in acid ceramidase in MPS animals, a likely response to elevated ceramide released by sphingomyelinase in response to chondrocyte apoptosis and cartilage degradation; acid sphingomyelinase plays a role in inflammatory macrophage activation and cellular apoptosis, two processes that may contribute to MPS joint disease [18,19]. Aliquots of articular fluid from baseline, 1, and 2 months post-injection had undergone several freeze/thaw cycles and subsequently enzymatic assays could not be accurately performed. Aliquots from later time points were thawed on ice, proteins were quantified (Bio-Rad, Hercules, CA, USA), and subjected to acid ceramidase and acid sphingomyelinase enzymatic activity measurement using unpublished modifications of previously described methods [20,21].
2.11 IDUA quantification in synovium
2.11.1
A portion of synovium from each biopsied joint was snap-frozen in liquid nitrogen. After thawing, the specimens were weighed, immersed in 3 volumes of PAD (10mM phosphate-buffer pH 5.8, 0.02% azide and 0.1 mM dithiothreitol) + 0.1% Triton X-100, then homogenized. α-iduronidase levels were determined using a validated assay with the artificial substrate 4-methylumbelliferyl (4MU) α-L-iduronide [13]. Net fluorescence was determined by fluorometry at 365 nm excitation and 440 nm emissions. One unit of IDUA enzyme activity is equivalent to a catalytic activity of 1 micromole of 4MU substrate cleaved per hour per mg protein at 37 °C.
2.12 Statistical analysis
2.12.1
Mean±standard deviation of the following parameters were calculated: synovial GAG concentration; synovial α-iduronidase enzyme activity; synovial fluid acid sphingomyelinase and acid ceramidase enzyme activities; scoring of synoviocyte and chondrocyte lysosomal storage; and scoring of synovial plasma cell and macrophage infiltrates. Normality of data was assessed with the K-S test; differences in means were compared with Student's T-test or Wilcoxon rank-sum test, depending on normality of data. We were fully aware that comparisons were constrained by the small number of animals, but preliminary data indicated that anticipated differences in means and standard deviations for treated versus untreated would be sufficient enough to generate meaningful statistical comparisons.
3. RESULTS
3.1 Safety / Tolerability
3.1.1
Each dog tolerated all injections well, and all ambulated normally after recovery from anesthesia. There were no local or systemic adverse reactions to the injections. None experienced joint erythema, effusions, or infection. No dog developed fever, hypotension, respiratory distress, or anaphylaxis. Their weights remained stable throughout the study. None of the animals demonstrated any change in joint laxity. Following the initial joint biopsies, one dog (sph) developed reduction of hind limb mobility secondary to spinal cord compression and received a 23-day tapering course of prednisone. Two dogs (oks, spi) experienced infection of interdigital cysts on their paws and were treated with oral antibiotics (clavamox, cephalexin).
3.1.2
Electrolytes and chemistries remained within normal limits for the duration of the study, except for spo, whose amylase level was elevated at baseline (1156 IU/L, reference range 290 – 1125 IU/L per testing laboratory), ranged from 1009 to a peak of 1482 IU/L (prior to the sixth injection) during the study, and ended at 996 IU/L. The elevations were asymptomatic and spo had good appetite without vomiting, abdominal distension, or discomfort. The dogs' mean platelet count was above upper limit of canine normal (per testing laboratory, reference range 170 – 400 k/μL) at baseline (427 ± 127 k/μL) and remained elevated for the duration of the study (610 ± 127 k/μL at study-end). Three of four dogs experienced mild monocytosis (maximum 1430 / μL; reference range < 840 / μL) at various time points throughout the study. Synovial fluid protein was elevated prior to the first injection: 2.9 ± 0.14 g/dL in the joints to be treated with rhIDUA, and 2.67 ± 0.15 g/dL in the joints to be treated with saline (reference range ≤ 2.5 g/dL), and remained at or above the normal limit for the duration of the study. At each time point, synovial fluid red blood cell counts remained within reference range of ≤ 3000/mm3 with the exception of a bloody elbow tap in animal spi at baseline (RBC 33,000/mm3 with WBC of 6/mm3) that normalized at the next measurement. Synovial fluid white blood cell counts also remained within reference range of ≤ 3000/mm3 with the exceptions of the rhIDUA-treated elbow (3663/mm3) and rhIDUA-treated knee (3285/mm3) in spi at study-end.
3.1.3
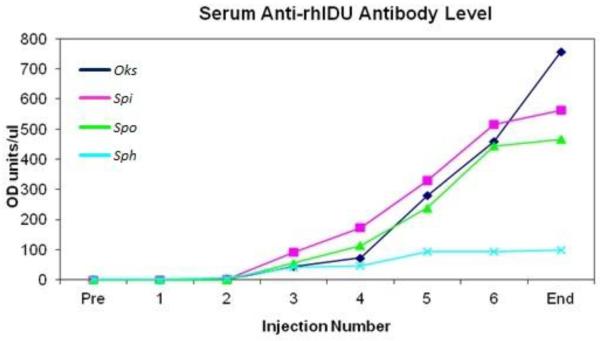
Circulating anti-rhIDUA antibody levels were undetectable at baseline, began to appear at the third IA injection (101 ± 55 O.D. units / μL), and continued to increase every month thereafter, reaching 471 ± 277 O.D. units / μL at the time of necropsy. Of note, sph, the dog who received the prednisone taper at the beginning of the study, had the lowest anti-rhIDUA levels, remaining below 100 O.D. units / μL for the study duration (Figure 1). Similarly, synovial fluid anti-rhIDUA antibody levels were also near zero at baseline (0 – 2.7 O.D. units / μL). At study-end, synovial fluid anti-rhIDUA antibody levels in rhIDUA-treated joints (1025 ± 432 O.D. units / μL) were higher than levels in saline-treated joints (129 ± 89 O.D. units / μL); please refer to Table 1 for details.
Figure 1.
Circulating anti-rhIDUA antibody levels were absent at baseline but began to increase steadily beginning at the third IA-rhIDUA injection. Anti-rhIDUA levels in the prednisone-treated animal (sph, light blue) remained at low levels throughout the study.
Table 1.
Anti-rhIDUA antibody titer measurements in synovial fluid demonstrated near-zero titers in fluid from all joints at baseline. At the end of the study, the rhIDUA-treated joints demonstrated the highest anti-rhIDUA antibody titers, while the saline-treated joints had elevated anti-rhIDUA antibody titers, but below circulating serum titer levels. Of note, Sph, the animal treated with prednisone prior to study initiation, had the lowest saline-treated titer level and the second lowest rhIDUA-treated titer level.
| Synovial Fluid | OD Units/ul |
||
|---|---|---|---|
| Baseline | Post-treatment | ||
| Oks | Saline-treated | 0.180 | 98.8 |
|
|
|||
| Spi | Saline-treated | 0 | 229 |
|
|
|||
| Spo | Saline-treated | 0 | 167 |
|
|
|||
| Sph | Saline-treated | 0.140 | 21.1 |
|
| |||
| Mean ± SD | 0.08 ± 0.09 | 129 ± 89 | |
|
| |||
| Oks | rhIDUA-treated | 0 | 613 |
|
|
|||
| Spi | rhIDUA-treated | 2.70 | 1500 |
|
|
|||
| Spo | rhIDUA-treated | 0.540 | 1280 |
|
|
|||
| Sph | rhIDUA-treated | 0 | 708 |
|
| |||
| Mean ± SD | 0.81 ± 1.29 | 1025 ± 432 | |
3.1.4
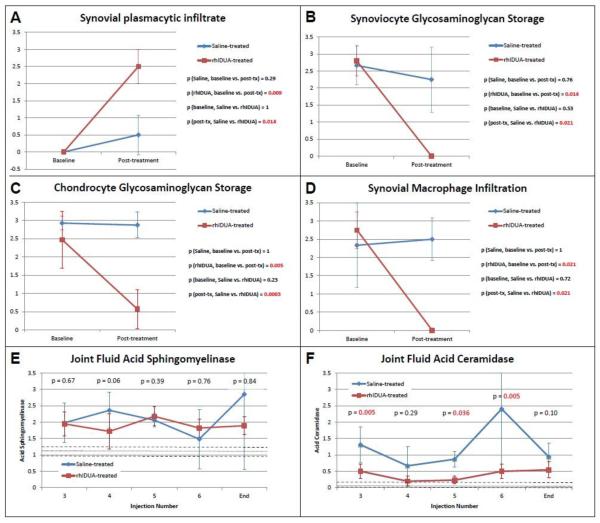
At the beginning of the study, neither rhIDUA-treated nor saline-treated joints contained any plasma cells (both scored 0); at study-end, plasmacytic infiltrate developed in the synovial tissues of the rhIDUA-treated joints (Figure 2 B, arrows) with a mean score of 2.5 ± 0.5. This was statistically significant compared to the rhIDUA-treated joints at baseline (p = 0.009) and to saline-treated joints at study-end (0.5 ± 0.58, p = 0.014) (Figure 3 A).
Figure 2.
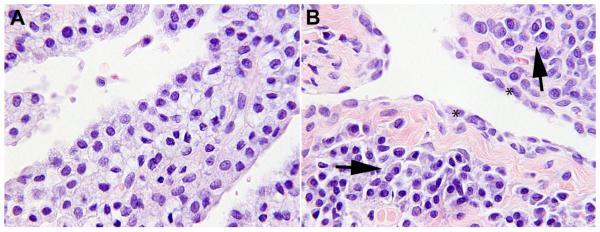
A: Knee synovium, baseline: Synoviocytes are foamy-appearing and swollen with storage material.
B: Knee synovium, IA-rhIDUA treated: Synoviocytes (*) no longer demonstrate storage and have adopted a normal, flattened morphology. Arrows: intra-synovial lymphocytic and plasma cell infiltrate.
Figure 3.
A: Plasma cell infiltrate within the synovium was absent at baseline and developed only in rhIDUA-treated joints.
B: At baseline, synoviocyte GAG storage was equivalent in all joints. Significant study-end reduction of storage was observed only in rhIDUA-treated joints, not in saline-treated joints.
C: Similarly, chondrocyte GAG storage was equivalent in all joints at baseline, and was significantly reduced only in rhIDUA-treated joints, not in saline-treated joints.
D: Inflammatory macrophages were prominently observed at baseline in all joints. Significant reduction in macrophages was observed only in rhIDUA-treated joints, not in saline-treated joints.
E: Synovial fluid acid sphingomyelinase remained elevated in both saline and rhIDUA-treated joints throughout the study compared to levels measured in normal dogs (n = 7). Dashed lines: range of acid sphingomyelinase levels measured in normal dogs. Dotted line: mean enzyme level measured in normal dogs.
F: Synovial fluid acid ceramidase levels were significantly reduced to near-normal levels in IA-rhIDUA treated joints, but remained significantly higher in saline-treated joint fluid throughout the study. Dashed lines: range of synovial fluid acid ceramidase levels measured in normal dogs (n = 7). Dotted line: mean enzyme level measured in normal dogs.
3.2 Efficacy
3.2.1
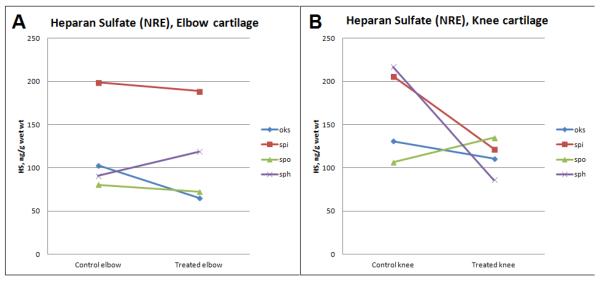
Synovial IDUA activity was still measurable (4.37 ± 4.05 units / mg protein) in rhIDUA-treated joints one month after the last enzyme injection. There was negligible enzymatic activity (0.002 ± 0.006 units / mg protein) in saline-treated joints; p = 0.0008. Mean joint synovial GAG levels were reduced in rhIDUA-treated joints by 60% at study end: mean saline-treated synovial GAG content was 21.6 ± 10.4 μg/mg dry weight, and the mean rhIDUA-treated synovial GAG content was 8.62 ± 5.86 μg/mg dry weight; p = 0.083. Mean cartilage heparan sulfate NRE content was also reduced in the rhIDUA-treated joints (113 ± 39.5 ng/g wet weight) compared to saline-treated joints (142 ± 56.4 ng/g wet weight), showing a trend towards heparan sulfate clearance in treated joints (Figure 4). Mean cartilage heparan sulfate NRE level in normal dogs was 2.44 ng/g wet weight, and 154 ng/g wet weight in untreated MPS I dogs (unpublished data).
Figure 4.
Cartilage heparan sulfate levels, quantified via tandem mass spectroscopy of Non-Reducing End (NRE) disaccharides, were reduced in rhIDUA-treated elbows (A) and knees (B). Cartilage heparan sulfate from normal dogs was 2.44 ng/g wet weight.
3.2.2
Synoviocytes from pre-treatment biopsies were swollen and foamy-appearing due to glycosaminoglycan storage (Figure 2 A), while rhIDUA-treated synoviocytes had adopted a normal, flattened morphology without cytoplasmic storage (Figure 2 B). Prior to treatment initiation, there was no difference in synoviocyte GAG scoring between joints to be treated with saline (2.67 ± 0.58) and those to be treated with rhIDUA (2.8 ± 0.45); p = 0.76. Study-end rhIDUA-treated joints had significantly reduced synovial GAG storage (0 ± 0) compared to baseline; p = 0.014, and to study-end saline-treated synoviocytes (2.25 ± 0.96); p = 0.021. On the other hand, synoviocyte storage was equivalent in baseline and study-end saline-treated joints; p = 0.53. These scoring results are summarized in Figure 3 B.
3.2.3
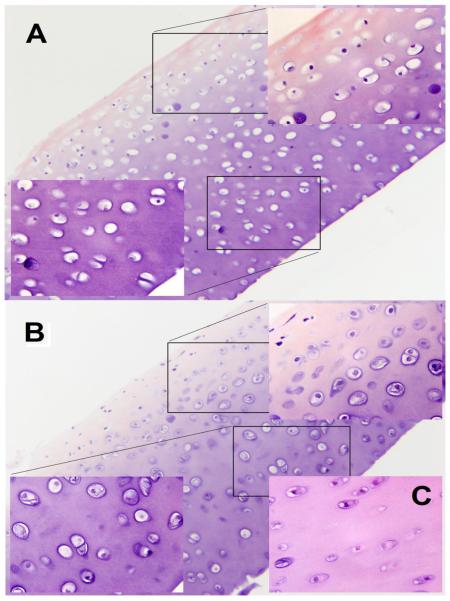
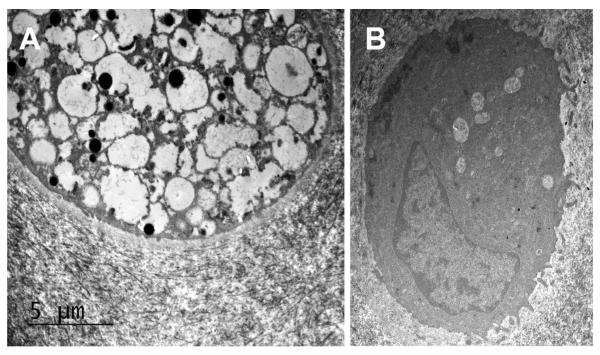
At baseline, chondrocytes were markedly distended with foamy storage material that pushed nuclei to the cell periphery. Saline-treated chondrocytes appeared similar to baseline (Figure 5 A). Enzyme-treated chondrocytes, including those deep within the articular cartilage, were smaller, demonstrated reduced storage material (Figure 5 B), and looked more similar to age-matched normal chondrocytes (Figure 5 C). Results of chondrocyte GAG storage were similar to synoviocyte scoring. At the beginning of the study, there were no differences in scoring between joints to be treated with enzyme (2.47 ± 0.78) and those to be treated with saline (2.93 ± 0.19); p = 0.23. Study-end rhIDUA-treated joints showed a significant reduction in chondrocyte GAG storage (0.57 ± 0.53) compared to baseline; p = 0.005, and to study-end saline-treated (2.88 ± 0.35) joints; p = 0.0003. There was no difference in chondrocyte scoring between baseline (3 ± 0) and study-end (3 ± 0) saline-treated joints; p = 1. These results are summarized in figure 3 C. Transmission electron micrographs of saline-treated (Figure 6 A) and rhIDUA-treated (Figure 6 B) chondrocytes showed that the latter demonstrated significantly reduced lysosomal storage granules and restoration of normal cellular structures.
Figure 5.
A: Full-thickness view of knee cartilage, treated with saline for six months (Storage score: 3). The sample is oriented with articular surface to the upper left, and the deep zone to the lower right. Higher-power views of the articular surface and deep zone demonstrate chondrocytes filled with foamy storage material.
B: Full-thickness view of knee cartilage, treated with rhIDUA for six months (Storage score: 1). The sample is oriented like A, with higher-power views of articular surface and deep zone. Chondrocytes both at the surface and deep zone demonstrate more cytoplasm and much less storage than saline-treated chondrocytes.
C: Knee cartilage from a similarly aged, wild-type dog for reference.
Figure 6.
Transmission electron micrographs of chondrocytes taken from cartilage at baseline (A) and following treatment with IA-rhIDUA (B). Notice the large amount of lysosomal storage vacuoles in the untreated chondrocyte. The treated chondrocyte demonstrates a small number of residual lysosomal storage vacuoles but has normal-appearing cytoplasm and morphology.
3.2.4
Synovial macrophage infiltration was not visible in enzyme-treated joints (Figure 3 D). At the beginning of the study, the joints to be treated with rhIDUA (2.75 ± 0.5) and joints to be treated with saline (2.33 ± 1.15) showed no significant differences in synovial macrophage scoring; p = 0.72. At the conclusion of the study, enzyme-treated joints demonstrated no macrophage infiltration (0 ± 0), which was statistically significant compared to baseline; p = 0.021 and to study-end saline-only joints (2.5 ± 0.58); p = 0.21. Saline-treated joints showed no significant difference in macrophage scoring from baseline to study-end; p = 1. Blinded scoring of normal canine synovial GAG, chondrocyte GAG, synovial plasma cells, and synovial macrophages demonstrated scores of `0' in all four categories. While synovial fluid acid sphingomyelinase levels remained essentially constant and elevated above the normal range of 0.976 – 1.24 pmol/mcg protein/hr (unpublished data, n = 7 dogs) without appreciable differences between saline-treated and enzyme-treated joints (Figure 3 E), fluid from the rhIDUA-treated joints consistently maintained lower acid ceramidase levels compared to control joints (Figure 3 F) that approached normal range of 0.01 – 0.195 pmol/mcg protein/hr (unpublished data, n = 7 dogs).
4. DISCUSSION
4.1
Neither HSCT, which in 1981 was first reported as treatment for MPS I, nor IV rhIDUA despite its approval for clinical use more than 11 years ago, have been able to prevent the progression of joint disease in human MPS I patients [5,6]. The similar inability of IV recombinant human arylsulfatase B (rhARSB) to treat MPS VI joint disease led to a series of studies in the feline MPS VI model, demonstrating clearance of lysosomal storage in synoviocytes and chondrocytes following treatment with intra-articular arylsulfatase B [3,4,7]. We based our canine MPS I IA rhIDUA study upon the feline MPS VI IA ARSB experience, utilizing an increased catalytic equivalent dose of 100 rhIDUA enzyme units (0.58 mg) per joint per month as recurrent chondrocyte storage was seen at the maximum rhARSB dose of 35 enzyme units (0.50 mg) per joint per month. The results from our study indicate successful proof-of-concept of intra-articular enzyme replacement therapy as a viable treatment modality for canine MPS I joint disease, and possibly human MPS I joint disease. Direct administration of rhIDUA into canine intra-articular spaces resulted in reduction of GAG storage not only in the synovium and chondrocytes in the articular surface of the joint cartilage, but also in the deeper chondrocytes further away from the articular surface. This demonstrated the ability of IA-ERT to bypass the therapeutic obstacles of IV-ERT resulting from the avascularity of articular cartilage and the synovial dysfunction caused by GAG storage. Measurable IDUA enzyme activity was detected in joint tissues of these non-tolerized animals, even one month following IA injection, at levels comparable to those found in immunotolerized, high-dose intravenous ERT treated canines 48 hours post-treatment [12]. The 0.58 mg IA injection dose of rhIDUA is therefore able to penetrate deep into the articular cartilage and remain biologically active for an extended period of time after administration.
4.2
The reduction of macrophages in rhIDUA-treated synovium and levels of articular fluid acid ceramidase, a marker of inflammation [22], in treated joints but not control joints suggest that rhIDUA IA-ERT also successfully mitigated downstream toxic effects of GAG storage within joint tissues. The pathogenesis of joint disease in MPS has been linked to activation of the innate immune system via Toll-Like Receptor 4 (TLR4). Free oligosaccharides derived from heparan sulfate and other GAGs mimic the structure of bacterial lipopolysaccharide (LPS), the native epitope of TLR4, and trigger an inflammatory signal transduction cascade. Downstream effects include upregulated synthesis of tumor necrosis factor-α (TNFα), matrix metalloproteinase and other proteases, activation of macrophages, and release of pro-apoptotic factors such as ceramide with compensatory increases in acid ceramidase [23–26]. The pro-inflammatory milieu in the MPS joint stimulates synovial hyperplasia while promoting apoptosis of chondrocytes, resulting in shorter, poorly organized columns in the proliferative and hypertrophic zones of the growth plate [24,27,28]. Endochondral ossification of the abnormal epiphyses and abnormal bone remodeling are responsible for the vertebral and long bone dysplasia in MPS known as dysostosis multiplex. MPS VII / TLR4 double knockout mice had more organized chondrocyte columns, normal linear growth, and reduced circulating TNFα levels compared to MPS VII knockout mice [25]. Blockade of TNFα in MPS VI rats via treatment with Infliximab, a monoclonal antibody against TNFα, normalized levels of serum TNFα and pro-apoptotic ceramide and reduced chondrocyte apoptosis to near-normal levels [25].
4.3
If IA-ERT can reduce inflammation and apoptosis in the canine MPS I joint, presumably by elimination of GAG-derived oligosaccharides and their downstream effects on TLR4 signaling, this treatment represents a possible method to slow down or halt the progression of osteoarthritis in human MPS I. Further, the effects of interrupted TLR4 or TNFα-mediated signaling upon murine MPS I joint phenotypes, suggest that initiation of IA-ERT in younger children with MPS I may reduce dysostosis multiplex, improve growth plate structure and subsequent linear growth.
4.4
Grossly, the articular cartilage did not appear to have any evidence of degenerative osteoarthritis. The synovium in rhIDUA IA-ERT treated joints, and to a much lesser extent the saline-treated joints, developed lymphocytic and plasma cell infiltrates. This phenomenon, which is likely a cellular immune response to the IA-administered enzyme, has been observed before in the spinal meninges of dogs treated with intrathecal rhIDUA and was not accompanied by meningitis or clinically apparent symptoms [9]. The cellular infiltrates did not correspond with any evidence of local inflammation as evidenced by stability of synovial fluid protein levels, white blood cell count, and red blood cell counts in the rhIDUA-treated joints during the study. In fact, the reduction in macrophage counts and ceramidase levels in the treated joints indicates an overall reduction of joint inflammation. Our dogs did not demonstrate any systemic or jointspecific adverse effects, even though they developed circulating anti-rhIDUA titers that approximated levels seen in nontolerized dogs treated with IV-ERT [15]. Interestingly, post-treatment synovial fluid anti-rhIDUA titers were highest in the synovial fluid from rhIDUA-treated joints. This mirrors the CSF antibody response observed in MPS I dogs receiving intrathecal rhIDUA [9]. Although study-end titers in synovial fluid from saline-treated joints were also elevated, they were below titers measured in rhIDUA-treated joints and serum, likely a reflection of the mild plasma cell infiltrate in the saline-treated synovium.
4.5
In addition, a humoral response of the kind observed in the study dogs is not surprising. The formation of circulating antibodies would be expected to occur in canines treated with human α-L-iduronidase, especially with the large intra-compartmental doses that were administered during the course of the study. Interestingly, the one animal who received corticosteroids for treatment of spinal cord compression developed lower serum and synovial fluid anti-rhIDUA antibody titers while demonstrating the highest degree of synovial GAG clearance amongst the four animals, indicating that IA-ERT may be more effective in immunotolerized MPS I canines. Since most MPS I patients who have undergone HSCT or receive IV-ERT are immunotolerized [29], this observation indicates that IA-ERT may be more effective as an adjunctive treatment after a patient has undergone initial, systemic therapy and become tolerized.
4.6
Because the study utilized older dogs, we were unable to assess for any effects of rhIDUA IA-ERT upon the epiphyses, specifically the organization and number of chondrocytes populating the columns in the proliferative and hypertrophic zones. Their lack of immunotolerization resulted in the brisk anti-rhIDUA antibody response, possibly obscuring the full efficacy of the treatment, as anti-rhIDUA antibody interferes with mannose-6-phosphate receptor-mediated uptake of enzyme into cells [12] We also could not accurately extrapolate the functional effects of IA-ERT in canine joints to human MPS I joint restriction, because the MPS I canine model manifests joint hyperlaxity.
4.7 Conclusions
4.7.1
Despite these challenges, the results of this study indicate that IA-ERT with rhIDUA in the canine MPS I model system is safe, well-tolerated, and successfully reduces GAG storage and inflammatory markers in joint tissues. As the feline rhARSB IA-ERT studies led to the treatment of two human MPS VI patients with IA rhARSB [30], we anticipate the need for studies of rhIDUA IA-ERT in human patients with MPS I to assess the efficacy of multimodal ERT in reducing or preventing the debilitating orthopedic complications of the disorder.
Clinical Relevance.
The MPS I canine IA-rhIDUA results suggest that clinical studies should be performed to determine if IA-rhIDUA is a viable approach to ameliorating refractory orthopedic disease in human MPS I.
Highlights.
Four mucopolysaccharidosis I dogs received monthly intra-articular iduronidase.
Injections were safe without adverse reactions and caused no change in lab values.
Anti-iduronidase antibodies increased but were mitigated in one steroid-treated dog.
Treated joints demonstrated reduction in glycosaminoglycan storage versus control.
Treated joints demonstrated reduction in inflammatory markers versus control.
ACKNOWLEDGEMENTS
The project described was supported by the National Institutes of Health (GM 093131), MPS1 Research Foundation, Children's Hospital of Orange County Pediatric Subspecialty Faculty, University of Pennsylvania – Improved Therapies for MPS I Grant Program, and National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124.The rhIDUA enzyme was generously provided by Genzyme-Sanofi. The authors thank Dr John Dunlap (Advanced Microscopy and Imaging Center, University of Tennessee) for assistance in preparing/evaluating biopsy samples. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICTS OF INTEREST RW: pro bono member of the Genzyme-Sanofi North American MPS I Registry Board of Advisors. PID: research support from BioMarin and Genzyme. MM, NME, LS, JE: none. The Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center (PID) and Iowa State University (NME) receive institutional support from Genzyme-Sanofi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Los Angeles Biomedical Research Institute and the Department of Pediatrics at Harbor-UCLA Medical Center have a financial interest in recombinant α-L-iduronidase.
REFERENCES
- [1].Kakkis ED, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, Hasson CW, O'Malley T, Weil MA, Aguirre GA, Brown DE, Haskins ME. Enzyme replacement therapy in feline mucopolysaccharidosis I. Mol. Genet. Metab. 2001;72:199–208. doi: 10.1006/mgme.2000.3140. [DOI] [PubMed] [Google Scholar]
- [2].Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, Bleyzac N, Mulier C, Durin A, Kebaili K, Galambrun C, Bertrand Y, Froissart R, Dorche C, Gebuhrer L, Garin C, Berard J, Guibaud P. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- [3].Byers S, Crawley AC, Brumfield LK, Nuttall JD, Hopwood JJ. Enzyme replacement therapy in a feline model of MPS VI: modification of enzyme structure and dose frequency. Pediatr. Res. 2000;47:743–749. doi: 10.1203/00006450-200006000-00010. [DOI] [PubMed] [Google Scholar]
- [4].Auclair D D, Hein LK, Hopwood JJ, Byers S. Intra-articular enzyme administration for joint disease in feline mucopolysaccharidosis VI: enzyme dose and interval. Pediatr. Res. 2006;59:538–543. doi: 10.1203/01.pdr.0000203090.41012.a6. [DOI] [PubMed] [Google Scholar]
- [5].van der Linden MH, Kruyt MC, Sakkers RJ, de Koning TJ, Oner FC, Castelein RM. Orthopaedic management of Hurler's disease after hematopoietic stem cell transplantation: a systematic review. J. Inherit. Metab. Dis. 2011;34:657–669. doi: 10.1007/s10545-011-9304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Taylor C, Brady P, O'Meara A, Moore D, Dowling F, Fogarty E. Mobility in Hurler syndrome. J. Pediatr. Orthop. 2008;28:163–168. doi: 10.1097/BPO.0b013e3181649e25. [DOI] [PubMed] [Google Scholar]
- [7].Auclair D, Hopwood JJ, Lemontt JF JF, Chen L, Byers S. Long-term intra-articular administration of recombinant human N-acetylgalactosamine-4-sulfatase in feline mucopolysaccharidosis VI. Mol. Genet. Metab. 2007;91:352–361. doi: 10.1016/j.ymgme.2007.04.009. [DOI] [PubMed] [Google Scholar]
- [8].Kakkis ED, McEntee MF, Schmidtchen A, Neufeld EF, Ward DA, Gompf RE, Kania S, Bedolla C, Chien SL, Shull RM. Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. Biochem. Mol. Med. 1996;58:156–167. doi: 10.1006/bmme.1996.0044. [DOI] [PubMed] [Google Scholar]
- [9].Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol. Genet. Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- [10].Dickson PI, Hanson S, McEntee MF, Vite CH, Vogler CA, Mlikotic A, Chen AH, Ponder KP, Haskins ME, Tippin BL, Le SQ, Passage MB, Guerra C, Dierenfeld A, Jens J, Snella E, Kan SH, Ellinwood NM. Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol. Genet. Metab. 2010;101:115–122. doi: 10.1016/j.ymgme.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Menon KP, Tieu PT, Neufeld EF. Architecture of the canine IDUA gene and mutation underlying canine mucopolysaccharidosis I. Genomics. 1992;14:763–768. doi: 10.1016/s0888-7543(05)80182-x. [DOI] [PubMed] [Google Scholar]
- [12].Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J. Clin. Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, Hanson S, Passage M, Kakkis E. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol. Genet. Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lipowitz Alan. Synovial Fluid. In: Newton CD, Nunamaker DM, editors. Textbook of small animal orthopedics. 3rd edition Elsevier Science; Philadelphia: 2003. [Google Scholar]
- [15].Kakkis E, Lester T, Yang R, Tanaka C, Anand V, Lemontt J, Peinovich M, Passage M. Successful induction of immune tolerance to enzyme replacement therapy in canine mucopolysaccharidosis I. Proc. Natl. Acad. Sci. U. S. A. 2004;101:829–834. doi: 10.1073/pnas.0305480101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J. Biol. Chem. 2008;283:33674–33684. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons GJ, Esko JD, Crawford BE. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat. Chem. Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simonaro C, Sachot S, Ge Y, He X, DeAngelis VA, Eliyahu E, Leong DJ, Sun HB, Mason JB, Haskins ME, Richardon DW, Schuchman EH. Acid ceramidase maintains the chondrogenic phenotype of expanded primary chondrocytes and improves the chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. PLoS One. 2013;8:e62715. doi: 10.1371/journal.pone.0062715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 2008;22:3419–3431. doi: 10.1096/fj.08-108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].He X, Li CM, Park JH, Dagan A, Gatt S, Schuchman EH. A fluorescence-based high performance liquid chromatographic assay to determine acid ceramidase activity. Anal. Biochem. 1999;275:264–269. doi: 10.1006/abio.1999.4284. [DOI] [PubMed] [Google Scholar]
- [21].He X, Chen F, Gatt S, Schuchman EH. An enzymatic method for quantifying sphingomyelin in tissues and plasma from humans and mice with Niemann-Pick disease. Anal. Biochem. 2001;293:204–211. doi: 10.1006/abio.2001.5108. [DOI] [PubMed] [Google Scholar]
- [22].Canals D, Perry DM, Jenkins RW, Hannun YA. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br. J. Pharmacol. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Simonaro CM, D'Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr. Res. 2005;57:701–707. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- [24].Simonaro CM, D'Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am. J. Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Simonaro CM, Ge Y, Eliyahu E, He X, Jepsen KJ, Schuchman EH. Involvement of the Toll-like receptor 4 pathway and use of TNF-alpha antagonists for treatment of the mucopolysaccharidoses. Proc. Natl. Acad. Sci. U. S. A. 2010;107:222–227. doi: 10.1073/pnas.0912937107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ausseil J, Desmaris N, Bigou S, Attali R, Corbineau S, Vitry S, Parent M, Cheillan D, Fuller M, Maire I, Vanier MT, Heard JM. Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice. PLoS One. 2008;3:e2296. doi: 10.1371/journal.pone.0002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abreu S, Hayden J, Berthold P, Shapiro IM, Decker S, Patterson D, Haskins M. Growth plate pathology in feline mucopolysaccharidosis VI. Calcif. Tissue. Int. 1995;57:185–190. doi: 10.1007/BF00310256. [DOI] [PubMed] [Google Scholar]
- [28].Metcalf JA, Zhang Y, Hilton MJ, Long F, Ponder KP. Mechanism of shortened bones in mucopolysaccharidosis VII. Mol. Genet. Metab. 2009;97:202–211. doi: 10.1016/j.ymgme.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kakavanos R, Turner CT, Hopwood JJ, Kakkis ED, Brooks DA. Immune tolerance after long-term enzyme replacement therapy among patients who have mucopolysaccharidosis type I. Lancet. 2003;361:1608–1613. doi: 10.1016/S0140-6736(03)13311-9. [DOI] [PubMed] [Google Scholar]
- [30].Auclair D, Ketteridge D, Oates S, Hopwood JJ, Byers S. An overview of intra-articular therapy for mucopolysaccharidosis VI. J. Pediatr. Rehabil. Med. 2010;3:3–6. doi: 10.3233/PRM-2010-0101. [DOI] [PubMed] [Google Scholar]