Introduction
Disorders involving uric acid including gout, hyperuricemia and resultant kidney failure are prevalent. There are three main groups of pharmaceuticals used for modulating uric acid: 1. Drugs that reduce the generation of uric acid (allopurinol and febuxistat), 2. Those that increase the removal of uric acid (rasburicase, pegloticase), (both depicted in Figure 1) or 3. Those that inhibit the reabsorption of uric acid (depicted in Figure 3). This summary briefly describes the mechanisms of action and the candidate genes and genetic variants associated with response to these drugs. Clinically relevant genetic variants have been identified for allopurinol and rasburicase, and CPIC guidelines have been published that recommend selection of alternative treatments for some individuals. A more extensive description and interactive version can be found for each pathway at www.pharmgkb.org.
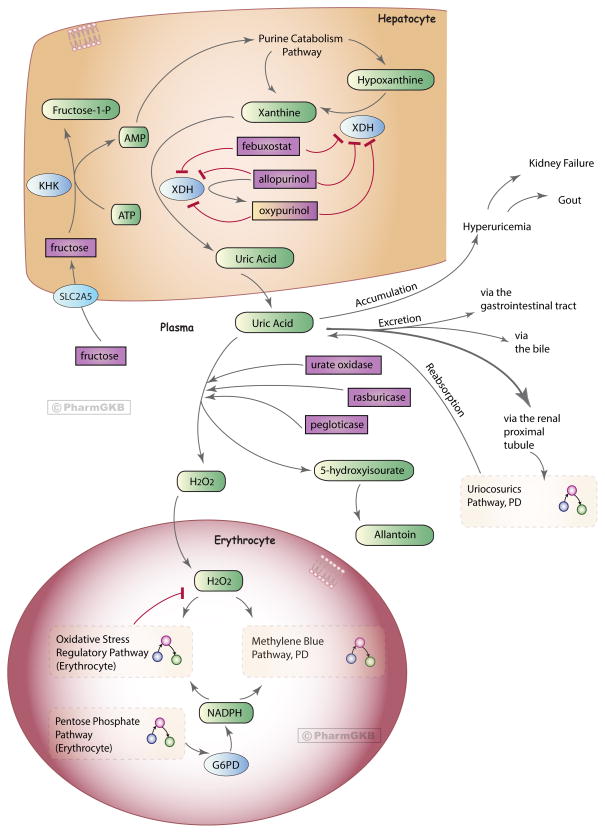
Figure 1. Uric acid-lowering drugs pathway, Pharmacodynamics.
Uric acid is the end product of the purine catabolism pathway in humans. Here is a representation of the drugs involved in lowering plasma uric acid levels, depicting their mechanism of action by either 1) preventing the formation of uric acid in hepatocytes by inhibition of xanthine dehydrogenase enzyme (XDH): allopurinol and febuxostat, or 2) breaking down uric acid in the plasma to a more soluble form: urate oxidase, rasburicase and pegloticase. Proteins/enzymes are represented by their genes (using HGNC approved symbols). Drugs involved in preventing the reaborption of uric acid in the renal proximal tubule are depicted in the interconnecting Uricosurics Pathway (Figure 3). The majority of uric acid found at physiological pH is in the form of urate anion [68]; here we used the term ‘uric acid’ to encompass urate. The mechanisms depicted in the erythrocyte show the neutralization of hydrogen peroxide (H2O2), produced in the break down of uric acid. In G6PD deficient erythrocytes this is defective, and may ultimately result in methemoglobinemia and or hemolysis. See the main text description and the three interconnecting pathways for further details. An interactive version of this pathway can be found at http://www.pharmgkb.org/pathway/PA165980774.
Abbreviations:
AMP = adenosine monophosphate; ATP = adenosine triphosphate; ER = endoplasmic reticulum; G6PD = glucose-6-phosphate dehydrogenase; H202 = hydrogen peroxide; HGNC = HUGO Gene Nomenclature Committee; HLA-B = major histocompatibility complex, class I, B; KHK = ketohexokinase (fructokinase); NADPH = nicotinamide adenine dinucleotide phosphate; PD = pharmacodynamic; PharmGKB = Pharmacogenomics Knowledgebase; SLC2A5 = solute carrier family 2 (facilitated glucose/fructose transporter) member 5; XDH = xanthine dehydrogenase.
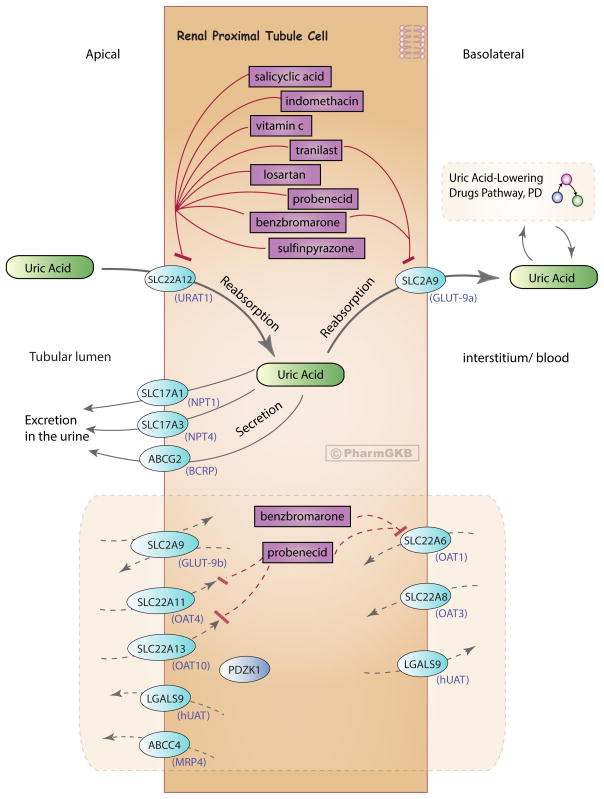
Figure 3. Uricosurics pathway, Pharmacodynamics.
A representative diagram of a renal proximal tubule epithelial cell and the drugs that are involved in preventing the reabsorption of uric acid into the body. Uric acid is reabsorbed into the interstitium/blood by transport from the tubular lumen across the apical membrane into the cell and then across the basolateral membrane. It is transported across the apical membrane for excretion in the urine. Depicted are genes that represent the transporters involved in uric acid transport across the apical and basolateral membrane of the cell. Commonly used names for the transporter proteins are indicated in parenthesis under the gene name. Uric acid in the blood links to the interconnecting Uric Acid-Lowering Drugs Pathway (Figure 1). Genes/proteins that are putatively involved in uric acid transport are also shown in the shaded bottom half of the diagram, representing potential novel drug targets or the potential for repurposing of drugs known to target these proteins. An interactive version of this pathway can be found at http://www.pharmgkb.org/pathway/PA166114721. The majority of uric acid found at physiological pH is in the form of urate anion [68]; here we used the term ‘uric acid’ to encompass urate.
1) Drugs that reduce the generation of uric acid: allopurinol and febuxostat
Pharmacodynamics
Allopurinol is a drug indicated for the treatment of gout, prophylaxis of hyperuricemia in patients undergoing chemotherapy, and prevention of kidney stones recurrence [1]. Allopurinol and its metabolite oxypurinol are analogues of hypoxanthine and xanthine, respectively, and work by binding to and inhibiting xanthine dehydrogenase (XDH), preventing the formation of uric acid (Figure 1) [1–5]. Hypoxanthine and xanthine are cleared in the urine or reutilized in the synthesis of nucleotides and nucleic acids [1, 6]. Febuxostat (Uloric®) is a non-purine inhibitor of XDH that can bind to and inhibit both oxidized or reduced forms of XDH (Figure 1) [2, 7, 8]. It is indicated for the treatment of hyperuricemia in patients with gout (but not for asymptomatic hyperuricemia) [9], and is an alternative therapy for patients where allopurinol has been contraindicated due to allergic responses [7, 8, 10]. A downside to allopurinol/febuxostat treatment is that existing high levels of plasma uric acid are not cleared and, as xanthine is less soluble than uric acid, xanthine kidney stones or xanthine nephropathy may result [3, 6].
Pharmacogenomics
A small proportion (0.1 to 0.4%) of patients receiving allopurinol develop life-threatening severe cutaneous adverse reactions (SCARs) that include drug hypersensitivity syndrome (DRESS), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) [11]. The allopurinol hypersensitivity reaction is completely distinct from the drug’s intended pharmacodynamic pathway: SJS and TEN are thought to occur via cytotoxic T cell-induced keratinocyte death by a drug-specific, HLA class I-restricted and granulysin-mediated pathway [12, 13]. NK cells may also be involved [13].
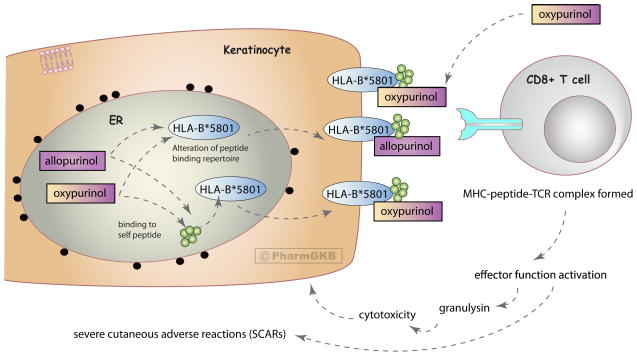
The HLA-B*5801 allele is currently the most well-established genetic association with allopurinol-induced SCAR and has been demonstrated in numerous populations (Table 1). The human HLA-B gene is the most polymorphic in the genome, with over 1500 alleles that are made up of many variants [14–16]. Historically, these alleles were identified by antigen binding assays but more recently sequencing and genotype techniques are utilized (see Table 1). Single Nucelotide Polymorphisms (SNPs) that tag the HLA-B*5801 allele differ in different populations [17]. The mechanism behind HLA-B*5801-specific allopurinol-induced hypersensitivity is not fully understood, however several hypotheses proposed for HLA-dependent T cell stimulation by drugs could be applicable (depicted in Figure 2) [11, 12, 18, 19]. Allopurinol or oxypurinol may bind self-protein/peptide to create a haptenated product which undergoes antigen processing and is presented specifically by HLA-B*5801 to activate antigen-specific T cells (the hapten concept) [18]. They may interact with the HLA-B*5801-MHC-peptide complex and TCR directly at the cell surface, rather than undergoing antigen processing (the pi concept) [18, 20]. During folding of HLA-B*5801 in the endothelium reticulum, allopurinol/oxypurinol may become incorporated into the peptide-binding groove, potentially changing the repertoire of self-peptides HLA-B*5801 is able to present, resulting in alloreactivity due to the presentation of novel peptides (the anchor site modification/occupation model) [18]. A recent in vitro study suggests evidence for the pi concept, and that oxypurinol has higher affinity for the HLA-*5801 molecule compared to allopurinol in docking experiments [20].
Table 1.
Reported associations between HLA-B*58:01 and risk of allopurinol-induced SCARs
| Study Type | Cases | Controls | OR (95% C.I.) |
P value | Patient Population |
Race/ethnicity | Typing and genotyping method |
Reference |
|---|---|---|---|---|---|---|---|---|
| Case/control study | n=7 (SJS, SJS ocular type, erythema exudativum multiforme (EEM) minor). | n=25 (no ADRs). | 65.6 (2.9–1497) | 9.733×10−4 | Hyperuricemia. | Japanese. | PCR-rSSO and PCR-SBT. | PMID: 23669020 [99] |
| Case/control study | n=25 (SJS, SJS/TEN, DRESS). | n=23 allopurinol- tolerant. | 39.11 (4.49–340.51) | 5.9×10−4 | Asymptomatic hyperuricemia, gouty arthritis or nephropathy. | Portuguese. | PCR-rSSO and SSO-HR typing kit. | PMID: 23600531 [100] |
| Observational study in family members | n=1 male who developed SJS. | n=1 the brother of the case, allopurinol-tolerant. | NA | NA | Gout and essential hypertension. | Self-described Han Chinese. | 4-digit, high resolution DNA sequencing. | PMID: 23280169 [101] |
| Case/control study | n=20 (SJS, DRESS, TEN, EMM). | n=30 allopurinol-tolerant, treated for at least 1 year (not matched to cases). | 123.5 (12.8–1195.1) (OR was higher when EMM case was excluded). | <1×10−4 | NA | Han Chinese (Hong Kong). | Sequence-based typing. | PMID: 22348415 [102] |
| Case-control study | n= 51 patients with allopurinol-induced SCAR (SJS, TEN or HSS). | n= 135 allopurinol-tolerant (at least 6 months of treatment). | 580.3 (34.4–9780.9) | 4.7×10−24 | Hyperuricemia. Chronic renal insufficiency was significantly higher in cases vs controls, and gouty arthritis was the converse. | Han Chinese residing in Taiwan. | Sequence-specific oligonucleotide reverse lineblot. | PMID: 15743917 [24] |
| Comparison of allele frequencies in a population. | n=31 patients with allopurinol-induced SJC or TEN. | NA | 61 (32–118) for the allele frequency of cases compared to the allele frequency found in the general European population. | <10−8 | Mostly hyperuricemia. | Mixed population, mostly European. | OLERSUP SSP HLA-B kit, sequencing in three cases. | PMID: 18192896 [103] |
| Comparison of allele frequencies in a population. | n=10 patients with allopurinol-induced SJS, TEN (one also treated with carbamazepine, another also treated with phenytoin). | NA | 40.83 (10.53–158.9) allele frequency compared to the frequency in n=493 healthy Japanese subjects. | <0.0001 | NA | Japanese. | Sequencing. | PMID: 19018717 [104] |
| Case-control | n= 27 patients with allopurinol-induced SJS or TEN (within 3 months of treatment). | n=54 allopurinol-tolerant patients (>6 months treatment) from the same hospitals. | 348.3 (19.2–6336.9) | 1.6×10−13 | Hyperuricemia, some patients with gouty arthritis. | Self-identified Thai or Thai-Chinese. | PCR with sequence specific primers, and sequence-based typing. | PMID: 19696695 [105] |
| Case/control | n=25 patients with allopurinol-induced SCARs (20 with DIHS, 5 with SJS/TEN). | n=57 allopurinol-tolerant patients. | 97.8 (18.3–521.5) (cases verses tolerant control). | 2.45×10−11 | Cases = patients with gouty arthritis or hyperuricemia related to chronic renal failure. Controls = patients with chronic renal failure. | Korean. | Direct DNA sequencing analysis. | PMID: 21301380 [28] |
| Comparison of allele frequency in healthy individuals. | n=7 allopurinol-induced SJS/TEN patients. | n=115 healthy individuals. | 13.625 (2.774–69.448) | 0.248 after correction for multiple comparisons. | NA | Caucasian, Northern Italy. | 4-digit allele level within the antigen binding domain. PCR-SSP. | PMID: 21545408 [29] |
| Case/control | n=16 patients with an allopurinol hypersensitivity reaction (9 had SCARs, 7 had simple rashes). | n=432 allopurinol-tolerant patients (≥60 days). | 179.24 (10.19–3151.74) SCARs patients vs tolerant. | <0.001 | Patients with chronic renal insufficiency who took allopurinol. | Korean. | Serologic HLA typing by microlymphoctotocity method for HLA-B*58. | PMID: 21393610 [106] |
| Case/ control | n=38 allopurinol- induced MPE, DRESS, SJS/TEN (within first 2 months of exposure). | n=63 allopurinol- tolerant (treated for >3 months with no cuteanous manifestations). | 580.07 (32.18–10456.80) | 7.01×10−18 | Hyperuricemia and gouty arthritis. (A higher frequency of chronic renal insufficiency was seen in cases). | Cases = from the Southern region of China, control = all Han Chinese. | Direct DNA sequencing. | PMID: 22909208 [107] |
| Case report | n=1, an 8-year old girl who developed TEN. | NA | NA. A test revealed she had HLA-B*5801. |
NA | Developed asymptomatic hyperuricemia due to anti-TB treatment and was treated with allopurinol. | German, Kenyan parents. | Not described. | PMID: 19483528 [108] |
| Case report | n=1, a 65-year old male who developed DRESS 1 month after allopurinol treatment initiation. | NA | NA. A test revealed he had a HLA-B*5801 positive genotype. |
NA | Hyperuricemia. | Han Chinese. | Method not described. | PMID: 22901319 [109] |
| Study identified patients across Australia with allopurinol hypersensitivity and carried out genotyping. | N=11 patients with allopurinol hypersensitivity including SJS/TEN and DRESS/DIHS, n=12 patients with MPE. | NA | NA. HLA-B*5801 was found in 6/5 cases with SJS/Ten, 1/5 cases with DRESS/DIHS and none of the patients with MPE. |
NA | Not described. | Australian, mixed population: Caucasian and South-East Asian. | Four-digit high resolution DNA sequence-based HLA typing. | PMID: 21790926 [110] |
| Case report | n=1, a 57-year old male who was diagnosed with SJS 10 days after allopurinol treatment. | NA | NA. Typing showed he had HLA-A31, A33, B51 and B58. |
NA | Not described. | Not described. | Reverse sequence-specific oligonucleotide with PCR for serological HLA typing. | PMID: 17587850 [111] |
| Case report | n=1, a 77 year-old male who was diagnosed with allopurinol-induced DIHS. | NA | NA. Typing showed he had HLA-A31, A33, B39 and B58. |
NA | Not described. | Not described. | Not described. | PMID: 17587850 [111] |
| Case report | n=1, a 93-year old male diagnosed with Allopurinol-induced SJS/TEN (a year earlier he had previously experienced maculopapular-type eruption and fever within 1 month of allopurionol treatment). | NA | NA. He had HLA-A24, A33, B52 and B58. |
NA | Not described. | Not described. | Not described. | PMID: 17587850 [111] |
| Drug-surveillance programme assessing Allopurinol cutaneous ADRs | n=84 cases, including maculopapular eruptions, SJS, TEN and DRESS. Testing for HLA-B*5801 was only done in a subgroup of patients with SJS/TEN. | Allele frequency in a general European population of 0.015 was compared. | 18/18 cases of SJS/TEN in whom the assay was performed carried HLA-B*5801. OR=65.07 (30.66–138.09) compared to the general European population allele frequency. | <0.0000. | Asymptomatic hyperuricemia, gout or secondary hyperuricemia (many also with hypertensive heart disease and renal failure). | Southern Sardinia, Italian, European. | PCR-SSO/PCR-SSP. | PMID: 22017528 [112] |
Abbreviations
ADRs = adverse drug reactions
DIHS= drug-induced hypersensitivity syndrome
DRESS = drug reaction with eosinophilia and systemic symptoms
EMM = erythema multiforme major
HSHSS= hypersensitivity syndrome
NA = not applicable
MPE = maculopapular exanthema
PCR = polymerase chain reaction
PCR-rSSO = PCR- reverse sequence-specific oligonucleotide
PCR-SBT = PCR-sequence based typing
SJS = Steven’s Johnson Syndrome
SSP = sequence-specific primers
TEN = toxic epidermal necrolysis
OR = odds ratio
CI = confidence interval
HLA = human leukocyte antigen
Figure 2. Potential mechanisms underlying allopurinol-induced SCARs.
Dashed arrows represent hypothesized mechanisms of action that may underly allopurinol-induced severe cutaneous adverse reactions (SCARs) linked to the HLA-B*5801 allele (see main text for further description).
HLA = human leukocyte antigen
MHC = major histocompatibility complex
TCR = T cell receptor
A meta-analysis calculated the odds ratios for allopurinol induced SJS/TEN in HLA-B*5801 carriers as 96.6 compared to allopurinol-tolerant matched controls, or 79 compared to population controls [21]. The American College of Rheumatology (ACR) guidelines recommend screening only patients who are in high risk populations (Koreans with stage 3 chronic kidney disease, Han Chinese and Thai patients), and those found to be positive for HLA-B*5801 should be prescribed an alternative drug [PMID: 23024028] [22]. The Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines also recommend not using allopurinol in patients who are known carriers of HLA-B*5801 [11]. The FDA-approved drug label for allopurinol currently carries no information regarding HLA genotype, despite FDA scientists publishing a study assessing the clinical usefulness of allopurinol pharmacogenetics and reporting a strong and highly significant association between HLA-B*5801 and likelihood of allopurinol-associated SCAR [1, 23].
In some populations (Han Chinese, Thai, Korean) HLA-B*5801 explains 80–100% of the SCAR cases, whereas in other populations (Japanese, European) HLA-B*5801 explains around 55% of the cases [24–30] suggesting that there are additional risk alleles and genetic variants that are also important for SCAR. In vitro experiments also suggest that allopurinol/oxypurinol-induced reactions are not strictly restricted to the HLA-B*5801 molecule [20]. Additional alleles associated with allopurinol-induced SCAR include: rs9263726 A (which is in complete linkage with HLA-B*5801 in Japanese patients), rs2734583, rs3094011, GA005234, rs2844665 C, rs3815087 A, rs3130931 C, rs3130501 G, rs3094188 A, rs9469003 C, HLA-A*33:03, HLA-C*03:02, HLA-C*08:01, HLA-Cw3, HLA-A33, HLA-DR13, HLA-D3, HLA-Cw*0302, HLA-A*3303, HLA-DRB1*0301, rs3117583, rs1150793, rs2855804, rs2268791, rs1594, rs2304224 (details of each study are available at (http://www.pharmgkb.org/drug/PA448320) [24, 28–31].
Febuxostat was associated with hypersensitivity in two reported cases of patients who previously had adverse reactions to allopurinol [32, 33] – to our knowledge the genetic mechanisms behind this have not been described, as yet. No pharmacogenetic studies related to febuxostat are currently found.
2) Drugs that increase the removal of uric acid: urate oxidase (uricase), rasburicase, pegloticase
Pharmacodynamics
One strategy for enhancing uric acid excretion is to add exogenous urate oxidase enzyme not expressed in humans. Urate oxidase breaks down uric acid to 5-hydroxyisourate, which is then spontaneously degraded to allantoin without the aid of enzymes (Figure 1) [2–4]. Allantoin has 5–10 fold increased solubility compared to uric acid, and thus is more readily excreted via the kidneys [3, 4, 6]. Uricozyme® is urate oxidase extracted from Aspergillus flavus, however due to product-related impurities it is associated with acute hypersensitivity reactions [2, 6]. A recombinant form of urate oxidase, rasburicase, was developed to reduce the occurrence of these reactions [2, 6]. Rasburicase rapidly reduces uric acid plasma concentrations and exposure [2, 34–37]. Rasburicase however is unsuitable for the treatment of gout due to its short half-life, thus pegloticase (or PEG uricase, Krystexxa®) is recombinant urate oxidase conjugated to polyethylene glycol that is thought to result in an increased half-life compared to rasburicase [2, 38].
Pharmacogenomics
Rasburicase and pegloticase (forms of urate oxidase) are contraindicated in G6PD deficient individuals due to an increased risk of hemolytic anemia and methemoglobinemia, conditions involving red blood cells (RBCs) [39–41]. This increased risk stems from G6PD’s role in producing NADPH in RBCs (see the PharmGKB Pentose Phosphate Pathway) [42]. When rasburicase breaks down uric acid into allantoin, hydrogen peroxide (H2O2) is released as a by-product, a reactive oxygen species that can cause damage in RBCs and may ultimately result in cell turnover (hemolysis). Under normal conditions, the H2O2 is reduced to water and oxygen molecules by regulatory mechanisms, many of which require NADPH (See the PharmGKB Oxidative Stress Regulatory Pathway). Methemoglobin (MetHb) is formed through the oxidation of heme iron atoms in hemoglobin and cannot transport oxygen or carbon dioxide; it is maintained at levels of around 1% in order to prevent methemoglobinemia (>1% MetHb), which can lead to cyanosis and tissue hypoxia (see the PharmGKB Methylene Blue Pathway, PD) [42]. G6PD deficient RBCs are unable to enhance NADPH production and are thus more susceptible to oxidative damage that can ultimately result in methemoglobinemia and/or hemolysis [39, 43–49]. More than 180 genetic variants within the G6PD gene have been described to date, and many confer deficiency of the G6PD enzyme in RBCs (see the PharmGKB G6PD VIP summary for more detailed information www.pharmgkb.com/vip/PA28469) [44, 50–53]. Several cases of methemoglobinemia and hemolytic anemia subsequent to treatment by rasburicase or urate oxidase have been reported in G6PD deficient individuals, though the underlying G6PD variant is often not reported and few studies report genotyping (Table 2). CPIC guidelines recommend avoiding the use of rasburicase in patients homo/hemizygous for G6PD variants that confer deficiency – in all other patients an enzyme test for G6PD deficiency is recommended prior to rasburicase use [54].
Table 2.
Urate oxidase/rasburicase-induced adverse reactions associated with G6PD deficiency.
| Patient details | Reason for urate oxidase/rasburicase treatment | Consequence of urate oxidase/rasburicase treatment | Genetic or enzyme screening | Reference |
|---|---|---|---|---|
| 16 year-old African American male. | Hyperuricemia due to Burkitt’s lymphoma. | Developed methemoglobinemia. This was reversed by discontinuation of rasburicase, blood transfusion and oxygen delivery. | Diagnosed with G6PD deficiency because of known sensitivity - no screening to confirm was carried out and no genetic information was provided. | PMID: 22190578 (Ng et al, 2011) [113] |
| Adult case report. | Cancer patients at high risk of developing TLS. | Patients with known G6PD deficiency were excluded from the study, however one patient developed methemoglobinemia and hemolytic anemia after the first dose of rasburicase, which was discontinued. Methylene blue treatment exacerbated hemolysis, and the patient was identified as having G6PD deficiency. | Diagnosis method not reported and no genetic information provided. | PMID: 22015451 (Vadhan-Raj et al, 2011) [114] |
| Male adult case report | A male with Burkitt’s lymphoma prescribed rasburicase before initiating chemotherapy. | Developed acute intravascular hemolysis. | The initial assay obtained soon after hemolysis was normal, however A- G6PD deficiency was later confirmed by a subsequent assay. | PMID: 20196170 (Bain et al, 2010) [115] |
| 12 year old Laotian male with T-cell ALL. | Hyperuricemia. | Developed methemoglobinemia and was treated with methylene blue. This did not improve methemoglobin levels or oxygen saturation levels and an exchange transfusion was undertaken to remove methemoglobin. | G6PD activity assay revealed a deficiency (genotyping not reported). | PMID: 18561168 (Bhat et al, 2008) [116] |
| African American male adult. | Acute renal failure secondary to hyperuricemia. | Developed methemoglobinemia and hemolytic anemia. Treated with daily packed red blood cell transfusions for 3 days, with resolution of methemoglobinemia and hemolysis. Rasburicase was thought to be the probable cause, as indicated by the Naranjo probability scale. | G6PD deficiency was confirmed by G6PD activity assay (no genotyping reported). | PMID: 16204390 (Browning and Kruse, 2005) [49] |
| Male adult. | Long term treatment with an i.v. of urate oxidase every other day for hyperuricemia several months following a kidney transplant. | 9 months later treated for 3 consecutive days with urate oxidase and subsequently diagnosed with hemolytic anemia, for which the patient received hemodialysis and recovered. | G6PD deficiency was later confirmed. The patient was classified as having the Mediterranean variant because of ethnic origin (born in Italy), although this was not confirmed by electrophoresis or genotyping. | PMID: 2019023 (Ducros et al, 1991) [117] |
| Pediatric case (mentioned in the discussion as an unpublished observed case). | Undergoing chemotherapy. | Hemolytic anemia. | Not detailed. | PMID: 2019023 (Ducros et al, 1991) [117] |
| A 12 year old African American male with ALL. | Patients with known G6PD deficiency were excluded from the study. | Developed methemoglobinemia after administration of non-recombinant urate oxidase (Uricozyme) and was later diagnosed with G6PD deficiency. | No test for diagnosis or genotyping reported. | PMID: 9369411 (Pui et al, 1997) [34] |
| A single case reported in the cohort of n=410. | A retrospective study of medical records of patients with B-cell lymphoma or ALL. | A single patient developed hemolysis with urate-oxidase treatment, ‘revealing a previously unknown G6PD deficiency’. | No tests confirming the diagnosis of G6PD deficiency were mentioned. | PMID: 12075750 (Patte et al, 2002) [118] |
| African American adult male with multiple myeloma admitted for chemotherapy. | Hyperuricemia and acute kidney injury. | Methemoglobinemia. As G6PD status was unknown, ascorbic acid was administered to treat the methemoglobinemia rather than methylene blue. The patient developed hemolysis, and required red blood cell transfusion. | Test results on day 3 revealed the patient was G6PD deficient (by enzyme activity level, measured in U/g Hb). Both methemoglobinemia and hemolysis were attributed to G6PD deficiency (genotyping not reported). | PMID: 22573495 (Sonbol et al, 2012) [119] |
| A 14 year old Cambodian male diagnosed with Burkitt’s lymphoma. | Received allopurinol for hyperuricemia, and furosemide and calcitonin for hypercalcemia, Rasburicase was administered before induction of chemotherapy. | Developed methemoglobinemia and subsequent hemolysis and required 3 red blood cell transfusions. | On day 3, G6PD deficiency was confirmed by quantitative assay (prior to the first transfusion). | PMID: 17387701 (Borinstein et al, 2007) [120] |
| A pediatric case in a cohort of n=278 pediatric and adult patients. | Rasburicase was administered in patients at risk of tumor lysis during initiation of chemotherapy in a compassionate use study. Patients with known G6PD deficiency were excluded. | A pediatric patient developed methemoglobinemia and was subsequently diagnosed with G6PD deficiency. | Testing method was not reported. | PMID: 12942574 (Bosly et al, 2003) [121] |
| A newborn male who was born at 30 weeks gestation by cesarean. | Among other complications, he was treated with rasburicase for a high uric acid level. | Hemolysis developed and he subsequently died. | A postmortem blood analysis showed a deficiency in G6PD enzyme, and genetic analysis revealed he was hemizygous for the G6PD Mediterranean variant. | PMID: 23209099 (Zaramella et al, 2012) [122] |
| 46-year old man, mixed Mauritian-Chinese background. | Treated for chronic lymphocytic leukemia. | 12 hours after rasburicase administration methemoglobinemia and hemolytic anemia developed. He was transfused with packed red blood cells, fluids and ascorbic acid. | Screening test revealed he had abnormal G6PD deficiency (variant not described). | PMID: 23860572 (Cheah et al, 2013) [123] |
| Case report of an adult male. | Part of a chemotherapy protocol. | A deficiency in G6PD was revealed. | Mediterranean variant determined by genotyping. | PMID: 19654083 (article in French) (Joly et al, 2009) [124]. |
Abbreviations
ALL = acute lymphoblastic leukemia; G6PD = glucose-6-phosphate dehydrogenase; TLS = tumor Lysis Syndrome.
Deficiencies in anti-oxidant mechanism pathways, not just in the G6PD enzyme, may further contribute to risk of rasburicase-induced methemoglobinemia/hemolysis [42, 55]. Individuals with acatalasemia are homozygous for a catalase gene (CAT) variant that affects catalase mRNA expression (Japanese Type I; OMIM 115500.0001, II; OMIM 115500.0002, Hungarian Type A; OMIM 115500.0003, B-D) or have the unstable Swiss protein variant, resulting in minimal catalase activity in erythrocytes [56, 57]. Heterozygotes of Japanese and Hungarian CAT variants have hypocatalasemia, with around half of the normal catalase blood levels [56, 57]. Takahara’s disease can develop in patients with acatalasemia from the production of H2O2 by oral microorganisms [56]. Higher levels of methememoglobin are seen in Japanese acatalasemia erythrocytes compared to normal erythrocytes when exposed to nitrogen monoxide or dioxide [57], and treatment of murine acatalasemia erythrocytes with H2O2 induces hemolysis in vitro [58]. Methemoglobinemia developed in a patient with the CAT Japanese Type genetic variant when H2O2 disinfectant was used pre-surgery [59]. In conclusion, catalase deficient patients may be more susceptible to methemoglobinemia and hemolysis with rasburicase treatment due to the release of H2O2, though direct cases have yet to be reported to our knowledge [55]. Cases of individuals with combined G6PD and catalase deficiency have been reported [56] and thus these individuals may be at a higher risk of rasburicase–induced methemoglobinemia and/or hemolysis. Patients with cytochrome b5 reductase (CYB5R3, NADH-dependent methemoglobin reductase) deficiency are less able to reduce methemoglobin, and may be more susceptible to drug-induced methemoglobinemia and hemolysis [60–64], along with individuals with hemoglobin variants e.g. Hasharon [62, 65], or deficiency in glutathione reductase (GSR) [65].
3) Drugs that inhibit the reabsorption of uric acid: uricosurics
Pharmacodynamics
Uricosuric drugs (e.g. probenecid, benzbromarone, sulfinpyrazone) inhibit reabsorption of uric acid in the renal proximal tubule by targeting transporters (Figure 3) [2, 5]. Probenecid is recommended by the ACR as an alternative first line therapy in patients with gout in whom allopurinol/febuxostat are contraindicated [22]. Numerous transporters have a putative role in the secretion and reabsorption of uric acid across the luminal apical membrane of the renal proximal tubule and across the basolateral interstitual membrane; however, a clear picture of the mechanisms of uric transport has still to be defined [66, 67]. Recent genetic analyses such as genome wide association studies (GWAS) have identified variants in novel genes associated with uric acid levels or gout and have helped in the elucidation of which proteins may be important in uric acid transport and plasma levels in humans [66–73]. The current model involves a complex of proteins: the urate or uric acid ‘transportasome’ [66, 67, 69]. The transporters of the transportasome are involved in the transport of other compounds; however, here we focus on uric acid.
URAT1 (SLC22A12) is a transporter protein found on the apical surface of renal proximal tubule epithelial cells with a major role in the uptake of uric acid from the lumen [66, 68, 69, 74, 75]. Variants in the SLC22A12 gene identified in renal hypouricemia patients lack uric acid transport activity or display significantly decreased activity compared to wild-type URAT1 in vitro [74, 76]. Also expressed on the apical membrane of renal proximal tubule cells with a role in uric acid secretion into the glomerular filtrate/lumen are transporters encoded by SLC17A1 (NPT1), SLC17A3 (NPT4) and ABCG2 (BCRP) [66, 68, 69]. GLUT9 (SLC2A9) is expressed on the basolateral membrane, with a chief role in the transport of uric acid into the interstitium and blood [66, 68, 69]. Evidence suggests that two isoforms of GLUT9 exist – GLUT-9a (isoform 1) on the basolateral side, and GLUT-9b (isoform 2 or GLUT9ΔN) on the apical side, though the role of the latter remains unclear [66, 68, 69]. In vitro, both isoforms are capable of transporting uric acid in stably expressing cells [77]. Validating their role in uric acid transport, two recent meta-analyses of different GWAS studies (both in more than 28,000 individuals of European descent/White ethnicity), found SNPs associated with serum uric acid levels in loci containing the SLC22A12, SLC2A9, SLC17A1, SLC17A3 and ABCG2 genes, amongst other novel loci [78, 79]. A GWAS meta-analysis examining four phenotypes related to kidney function verified SLC2A9, ABCG2 and SLC22A12 loci were associated with uric acid concentration in 33,074 East Asian individuals [73]. GWAS meta-analysis of >140,000 individuals of European descent from the Global Urate Genetics Consortium (GUGC) identified 10 previously known and 16 novel loci associated with serum urate concentrations at genome-wide significance [72]. These loci explain 7% of the variance in urate concentrations, with SLC2A9 and ABCG2 loci contributing to 3.4% of this [72]. 17 of the loci associated with urate concentrations were also associated with gout [72], as has been shown previously for SLC2A9 and ABCG2 loci [79]. Many of the SNPs associated with uric acid concentrations in the European analysis were also associated in cohorts of African-Americans (n=5,820), Japanese (n=15,286) and individuals of Indian ancestry (n=8,340), though differences are observed and may be due to variation in allele frequencies between the four different populations [72]. Interestingly, novel loci associated with uric acid concentrations reported in recent GWAS meta-analyses include genes encoding transcription factors and genes implicated in glucose homeostasis and kidney function, revealing possible new avenues for drug discovery for reducing urate levels and preventing kidney disease [72, 73, 78, 79].
Several other genes shown in the shaded section of Figure 3 have a potential role in uric acid transport in the human renal proximal tubule. These all have in vitro evidence, and some have also been identified in GWAS related to serum uric acid concentrations and/or gout [66–69, 72, 78–83].
Probenecid prevents the reabsorption of organic anions such as uric acid from the renal proximal tubule predominantly by inhibiting URAT1 (SLC22A12) transporter protein activity [5, 66, 74, 75, 84]. It may also act upon OAT1 (SLC22A6), OAT4 (SLC22A11) and OAT10 [83–85]. In vitro probenecid does not seem to have an effect on GLUT9 (SLC2A9) at an effective pharmacological concentration of 1mM [77]. Benzbromarone inhibits uric acid transport by URAT1 (SLC22A12) and GLUT-9 (SLC2A9) in vitro, though at therapeutic doses its action upon GLUT-9 may be minimal [69, 74, 75, 77, 84, 86, 87]. It also displays action against uric acid uptake by OAT1 (SLC22A6) in vitro [85]. Sulfinpyrazone inhibits uric acid uptake by URAT1 (SLC22A12) in vitro, and reduces serum uric acid in vivo [74, 88, 89].
Used for hypertension treatment, losartan is a unique angiotensin II receptor antagonist in that it also increases uric acid secretion and significantly decreases plasma levels by targeting URAT1 (SLC22A12) [74, 86, 90]. Tranilast is an anti-inflammatory, however also exhibits uricosuric properties by inhibiting URAT1 and GLUT9, thus these combined effects make it a potential gout therapeutic [84]. Vitamin c is thought to act as a uricosuric by inhibiting reabsorption of uric acid by URAT1 (SLC22A12) [91]. Studies have revealed that doses of 500mg/daily may reduce serum uric acid and doses of >1000mg/daily may reduce risk of gout [38]. Salicylate/salicyclic acid and indomethacin also inhibit uric acid uptake by URAT1 (SLC22A12) in vitro [74, 75].
Pharmacogenomics
As well as influencing plasma uric acid levels and risk of gout, polymorphisms in transporter genes may also affect the efficacy of uricosurics to inhibit reabsorption. However, currently there are few published pharmacogenetic studies investigating these drugs; below are the genetic associations with uricosurics found in an extensive literature search. Figure 3 illustrates genes that express proteins involved in the regulation of uric acid transport, highlighting potential novel drug targets and potential genes of interest for future pharmacogenetic studies. Polymorphisms in genes involved in the metabolism of these drugs may also influence their toxicity and efficacy.
Numerous variants within SLC22A12 have been identified in patients with hypouricemia, some of which display reduced uric acid uptake in vitro [74, 76, 92, 93]. There is some evidence to suggest that these variants may affect response to uricosuric and anti-uricosuric drugs that act upon URAT1. Amongst hypertensive patients (without hypouricemia) with wildtype SLC22A12, benzbromarone or losartan treatments significantly increase in uric acid clearance (Cur)/creatinine clearance (Ccr) ratio (indicating a decrease in serum uric acid). In patients with hypertension and hypouricemia who were homozygous or compound heterozygous for variants in the SLC22A12 gene, benzbromarone or losartan treatment had no effect on the Cur/Ccr ratio (indicating a lack of effect on serum uric acid levels). Losartan was still able to maintain its hypotensive effect on blood pressure in these patients [86]. These variants included rs121907896 G269A (Arg90His) and rs121907892 G774A Trp258Ter (c.NM_144585.2); both identified in patients with idiopathic renal hypouricemia, associated with reduced uric acid uptake in vitro and lower residuals of serum uric acid levels compared to wild-type in subjects [74, 76, 93]. This lack of response to benzbromarone in hypouricemia patients with SLC22A12 variants that reduce uric acid transport is supported by another study, which also observed an effect on the anti-uricosuric activity of pyrazinamide [76].
Due to reports of fatal hepatotoxicity, benzbromarone was withdrawn from the market by one of its main manufacturers, though it is still available from other drug companies in several countries across the globe [87, 94]. Genetics could be a factor that influences toxicity risk. Benzbromarone is metabolized by CYP2C9 to form the active metabolite 6-hydroxybenzbromarone (which has inhibitory activity upon URAT1 uric acid uptake) and by CYP3A4 to form 1′-hydroxybenzbromarone [75, 87, 94, 95]. 6-hydroxybenzbromarone is further metabolized to 5,6-dihydroxybenzbromarone by CYP2C9 and CYP1A2 [94]. A small study in 20 healthy individuals saw reduced metabolism and clearance of benzbromarone in an individual with the CYP2C9*3/*3 genotype compared to those with *1/*1 or *1/*3 genotypes, and a significantly higher elimination half life of 6-hydroxybenzbromarone in patients with the *1/*3 genotype compared to *1/*1 [96]. Whether CYP2C9 genotype may have a clinically relevant effect on benzbromarone pharmacodynamics is unknown; the study saw no differences in uric acid excretion or plasma concentrations [96]. Polymorphisms in CYP2C9 that result in higher plasma levels of benzbromarone and reduced metabolism to 6-hydroxybenzbromarone could contribute to toxicity: benzbromarone, 1′-hydroxybenzbromarone and 5,6-dihydroxybenzbromarone have been associated with toxicity in vitro [95, 97]. The CYP2C9 dependent sequential oxidations forming the 5,6-catechol has the potential for forming a reactive quinone, which is supported by forming glutathione adducts in vitro [87, 95]. This reaction profile is typical of several other hepatotoxic drugs. It has been recommended that CYP2C9 genotyping be carried out in cases of benzbromarine-induced hepatotoxicity [87], in order to begin to capture more evidence for whether an asssociation between CYP2C9 genotype and benzbromarone toxicity exists.
Intrinsic clearance of sulfinpyrazone by UGT1A9 glucuronidation was shown to be significantly lower in cells expressing the UGT1A9 variant Met33Thr (rs72551330 allele C) compared to Met33 (allele T) [98].
Conclusions
Unlike other animals, humans and some apes do not have the ability to break down uric acid into a more soluble form; high levels of plasma uric acid are a feature of pathologies such as gout and Tumor Lysis Syndrome and can result in kidney failure. Pharmaceuticals have been developed to either inhibit the formation of uric acid, increase its excretion or inhibit its reabsorption. Here we have outlined the mechanisms of action of uric acid-lowering drugs, the genes that express proteins targeted in these pathways and genetic variants associated with response to these drugs. Allopurinol and rasburicase are not recommended in individuals with particular genetic polymorphisms due to adverse reactions. Few pharmacogenetic studies are published for uricosurics and a full picture of the uric acid transportasome is still emerging; further research into the effects of polymorphisms in transporter genes on the uricosuric action of these drugs may provide insights into treatment efficacy or novel targets for drug development.
Acknowledgments
This work is supported by the NIH/NIGMS (R24 GM61374). Thank you to Dr Michael S. Hershfield for valuable feedback and suggestions.
Footnotes
Conflicts of Interest
None to declare.
References
- 1.Allopurinol tablet [Accord Healthcare Inc. [Accessed Oct 9th 2013]; available on the DailyMed website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=682dd8b8-fc6e-47c5-95b7-82d7ad96b750.
- 2.Whelton A. Current and future therapeutic options for the management of gout. Am J Ther. 2010;17:402–417. doi: 10.1097/MJT.0b013e3181df8ad2. [DOI] [PubMed] [Google Scholar]
- 3.Navolanic PM, Pui CH, Larson RA, Bishop MR, Pearce TE, Cairo MS, Goldman SC, Jeha SC, Shanholtz CB, Leonard JP, McCubrey JA. Elitek-rasburicase: an effective means to prevent and treat hyperuricemia associated with tumor lysis syndrome, a Meeting Report, Dallas, Texas, January 2002. Leukemia. 2003;17:499–514. doi: 10.1038/sj.leu.2402847. [DOI] [PubMed] [Google Scholar]
- 4.Oldfield V, Perry CM. Rasburicase: a review of its use in the management of anticancer therapy-induced hyperuricaemia. Drugs. 2006;66:529–545. doi: 10.2165/00003495-200666040-00008. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Yuan Y, Zhan CG, Liao F. Uricases as therapeutic agents to treat refractory gout: Current states and future directions. Drug Dev Res. 2012;73:66–72. doi: 10.1002/ddr.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pui CH. Rasburicase: a potent uricolytic agent. Expert Opin Pharmacother. 2002;3:433–442. doi: 10.1517/14656566.3.4.433. [DOI] [PubMed] [Google Scholar]
- 7.Pascual E, Sivera F, Yasothan U, Kirkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8:191–192. doi: 10.1038/nrd2831. [DOI] [PubMed] [Google Scholar]
- 8.Jansen TL, Richette P, Perez-Ruiz F, Tausche AK, Guerne PA, Punzi L, Leeb B, Barskova V, Uhlig T, Pimentao J, et al. International position paper on febuxostat. Clin Rheumatol. 2010;29:835–840. doi: 10.1007/s10067-010-1457-8. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed 9th Oct 2013];Uloric (febuxostat) drug label available on the DailyMed website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=54de10ef-fe5f-4930-b91d-6bbb04c664bd.
- 10.Hilmi BA, Asmahan MI, Rosman A. Use of newly available febuxostat in a case of chronic tophaceous gout contraindicated to allopurinol and probenecid. Med J Malaysia. 2012;67:125–126. [PubMed] [Google Scholar]
- 11.Hershfield MS, Callaghan JT, Tassaneeyakul W, Mushiroda T, Thorn CF, Klein TE, Lee MT. Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 2013;93:153–158. doi: 10.1038/clpt.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung WH, Hung SI, Chen YT. Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2007;7:317–323. doi: 10.1097/ACI.0b013e3282370c5f. [DOI] [PubMed] [Google Scholar]
- 13.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–1350. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 14.Robinson J, Mistry K, McWilliam H, Lopez R, Parham P, Marsh SG. The IMGT/HLA database. Nucleic Acids Res. 2011;39:D1171–1176. doi: 10.1093/nar/gkq998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Fernandez-Vina M, Geraghty DE, Holdsworth R, Hurley CK, et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HLA Nomenclature. http://hla.alleles.org/
- 17.de Bakker PI, McVean G, Sabeti PC, Miretti MM, Green T, Marchini J, Ke X, Monsuur AJ, Whittaker P, Delgado M, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharadwaj M, Illing P, Theodossis A, Purcell AW, Rossjohn J, McCluskey J. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu Rev Pharmacol Toxicol. 2012;52:401–431. doi: 10.1146/annurev-pharmtox-010611-134701. [DOI] [PubMed] [Google Scholar]
- 19.Yun J, Adam J, Yerly D, Pichler WJ. Human leukocyte antigens (HLA) associated drug hypersensitivity: consequences of drug binding to HLA. Allergy. 2012;67:1338–1346. doi: 10.1111/all.12008. [DOI] [PubMed] [Google Scholar]
- 20.Yun J, Marcaida MJ, Eriksson KK, Jamin H, Fontana S, Pichler WJ, Yerly D. Oxypurinol Directly and Immediately Activates the Drug-Specific T Cells via the Preferential Use of HLA-B*58:01. J Immunol. 2014 doi: 10.4049/jimmunol.1302306. [DOI] [PubMed] [Google Scholar]
- 21.Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet. 2011;12:118. doi: 10.1186/1471-2350-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zineh I, Mummaneni P, Lyndly J, Amur S, La Grenade LA, Chang SH, Rogers H, Pacanowski MA. Allopurinol pharmacogenetics: assessment of potential clinical usefulness. Pharmacogenomics. 2011;12:1741–1749. doi: 10.2217/pgs.11.131. [DOI] [PubMed] [Google Scholar]
- 24.Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, Lan JL, Yang LC, Hong HS, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, Naldi L, Bouwes-Bavinck JN, Sidoroff A, de Toma C, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 26.Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, Sawada J, Furuya H, Takahashi Y, Muramatsu M, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–1622. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 27.Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, Chucherd P, Konyoung P, Vannaprasaht S, Choonhakarn C, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19:704–709. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 28.Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, Park HW, Chang YS, Jang IJ, Cho SH, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011;21:303–307. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- 29.Cristallo AF, Schroeder J, Citterio A, Santori G, Ferrioli GM, Rossi U, Bertani G, Cassano S, Gottardi P, Ceschini N, et al. A study of HLA class I and class II 4-digit allele level in Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Immunogenet. 2011;38:303–309. doi: 10.1111/j.1744-313X.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 30.Tohkin M, Kaniwa N, Saito Y, Sugiyama E, Kurose K, Nishikawa J, Hasegawa R, Aihara M, Matsunaga K, Abe M, et al. A whole-genome association study of major determinants for allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2011.41. [DOI] [PubMed] [Google Scholar]
- 31.Genin E, Schumacher M, Roujeau JC, Naldi L, Liss Y, Kazma R, Sekula P, Hovnanian A, Mockenhaupt M. Genome-wide association study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Europe. Orphanet J Rare Dis. 2011;6:52. doi: 10.1186/1750-1172-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abeles AM. Febuxostat hypersensitivity. J Rheumatol. 2012;39:659. doi: 10.3899/jrheum.111161. [DOI] [PubMed] [Google Scholar]
- 33.Chohan S. Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J Rheumatol. 2011;38:1957–1959. doi: 10.3899/jrheum.110092. [DOI] [PubMed] [Google Scholar]
- 34.Pui CH, Relling MV, Lascombes F, Harrison PL, Struxiano A, Mondesir JM, Ribeiro RC, Sandlund JT, Rivera GK, Evans WE, Mahmoud HH. Urate oxidase in prevention and treatment of hyperuricemia associated with lymphoid malignancies. Leukemia. 1997;11:1813–1816. doi: 10.1038/sj.leu.2400850. [DOI] [PubMed] [Google Scholar]
- 35.Pui CH, Mahmoud HH, Wiley JM, Woods GM, Leverger G, Camitta B, Hastings C, Blaney SM, Relling MV, Reaman GH. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients With leukemia or lymphoma. J Clin Oncol. 2001;19:697–704. doi: 10.1200/JCO.2001.19.3.697. [DOI] [PubMed] [Google Scholar]
- 36.Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, Tou C, Harvey E, Morris E, Cairo MS. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998–3003. doi: 10.1182/blood.v97.10.2998. [DOI] [PubMed] [Google Scholar]
- 37.Pui CH. Urate oxidase in the prophylaxis or treatment of hyperuricemia: the United States experience. Semin Hematol. 2001;38:13–21. doi: 10.1016/s0037-1963(01)90039-3. [DOI] [PubMed] [Google Scholar]
- 38.Suresh E, Das P. Recent advances in management of gout. QJM. 2012;105:407–417. doi: 10.1093/qjmed/hcr242. [DOI] [PubMed] [Google Scholar]
- 39.Sivilotti ML. Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev. 2004;23:169–188. doi: 10.2165/00139709-200423030-00004. [DOI] [PubMed] [Google Scholar]
- 40.Elitek (rasburicase) drug label. Sanofi-aventis U.S. LLC; [accessed 27th July 2012]. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0ae10bc4-6b65-402f-9db5-2d7753054922. [Google Scholar]
- 41.Krystexxa (Pegloticase) drug label. Savient Pharmaceuticals Inc; [accessed 27th July 2012]. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5f4574d1-401f-4647-83e5-28c0f4a122a7. [Google Scholar]
- 42.McDonagh EM, Bautista JM, Youngster I, Altman RB, Klein TE. PharmGKB summary: methylene blue pathway. Pharmacogenet Genomics. 2013;23:498–508. doi: 10.1097/FPC.0b013e32836498f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaetani GD, Parker JC, Kirkman HN. Intracellular restraint: a new basis for the limitation in response to oxidative stress in human erythrocytes containing low-activity variants of glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1974;71:3584–3587. doi: 10.1073/pnas.71.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67:601–611. [PMC free article] [PubMed] [Google Scholar]
- 45.Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J. 2011;104:757–761. doi: 10.1097/SMJ.0b013e318232139f. [DOI] [PubMed] [Google Scholar]
- 46.Curry S. Methemoglobinemia. Ann Emerg Med. 1982;11:214–221. doi: 10.1016/s0196-0644(82)80502-7. [DOI] [PubMed] [Google Scholar]
- 47.Gaetani GF, Rolfo M, Arena S, Mangerini R, Meloni GF, Ferraris AM. Active involvement of catalase during hemolytic crises of favism. Blood. 1996;88:1084–1088. [PubMed] [Google Scholar]
- 48.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 49.Browning LA, Kruse JA. Hemolysis and methemoglobinemia secondary to rasburicase administration. Ann Pharmacother. 2005;39:1932–1935. doi: 10.1345/aph.1G272. [DOI] [PubMed] [Google Scholar]
- 50.Minucci A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of the “old” and update of the new mutations. Blood Cells Mol Dis. 2012;48:154–165. doi: 10.1016/j.bcmd.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: the genotype-phenotype association. Blood Rev. 2007;21:267–283. doi: 10.1016/j.blre.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida A, Beutler E, Motulsky AG. Human glucose-6-phosphate dehydrogenase variants. Bull World Health Organ. 1971;45:243–253. [PMC free article] [PubMed] [Google Scholar]
- 53.McDonagh EM, Thorn CF, Bautista JM, Youngster I, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for G6PD. Pharmacogenet Genomics. 2012;22:219–228. doi: 10.1097/FPC.0b013e32834eb313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Relling MV, McDonagh EM, Chang T, Caudle KE, McLeod HL, Haidar CE, Klein TE, Luzzatto L. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for Rasburicase Therapy in the context of G6PD Deficiency Genotype. Clin Pharmacol Ther. 2014 May 2; doi: 10.1038/clpt.2014.97. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goth L, Bigler NW. Catalase deficiency may complicate urate oxidase (rasburicase) therapy. Free Radic Res. 2007;41:953–955. doi: 10.1080/10715760701482451. [DOI] [PubMed] [Google Scholar]
- 56.Ogata M. Acatalasemia. Hum Genet. 1991;86:331–340. doi: 10.1007/BF00201829. [DOI] [PubMed] [Google Scholar]
- 57.Ogata M, Wang DH, Ogino K. Mammalian acatalasemia: the perspectives of bioinformatics and genetic toxicology. Acta Med Okayama. 2008;62:345–361. doi: 10.18926/AMO/30951. [DOI] [PubMed] [Google Scholar]
- 58.Masuoka N, Sugiyama H, Ishibashi N, Wang DH, Masuoka T, Kodama H, Nakano T. Characterization of acatalasemic erythrocytes treated with low and high dose hydrogen peroxide. Hemolysis and aggregation. J Biol Chem. 2006;281:21728–21734. doi: 10.1074/jbc.M513818200. [DOI] [PubMed] [Google Scholar]
- 59.Hamada Y, Kameyama Y, Iizuka T, Ishizaki T, Nishiyama T, Isshiki A. Methemoglobinemia from hydrogen peroxide in a patient with acatalasemia. Anesthesiology. 2004;101:247–248. doi: 10.1097/00000542-200407000-00038. [DOI] [PubMed] [Google Scholar]
- 60.Karadsheh NS, Shaker Q, Ratroat B. Metoclopramide-induced methemoglobinemia in a patient with co-existing deficiency of glucose-6-phosphate dehydrogenase and NADH-cytochrome b5 reductase: failure of methylene blue treatment. Haematologica. 2001;86:659–660. [PubMed] [Google Scholar]
- 61.Sannes LJ, Hultquist DE. Effects of hemolysate concentration, ionic strength and cytochrome b5 concentration on the rate of methemoglobin reduction in hemolysates of human erythrocytes. Biochim Biophys Acta. 1978;544:547–554. doi: 10.1016/0304-4165(78)90329-x. [DOI] [PubMed] [Google Scholar]
- 62.Ganer A, Knobel B, Fryd CH, Rachmilewitz EA. Dapsone-induced methemoglobinemia and hemolysis in the presence of familial hemoglobinopathy Hasharon and familial methemoglobin reductase deficiency. Isr J Med Sci. 1981;17:703–704. [PubMed] [Google Scholar]
- 63.Daly JS, Hultquist DE, Rucknagel DL. Phenazopyridine induced methaemoglobinaemia associated with decreased activity of erythrocyte cytochrome b5 reductase. J Med Genet. 1983;20:307–309. doi: 10.1136/jmg.20.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen RJ, Sachs JR, Wicker DJ, Conrad ME. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N Engl J Med. 1968;279:1127–1131. doi: 10.1056/NEJM196811212792102. [DOI] [PubMed] [Google Scholar]
- 65.Ingram J, Weitzman S, Greenberg ML, Parkin P, Filler R. Complications of indwelling venous access lines in the pediatric hematology patient: a prospective comparison of external venous catheters and subcutaneous ports. Am J Pediatr Hematol Oncol. 1991;13:130–136. doi: 10.1097/00043426-199122000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. 2012;14:179–188. doi: 10.1007/s11926-012-0240-z. [DOI] [PubMed] [Google Scholar]
- 67.Anzai N, Jutabha P, Amonpatumrat-Takahashi S, Sakurai H. Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies. Clin Exp Nephrol. 2012;16:89–95. doi: 10.1007/s10157-011-0532-z. [DOI] [PubMed] [Google Scholar]
- 68.Wright AF, Rudan I, Hastie ND, Campbell H. A ‘complexity’ of urate transporters. Kidney Int. 2010;78:446–452. doi: 10.1038/ki.2010.206. [DOI] [PubMed] [Google Scholar]
- 69.Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2012;8:610–621. doi: 10.1038/nrrheum.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le MT, Shafiu M, Mu W, Johnson RJ. SLC2A9--a fructose transporter identified as a novel uric acid transporter. Nephrol Dial Transplant. 2008;23:2746–2749. doi: 10.1093/ndt/gfn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–442. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 72.Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, Pistis G, Ruggiero D, O’Seaghdha CM, Haller T, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45:145–154. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okada Y, Sim X, Go MJ, Wu JY, Gu D, Takeuchi F, Takahashi A, Maeda S, Tsunoda T, Chen P, et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat Genet. 2012;44:904–909. doi: 10.1038/ng.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 75.Shin HJ, Takeda M, Enomoto A, Fujimura M, Miyazaki H, Anzai N, Endou H. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology (Carlton) 2011;16:156–162. doi: 10.1111/j.1440-1797.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 76.Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15:164–173. doi: 10.1097/01.asn.0000105320.04395.d0. [DOI] [PubMed] [Google Scholar]
- 77.Caulfield MJ, Munroe PB, O’Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5:e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, Chasman DI, Aspelund T, Eiriksdottir G, Harris TB, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3:523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato M, Iwanaga T, Mamada H, Ogihara T, Yabuuchi H, Maeda T, Tamai I. Involvement of uric acid transporters in alteration of serum uric acid level by angiotensin II receptor blockers. Pharm Res. 2008;25:639–646. doi: 10.1007/s11095-007-9401-6. [DOI] [PubMed] [Google Scholar]
- 81.Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94:297–304. doi: 10.1254/jphs.94.297. [DOI] [PubMed] [Google Scholar]
- 82.Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2011:29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]
- 83.Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, Burckhardt G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13) J Biol Chem. 2008;283:16332–16341. doi: 10.1074/jbc.M800737200. [DOI] [PubMed] [Google Scholar]
- 84.Mount DB. The kidney in hyperuricemia and gout. Curr Opin Nephrol Hypertens. 2013;22:216–223. doi: 10.1097/MNH.0b013e32835ddad2. [DOI] [PubMed] [Google Scholar]
- 85.Ichida K, Hosoyamada M, Kimura H, Takeda M, Utsunomiya Y, Hosoya T, Endou H. Urate transport via human PAH transporter hOAT1 and its gene structure. Kidney Int. 2003;63:143–155. doi: 10.1046/j.1523-1755.2003.00710.x. [DOI] [PubMed] [Google Scholar]
- 86.Hamada T, Ichida K, Hosoyamada M, Mizuta E, Yanagihara K, Sonoyama K, Sugihara S, Igawa O, Hosoya T, Ohtahara A, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT 1) in hypertensive patients. Am J Hypertens. 2008;21:1157–1162. doi: 10.1038/ajh.2008.245. [DOI] [PubMed] [Google Scholar]
- 87.Lee MH, Graham GG, Williams KM, Day RO. A benefit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interest of patients? Drug Saf. 2008;31:643–665. doi: 10.2165/00002018-200831080-00002. [DOI] [PubMed] [Google Scholar]
- 88.Nagy F. Use of Rabenid for different indications. Ther Hung. 1991;39:90–92. [PubMed] [Google Scholar]
- 89.Pfister B, Imhof P, Wirz H. Effect of sulphinpyrazone (Anturan) on uric acid excretion and plasma uric acid concentration in healthy volunteers. Eur J Clin Pharmacol. 1978;13:263–265. doi: 10.1007/BF00716361. [DOI] [PubMed] [Google Scholar]
- 90.Burnier M, Roch-Ramel F, Brunner HR. Renal effects of angiotensin II receptor blockade in normotensive subjects. Kidney Int. 1996;49:1787–1790. doi: 10.1038/ki.1996.268. [DOI] [PubMed] [Google Scholar]
- 91.Torralba KD, De Jesus E, Rachabattula S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: current and future directions. Int J Rheum Dis. 2012;15:499–506. doi: 10.1111/1756-185X.12010. [DOI] [PubMed] [Google Scholar]
- 92.Ichida K, Hosoyamada M, Kamatani N, Kamitsuji S, Hisatome I, Shibasaki T, Hosoya T. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet. 2008;74:243–251. doi: 10.1111/j.1399-0004.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 93.Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H. A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int. 2004;66:935–944. doi: 10.1111/j.1523-1755.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 94.Kobayashi K, Kajiwara E, Ishikawa M, Oka H, Chiba K. Identification of CYP isozymes involved in benzbromarone metabolism in human liver microsomes. Biopharm Drug Dispos. 2012;33:466–473. doi: 10.1002/bdd.1813. [DOI] [PubMed] [Google Scholar]
- 95.McDonald MG, Rettie AE. Sequential metabolism and bioactivation of the hepatotoxin benzbromarone: formation of glutathione adducts from a catechol intermediate. Chem Res Toxicol. 2007;20:1833–1842. doi: 10.1021/tx7001228. [DOI] [PubMed] [Google Scholar]
- 96.Uchida S, Shimada K, Misaka S, Imai H, Katoh Y, Inui N, Takeuchi K, Ishizaki T, Yamada S, Ohashi K, et al. Benzbromarone pharmacokinetics and pharmacodynamics in different cytochrome P450 2C9 genotypes. Drug Metab Pharmacokinet. 2010;25:605–610. doi: 10.2133/dmpk.dmpk-10-nt-040. [DOI] [PubMed] [Google Scholar]
- 97.Kobayashi K, Kajiwara E, Ishikawa M, Mimura H, Oka H, Ejiri Y, Hosoda M, Chiba K. Cytotoxic effects of benzbromarone and its 1′-hydroxy metabolite in human hepatocarcinoma FLC4 cells cultured on micro-space cell culture plates. Drug Metab Pharmacokinet. 2013;28:265–268. doi: 10.2133/dmpk.dmpk-12-nt-105. [DOI] [PubMed] [Google Scholar]
- 98.Korprasertthaworn P, Rowland A, Lewis BC, Mackenzie PI, Yoovathaworn K, Miners JO. Effects of amino acid substitutions at positions 33 and 37 on UDP-glucuronosyltransferase 1A9 (UGT1A9) activity and substrate selectivity. Biochem Pharmacol. 2012;84:1511–1521. doi: 10.1016/j.bcp.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 99.Niihara H, Kaneko S, Ito T, Sugamori T, Takahashi N, Kohno K, Morita E. HLA-B*58:01 strongly associates with allopurinol-induced adverse drug reactions in a Japanese sample population. J Dermatol Sci. 2013;71:150–152. doi: 10.1016/j.jdermsci.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Goncalo M, Coutinho I, Teixeira V, Gameiro AR, Brites MM, Nunes R, Martinho A. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol. 2013;169:660–665. doi: 10.1111/bjd.12389. [DOI] [PubMed] [Google Scholar]
- 101.Lee MH, Stocker SL, Williams KM, Day RO. HLA-B*5801 should be used to screen for risk of Stevens-Johnson syndrome in family members of Han Chinese patients commencing allopurinol therapy. J Rheumatol. 2013;40:96–97. doi: 10.3899/jrheum.120803. [DOI] [PubMed] [Google Scholar]
- 102.Chiu ML, Hu M, Ng MH, Yeung CK, Chan JC, Chang MM, Cheng SH, Li L, Tomlinson B. Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol. 2012;167:44–49. doi: 10.1111/j.1365-2133.2012.10894.x. [DOI] [PubMed] [Google Scholar]
- 103.Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, Naldi L, Bouwes-Bavinck JN, Sidoroff A, de Toma C, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 104.Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, Sawada J, Furuya H, Takahashi Y, Muramatsu M, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–1622. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 105.Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, Chucherd P, Konyoung P, Vannaprasaht S, Choonhakarn C, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19:704–709. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 106.Jung JW, Song WJ, Kim YS, Joo KW, Lee KW, Kim SH, Park HW, Chang YS, Cho SH, Min KU, Kang HR. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011;26:3567–3572. doi: 10.1093/ndt/gfr060. [DOI] [PubMed] [Google Scholar]
- 107.Cao ZH, Wei ZY, Zhu QY, Zhang JY, Yang L, Qin SY, Shao LY, Zhang YT, Xuan JK, Li QL, et al. HLA-B*58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics. 2012;13:1193–1201. doi: 10.2217/pgs.12.89. [DOI] [PubMed] [Google Scholar]
- 108.Kemen C, Lemke J, Hoeger PH, Beckmann C, Hennenberger A, Detjen A, Magdorf K. Human leukocyte antigen-related risk factors for toxic epidermal necrosis. Pediatr Infect Dis J. 2009;28:552. doi: 10.1097/INF.0b013e31819f3610. [DOI] [PubMed] [Google Scholar]
- 109.Huang YC, Shih PY, Chin SY, Chiang YY. Allopurinol-induced drug rash with eosinophilia and systemic symptoms mimicking acute generalized exanthematous pustulosis. J Dermatol. 2012;39:1077–1078. doi: 10.1111/j.1346-8138.2012.01651.x. [DOI] [PubMed] [Google Scholar]
- 110.Lee MH, Stocker SL, Anderson J, Phillips EJ, Nolan D, Williams KM, Graham GG, Sullivan JR, Day RO. Initiating allopurinol therapy: do we need to know the patient’s human leucocyte antigen status? Intern Med J. 2012;42:411–416. doi: 10.1111/j.1445-5994.2011.02567.x. [DOI] [PubMed] [Google Scholar]
- 111.Dainichi T, Uchi H, Moroi Y, Furue M. Stevens-Johnson syndrome, drug-induced hypersensitivity syndrome and toxic epidermal necrolysis caused by allopurinol in patients with a common HLA allele: what causes the diversity? Dermatology. 2007;215:86–88. doi: 10.1159/000102045. [DOI] [PubMed] [Google Scholar]
- 112.Atzori L, Pinna AL, Mantovani L, Ferreli C, Pau M, Mulargia M, Aste N. Cutaneous adverse drug reactions to allopurinol: 10 year observational survey of the dermatology department--Cagliari University (Italy) J Eur Acad Dermatol Venereol. 2012;26:1424–1430. doi: 10.1111/j.1468-3083.2011.04313.x. [DOI] [PubMed] [Google Scholar]
- 113.Ng JS, Edwards EM, Egelund TA. Methemoglobinemia induced by rasburicase in a pediatric patient: A case report and literature review. J Oncol Pharm Pract. 2011 doi: 10.1177/1078155211429385. [DOI] [PubMed] [Google Scholar]
- 114.Vadhan-Raj S, Fayad LE, Fanale MA, Pro B, Rodriguez A, Hagemeister FB, Bueso-Ramos CE, Zhou X, McLaughlin PW, Fowler N, et al. A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumor lysis syndrome. Ann Oncol. 2012;23:1640–1645. doi: 10.1093/annonc/mdr490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bain BJ. A ghostly presence-G6PD deficiency. Am J Hematol. 2010;85:271. doi: 10.1002/ajh.21660. [DOI] [PubMed] [Google Scholar]
- 116.Bhat P, Sisler I, Collier AB., 3rd Exchange transfusion as treatment for rasburicase induced methemoglobinemia in a glucose-6-phosphate dehydrogenase deficient patient. Pediatr Blood Cancer. 2008;51:568. doi: 10.1002/pbc.21582. [DOI] [PubMed] [Google Scholar]
- 117.Ducros J, Saingra S, Rampal M, Coulange C, Barbe MC, Verzetti G. Hemolytic anemia due to G6PD deficiency and urate oxidase in a kidney-transplant patient. Clin Nephrol. 1991;35:89–90. [PubMed] [Google Scholar]
- 118.Patte C, Sakiroglu C, Ansoborlo S, Baruchel A, Plouvier E, Pacquement H, Babin-Boilletot A. Urate-oxidase in the prevention and treatment of metabolic complications in patients with B-cell lymphoma and leukemia, treated in the Societe Francaise d’Oncologie Pediatrique LMB89 protocol. Ann Oncol. 2002;13:789–795. doi: 10.1093/annonc/mdf134. [DOI] [PubMed] [Google Scholar]
- 119.Sonbol MB, Yadav H, Vaidya R, Rana V, Witzig TE. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am J Hematol. 2012 doi: 10.1002/ajh.23182. [DOI] [PubMed] [Google Scholar]
- 120.Borinstein SC, Xu M, Hawkins DS. Methemoglobinemia and hemolytic anemia caused by rasburicase administration in a newly diagnosed child with Burkitt lymphoma/leukemia. Pediatr Blood Cancer. 2008;50:189. doi: 10.1002/pbc.21193. [DOI] [PubMed] [Google Scholar]
- 121.Bosly A, Sonet A, Pinkerton CR, McCowage G, Bron D, Sanz MA, Van den Berg H. Rasburicase (recombinant urate oxidase) for the management of hyperuricemia in patients with cancer: report of an international compassionate use study. Cancer. 2003;98:1048–1054. doi: 10.1002/cncr.11612. [DOI] [PubMed] [Google Scholar]
- 122.Zaramella P, De Salvia A, Zaninotto M, Baraldi M, Capovilla G, De Leo D, Chiandetti L. Lethal effect of a single dose of rasburicase in a preterm newborn infant. Pediatrics. 2013;131:e309–312. doi: 10.1542/peds.2011-1580. [DOI] [PubMed] [Google Scholar]
- 123.Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase Causing Severe Oxidative Hemolysis and Methemoglobinemia in a Patient with Previously Unrecognized Glucose-6-Phosphate Dehydrogenase Deficiency. Acta Haematol. 2013;130:254–259. doi: 10.1159/000351048. [DOI] [PubMed] [Google Scholar]
- 124.Joly P, Bon C, Francina A, Gelineau MC, Lacan P, Orfeuvre H. A severe G6PD deficiency revealed during a chemotherapy protocol including rasburicase. Ann Biol Clin (Paris) 2009;67:432–436. doi: 10.1684/abc.2009.0353. [DOI] [PubMed] [Google Scholar]