Abstract
The Continuous Performance Task (CPT) is a widely-used measure of sustained attention and impulsivity. Deficits in CPT performance have been found in several psychiatric disorders, such as Attention-Deficit/hyperactivity disorder (ADHD) and schizophrenia. Molecular genetic studies of CPT performance are currently limited and have generally revealed inconsistent findings. The current study tested the associations of the COMT val108/158met polymorphism with AX-CPT indices (i.e., omission and commission errors, d′, and lnβ), as well as the variability of these indices across blocks, in a sample of clinic-referred and non-referred children (N = 380). We found significant associations between COMT and variability in the Signal Detection Theory (SDT) indices d′ and lnβ across blocks, as well as a statistical trend for association between COMT and commission errors. Higher externalizing psychopathology was associated with general impairment on AX-CPT performance, and for some indices (i.e., d′ variability and lnβ variability) the effect of COMT was stronger at higher levels of psychopathology. Our findings support the role of COMT in components of CPT performance and highlight the potential utility of using SDT indices, particularly in relation to variability in performance. Moreover, our results suggest that for some indices the effect of COMT is stronger at higher levels of externalizing psychopathology. Our study yields some preliminary insights regarding the neurobiology of CPT performance, which may elucidate the mechanisms by which specific genes confer risk for various cognitive deficits, as well as relevant disorders characterized by these deficits.
Keywords: COMT, Dopamine, Continuous Performance Task, CPT, Endophenotype, ADHD
1. Introduction
The continuous performance task (CPT) is one of the most widely used neuropsychological measures hypothesized to assess sustained attention and impulsivity (e.g., Davies & Parasuraman, 1982). In a CPT, participants view a continuous presentation of changing stimuli and must make a response, usually a button press, to a specified target. Distinct indices are collected to analyze different aspects of CPT performance, which have implications for the underlying cognitive mechanisms. Traditionally, commission errors (i.e., responses to non-targets) are posited to index impulsivity or deficits in inhibition, and omission errors (i.e., failures to respond to target stimuli) are believed to index inattention (Riccio, Reynolds, & Lowe, 2001). Signal Detection Theory (SDT) indices have also been increasingly used to quantify and distinguish sensitivity (d′) from response bias (lnβ) (McNicol, 1972). The d′ index measures the degree to which targets are successfully discriminated from nontargets and reflects attentional capacity (Swets, Tanner, & Birdsall, 1961). The lnβ index measures response style, where a tendency to over-respond (i.e., lower lnβ) indicates an impulsive, risk-taking response style and a tendency to under-respond (i.e., higher lnβ) suggests a cautious response style (Keilp, Sackeim, & Mann, 2005).
There are various versions of the CPT that differ in the type of stimuli used (e.g., numbers, letters, sounds), signal probability (e.g., proportion of target to non-target stimuli), and event rate (e.g., duration of stimulus presentation). The AX-CPT (Cohen, Barch, Carter, & Servan-Schreiber, 1999; Halperin et al., 1988; Servan-Schreiber, Cohen, & Steingard, 1996), a modified version of the classic CPT (Rosvold, Mirsky, Sarason, Bransome, & Beck, 1956), requires participants to respond to the target letter ‘X’, but only if it immediately follows the letter ‘A’ (the cue). In addition to measuring components of sustained attention and impulsivity (e.g., Davies & Parasuraman, 1982), the AX-CPT differs from simpler versions in that it also indexes components of working memory, a set of cognitive processes involved in actively maintaining and manipulating relevant information to guide goal-directed behaviors (Braver & Cohen, 2000; O’Reilly, Braver, & Cohen, 1999; van den Bosch, Rombouts, & van Asma, 1996). In particular, the AX-CPT measures context processing, a specific component in the domain of working memory that is involved in the active representation and maintenance of context information (provided by the cue stimulus ‘A’), which refers to prior task-relevant information that is internally represented in such a form that it can be used to mediate appropriate behavioral responses (Braver & Cohen, 2000; Cohen et al., 1999; Servan-Schreiber et al., 1996).
Deficits in CPT performance have been reported in various complex psychiatric disorders, including Attention-Deficit/Hyperactivity Disorder (ADHD) (e.g., Losier, McGrath, & Klein, 1996) and schizophrenia (e.g., Nuechterlein & Dawson, 1984). The dorsolateral prefrontal cortex (dlPFC) has been shown to play a critical role in CPT performance (e.g., Brooks et al., 2006; Sax et al., 1999). Specifically, computational models and neuroimaging studies of AX-CPTs implicate the dlPFC in the active maintenance and regulation of context information in working memory (Barch et al., 1997; Barch et al., 2001; Braver, Barch, & Cohen, 1999; Braver & Cohen, 2000; Cohen et al., 1997; Cohen & Servan-Schreiber, 1992; Servan-Schreiber et al., 1996). Interestingly, structural and functional abnormalities in this region have been consistently found in disorders such as ADHD (e.g., Konrad & Eickhoff, 2010) and schizophrenia (e.g., Breier et al., 1992), both of which are characterized by deficits in CPT performance. For example, altered prefrontal dopaminergic neurotransmission has been implicated in the pathophysiology of ADHD (Castellanos, 1997; Davids, Zhang, Tarazi, & Baldessarini, 2003), as well as impairment in various cognitive functions such as CPT performance (Braver et al., 1999; Gasparini, Fabrizio, Bonifati, & Meco, 1997), although the exact underlying neurobiological mechanisms are still unclear. As CPT performance has also been shown to be heritable (e.g., Cornblatt, Risch, Faris, Friedman, & Erlenmeyer-Kimling, 1988), genes involved in regulating prefrontal function, such as the catechol-O-methyltransferase gene (COMT), represent promising candidates for understanding deficits in CPT performance and elucidating the etiology of relevant disorders.
COMT is predominantly expressed in the dlPFC, and COMT enzymatic activity is especially important for regulating dopamine levels in this region due to sparse expression of the dopamine transporter protein, which is a primary determinant of dopamine reuptake in most dopaminergic neurons (Matsumoto et al., 2003a, b; Sesack, Hawrylak, Matus, Guido, & Levey, 1998). COMT contains a widely-studied single nucleotide polymorphism (SNP) (val108/158met) that codes for the substitution of valine (val) by methionine (met) (Lachman et al., 1996). This specific marker is highly functional, with the enzymatic activity of the val allele being three to four times higher than that of the met allele (Chen et al., 2004; Lachman et al., 1996). Accordingly, the val allele is associated with increased dopamine catabolism (Lachman et al., 1996), and the consequent lower dopamine availability in val allele carriers appears to be related to decreased efficiency in dlPFC activity, which may contribute to impairment in prefrontally-mediated cognitive functions (e.g., Meyer-Lindenberg et al., 2005) and relevant disorders such as ADHD (Eisenberg et al., 1999). Nonetheless, a recent meta-analysis suggests that there is no association between this polymorphism in COMT and ADHD (Gizer, Ficks, & Waldman, 2009) and the few studies thus far that have specifically examined the association between COMT and cognitive functions in ADHD populations have also been inconclusive (Bellgrove et al., 2005; Matthews et al., 2012; Mills et al., 2004; Taerk et al., 2004). This warrants further investigation for a better understanding of the pathophysiological mechanisms (e.g., the nature of abnormalities in prefrontal dopamine neurotransmission) underlying specific cognitive deficits in relevant disorders such as ADHD (e.g., Faraone & Biederman, 2002; Kirley et al., 2002).
There is limited research investigating the influence of COMT val108/158met polymorphism on CPT performance and thus far, the findings have been mixed (Caldú et al., 2007; Eisenberg et al., 1999; Liao et al., 2009; MacDonald, Carter, Flory, Ferrell, & Manuck, 2007; Mills et al., 2004). Importantly, the exact nature of the role of COMT in cognitive function is unclear with regard to specificity. For example, there is evidence that COMT is related to cognitive stability that reflects the maintenance of representations in working memory in CPT performance (Stefanis et al., 2005) and that accordingly, COMT may be more closely associated with a specific context processing deficit measured by modified versions of AX-CPTs, rather than a more generalized cognitive deficit (MacDonald et al., 2007). These specific cognitive functions likely depend considerably on efficient prefrontal dopaminergic signaling (MacDonald et al., 2007; Seamans & Yang, 2004; Stefanis et al., 2005) and thus AX-CPT performance indices that more precisely capture these functions may be particularly informative for elucidating the role of COMT in cognition and relevant disorders. For instance, proficient context processing is largely reflected in the d′ index (Cohen et al., 1999) and cognitive stability can be indexed by the variability in performance across blocks of the task (Stefanis et al., 2005). Relatedly, recent evidence suggests that in children with ADHD, COMT specifically influences these aforementioned cognitive processes, but not other aspects of cognitive performance (e.g., updating of information) (Matthews et al., 2012), which may also suggest that the effect of COMT on these processes may be stronger in such disorders that are characterized by dysfunctional prefrontal dopamine neurotransmission compared to controls (del Campo, Chamberlain, Sahakian, & Robbins, 2011; Solanto, 2002; Swanson et al., 2007). Nonetheless, the role of COMT in specific components of CPT performance, particularly as it relates to performance across blocks of the task, remains elusive. For instance, while variability in response time (RT) has been shown to be associated with COMT (Stefanis et al., 2005), the stability of other indices across the task is unknown. Moreover, the role of COMT in cognitive processes in children is especially unclear and given the prolonged structural and functional changes that occur in prefrontal regions throughout childhood and adolescence (Casey, Giedd, & Thomas, 2000), COMT may differentially influence cognitive functions underlying CPT performance throughout development.
In the current study we tested the association between AX-CPT performance indices (i.e., omission and commission errors, d′, lnβ) and the COMT val108/158met polymorphism in children. We hypothesized that COMT would be associated with these performance indices, with the SDT indices (i.e., d′, lnβ) showing the strongest associations. We also tested the association between COMT and the AX-CPT performances indices across blocks (i.e., the variability of each of the AX-CPT performance indices). We hypothesized that COMT would be associated with the variability of these indices across blocks, with d′ variability and lnβ variability showing the strongest associations. Further, we examined the effect of externalizing psychopathology (i.e., as operationalized in this study by clinic-referred status for disruptive disorders such as ADHD) on AX-CPT performance indices and whether the effects of COMT on AX-CPT indices were moderated by differing levels of externalizing psychopathology. We hypothesized that the effects of COMT would be stronger in children with higher levels of externalizing psychopathology.
2. Method
All assessment procedures were approved by the Emory University Institutional Review Board. Parents read and signed an informed consent form prior to study participation, and verbal assent was obtained from the children.
2.1. Participants
Participants included a clinic-referred sample of children and their siblings (N = 224) from 138 families and a twin sample (N = 156) from 83 families, with a mean age of 12.2 (SD = 3.2) years. Additional sample characteristics are provided in Table 1. Participants in the clinic-referred sample were recruited through the Center for Learning and Attention Deficit Disorders (CLADD) at the Emory University School of Medicine and the Emory University Psychological Center in Atlanta, Georgia. Both clinics specialize in the assessment and treatment of childhood learning disabilities and externalizing disorders. The clinic-referred participants consisted of probands and their siblings, where probands refer to members of a family who brought the family to the attention of researchers because they were clinically-referred for assessment of criteria for an externalizing disorder (e.g., Attention-Deficit/Hyperactivity Disorder, Conduct Disorder, Oppositional Defiant Disorder). Participants in the twin sample were drawn from the Georgia Twin Registry, a sample of twins from the general population of Georgia and recruited through state birth records. Children diagnosed with autism, traumatic brain injury, or neurological conditions (e.g., epilepsy) were excluded, as were children with IQs < 75. Other diagnoses remained confidential and did not influence inclusion in the study.
Table 1.
Sample Characteristics
| Clinic-Referred Sample | Twin Sample | Total | |
|---|---|---|---|
| Sample Size | |||
| # of families | 138 | 83 | 221 |
| Total children | 224 | 156 | 380 |
| # of probands | 135 | NA | 135 |
| # of siblings | 89 | 53 | 142 |
| # of controls | NA | 103 | 103 |
| Diagnostic Information | |||
| Probands | |||
| With ADHD | 104 (I-43, H-5, C-56) | NA | 104 (I-43, H-5, C-56) |
| With ODD | 41 | NA | 41 |
| With CD | 3 | NA | 3 |
| Siblings | |||
| With ADHD | 16 (I-9, H-5, C-2) | 34 (I-20, H-5, C-9) | 50 (I-29, H-10, C-11) |
| With ODD | 30 | 0 | 30 |
| With CD | 4 | 0 | 4 |
| Demographic Characteristics | |||
| Age*a | 11.2 (3.3) | 13.5 (2.4) | 12.2 (3.2) |
| Gender*b | 144 M (64%), 80 F | 61 M (39%), 95 F | 205 M, 175 F |
| Ethnicity | |||
| Hispanic | 2 (1%) | 0 (0%) | 2 |
| European-American | 170 (76%) | 134 (86%) | 304 |
| African-American*c | 18 (8%) | 4 (3%) | 22 |
| Other*d | 23 (10%) | 2 (1%) | 25 |
| Missing | 11 (5%) | 16 (10%) | 27 |
Note. ADHD = Attention-Deficit/Hyperactivity Disorder; ADHD diagnostic subtype information – I = Predominantly Inattentive type, H = Predominantly Hyperactive-Impulsive type, C = Combined type; ODD = Oppositional Defiant Disorder; CD = Conduct Disorder; Gender information – M = Male, F = Female;
Clinic-referred and twin samples significantly differed;
t(378) = 7.80, p < .001;
χ2(1, N = 380) = 23.47, p < .001;
χ2(1, N = 380) = 5.03, p = .025;
χ2(1, N = 380) = 8.73, p = .003
2.2. Genotyping
DNA collection, extraction, and amplification were performed by use of previously published procedures (Gizer & Waldman, 2012). The val108/158met polymorphism of COMT was genotyped along with 23 other SNPs in COMT on the Sequenom iPlex genotyping platform by the company (i.e., Sequenom) and at the Broad Institute of Harvard and MIT. Genotypes were called using the Sequenom iPlex chemistries and the MassARRAY system (Sequenom Inc., San Diego, CA, USA).
2.3. The A-X Continuous Performance Task (AX-CPT)
The AX-CPT was programmed according to the parameters outlined by Halperin and colleagues (1988). Stimuli comprised 11 letters, presented for 200 ms each, with an interstimulus interval of 1500 ms. Participants were to respond (i.e., press the space bar) whenever the target sequence “A-X” (i.e., an A followed by an X) appeared. There were 40 target trials distributed across 400 trials during the 12-minute test, which was divided into 4 blocks. Subjects underwent a brief practice session prior to the test.
Omission errors (misses, or non-response to the target) and commission errors (responses made to nontargets) were calculated according to procedures described by Halperin and colleagues (1988). Signal detection indices for sensitivity (d′) and response bias (lnβ) were calculated for analyses according to McNicol (1972). Intra-individual variability in CPT performance indices was calculated by taking the within-person standard deviation (SD) of each index across the four blocks of the task (Li, Huxhold, & Schmiedek, 2004; MacDonald, Li, & Bäckman, 2009).
2.4. Procedures
All testing was conducted in the subjects’ homes in a quiet room free of distractions using a laptop computer. Parents were instructed to withhold their child’s stimulant medications for the day of testing and compliance was confirmed verbally prior to testing. The time of day of testing varied and was not controlled for.
2.5. Quality Control Analyses
Reliability of genotyping was assessed by examining the concordance of genotypes across the different platforms. There was acceptable genotyping between the two platforms at 91% (ϕc = .86, p < .001). Departure from Hardy-Weinberg equilibrium (HWE) was also used to evaluate genotyping quality. The genotype frequencies for our sample were as follows: met/met, 25%; val/met, 47%; and val/val, 29%, and were consistent with HWE (p = .295).
2.6. Data Analyses
We tested for differences in AX-CPT performance (i.e., individual indices and variability of the indices) across the three COMT genotypes (i.e., met/met, val/met, and val/val) using Generalized Linear Modeling analyses with generalized estimating equations (GEEs). Generalized Linear Modeling allows for the use of alternative distributions other than the normal distribution to account for non-normality in the outcome variables, while GEE takes into account the nested data structure due to the clustering of children (i.e., multiple siblings) within families (Liang & Zeger, 1986; Zeger, Liang, & Albert, 1988). Given the distributions of the outcome variables examined (i.e., CPT indices), we modeled them using a negative binomial distribution with a log link function to accommodate any overdispersion (i.e., the variance being greater than the mean) (Nelder & Wedderburn, 1972). The Generalized Linear Modeling analyses yield a Wald χ2 statistic that was used in hypothesis testing and converted into the effect size index R2 using the formula χ2/N, where N = the number of children included in the analysis (Rosenthal, 1991). In our analyses, we controlled for ethnicity, sex, age, and age2 (the non-linear term for age). Ethnicity was coded as a continuous variable, indicating the percentage of European-American ethnicity, for each individual. Age2 was included to account for potential non-linear (e.g., quadratic) effects of age on the CPT indices (Cohen, Cohen, West, & Aiken, 2003). These covariates were included because they had significant effects on several of the CPT performance indices.
We also conducted these analyses (examining associations between COMT and AX-CPT performance indices) including clinical status (i.e., clinic-referred or non-referred subsample) in the model, and included the interaction term between COMT and clinical status to examine whether the effects of COMT on AX-CPT performance were moderated by clinical status. If interaction effects were present, we proceeded to examine the associations between COMT and AX-CPT indices in the non-referred and clinic-referred subsamples separately.
3. Results
3.1. Tests of Association between COMT and AX-CPT Indices
Table 2 summarizes the results from the association analyses between COMT and the AX-CPT indices. First, for omission errors there were significant effects of covariates, including ethnicity (p = .029, R2 = 1%), age (p < .001, R2 = 34%) and age2 (p = .032, R2 = 1%). Specifically, omission errors decreased quadratically with increasing age. When the main effect of clinical status was included in the model as a covariate, there was a significant effect of clinical status on omission errors (p < .001, R2 = 4%), such that higher omission errors were found in the clinic-referred subsample.
Table 2.
Association of COMT and AX-CPT Indices
| Omission errors | Commission errors | d′ | lnβ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Wald χ2 | df | p | R2 | Wald χ2 | df | p | R2 | Wald χ2 | df | p | R2 | Wald χ2 | df | p | R2 |
| Ethnicitya | 4.76 | 1 | .029 | 1% | 2.43 | 1 | .119 | 0% | 2.80 | 1 | .094 | 1% | 1.06 | 1 | .302 | 0% |
| Sex | 1.28 | 1 | .258 | 0% | 7.93 | 1 | .005 | 2% | 5.39 | 1 | .020 | 1% | 3.90 | 1 | .048 | 1% |
| Age | 130.13 | 1 | <.001 | 34% | 94.61 | 1 | <.001 | 25% | 81.59 | 1 | <.001 | 21% | 0.04 | 1 | .847 | 0% |
| Age2 | 4.61 | 1 | .032 | 1% | 1.00 | 1 | .318 | 0% | 31.52 | 1 | <.001 | 8% | 0.18 | 1 | .675 | 0% |
| Clinical status | 14.08 | 1 | <.001 | 4% | 27.57 | 1 | <.001 | 7% | 13.05 | 1 | <.001 | 3% | <0.01 | 1 | .954 | 0% |
| COMTb | 2.58 | 2 | .275 | 1% | 4.60 | 2 | .100 | 1% | 3.11 | 2 | .212 | 1% | 1.55 | 2 | .461 | 0% |
| COMT val/val vs. val/met & met/met combinedb | 1.89 | 1 | .169 | 0% | 4.32 | 1 | .038 | 1% | 3.10 | 1 | .078 | 1% | 0.02 | 1 | .900 | 0% |
| COMT val/met vs. met/metb | 1.02 | 1 | .313 | 0% | 0.18 | 1 | .670 | 0% | 0.02 | 1 | .886 | 0% | 1.13 | 1 | .288 | 0% |
| COMTc | 2.83 | 2 | .243 | 1% | 3.78 | 2 | .151 | 1% | 2.95 | 2 | .229 | 1% | 1.62 | 2 | .444 | 0% |
| COMT val/val vs. val/met & met/met combinedc | 1.34 | 1 | .247 | 0% | 3.15 | 1 | .076 | 1% | 2.92 | 1 | .088 | 1% | 0.02 | 1 | .897 | 0% |
| COMT val/met vs. met/metc | 1.67 | 1 | .196 | 0% | 0.25 | 1 | .618 | 0% | 0.08 | 1 | .783 | 0% | 1.13 | 1 | .287 | 0% |
| COMT × Clinical status | 0.58 | 2 | .747 | 0% | 4.69 | 2 | .096 | 1% | 3.69 | 2 | .158 | 1% | 1.91 | 2 | .384 | 1% |
Note.
Percentage of European-American ethnicity;
Clinical status not included in model;
Clinical status included in model
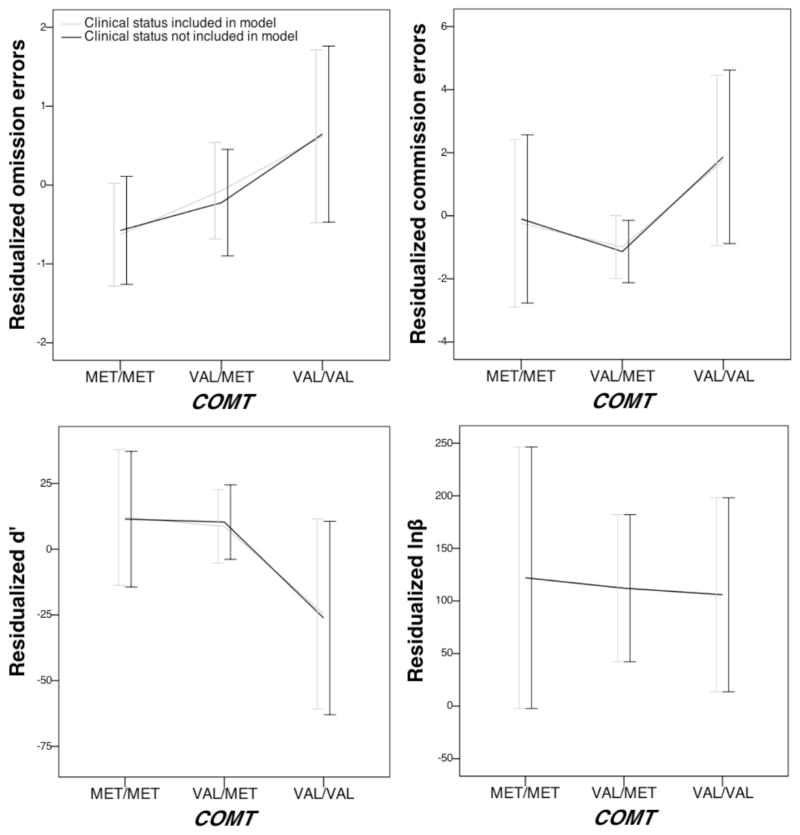
Second, for commission errors there was a statistical trend for association for the main effect of COMT (p = .100; see Figure 1), and thus findings from specific genotype contrasts should be interpreted with caution. Nonetheless, there was a significant difference in commission errors across COMT genotypes (p = .038, R2 = 1%), such that individuals with two valine alleles (i.e., the val/val genotype) tended to make more commission errors compared to those with at least one methionine allele (i.e., the val/met and met/met genotypes). There were also significant effects of covariates on commission errors, including sex (p = .005, R2 = 2%) and age (p < .001, R2 = 25%). Specifically, commission errors decreased linearly with increasing age, and higher commission errors were found in males. When the main effect of clinical status was included in the model as a covariate, there was a significant effect of clinical status on commission errors (p < .001, R2 = 7%), such that higher commission errors were found in the clinic-referred subsample.
Figure 1.
Association of COMT and residualized AX-CPT indices.
Third, for d′ there were significant effects of covariates, including sex (p = .020, R2 = 1%), age (p < .001, R2 = 21%), and age2 (p < .001, R2 = 8%). Specifically, d′ increased quadratically with increasing age, and higher d′ values were found in females. When the main effect of clinical status was included in the model as a covariate, there was a significant effect of clinical status on d′ (p < .001, R2 = 3%), such that higher d′ values were found in the non-referred subsample. Lastly, for lnβ there was a significant effect of sex (p = .048, R2 = 1%) on lnβ, such that higher lnβ values were found in males.
3.2. Tests of Association between COMT and the Variability of CPT Indices Across Blocks
Table 3 summarizes the results from the association analyses between COMT and the variability of AX-CPT indices across blocks. First, for omission error variability there was a significant effect of age (p < .001, R2 = 25%), such that omission error variability decreased linearly with increasing age. When the main effect of clinical status was included in the model as a covariate, there was a significant effect of clinical status on omission error variability (p < .001, R2 = 4%), such that higher omission error variability was found in the clinic-referred subsample.
Table 3.
Association of COMT and Variability of AX-CPT Indices Across Blocks
| Omission error variability | Commission error variability | d′ variability | lnβ variability | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Wald χ2 | df | p | R2 | Wald χ2 | df | p | R2 | Wald χ2 | df | p | R2 | Wald χ2 | df | p | R2 |
| Ethnicitya | 2.26 | 1 | .133 | 1% | 3.44 | 1 | .064 | 1% | 6.44 | 1 | .011 | 2% | 6.59 | 1 | .010 | 2% |
| Sex | 0.76 | 1 | .385 | 0% | 7.76 | 1 | .005 | 2% | 6.58 | 1 | .010 | 2% | 7.92 | 1 | .005 | 2% |
| Age | 93.75 | 1 | <.001 | 25% | 55.54 | 1 | <.001 | 15% | 155.97 | 1 | <.001 | 41% | 113.97 | 1 | <.001 | 30% |
| Age2 | 1.73 | 1 | .188 | 0% | 0.96 | 1 | .328 | 0% | 1.74 | 1 | .188 | 0% | 8.18 | 1 | .004 | 2% |
| Clinical status | 15.10 | 1 | <.001 | 4% | 13.22 | 1 | <.001 | 3% | 29.44 | 1 | <.001 | 8% | 37.81 | 1 | <.001 | 10% |
| COMTb | 2.13 | 2 | .344 | 1% | 1.48 | 2 | .477 | 0% | 6.16 | 2 | .046 | 2% | 9.48 | 2 | .009 | 2% |
| COMT val/val vs. val/met & met/met combinedb | 0.18 | 1 | .669 | 0% | 1.48 | 1 | .224 | 0% | 6.06 | 1 | .014d | 2% | 9.24 | 1 | .002 | 2% |
| COMT val/met vs. met/metb | 2.00 | 1 | .157 | 1% | 0.03 | 1 | .874 | 0% | 0.17 | 1 | .678e | 0% | 0.09 | 1 | .759 | 0% |
| COMTc | 2.65 | 2 | .266 | 1% | 1.00 | 2 | .606 | 0% | 4.46 | 2 | .107 | 1% | 8.66 | 2 | .013 | 2% |
| COMT val/val vs. val/met & met/met combine dc | 0.04 | 1 | .841 | 0% | 0.84 | 1 | .360 | 0% | 3.51 | 1 | .061d | 1% | 7.80 | 1 | .005 | 2% |
| COMT val/met vs. met/metc | 2.47 | 1 | .116 | 1% | 0.04 | 1 | .839 | 0% | 0.01 | 1 | .912e | 0% | 0.18 | 1 | .671 | 0% |
| COMT × Clinical status | 0.83 | 2 | .660 | 0% | 9.29 | 2 | .010 | 2% | 9.85 | 2 | .007 | 3% | 8.03 | 1 | .018 | 2% |
Note.
Percentage of European-American ethnicity;
Clinical status not included in model;
Clinical status included in model;
COMT met/met vs. val/met & val/val combined;
COMT val/met vs. val/val.
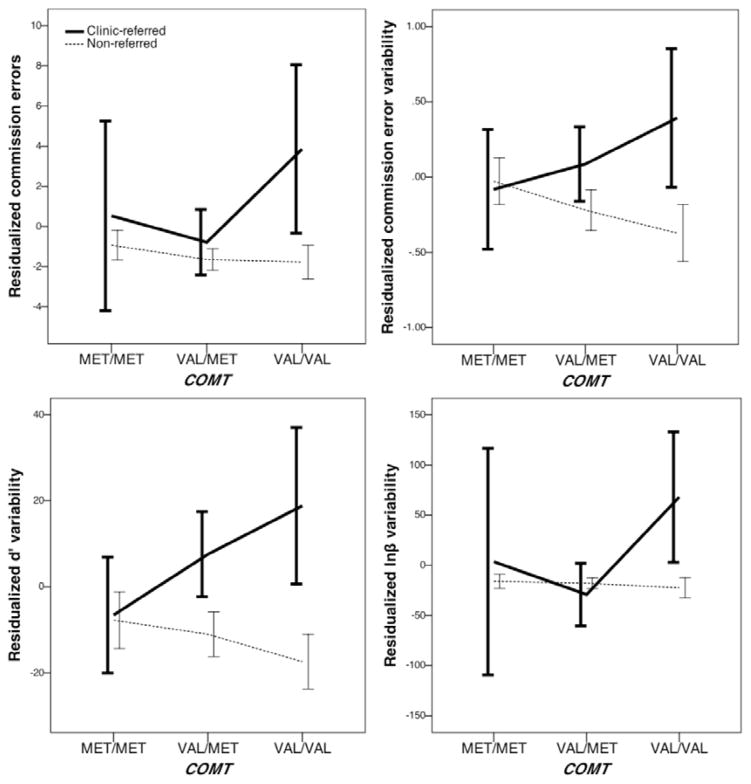
Second, for commission error variability there were significant effects of covariates, including sex (p = .005, R2 = 2%) and age (p < .001, R2 = 15%). Specifically, commission error variability decreased linearly with increasing age and higher commission error variability was found in males. When the main effect of clinical status was included in the model as a covariate, there was a significant effect of clinical status on commission error variability (p < .001, R2 = 3%), such that higher commission error variability was found in the clinic-referred subsample. The COMT × clinical status interaction term was significant for commission error variability (p = .010, R2 = 2%). The effects of the COMT genotype contrast appeared to go in opposite directions in the clinic-referred and non-referred subsamples (see Figure 2).
Figure 2.
Association of COMT and residualized AX-CPT indices, moderated by clinical status
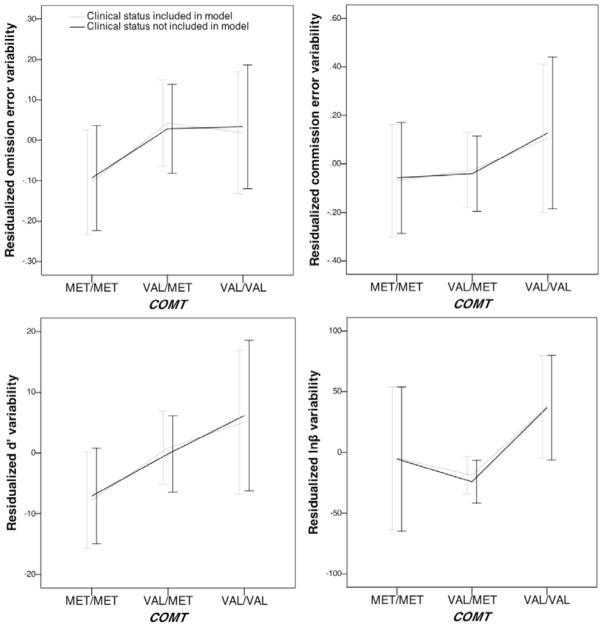
Third, for d′ variability there was a significant main effect of COMT (p = .046, R2 = 2%), such that children with two methionine alleles (i.e., the met/met genotype) showed less variability in sensitivity (d′) compared to children with at least one valine allele (i.e., the val/met and val/val genotypes) (p = .014, R2 = 2%; see Figure 3). There were also significant effects of covariates on d′ variability, including ethnicity (p = .011, R2 = 2%), sex (p = .010, R2 = 2%), and age (p < .001, R2 = 41%). Specifically, d′ variability decreased linearly with increasing age and higher d′ variability was found in males. When the main effect of clinical status was included in the model as a covariate, there was a significant effect of clinical status on d′ variability (p < .001, R2 = 8%), such that higher d′ variability was found in the clinic-referred subsample. The COMT × clinical status interaction term was significant for d′ variability (p = .007, R2 = 3%). Follow-up analyses showed a non-significant association for the main effect of COMT on d′ variability in the non-referred subsample (p = .165), but a significant effect in the clinic-referred subsample (p = .001, R2 = 6%) (see Figure 2). Findings from the subsequent specific genotype contrasts in the clinic-referred subsample was significant (p < .001, R2 = 6%), such that children with two methionine alleles (i.e., the met/met genotype) showed less variability in sensitivity (d′) compared to children with at least one valine allele (i.e., the val/met and val/val genotypes).
Figure 3.
Association of COMT and residualized variability of AX-CPT indices across blocks.
Lastly, for lnβ variability there was a significant main effect of COMT (p = .009, R2 = 2%), such that children with two valine alleles (i.e., the val/val genotype) showed greater variability in response bias (lnβ) compared to children with at least one methionine allele (i.e., the val/met and met/met genotypes) (p = .002, R2 = 2%; see Figure 3). There were also significant effects of covariates on lnβ variability, including ethnicity (p = .010, R2 = 2%), sex (p = .005, R2 = 2%), age (p < .001, R2 = 30%), and age2 (p = .004, R2 = 2%). Specifically, lnβ variability decreased quadratically with increasing age, and higher lnβ variability was found in males. When the main effect of clinical status was included in the model as a covariate, the association between COMT and lnβ variability became less significant (p = .013, R2 = 2%), as well as the findings from the specific genotype contrasts (p = .005, R2 = 2%) (see Figure 3). There was a significant effect of clinical status on lnβ variability (p < .001, R2 = 10%), such that higher lnβ variability was found in the clinic-referred subsample. The COMT × clinical status interaction term was significant for lnβ variability (p = .018, R2 = 2%). Follow-up analyses showed a non-significant association for the main effect of COMT on lnβ variability in the non-referred subsample (p = .366), but a significant effect in the clinic-referred subsample (p = .009, R2 = 4%) (see Figure 2). Findings from the subsequent specific genotype contrasts in the clinic-referred subsample was significant (p = .003, R2 = 4%), such that children with two valine alleles (i.e., the val/val genotype) showed greater variability in response bias (lnβ) compared to children with at least one methionine allele (i.e., the val/met and met/met genotypes).
4. Discussion
In the present study, we investigated the effects of the COMT val108/158met polymorphism on AX-CPT performance indices. Our analyses revealed a significant association between COMT and variability in the Signal Detection Theory (SDT) indices, d′ and lnβ, across blocks, which represents a novel finding in the literature. Further, we found an overall trend for COMT to be associated with commission errors, consistent with previous studies (i.e., Caldú et al., 2007; Eisenberg et al., 1999), and specific contrast analyses revealed a significant difference between the val/val genotype and methionine carriers (i.e., the val/met and met/met genotypes). It is important to mention that the previous studies by Caldú and colleagues (2007) and Eisenberg and colleagues (1999) specifically found an effect of the COMT val allele (comparing met/met homozygotes to val carriers) on commission errors, whereas our study found a significant effect when comparing val/val homozygotes to met carriers. This difference may reflect the use of different CPT task versions, and/or specific cognitive mechanisms measured within tasks, that may have differential sensitivity to dopamine levels in the prefrontal cortex (Landi et al., 2013; Stokes, Rhodes, Grasby, & Mehta, 2011; Tan et al., 2007). Accordingly there is evidence that val/val homozygosity (compared to met carriers) is associated with a specific context processing deficit, as measured by a modified AX-CPT (MacDonald et al., 2007), and a recent meta-analysis of neural substrates associated with COMT (Mier, Kirsh, & Meyer-Lindenberg, 2010) found that performance on cognitive tasks that primarily measure attention and working memory was better in met allele carriers, while performance on emotional processing tasks was better for val allele carriers, suggesting differential effects of the COMT val/met alleles on different aspects of task performance (Landi et al., 2013; Mier et al., 2010). In addition, differences in sample characteristics across studies (e.g., age, psychiatric diagnostic status) may also affect dopamine signaling, which in turn may influence how variations in COMT affects aspects of cognition. In particular, Caldú and colleagues (2007) studied undergraduate adults whereas Eisenberg and colleagues (1999) recruited children (probands) diagnosed with Attention-Deficit/Hyperactivity Disorder (ADHD). Tonic and phasic levels of dopamine in prefrontal regions change across age, which likely impact differential effects of COMT on cognitive performance throughout development (Mechelli et al., 2009; Wahlstron, White, & Luciana, 2010). Further, abnormal dopaminergic signaling has also been suggested in ADHD (del Campo, Chamberlain, Sahakian, & Robbins, 2011; Solanto, 2002; Swanson et al., 2007), which may also contribute to differences in the effects of the COMT polymorphism on prefrontally-mediated cognition (Bellgrove et al., 2005; Diamond, Briand, Fossella, & Gehlbach, 2004). Thus, as variations in COMT genotype may be influenced by task parameters, age, and various psychiatric conditions, future research should aim to more comprehensively explore potential sources of differences in dopaminergic signaling and its implications for COMT’s effects on cognition.
Our study also found that the clinic-referred subsample with higher levels of externalizing psychopathology tended to show general impairment in AX-CPT performance. Further, for d′ variability and lnβ variability, COMT had a significant effect in the clinic-referred subsample but not in the non-referred subsample, whereas for commission error variability the effects of COMT went in the opposite direction in the two subsamples. These findings suggest that the effects of COMT on several CPT performance indices differ by levels of externalizing psychopathology, and that for some AX-CPT indices, particularly the variability indices across blocks, the effect of COMT was stronger at higher levels of externalizing psychopathology. This may suggest that dopaminergic genes, such as COMT, that are crucially involved in regulating prefrontal dopamine levels may be especially important for cognitive functions in disorders such as ADHD that are characterized by dysfunction in prefrontal dopaminergic signaling (del Campo, Chamberlain, Sahakian, & Robbins, 2011; Solanto, 2002; Swanson et al., 2007). Specifically, prefrontal dopaminergic hypo-function has been implicated in the pathophysiology of ADHD (e.g., Krause, Dresel, Krause, la Fougere, & Ackenheil, 2003) and due to lower prefrontal dopamine levels, cognitive performance in individuals with ADHD may be more strongly influenced by the increased dopamine catabolism associated with the val allele of COMT (Lachman et al., 1996) compared to that in controls. Nonetheless, the exact nature of the abnormalities in prefrontal dopamine neurotransmission in ADHD is still unclear and debated (e.g., Bellgrove et al., 2005) and further research is needed to examine the nature of the relation between ADHD and prefrontal function (e.g., differential activation patterns) and the relevant role of COMT in the context of various cognitive impairments (Congdon & Canli, 2005). For instance, there is some recent evidence that in children with ADHD, the val allele of COMT may have a stronger effect on specific cognitive functions that depend more crucially on prefrontal dopamine function, such as the stable maintenance of representations in working memory, and that this may represent an important mechanism by which COMT confers susceptibility to ADHD (Matthews et al., 2012). Although these the current findings regarding stronger effects of COMT at higher levels of externalizing psychopathology are interesting, it is important to note that these are novel, exploratory results and need to be replicated in independent datasets in future studies.
Our results yield some preliminary insights regarding the precise function of COMT in distinct components of CPT performance. These effects, particularly on the variability of SDT indices across blocks, may be specific to COMT compared to other candidate genes in the dopaminergic system. For example, supplementary results from the current sample indicated no significant association between the dopamine transporter gene (DAT1) and variability of AX-CPT performance indices across blocks (p-values ranged from .481 to .861, R2 ≈ 0%), supporting the specificity of COMT’s influence on cognitive stability, or the active maintenance of representations in working memory (Stefanis et al., 2005). Interestingly, while COMT was not associated with lnβ, results from our sample indicated a significant but modest effect of DAT1 on lnβ (p = .037, R2 = 1%), which has also been found in a previous study (Loo et al., 2003). In addition, similar to COMT, DAT1 was not significantly associated with omission errors (p = .555, R2 ≈ 0%) or d′ (p = .692, R2 ≈ 0%). Nonetheless, there also is some evidence of similar effects on AX-CPT performance of COMT and DAT1. In particular, findings from the current sample showed a significant association between DAT1 and commission errors (p = .014, R2 = 2%), albeit to a greater degree compared to COMT (p = .100, R2 = 1%). The association between DAT1 and commission errors has also been supported in the previous literature (Caldú et al., 2007; Gizer & Waldman, 2012; Loo et al., 2003). While in need of replication, our results provide some support for the unique effects of COMT and DAT1 on distinct AX-CPT performance indices, in addition to having some common effects, and thus have important implications for the neurobiological mechanisms underlying CPT performance.
Although both DAT1 and COMT are involved in terminating the action of dopamine in the brain, they are predominantly expressed in different regions. Specifically, COMT is primarily expressed in the dlPFC with minimal expression in subcortical regions (Matsumoto et al., 2003a, b), whereas DAT1 is abundantly expressed in subcortical areas (e.g., striatum and midbrain), with minimal expression in frontal cortical regions (e.g., Ciliax et al., 1999; Sesack et al., 1998). These brain regions form components of separate, but interdependent neural circuits that underlie different elements of cognitive and affective control involved in the pathophysiology of disorders such as ADHD (Alexander, Crutcher, DeLong, 1991; Nigg & Casey, 2005). Thus, in addition to this neuroanatomical dissociation, these two neural pathways may also underlie distinct cognitive mechanisms, also suggesting a functional dissociation. For AX-CPTs in particular, computational models of prefrontal and subcortical interactions have been proposed, implicating prefrontal regions in the active maintenance of context information (provided by the cue stimulus ‘A’ before the ‘X’) and subcortical regions in the flexible and selective updating of representations (Braver & Cohen, 2000). Accordingly, COMT has been shown to be related to prefrontal cortical underpinnings of attention and working memory (e.g., Egan et al., 2001), as well as cognitive stability (e.g., Stefanis et al., 2005), consistent with our findings of associations between COMT and variability in performance (i.e., SDT indices across blocks). In contrast, DAT1 has been implicated in inhibitory control processes (Cornish et al., 2005) that more strongly reflect aspects of impulsivity (Logan, Schachar, & Tannock, 1997). Consistent with this, supplementary results from our sample suggest associations between DAT1 and lnβ and commission errors, indices which have been shown to reflect impulsivity (e.g., Brooks et al., 2006; Gizer & Waldman, 2012; Keilp, Sackeim, & Mann, 2005). Thus, findings from our sample provide preliminary evidence for dissociable effects of COMT and DAT1 on AX-CPT performance and their corresponding underlying neural mechanisms, which warrants further investigation in the literature.
Our findings further lend support to the potential utility of using the SDT indices, and in particular, highlight the specific role of COMT in the variability of SDT indices across the task, which may provide further insights regarding the core cognitive deficits in relevant psychiatric disorders such as ADHD. In particular, given that genetic associations with specific diagnoses (e.g., ADHD) have often failed to replicate and have shown small effect sizes (e.g., Faraone et al., 2005; Gizer et al., 2009), valid intermediate phenotypes (i.e., endophenotypes) may be helpful in identifying susceptibility loci for disorders by more directly assessing the biological mechanisms hypothesized to underlie those disorders (Doyle et al., 2005; Gottesman & Gould, 2003; Waldman, 2005). Accordingly, there is evidence that CPT indices may represent useful endophenotypes for ADHD (Doyle et al., 2005; Gizer & Waldman, 2012; Waldman, 2005), consistent with our findings. Our results go beyond previous findings to suggest that the SDT indices, particularly in relation to cross-block variability in performance, may represent especially useful endophenotypes for molecular genetic studies. Thus our large sample consisting of children spanning a range of psychopathology allows for increased statistical power (Lohmueller, Pearce, Pike, Lander, & Hirschhorn, 2003) to 1) detect the effects of COMT on the putative endophenotype measures (i.e., AX-CPT indices) and 2) to examine how these effects vary as a function of clinical status, such that these effects tend to be stronger at higher levels of externalizing psychopathology for several indices. Given that there has been a lack of support for association between COMT and ADHD (Gizer et al., 2009), utilizing such putative endophenotypes may be especially informative for elucidating the underlying neurobiological pathways by which specific genes confer risk for ADHD.
Although the present findings have important implications for the role of COMT on CPT performance and relevant disorders, our study has several limitations. Given the novelty of our results, they need to be replicated in independent samples. Moreover we did not control for multiple testing in the current study. It has been proposed that adjustments for multiple comparisons may not be strictly required for exploratory methods, as in the current investigation, that primarily aim to identify preliminary evidence to inform future research, as such adjustments may potentially dismiss important relations by decreasing statistical power (Bender & Lange, 2001; Cohen, 1994; Cole, 1979, 1993; Michels & Rosner, 1996; Poole, 1991; Rothman, 1990; Savitz & Olshan, 1995). Exploratory methods with flexible statistical approaches have been proposed as a good tool for understanding and gaining preliminary information from the data, instead of relying solely on strict criterion for significance testing (Cohen, 1994). In addition, the current investigation focused on omnibus tests of association, which diminishes the multiple testing problem as it involves fewer overall tests being conducted (Cohen, Cohen, West, & Aiken, 2003). Nonetheless, it is critical for these findings to be rigorously tested for replication in other independent datasets (Bender & Lange, 2001; Cohen, 1994).
Further, while our sample was relatively large compared to other genetic association studies of neurocognitive measures, larger samples are needed for sufficient statistical power (Lohmueller et al., 2003), especially for tests of multiple genetic markers. In addition, given that differences in CPT task parameters may differentially influence aspects of cognitive performance (Solanto, Etefia, & Marks, 2004), it is important to investigate the generalizability of our findings to other versions of CPTs and to explore other potentially valid performance indices (Halperin et al., 1988). In particular, previous research indicates that COMT may be more closely linked to versions of the AX-CPT in which certain parameters are modified to more specifically capture aspects of context processing (MacDonald et al., 2007). This suggests that COMT may play a more specific role in context processing deficits, rather than a more generalized deficit within the broader cognitive domain (e.g., working memory). Thus focusing on more specific functions like context processing may be advantageous for finding associations of cognitive deficits with specific genes and further assist in the understanding of the neurobiology of relevant psychiatric disorders (MacDonald et al., 2007). Future research should also explore other genes that influence the neurobiological pathways associated with CPT performance, such as DAT1 discussed above, for a more comprehensive understanding of distinct components of AX-CPT performance.
In conclusion, our results provide support for the influence of COMT on specific AX-CPT performance indices in children, which has been investigated only rarely in the extant literature, especially the variability of SDT indices, d′ and lnβ, across the task. Our study also suggests that the effects of COMT on specific AX-CPT performance indices differ by levels of externalizing psychopathology. As the neurobiology of the component indicators of AX-CPT performance indices becomes better understood, these findings will potentially allow for a more comprehensive conceptualization of relevant disorders that are characterized by specific deficits in CPT performance.
Highlights.
COMT was associated with variability in d′ and lnβ across blocks of the AX-CPT.
COMT showed a statistical trend for association with commission errors.
Higher externalizing psychopathology was associated with impaired CPT performance.
Some indices were more strongly related to COMT at higher levels of psychopathology.
CPT indices may represent useful endophenotypes for relevant disorders (e.g., ADHD).
Acknowledgments
This work was supported in part by NIMH grant K01-MH01818 to I.D.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia thalamocortical circuits: Parallel substrates for motor, oculomotor, prefrontal and limbic functions. Progress in Brain Research. 1991;85:119–145. http://dx.doi.org/10.1016/S0079-6123(08)62678-3. [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom L, Forsman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35(10):1373–1380. doi: 10.1016/s0028-3932(97)00072-9. http://dx.doi.org/10.1016/S0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, III, Noll DC, Cohen JD. Selective Deficits in Prefrontal Cortex Function in Medication- Naive Patients With Schizophrenia. Archives of General Psychiatry. 2001;58(3):280–288. doi: 10.1001/archpsyc.58.3.280. http://dx.doi.org/10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Domschke K, Hawi Z, Kirley A, Mullins C, Robertson IH, Gill M. The methionine allele of the COMT polymorphism impairs prefrontal cognition in children and adolescents with ADHD. Experimental Brain Research. 2005;163(3):352–360. doi: 10.1007/s00221-004-2180-y. http://dx.doi.org/10.1007/s00221-004-2180-y. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing--when and how? Journal of Clinical Epidemiology. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0. http://dx.doi.org/10.1016/S0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: A computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46(3):312–328. doi: 10.1016/s0006-3223(99)00116-x. http://dx.doi.org/10.1016/S0006-3223(99)00116-X. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 713–738. [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain Morphology and Schizophrenia, A Magnetic Resonance Imaging Study of Limbic, Prefrontal Cortex, and Caudate Structures. Archives of General Psychiatry. 1992;49(12):921–926. doi: 10.1001/archpsyc.1992.01820120009003. http://dx.doi.org/10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Brooks JO, Wang PW, Strong C, Sachs N, Hoblyn JC, Fenn R, Ketter TA. Preliminary evidence of differential relations between prefrontal cortex metabolism and sustained attention in depressed adults with bipolar disorder and healthy controls. Bipolar Disorders. 2006;8(3):248–254. doi: 10.1111/j.1399-5618.2006.00310.x. http://dx.doi.org/10.1111/j.1399-5618.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado MA, Serra-Grabulosa JM, Junqué C. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuro Image. 2007;37(4):1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. http://dx.doi.org/10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. http://dx.doi.org/10.1016/S0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clinical Pediatrics (Philadelphia) 1997;36(7):381–393. doi: 10.1177/000992289703600702. http://dx.doi.org/10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-o-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. The American Journal of Human Genetics. 2004;75(5):807–821. doi: 10.1086/425589. http://dx.doi.org/10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. The Journal of Comparative Neurology. 1999;409(1):38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. http://dx.doi.org/10.1002/(SICI)1096-9861(19990621)409:1%3C38::AID-CNE4%3E3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cohen J. The earth is round (p < .05) American Psychologist. 1994;49(12):997–1003. http://dx.doi.org/10.1037//0003-066X.49.12.997. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2003. [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108(1):120–133. doi: 10.1037//0021-843x.108.1.120. http://dx.doi.org/10.1037/0021-843X.108.1.120. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. http://dx.doi.org/10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychological Review. 1992;99(1):45–77. doi: 10.1037/0033-295x.99.1.45. http://dx.doi.org/10.1037/0033-295X.99.1.45. [DOI] [PubMed] [Google Scholar]
- Cole P. The evolving case-control study. Journal of Chronic Diseases. 1979;32(1–2):15–27. doi: 10.1016/0021-9681(79)90006-7. http://dx.doi.org/10.1016/0021-9681(79)90006-7. [DOI] [PubMed] [Google Scholar]
- Cole P. The hypothesis generating machine. Epidemiology. 1993;4(3):271–273. doi: 10.1097/00001648-199305000-00012. http://dx.doi.org/10.1097/00001648-199305000-00012. [DOI] [PubMed] [Google Scholar]
- Congdon E, Canli T. The endophenotype of impulsivity: reaching consilience through behavioral, genetic, and neuroimaging approaches. Behavioral and Cognitive Neuroscience Reviews. 2005;4(4):262–281. doi: 10.1177/1534582305285980. http://dx.doi.org/10.1177/1534582305285980. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. http://dx.doi.org/10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) and 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Molecular Psychiatry. 2005;10(7):686–698. doi: 10.1038/sj.mp.4001641. http://dx.doi.org/10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention- deficit hyperactivity disorder. Brain Research: Brain Research Reviews. 2003;42(1):1–21. doi: 10.1016/s0165-0173(02)00274-6. http://dx.doi.org/10.1016/S0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Davies DR, Parasuraman R. The Psychology of Vigilance. London, UK: Academic Press; 1982. [Google Scholar]
- del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The Roles of Dopamine and Noradrenaline in the Pathophysiology and Treatment of Attention-Deficit/Hyperactivity Disorder. Prefrontal Cortical Circuits Regulating Attention, Behavior and Emotion. 2011;69(12):e145–e157. doi: 10.1016/j.biopsych.2011.02.036. http://dx.doi.org/10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2004;161(1):125–132. doi: 10.1176/appi.ajp.161.1.125. http://dx.doi.org/10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, Pennington BF, Peart J, Biederman J. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? Journal of Child Psychology and Psychiatry. 2005;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. http://dx.doi.org/10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. http://dx.doi.org/10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg J, Mei-Tal G, Steinberg A, Tartakovsky E, Zohar A, Gritsenko I, Nemanov L, Ebstein RP. Haplotype relative risk study of catechol-O-methyltransferase (COMT) and attention deficit hyperactivity disorder (ADHD): association of the high-enzyme activity Val allele with ADHD impulsive-hyperactive phenotype. American Journal of Medical Genetics. 1999;88(5):497–502. http://dx.doi.org/10.1002/(SICI)1096-8628(19991015)88:5<497::AID-AJMG12>3.3.CO;2-6. [PubMed] [Google Scholar]
- Faraone SV, Biederman J. Pathophysiology of Attention Deficit Hyperactivity Disorder. In: Davis K, Charney D, Coyle JT, Nemeroff C, editors. ACNP’s Fifth Generation of Progress—Version 2. New York, NY: Lipponcott, Williams, and Wilkens; 2002. [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. http://dx.doi.org/10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Gasparini M, Fabrizio E, Bonifati V, Meco G. Cognitive improvement during tolcapone treatment in Parkinson’s disease. Journal of Neural Transmission. 1997;104(8–9):887–894. doi: 10.1007/BF01285556. http://dx.doi.org/10.1007/BF01285556. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. http://dx.doi.org/10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Waldman ID. Double dissociation between lab measures of inattention and impulsivity and the dopamine transporter gene (DAT1) and dopamine D4 receptor gene (DRD4) Journal of Abnormal Psychology. 2012;121(4):1011–1023. doi: 10.1037/a0028225. http://dx.doi.org/10.1037/a0028225. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. http://dx.doi.org/10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Wolf LE, Pascualvaca DM, Newcorn JH, Healey JM, O’Brien JD, Morganstein A, Young JG. Differential assessment of attention and impulsivity in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(3):326–329. doi: 10.1097/00004583-198805000-00010. http://dx.doi.org/10.1097/00004583-198805000-00010. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005;135(3):191–201. doi: 10.1016/j.psychres.2005.03.006. http://dx.doi.org/10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kirley A, Hawi Z, Daly G, McCarron M, Mullins C, Millar N, Waldman ID, Fitzgerald M, Gill M. Dopaminergic System Genes in ADHD: Toward a Biological Hypothesis. Neuropsychopharmacology. 2002;27(4):607–619. doi: 10.1016/S0893-133X(02)00315-9. http://dx.doi.org/10.1016/S0893-133X(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping. 2010;31(6):904–916. doi: 10.1002/hbm.21058. http://dx.doi.org/10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, la Fougere C, Ackenheil M. The dopamine transporter and neuroimaging in attention deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2003;27(7):605–613. doi: 10.1016/j.neubiorev.2003.08.012. http://dx.doi.org/10.1016/j.neubiorev.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. http://dx.doi.org/10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Landi N, Frost SJ, Mencl WE, Preston JL, Jacobsen LK, Lee M, Yrigollen C, Pugh KR, Grigorenko EL. The COMT Val/Met polymorphism is associated with reading related skills and consistent patterns of functional neural activation. Developmental Science. 2013;16(1):13–23. doi: 10.1111/j.1467-7687.2012.01180.x. http://dx.doi.org/10.1111/j.1467-7687.2012.01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Huxhold O, Schmiedek F. Aging and attenuated processing robustness: evidence from cognitive and sensorimotor functioning. Gerontology. 2004;50(1):28–34. doi: 10.1159/000074386. http://dx.doi.org/10.1159/000074386. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. http://dx.doi.org/10.1093/biomet/73.1.13. [Google Scholar]
- Liao SY, Lin SH, Liu CM, Hsieh MH, Hwang TJ, Liu SK, Guo SC, Hwu HG, Chen WJ. Genetic variants in COMT and neurocognitive impairment in families of patients with schizophrenia. Genes, Brain, and Behavior. 2009;8(2):228–237. doi: 10.1111/j.1601-183X.2008.00467.x. http://dx.doi.org/10.1111/j.1601-183X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–64. http://dx.doi.org/10.1111/j.1467-9280.1997.tb00545.x. [Google Scholar]
- Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nature Genetics. 2003;33(2):177–182. doi: 10.1038/ng1071. http://dx.doi.org/10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- Loo SK, Specter E, Smolen A, Hopfer C, Teale PD, Reite ML. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(8):986–993. doi: 10.1097/01.CHI.0000046890.27264.88. http://dx.doi.org/10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: a meta-analytic review. Journal of Child Psychology and Psychiatry. 1996;37(8):971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. http://dx.doi.org/10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Carter CS, Flory JD, Ferrell RE, Manuck SB. COMT Val158Met and executive control: a test of the benefit of specific deficits to translational research. Journal of Abnormal Psychology. 2007;116(2):306–312. doi: 10.1037/0021-843X.116.2.306. http://dx.doi.org/10.1037/0021-843X.116.2.306. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Li S, Bäckman L. Neural underpinnings of within-person variability in cognitive functioning. Psychology and Aging. 2009;24(4):792–808. doi: 10.1037/a0017798. http://dx.doi.org/10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003a;116(1):127–137. doi: 10.1016/s0306-4522(02)00556-0. http://dx.doi.org/10.1016/S0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003b;28(8):1521–1530. doi: 10.1038/sj.npp.1300218. http://dx.doi.org/10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- Matthews N, Vance A, Cummins TDR, Wagner J, Connolly A, Yamada J, Lockhart PJ, Panwar A, Wallace RH, Bellgrove MA. The COMT Val158 allele is associated with impaired delayed-match-to-sample performance in ADHD. Behavioral and Brain Functions. 2012;8(1):25. doi: 10.1186/1744-9081-8-25. http://dx.doi.org/10.1186/1744-9081-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol D. A primer of signal detection theory. London, UK: Allen & Unwin; 1972. [Google Scholar]
- Mechelli A, Tognin S, McGuire PK, Prata D, Sartori G, Fusar-Poli P, De Brito S, Hariri AR, Viding E. Genetic vulnerability to affective psychopathology in childhood: a combined voxel-based morphometry and functional magnetic resonance imaging study. Biological Psychiatry. 2009;66(3):231–237. doi: 10.1016/j.biopsych.2009.01.033. http://dx.doi.org/10.1016/j.biopsych.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nature Neuroscience. 2005;8:594–596. doi: 10.1038/nn1438. http://dx.doi.org/10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Michels KB, Rosner BA. Data trawling: to fish or not to fish. The Lancet. 1996;348(9035):1152–1153. doi: 10.1016/S0140-6736(96)05418-9. http://dx.doi.org/10.1016/S0140-6736(96)05418-9. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsh P, Meyer-Lindenberg A. Neural Substrates of Pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular Psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. http://dx.doi.org/10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Mills S, Langley K, Van den Bree M, Street E, Turic D, Owen MJ, O’Donovan MC, Thapar A. No evidence of association between Catechol-O-Methyltransferase (COMT) Val158Met genotype and performance on neuropsychological tasks in children with ADHD: a case-control study. BMC Psychiatry. 2004;7:4–15. doi: 10.1186/1471-244X-4-15. http://dx.doi.org/10.1186/1471-244X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder JA, Wedderburn RWM. Generalized linear models. Journal of the Royal Statistical Society Series A (General) 1972;135(3):370–384. http://dx.doi.org/10.2307/2344614. [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. http://dx.doi.org/10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information Processing and Attentional Functioning in the Developmental Course of Schizophrenic Disorders. Schizophrenia Bulletin. 1984;10(2):160–203. doi: 10.1093/schbul/10.2.160. http://dx.doi.org/10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Braver TS, Cohen JD. A Biologically Based Computational Model of Working Memory. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. New York, NY: Cambridge University Press; 1999. pp. 375–411. [Google Scholar]
- Poole C. Multiple comparisons? No problem! Epidemiology. 1991;2(4):241–243. Retrieved from http://www.jstor.org/stable/20065673. [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe PA. Clinical applications of continuous performance tests: Measuring attention and impulsive responding in children and adolescents. New York, NY: Wiley; 2001. [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: SAGE Publications; 1991. [Google Scholar]
- Rosvold H, Mirsky A, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. Journal of Consulting Psychology. 1956;20(5):343–350. doi: 10.1037/h0043220. http://dx.doi.org/10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. http://dx.doi.org/10.1097/00001648-199001000-00010. [PubMed] [Google Scholar]
- Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. American Journal of Epidemiology. 1995;142(9):904–908. doi: 10.1093/oxfordjournals.aje.a117737. Retrieved from http://aje.oxfordjournals.org/content/142/9/904.full.pdf. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman MW, DelBello MP, Keck PE, Jr, Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. American Journal of Psychiatry. 1999;156(1):139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology. 2004;74(1):1–57. doi: 10.1016/j.pneurobio.2004.05.006. http://dx.doi.org/10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context: a test of a theoretical model. Archives of General Psychiatry. 1996;53(12):1105–1113. doi: 10.1001/archpsyc.1996.01830120037008. http://dx.doi.org/10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. Journal of Neuroscience. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behavioural Brain Research. 2002;130(1–2):65–71. doi: 10.1016/s0166-4328(01)00431-4. http://dx.doi.org/10.1016/S0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Etefia K, Marks DJ. The utility of self-report measures and the continuous performance test in the diagnosis of ADHD in adults. CNS spectrums. 2004;9(9):649–659. doi: 10.1017/s1092852900001929. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Stefanis CN. Effect of COMT val158met polymorphism on the continuous performance test, identical pairs version: tuning rather than improving performance. American Journal of Psychiatry. 2005;162(9):1752–1754. doi: 10.1176/appi.ajp.162.9.1752. http://dx.doi.org/10.1176/appi.ajp.162.9.1752. [DOI] [PubMed] [Google Scholar]
- Stokes PR, Rhodes RA, Grasby PM, Mehta MA. The effects of the COMT val (108/158)met polymorphism on BOLD activation during working memory, planning, and response inhibition: a role for the posterior cingulate cortex? Neuropsychopharmacology. 2011;36(4):763–771. doi: 10.1038/npp.2010.210. http://dx.doi.org/10.1038/npp.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology Review. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. http://dx.doi.org/10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Swets JA, Tanner WP, Birdsall TG. Decision processes in perception. Psychological Review. 1961;68:301–340. http://dx.doi.org/10.1037/h0040547. [PubMed] [Google Scholar]
- Taerk E, Grizenko N, Ben Amor L, Lageix P, Mbekou V, Deguzman R, Torkaman-Zehi A, Ter Stepanian M, Baron C, Joober R. Catechol-O-methyltransferase (COMT) Val108/158 Met polymorphism does not modulate executive function in children with ADHD. BMC Medical Genetics. 2004;5(1):30. doi: 10.1186/1471-2350-5-30. http://dx.doi.org/10.1186/1471-2350-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicot JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. Journal of Neuroscience. 2007;27(49):13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14(4):1104–1106. doi: 10.1016/s0888-7543(05)80138-7. http://dx.doi.org/10.1016/S0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- van den Bosch RJ, Rombouts RP, van Asma MJ. What determines continuous performance task performance? Schizophrenia Bulletin. 1996;22(4):643–651. doi: 10.1093/schbul/22.4.643. http://dx.doi.org/10.1093/schbul/22.4.643. [DOI] [PubMed] [Google Scholar]
- Wahlstron D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience Biobehavioral Reviews. 2010;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. http://dx.doi.org/10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1347–1356. doi: 10.1016/j.biopsych.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. http://dx.doi.org/10.2307/2531734. [PubMed] [Google Scholar]