Abstract
Background
The absolute neutrophil count and the immature/total neutrophil ratio (I/T) provide information about the risk of early-onset sepsis in newborns. However, it is not clear how to combine their potentially overlapping information into a single likelihood ratio.
Methods
We obtained electronic records of blood cultures and of complete blood counts with manual differentials drawn <1 hour apart on 66,846 infants ≥34 weeks gestation and <72 hours old born at Kaiser Permanente Northern California and Brigham and Women’s Hospitals. We hypothesized that dividing the immature neutrophil count (I) by the total neutrophil count (T) squared (=I/T2) would provide a useful summary of the risk of infection. We evaluated the ability of the I/T2 to discriminate newborns with pathogenic bacteremia from other newborns tested using the area under the receiver operating characteristic curve (c).
Results
Discrimination of the I/T2 (c=0.79; 95% CI: 0.76, 0.82) was similar to that of logistic models with indicator variables for each of 24 combinations of the absolute neutrophil count and the proportion of immature neutrophils (c =0.80, 95% CI: 0.77, 0.83). Discrimination of the I/T2 improved with age, from 0.70 at <1 hour to 0.87 at ≥4 hours. However, 60% of I/T2 had likelihood ratios of 0.44 to 1.3, thus only minimally altering the pretest odds of disease.
Conclusions
Calculating the I/T2 could enhance prediction of early onset sepsiss, but the CBC will remain helpful mainly when done at >4 hours of age and when the pretest probability of infection is close to the treatment threshold.
Keywords: complete blood count, sepsis, neutrophils, leukocytes, sensitivity
A complete blood count (CBC) with white blood cell (WBC) differential is often done as part of an evaluation for sepsis in newborns. Beginning with Manroe et al1, many investigators have shown that a low WBC count, a low absolute neutrophil count (ANC) and a high proportion of immature neutrophils (I/T, where I is the immature and T is the total neutrophil count) predict infection.2-10 We recently showed that the CBC is more informative if its performance is deferred until at least 4 hours of age and the results are categorized into intervals, rather than simply dichotomized into “normal” and “abnormal” ranges.8 We provided likelihood ratios (LRs) for WBC counts, ANC and I/T results in 5 intervals for 3 time periods.8 However, we did not discuss the best way to combine the results of these tests.
If the WBC count, ANC and I/T were independent, one could simply multiply the LRs for their results together. The term “independent” means that in both those with and without disease, knowing the results of one of the tests does not provide information about the results of the other tests.11 However, because the ANC is a large component of the WBC count and is included in the denominator of the I/T, the tests are not independent. With a sufficient sample size, it would be possible to estimate interval LRs for each test result at each level of the others. However, obtaining such a large sample is not practical. Consequently, other approaches are needed.
Based on exploratory analyses of a previous dataset,7 we hypothesized a priori that the I/T divided by the total neutrophil count would capture information from both tests in a single number. The rationale for calculating the I/T2 is that a high I/T is more worrisome when the total neutrophil count is low.2, 12 By dividing the I/T by the total neutrophil count again, i.e., calculating I/T2, we hypothesized that we might capture this worrisome combination of a high I/T and low total neutrophil count with a single number.
Methods
The Institutional Review Boards for the Protection of Human Subjects of Kaiser Permanente Northern California (KPNC), the Brigham and Women’s Hospital (BWH) and the University of California, San Francisco approved the study protocol. We have previously described in detail the methods for obtaining the data analyzed for this study,8 and will summarize only briefly here.
Study Subjects
We used electronic medical records to identify infants born at ≥ 34 weeks gestation from January 1, 1995 through September 30, 2007 at KPNC hospitals, and from January 1, 1993 through December 31, 2007 at BWH. We included those who had a CBC done at <72 hours of age and within 1 hour of a blood culture. We kept the first positive blood culture and associated CBC for infants with positive cultures, and the first blood culture/CBC pair for other infants. CBCs and blood cultures were drawn according to the protocols and clinical judgment of clinicians at each site. From the 67,623 infants with paired CBC and blood culture results included in our previous study, we excluded 777 who did not have a manual microscopic WBC differential, leaving 66,846 subjects (including 244 of the 245 infants with infection) for the current study.
Predictor variables
CBCs were done using Beckman-Coulter or Sysmex hematology analyzers at KPNC hospitals and an Advia 120 Automated Hematology Analyzer at the BWH. The differential WBC count was done manually on a mean of 100.0 cells (SD 0.9) to allow for identification of bands. The ANC was calculated as the automated estimate of the WBC × (% segmented neutrophils + % bands)/100. The I/T was calculated as the total number of immature neutrophils (promyelocytes, myelocytes, metamyelocytes and bands) divided by the total number of cells in the neutrophilic cell line (immature plus segmented neutrophils). To account for changes in WBC over the first several hours after birth, we used 3 age strata, as defined in our previous study:8 < 1 hour, 1 to < 4 hours and ≥ 4 hours.
Outcome variable
The outcome variable was whether or not the blood culture paired with the CBC was positive for a presumed pathogen. We classified blood cultures without regard to CBC results using an algorithm based primarily on the organism and the time to culture positivity.8, 13 The predominant organisms were group B streptococcus (GBS ;56%) and Escherichia coli (21%).
Statistical analysis
We first compared models that included either the WBC count or the ANC, alone and combined with the I/T, to decide which of these two closely related counts to use for further analyses involving the I/T. We assessed performance by comparing discrimination, as measured by the area (c) under the ROC curve, and calibration, measured by the Hosmer-Lemeshow test of goodness of fit with 10 groups,14 using models in which the WBC count or ANC was included as either a continuous variable or as a group of indicator variables.
To explore the joint relationship between the ANC and the I/T, we grouped the results of the ANC and I/T into 5 categories each. We chose round number boundaries for these categories so that each category of cell counts had enough subjects to include at least 30 cases of bacteremia. Although the I/T2 was the only function of I and T that we hypothesized a priori, we used logistic regression with log(I) and log(T) to more generally examine predictors of the form IxTy , to see whether exponents different from x = 1 and y = −2 might lead to improved prediction of case status. These analyses excluded subjects with I = 0 because log(0) is undefined. Because the I/T2 was highly skewed, we used cutoffs that increased logarithmically to create single variables with 7 or 8 categories.
Although dichotomizing test results leads to loss of information,8, 11 another way to compare the I/T2 with the ANC and I/T is to compare specificity at a cutoff that yields a fixed sensitivity. To do this, we identified the I/T2 value associated with a LR of 1 by modeling log(I/T2) as a continuous variable in the whole cohort and in the 3 previously defined age groups.8 We calculated sensitivity and specificity of the I/T2 using that cutoff, then compared the specificity of the ANC and I/T at cutoffs that yielded that same sensitivity. We further compared the discrimination of the ANC, I/T and I/T2 in each age group by estimating the area under the ROC curve, dividing each variable into 7 intervals using round-number cutoffs.
To estimate the LR as a continuous function of the logarithm of the I/T2, we obtained predicted post-test probabilities of infection from the logistic regression model, used these to calculate the post-test odds of infection, and calculated the LR as the quotient of these post-test odds and the pre-test odds observed in this study.
Results
Of the 66,846 infants with paired CBC and blood cultures included in this study, 244 (0.37%) had bacteremia. About 70% of the infants were born at KPNC hospitals and 30% at BWH (Table 1). Limited additional information about the study subjects is provided in Table 1; more detailed information on this cohort has been previously published (except that infants with no manual differential were also included in the previous cohort).8
Table 1.
Description of the study participants
| Total Number |
% of Study Population |
Number with Infection |
Infection risk per 1000 |
P (Chi- squared test) |
|
|---|---|---|---|---|---|
| Year of birth | <0.001 | ||||
| 1993-1997 | 13,316 | 19.9% | 76 | 5.7 | |
| 1998-2002 | 24,688 | 36.9% | 109 | 4.4 | |
| 2003-2007 | 28,842 | 43.1% | 59 | 2.0 | |
|
Hospital/Healthcare
system of birth |
0.32 | ||||
|
Brigham and
Women’s Hospital |
20,225 | 30.3% | 81 | 4.0 | |
| Kaiser Permanente | 46,621 | 69.7% | 163 | 3.5 | |
| Gestational age | 0.1 | ||||
| 34 -36 6/7 wks | 11,274 | 16.9% | 29 | 2.6 | |
| 37 - 38 wks | 13,163 | 19.7% | 54 | 4.1 | |
| ≥ 39 wks | 42,409 | 63.4% | 161 | 3.8 | |
|
Age at the time of the
CBC |
<0.001 | ||||
| 0 - 0.99 hour | 10,119 | 15.1% | 63 | 6.2 | |
| 1 - 3.99 hours | 32,812 | 49.1% | 91 | 2.8 | |
| >= 4 hours | 23,915 | 35.8% | 90 | 3.8 | |
| Total | 66,846 | 100.0% | - | - |
Analyses comparing discrimination and calibration of the WBC count and the ANC, either alone or in combination with the I/T, showed that the WBC count and the ANC performed similarly, but that the ANC performed a little better in all models. We therefore selected the ANC (or the very closely related total neutrophil count, which includes metamyelocytes, myelocytes and promyelocytes as well as segmented neutrophils and bands) to use for subsequent analyses in combination with the I/T.
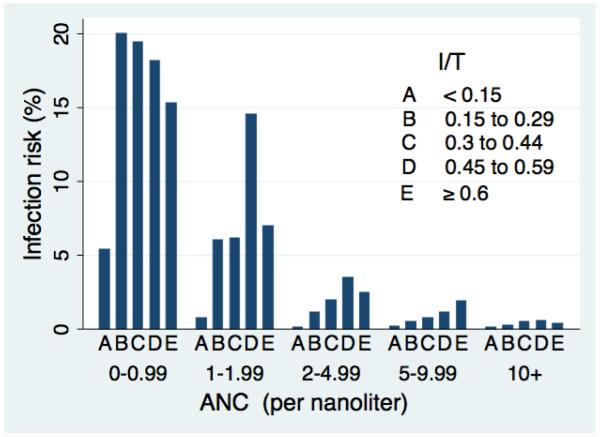
The 5 × 5 table showing the joint distribution of ANC and I/T yielded between 30 and 23,739 newborns per cell (Table 2). The risk of infection ranged from 0.1% to 20% (Figure 1). Newborns with the lowest ANC values were at high risk of infection regardless of their I/T, whereas those with the highest I/T were at not at high risk if their ANC was normal. The risk of infection increased monotonically with decreasing ANC at every level of I/T. In contrast, the effect of I/T at each level of ANC was more variable; the most consistent finding was that I/T <0.15 was the most reassuring result at all levels of ANC (Figure 1).
Table 2.
Joint distribution of Absolute Neutrophil Count (ANC) and proportion immature neutrophils (I/T) in 66,846 newborns evaluated for sepsis
| I/T | |||||||
|---|---|---|---|---|---|---|---|
| ANC (x 10^9/L) |
0 to 0.1499 |
0.15 to 0.299 |
0.3 to 0.449 |
0.45 to 0.599 |
0.6 to 1 |
Total | % in Row |
|
0 to
0.99 |
93 | 30 | 36 | 33 | 72 | 264 | 0.4% |
|
1 to
1.99 |
407 | 100 | 81 | 62 | 100 | 750 | 1.1% |
|
2 to
4.99 |
4,364 | 1,007 | 467 | 318 | 282 | 6,438 | 9.6% |
|
5 to
9.99 |
15,465 | 4,562 | 1,476 | 641 | 371 | 22,515 | 33.7% |
| ≥ 10 | 23,739 | 9,536 | 2,569 | 767 | 268 | 36,879 | 55.2% |
| Total | 44,068 | 15,235 | 4,629 | 1,821 | 1,093 | 66,846 | 100% |
|
% in
Column |
65.9% | 22.8% | 6.9% | 2.7% | 1.6% | 100% | |
Figure 1.
Risk of bacterial infection by proportion immature neutrophils (I/T) and absolute neutrophil count (ANC).
The logistic regression to identify optimal exponents x and y for a predictor of the form Ix/Ty yielded x = 0.93 and y = −1.98 when newborns with I = 0 were excluded. Adding 0.1 to the immature neutrophil count to include the 5,481 subjects (including 10 cases) with zero immature neutrophils yielded x = 0.99 and y = −2.03. These exponents are remarkably close to the values of x = 1 and y = −2 that we had hypothesized in proposing use of I/T2. We therefore continued analyses using the I/T2; the resulting 8-category variable had LRs that ranged from 0.17 to 91 (Table 3).
Table 3.
Prevalence of bacteremia and likelihood ratios for intervals of Immature neutrophils divided by total neutrophils squared (I/T2).
| I/T2× 100 Interval |
N | Cumulative % |
Cases of Infection |
Infection risk/1000 |
Likelihood Ratio for Interval (95% CI) |
|---|---|---|---|---|---|
| 0 * | 5,481 | 8% | 10 | 1.8 | 0.50 (0.27, 0.92) |
|
0.01 to
0.49 * |
14,767 | 30% | 9 | 0.6 | 0.17 (0.09, 0.32) |
| 0 to 0.49 * | 20,248 | 30% | 19 | 0.9 | 0.26 (0.17, 0.40) |
| 0.5 to 0.99 | 15,691 | 54% | 25 | 1.6 | 0.44 (0.30, 0.63) |
| 1 to 1.99 | 16,018 | 78% | 33 | 2.1 | 0.56 (0.41, 0.77) |
| 2 to 4.99 | 10,747 | 94% | 52 | 4.8 | 1.3 (1.0, 1.7) |
| 5 to 9.99 | 2,598 | 98% | 28 | 11 | 3.0 (2.1, 4.2) |
| 10 to 99.9 | 1,476 | 100% | 70 | 47 | 14 (11, 17) |
| ≥ 100 | 68 | 100% | 17 | 250 | 91 (53, 155) |
| Total | 66,846 | 100% | 244 | 3.7 |
Two methods of categorizing low values are shown; the top 2 rows are combined in the third row.
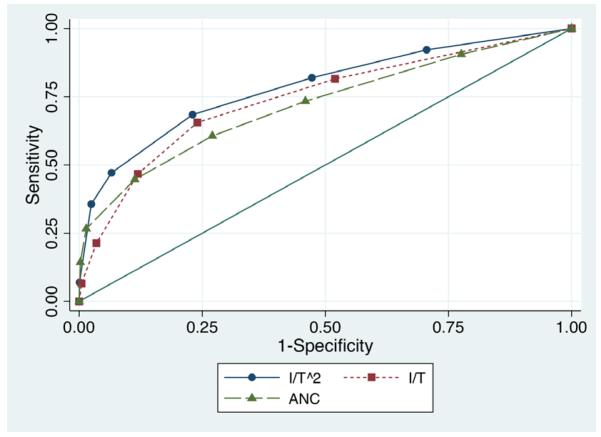
The LR for the 8% of subjects with I/T2=0 was 0.5, which was not as low as the LR for those with slightly higher immature neutrophil counts. This led to a poor fit when I/T2 was modeled as a continuous variable. Rather than allow LRs to change non-monotonically (which very slightly improved the discrimination but seemed biologically implausible), we combined the bottom two categories in Table 3 to create a 7-category I/T2 variable. The discrimination of this 7-category variable (c = 0.79; 95% CI: 0.76, 0.82) was significantly (P < 0.001) better than either the ANC alone (c = 0.72, 95% CI: 0.69, 0.76) or the I/T alone (c = 0.75; 95% CI: 0.72, 0.78; Figure 2) and almost as high as the value obtained (c = 0.80; 95% CI: 0.77, 0.83) with a total of 24 indicator variables representing every combination of 5-category ANC and I/T variables. Discrimination was similar for infections caused by GBS (c=0.80, 95% CI: 0.76, 0.84) and those caused by other organisms (c=0.78, 95% CI: 0.73, 0.83).
Figure 2.
Receiver Operating Characteristic (ROC) Curves for prediction of infection by I/T2, ANC and I/T.
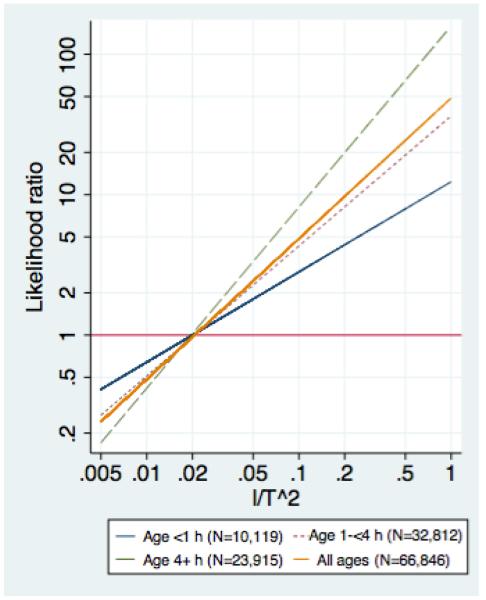
When modeled as a continuous variable the LR for the I/T2 was 1.0 at a value of 0.02 in the entire cohort and also separately in all 3 age groups studied. Dichotomized at 0.02, the I/T2 had sensitivity of 68% and specificity of 78%; the specificity was higher than either the ANC (63%) or I/T (73%) at that sensitivity (Table 4). The performance of the I/T2 improved with age, and was better than either the I/T or ANC at all ages except <1 hour, at which age the I/T was slightly better and the ANC was close to useless. For infants of all ages combined, the LR for the I/T2 was about 50 times the value of the I/T2; the equation for the line relating the LR to the I/T2 for all age groups combined had slope 49.3 and intercept −0.16. As expected, the LR increased less steeply for CBCs done at <1 hour and more steeply for those done at > 4 hours (Figure 3).
Table 4.
Test characteristics for dichotomized absolute neutrophil count (ANC), proportion of immature neutrophils (I/T) and I/T2 at different age groups.
| Sensitivity | Specificity | LR+ | LR− | C* (95% CI) | |
|---|---|---|---|---|---|
|
Age < 1 hour (63 cases) |
|||||
| ANC < 9.0 † | 62% | 43% | 1.08 | 0.89 | 0.55 (0.47, 0.63) |
| I/T > 0.2 | 62% | 73% | 2.31 | 0.52 | 0.71 (0.65, 0.78) |
| I/T2 > 0.02 | 62% | 67% | 1.88 | 0.57 | 0.70 (0.63, 0.76) |
|
Age 1-3.99 hours
(91 cases) |
|||||
| ANC < 8.9 | 64% | 58% | 1.53 | 0.62 | 0.68 (0.61, 0.74) |
| I/T > 0.172 | 64% | 72% | 2.26 | 0.5 | 0.72 (0.66, 0.78) |
| I/T2 > 0.02 | 64% | 76% | 2.64 | 0.48 | 0.75 (0.69, 0.81) |
|
Age ≥ 4 hours (90
cases) |
|||||
| ANC < 9.8 | 78% | 73% | 2.91 | 0.3 | 0.84 (0.78, 0.89) |
| I/T > 0.185 | 78% | 75% | 3.1 | 0.3 | 0.80 (0.75, 0.85) |
| I/T2 > 0.02 | 78% | 85% | 5.27 | 0.26 | 0.87 (0.83, 0.92) |
|
Total cohort (244 cases) |
|||||
| ANC < 9.03 | 68% | 63% | 1.83 | 0.5 | 0.72 (0.69, 0.76) |
| I/T > 0.18 | 68% | 73% | 2.54 | 0.43 | 0.75 (0.72, 0.78) |
| I/T2 > 0.02 | 68% | 78% | 3.1 | 0.41 | 0.79 (0.76, 0.82) |
Area under the ROC curve (c) is for the variable in the 7 categories shown in Table 3.
Cutoffs for ANC and I/T were chosen to match the sensitivity of I/T2 at a cutoff of 0.02.
LR, Likelihood Ratio
Figure 3.
Estimated likelihood ratios for the I/T2 overall and at different ages.
Discussion
This retrospective cross-sectional study included a single CBC on each of more than 60,000 term and near-term newborns, 244 of whom had culture-proven bacterial infections. As others 1-5, 9, 10 and we 6-8 have previously reported, both a high I/T and (especially) a low ANC were predictive of infection, mainly in infants > 4 hours old. Our novel result is that the best way to combine these two nonindependent tests is to calculate the I/T2. The I/T2 is a single number that had better specificity (78%) than either the ANC (63%) or I/T (73%) alone (at the same 68% sensitivity). As measured by the area under the ROC curve, it discriminated almost as well (c=0.79) as a multivariate model containing 25 possible combinations of the ANC and I/T (c=0.80).
Several features of the I/T2 make it potentially attractive for clinical use. First, it is easy to calculate. Although we anticipate that eventually an electronic medical record will calculate the I/T2 (and its age-specific likelihood ratio), for now clinicians calculating the I/T2 need only push the “divide” button on their calculators once more after obtaining the I/T. Second, the point at which the LR for the I/T2 is equal to 1.0, i.e., the point (about the 80th percentile) at which the result changes from reassuring to worrisome, is an easily remembered round number: 0.02. It is noteworthy that the I/T2 associated with a LR of 1.0 could have varied with age but did not. The fact that all 3 lines in Figure 3 cross close to where LR = 1 will make this index easier to use in practice. Third, a useful property of the I/T2 is that multiplying by 50 gives a rough estimate of the likelihood ratio. The actual LR depends on the age at which the CBC was done (with the LR slightly farther from 1 at older ages), but the I/T2 × 50 provides a useful anchoring point. Finally, use of the I/T2 allows use of information from both the ANC and the I/T, without the unwarranted assumption of independence and without a common oversimplification, which is to consider the CBC abnormal if any component of the CBC is abnormal.
While the large sample size is a strength of our study, its reliance on electronically available data is a limitation. We did not have data on method of blood sampling, which is known to affect WBC counts in newborns.15-17 However, in our previous study8 we found that using the deviation of the counts from those expected based on a variety of other factors also known to affect WBC counts, including the age at which they were drawn, the delivery method, maternal pre-eclampsia and gestational age,18, 19 did not improve the prediction of early-onset sepsis compared with using the counts themselves.
Another potential limitation is that the study period ended in 2007. Although recommendations for use of intrapartum antibiotics to prevent EOS have changed since that time,20, the distribution of organisms causing EOS in our study population has not.21 In addition, we found that the I/T2 performed similarly whether the infection was caused by GBS or other organisms. Thus we would not expect current I/T2 values to perform differently from those in this study. A strength of this study compared with other recent studies using electronic data9, 22 is that we had exact ages at which CBCs and blood cultures were drawn, allowing them to be matched more closely to one another. Combined with our focus on term and late preterm infants, this may account for the better predictive ability of the CBC in the current study.
Although the I/T2 appears to capture most of the ability of the CBC to predict bacteremia, the CBC still leaves much to be desired as a test for infection. More than 60% of newborns in this study had intermediate levels of I/T2, corresponding to LRs of 0.44 to 1.3, which are only minimally informative (Table 3). Even when most reassuring, the LR for the I/T2 is in the range of 0.25. This is not low enough to rule out infection in high-risk or symptomatic infants. Such infants should be treated without waiting for the results of the CBC.
The CBC is most likely to be helpful if obtained after 4 hours of age and when applied to “borderline” cases. Our team has recently described how to combine maternal factors23 with the evolving clinical examination.24 Thus, it is now possible to define “borderline” quantitatively. Future research should continue our examination of how best to combine different quantitative indices to obtain more refined and accurate estimates of a newborn’s risk for sepsis.
We conclude that the I/T2 shows promise as a simple way to capture the predictive ability of both the ANC and the I/T in a single number. Additional studies to validate this index in other populations are needed. In the meantime, even if it is only approximate, the simple rule that the LR is close to 50 × I/T2 may be a useful adjunct to CBC interpretation in newborns.
Acknowledgments
Funding source: This study was supported in part by a grant from the National Institute of General Medical Sciences, #R01-GM-80180
References
- 1.Manroe BL, Rosenfeld CR, Weinberg AG, Browne R. The differential leukocyte count in the assessment and outcome of early-onset neonatal group B streptococcal disease. J Pediatr. 1977 Oct;91(4):632–637. doi: 10.1016/s0022-3476(77)80522-2. [DOI] [PubMed] [Google Scholar]
- 2.al-Mulla ZS, Christensen RD. Neutropenia in the neonate. Clin Perinatol. 1995 Sep;22(3):711–739. [PubMed] [Google Scholar]
- 3.Berger C, Uehlinger J, Ghelfi D, Blau N, Fanconi S. Comparison of C-reactive protein and white blood cell count with differential in neonates at risk for septicaemia. Eur J Pediatr. 1995 Feb;154(2):138–144. doi: 10.1007/BF01991918. [DOI] [PubMed] [Google Scholar]
- 4.Da Silva O, Ohlsson A, Kenyon C. Accuracy of leukocyte indices and C-reactive protein for diagnosis of neonatal sepsis: a critical review. Pediatr Infect Dis J. 1995 May;14(5):362–366. doi: 10.1097/00006454-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg DN, Yoder BA. Changes in the differential white blood cell count in screening for group B streptococcal sepsis. Pediatr Infect Dis J. 1990 Dec;9(12):886–889. doi: 10.1097/00006454-199012000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Escobar GJ, Zukin T, Usatin MS, et al. Early discontinuation of antibiotic treatment in newborns admitted to rule out sepsis: a decision rule. Pediatr Infect Dis J. 1994 Oct;13(10):860–866. doi: 10.1097/00006454-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Escobar GJ, Li DK, Armstrong MA, et al. Neonatal sepsis workups in infants >/=2000 grams at birth: A population-based study. Pediatrics. 2000 Aug;106(2 Pt 1):256–263. doi: 10.1542/peds.106.2.256. [DOI] [PubMed] [Google Scholar]
- 8.Newman TB, Puopolo KM, Wi S, Draper D, Escobar GJ. Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics. 2010 Nov;126(5):903–909. doi: 10.1542/peds.2010-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornik CP, Benjamin DK, Becker KC, et al. Use of the Complete Blood Cell Count in Early-onset Neonatal Sepsis. Pediatr Infect Dis J. 2012 Aug;31(8):799–802. doi: 10.1097/INF.0b013e318256905c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy K, Weiner J. Use of leukocyte counts in evaluation of early-onset neonatal sepsis. Pediatr Infect Dis J. 2012 Jan;31(1):16–19. doi: 10.1097/INF.0b013e31822ffc17. [DOI] [PubMed] [Google Scholar]
- 11.Newman T, Kohn M. Evidence-Based Diagnosis. Cambridge University Press; New York: 2009. [Google Scholar]
- 12.Christensen RD, Bradley PP, Rothstein G. The leukocyte left shift in clinical and experimental neonatal sepsis. J Pediatr. 1981 Jan;98(1):101–105. doi: 10.1016/s0022-3476(81)80553-7. [DOI] [PubMed] [Google Scholar]
- 13.Bates DW, Lee TH. Rapid classification of positive blood cultures. Prospective validation of a multivariate algorithm. JAMA. 1992 Apr 8;267(14):1962–1966. [PubMed] [Google Scholar]
- 14.Statacorp. Stata, Release 9 . Reference Manual. Volume 2. Stata Corporation; (K-Q) College Station, TX: 2005. p. 90. [Google Scholar]
- 15.Christensen RD, Henry E, Jopling J, Wiedmeier SE. The CBC: reference ranges for neonates. Semin Perinatol. 2009 Feb;33(1):3–11. doi: 10.1053/j.semperi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Kayiran SM, Ozbek N, Turan M, Gurakan B. Significant differences between capillary and venous complete blood counts in the neonatal period. Clin Lab Haematol. 2003 Feb;25(1):9–16. doi: 10.1046/j.1365-2257.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 17.Peevy KJ, Grant PH, Hoff CJ. Capillary venous differences in neonatal neutrophil values. Am J Dis Child. 1982 Apr;136(4):357–358. doi: 10.1001/archpedi.1982.03970400075019. [DOI] [PubMed] [Google Scholar]
- 18.Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979 Jul;95(1):89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 19.Engle WD, Rosenfeld CR. Neutropenia in high-risk neonates. J Pediatr. 1984 Dec;105(6):982–986. doi: 10.1016/s0022-3476(84)80095-5. [DOI] [PubMed] [Google Scholar]
- 20.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010 Nov 19;59(RR-10):1–36. [PubMed] [Google Scholar]
- 21.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011 May;127(5):817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornik CP, Benjamin DK, Becker KC, et al. Use of the Complete Blood Cell Count in Late-onset Neonatal Sepsis. Pediatr Infect Dis J. 2012 Aug;31(8):803–807. doi: 10.1097/INF.0b013e31825691e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puopolo KM, Draper D, Wi S, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011 Nov;128(5):e1155–1163. doi: 10.1542/peds.2010-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escobar GJ, Puopolo KM, Wi S, et al. Quantitative stratification of the risk of early onset sepsis in newborns ≥ 34 weeks gestation. Pediatrics. In Press; doi: 10.1542/peds.2013-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]