Abstract
Acoustic communication requires gathering, transforming, and interpreting diverse sound cues. To achieve this, all the spatial and temporal features of complex sound stimuli must be captured in the firing patterns of the primary sensory neurons and then accurately transmitted along auditory pathways for additional processing. The mammalian auditory system relies on several synapses with unique properties in order to meet this task: the auditory ribbon synapses, the endbulb of Held, and the calyx of Held. Each of these synapses develops morphological and electrophysiological characteristics that enable the remarkably precise signal transmission necessary for conveying the miniscule differences in timing that underly sound localization. In this article, we review the current knowledge of how these synapses develop and mature to acquire the specialized features necessary for the sense of hearing.
Organization of neural circuits in the cochlea and auditory brainstem
Hearing begins with the detection of sound by hair cells in the cochlea of the inner ear. There are two types of hair cells in the sensory epithelium of the mammalian organ of Corti: a single row of inner hair cells (IHCs) and three to four rows of outer hair cells (OHCs). IHCs directly encode acoustic information (Nienhuys et al., 1978), whereas OHCs are responsible for mechanical amplification of sound-induced vibration (Ashmore et al., 1994). Both types of HCs convert the mechanical stimuli that are generated by sound waves into electrochemical signals, which are passed on to spiral ganglion neurons (SGNs). As the sole neurosensory link from the cochlea to the brain, SGNs transmit all sound information from IHCs to target neurons in the central nervous system (CNS). SGNs are bipolar neurons, with peripheral processes that project towards HCs and central processes that extend through the eighth nerve into the auditory brainstem (Fig. 1). They are grouped into two classes depending on their pattern of peripheral innervation: Type I neurons, which innervate IHCs and constitute 90–95% of the total SGN population, and Type II neurons, which form en passant and terminal contacts with multiple OHCs and represent the remaining 5 to 10% of SGNs (Simmons et al., 1988). HCs transmit frequency, intensity, and timing information to the SGNs via a specialized connection called the ribbon synapse (Fig. 1). The ribbon synapse contains a presynaptic ribbon, which is an electron-dense multi-protein structure tethering large clusters of synaptic vesicles (Khimich et al., 2005). It is suggested that the presynaptic ribbon supports a large pool of readily releasable vesicles, allows synchronous release of multiple vesicles, and promotes the replenishment of vesicles after exocytosis (Buran et al., 2010; Frank et al., 2010; Khimich et al., 2005). Therefore, the ribbon synapse is able to respond to graded changes in the HC membrane potential, and is capable of fast, sustained, and precise signaling (Buran et al., 2010; Khimich et al., 2005; Safieddine et al., 2012). These features allow us to sense sound over a dynamic range of several orders of magnitude in intensity with high temporal acuity.
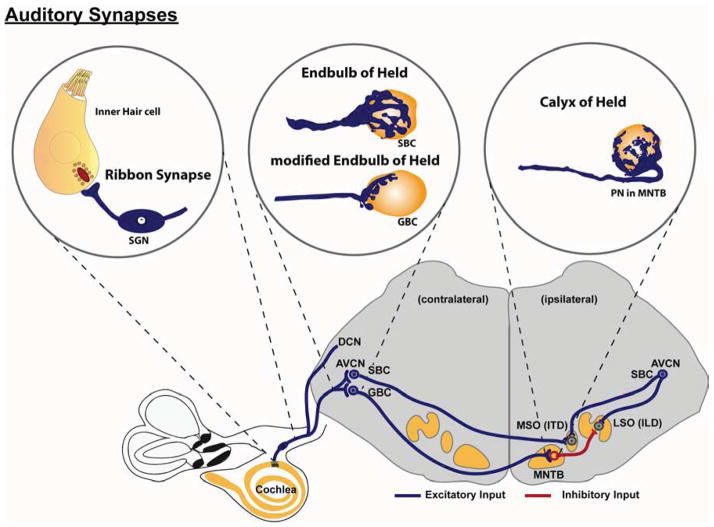
Fig. 1. Overview of the synapses that are specialized for transmission of signals along the auditory pathway.
Peripheral processes of spiral ganglion neurons (SGN) receive input from inner hair cells in the cochlea via the ribbon synapse. Central projections of SGNs bifurcate upon entering the brainstem. The ascending branch extends toward the anterior ventral cochlear nucleus (AVCN) and forms an endbulb of Held synaptic contact on spherical bushy cells (SBC) or smaller, modified endbulbs of Held on globular bushy cells (GBC). SBCs send bilateral projections to terminate on neurons of the contralateral and ipsilateral medial superior olive (MSO), which forms a pathway crucial for determining interaural time differences (ITD). Axons of GBCs project contralaterally to the medial nucleus of the trapezoid body (MNTB) and elaborate the calyx of Held synapse on principal neurons. The principal neurons of the MNTB provide glycinergic inhibitory inputs to neurons in the lateral superior olive (LSO), converging with excitatory inputs from SBCs of the ipsilateral AVCN. LSO neurons use contralateral inhibition from GBCs by way of the MNTB and ipsilateral excitation from SBCs to compute interaural level (intensity) differences (ILD). Computation of ITD and ILD permits binaural sound localization.
SGNs relay sound information to neurons in the auditory brainstem through their central processes. Upon entering the brainstem, the central axon of each individual SGN bifurcates (Fekete et al., 1984). The descending process projects through the posteroventral cochlear nucleus (PVCN) into the dorsal cochlear nucleus (DCN), extending branches that make both en passant and standard bouton contacts with a variety of target neurons. The ascending process sends a major projection into the anterior ventral cochlear nucleus (AVCN) and elaborates an extraordinarily large synaptic ending, known as the endbulb of Held, which envelops the cell body of the bushy cell neuron (Fig. 1) (Ryugo et al., 1982). Additional branches off the ascending process generate boutons, smaller complex terminals and up to two additional endbulbs of Held. There are two major types of bushy cell neurons - spherical and globular (Wu et al., 1984) – as defined by their appearance and location in the AVCN. Spherical bushy cells (SBCs) receive the largest endbulbs of Held, whereas globular bushy cells (GBCs) receive several smaller, modified endbulbs of Held (Rouiller et al., 1986). The large presynaptic terminal of the endbulb of Held harbors hundreds of release sites with a large number of synaptic vesicles (Nicol et al., 2002; Ryugo et al., 1996). These structural features of the endbulb of Held enable high frequency firing without depletion of the vesicle pool and facilitate rapid neurotransmission with high accuracy. These properties permit processing of the precise timing features necessary for interaural sound localization (Brenowitz et al., 2001) and speech perception, such as voice onset/offset, temporal gap and syllabic stress (Blackburn et al., 1990).
Within the auditory brainstem, GBCs and SBCs are responsible for passing auditory information from the AVCN to the superior olivary complex for additional processing largely related to sound localization. SBC axons form excitatory synapses ipsilaterally with neurons in the lateral superior olive (LSO) and bilaterally on neurons of the medial superior olive (MSO). MSO neurons help determine where each sound stimulus originates by detecting interaural time differences (ITD) between acoustic inputs from each ear (Fitzpatrick et al., 1997; Yin et al., 1990) (Fig. 1). In parallel, GBC axons cross the midline of the brainstem and terminate in the contralateral medial nucleus of the trapezoid body (MNTB), signaling via another giant synaptic ending, the calyx of Held (Fig. 1). With hundreds of active zones and a large readily releasable pool in the presynaptic ending (Taschenberger et al., 2002), the calyx of Held, similar to its smaller analogue, the endbulb of Held, allows signals to be relayed in the reliable and fast manner necessary for sound localization and speech recognition. The principal neurons in the MNTB are glycinergic and send inhibitory projections to the LSO. Therefore, the GBC-MNTB connection converts excitation originating from the contralateral cochlea into inhibition. The contralateral inhibitory input converges with the ipsilateral excitatory input from SBCs within the LSO, where post-synaptic neurons use these two inputs to compute interaural level differences (ILD) and provide another cue for sound localization (Glendenning et al., 1992; Sanes, 1990).
All of these auditory synapses – the ribbon synapse, the endbulb of Held, and the calyx of Held -- develop specialized structural and functional properties that ensure fast and high fidelity transmission of sound information and therefore play pivotal roles in relaying acoustic signals along the auditory pathway. In the following sections, we review what is known about the formation and maturation of these specialized synapses. We mainly focus on findings from mice, which currently provide the best genetic model system for human deafness, but include results from other mammals when no corresponding data from mice are available. Similar features are also found in the auditory circuits of birds (Kubke et al., 2000), emphasizing the need for specialized synapses for this kind of processing throughout the animal kingdom.
Development of the auditory ribbon synapse
Outgrowth, pathfinding, and innervation of IHCs by SGN peripheral projections
The first step in forming a synaptic contact is the growth of neuronal processes toward their target cells. We only briefly summarize early cochlear wiring events here, since they have been discussed extensively elsewhere (Appler et al., 2011; Coate et al., 2013; Defourny et al., 2011; Yang et al., 2011a). Around embryonic day (E) 9 in mice, neuroblasts begin to delaminate from the otocyst and form the cochlear-vestibular ganglion (Carney et al., 1983; Ma et al., 1998), which subsequently separates into distinct spiral and vestibular ganglia. SGNs exit the cell cycle along a base-to-apex gradient between E9.5 to 13.5 in mice (Koundakjian et al., 2007; Matei et al., 2005). Shortly after undergoing their final mitoses, SGNs become bipolar and extend their peripheral processes toward the developing sensory epithelium (Farinas et al., 2001). By E12.5, most SGNs have extended complex and branched peripheral processes towards the edge of the nascent spiral lamina. Over the next few days, these branches are lost, resulting in an orderly array of single peripheral processes terminating at the level of the immature hair cells (Koundakjian et al., 2007). In the developing sensory epithelium, hair cells are born later than SGNs, becoming post-mitotic around E11.5 to 14.5, in an apex-to-base progression (Matei et al., 2005). The initial guidance of SGN neurites to their pre-synaptic targets does not require differentiated HCs, as the afferent fibers still grow to the sensory epithelium in mice lacking Atoh1, a master transcription factor for HC differentiation (Fritzsch et al., 2005). It has been proposed that the earliest delaminated neuronal precursors project their axons back to their delamination site (Carney et al., 1983), providing a trail for later growing axons (Webber et al., 2006). However, little is known about the cues that regulate these early wiring events, though several neurotrophins, classical guidance molecules, and extracellular matrix proteins are clearly involved (Appler et al., 2011; Coate et al., 2013; Defourny et al., 2011; Yang et al., 2011a). In addition, intrinsic factors act in SGNs to influence these wiring events, with the transcription factor Gata3 playing a major role in the initiation and coordination of SGN differentiation (Appler et al., 2013; Duncan et al., 2013; Luo et al., 2013) and Prox1 ensuring that Type II SGN fibers are oriented toward the base of the cochlea (Fritzsch et al., 2010). Indeed, Type II afferents can be recognized as early as E16.5, based on their morphology, consistent with the idea that these initial guidance events are intrinsically controlled (Koundakjian et al., 2007).
As the gross wiring pattern of the cochlea is established, SGN afferent fibers arborize and send out temporary collateral branches towards both IHCs and OHCs (Echteler, 1992; Huang et al., 2007; Huang et al., 2012). At this early stage, some immature ribbon synapses are formed, but many of these contacts are subsequently eliminated during synaptic pruning and refinement steps (Huang et al., 2012; Sobkowicz et al., 1982). Over time, the arbors of individual SGN afferent fibers are refined, resulting in IHC innervation by Type I SGNs and OHCs by Type II SGNs. The molecular cues that establish this segregation of afferent innervation remain elusive, but a recent study suggests that Ephrin A5 in hair cells and EphA4 receptor in SGNs are involved (Defourny et al., 2013). In mice lacking Ephrin A5 or EphA4, a subset of type I SGNs aberrantly innervate the OHC region even after the onset of hearing, i.e. when the ear canal first opens, which is around postnatal day 12 in most mouse strains.
The auditory ribbon synapse
Hair cells are the primary detectors for sound information, yet lack an axon, underscoring the need for efficient signaling to the SGNs in order to capture biologically important features of the original sound stimulus. Hence, the synapses connecting IHCs and SGNs are morphologically and physiologically distinct from conventional synapses. At the morphological level, the defining characteristic is the presence of a prominent presynaptic ribbon in the IHC. The ribbon is an electron-dense, multi-protein structure that tethers ~10 to 20 synaptic vesicles to the active zone in mice (Frank et al., 2010) and is thought to provide a large pool of readily releasable vesicles for synchronous and sustained signaling (Buran et al., 2010; Khimich et al., 2005; Safieddine et al., 2012). The major protein component of the synaptic ribbon is RIBEYE, which is composed of a unique, ribbon-specific A domain and a B domain that is also present in the transcriptional co-repressor CtBP2 (Schmitz et al., 2000). The A domain is required for assembly of RIBEYE into a large complex, whereas the B domain binds NAD+ with high affinity and is proposed to mediate membrane fusion and fission during synaptic vesicle turnover through enzymatic activity. Because the B domain is shared with CtBP2, anti-CtBP2 immunostaining detects both RIBEYE (in synapses) and CtBP2 (in the nucleus) and is therefore frequently used to visualize the pre-synaptic machinery in hair cells. The ribbons are anchored to the active zone by Bassoon, which is also present in conventional synapses (Dick et al., 2003; Khimich et al., 2005). In Bassoon-deficient mice, ribbons are no longer properly localized to the active zone of the mouse IHC and have a diminished readily releasable pool of synaptic vesicles, leading to asynchronous synaptic transmission and hearing impairment (Khimich et al., 2005).
In addition to the unusual presence of a pre-synaptic ribbon, IHC ribbon synapses also rely on an unconventional combination of proteins for synaptic transmission. Like other types of synapses, ribbon synapses use several SNARE complex proteins, including syntaxin 1, SNAP-25 and synaptobrevin 1, to assemble the molecular machinery triggering vesicular fusion and exocytosis (Safieddine et al., 1999). However, mature ribbon synapses lack synaptotagmin 1 and 2, the two major calcium sensors found in typical CNS synapses (Safieddine et al., 1999; Uthaiah et al., 2010). Several other molecules important for neurotransmission at other synapses, such as synaptophysins, synapsins, and complexins are also missing from IHC ribbon synapses (Safieddine et al., 1999; Strenzke et al., 2009; Uthaiah et al., 2010). Interestingly, recent results suggest that exocytosis at the IHC ribbon synapse may operate without neuronal SNAREs (Nouvian et al., 2011). Instead, the IHC ribbon synapse seems to employ a specific calcium sensor, otoferlin (Roux et al., 2006), to mediate exocytosis, with CaV1.3 L-type Ca2+ channels controlling transmitter release (Platzer et al., 2000). Otoferlin is a multi C2-domain protein and has been identified as the genetic cause of a recessive, nonsyndromic form of human deafness, DFNB9 (Yasunaga et al., 1999). In otoferlin null IHCs, Ca2+ triggered exocytosis is almost completely abolished, and the mutant mice are profoundly deaf (Roux et al., 2006). Vesicular glutamate transporter 3 (Vglut3) is the principal glutamate transporter acting at the IHC ribbon synapse (Seal et al., 2008). Hence, glutamate release is impaired in Vglut3 null IHCs, which develop abnormally thin and elongated ribbons and are unable to elicit synaptic activity in the SGN afferent terminals.
To accommodate the peculiar signaling abilities of the presynaptic complex, SGN afferent terminals elaborate an unusually large and concave postsynaptic density (PSD). This PSD usually extends beyond the boundaries of the presynaptic active zone (Sobkowicz et al., 1982). It contains abundant clusters of AMPA-type receptors, including GluR2, GluR3 and GluR4, which are highly concentrated at the periphery of the PSD (Matsubara et al., 1996). The large number of receptors ensures rapid and sustained response to glutamate with minimal receptor saturation (Glowatzki et al., 2002), thereby enabling accurate transmission of the initial signal. To avoid the possible excitotoxic effects of glutamate accumulation in the synaptic cleft, glutamate is scavenged by glutamate transporters (GLAST) present in the surrounding supporting cells (Furness et al., 1997; Furness et al., 2003). The PSD of the IHC ribbon synapse also contains several molecules common to other synapses, such as PSD95, PSD93, and Shank (Davies et al., 2001; Huang et al., 2012). Several kainate (GluR5,6 and KA1,2) and NMDA (NR1 and NR2A-D) receptor subunits are also present (Niedzielski et al., 1995), but do not play a major role here (Glowatzki et al., 2002).
The composition and function of the OHC afferent synapse is poorly understood. Glutamate is also the primary neurotransmitter (Weisz et al., 2009), but Type II SGN terminals contain far fewer AMPA receptor subunits at the PSD than Type I SGN terminals (Huang et al., 2012; Matsubara et al., 1996). Accordingly, strong acoustic stimulation is required to produce a response in these neurons (Weisz et al., 2009). Additionally, Type II SGNs can be excited by ATP (Weisz et al., 2009). The molecular mechanism underlying auditory neurotransmission from the OHC afferent synapse may be fundamentally different from the IHC afferent synapse and remains to be clarified.
Formation and maturation of ribbon synapses
The precise onset of ribbon synapse formation in the mouse cochlea is still not clear. Physiologically, SGNs acquire action potential-generating capacity at E14 (Marrs et al., 2012) and membrane capacitance changes induced by evoked exocytosis can be recorded in IHCs as early as E16.5 (Johnson et al., 2005). Morphologically, immature afferent synapses containing presynaptic ribbon structures positioned opposite a PSD can be observed in the basal coil of the mouse cochlea at birth (Shnerson et al., 1981). Consistent with these observations, in newborn rats, spontaneous spiking activity in immature IHCs can trigger action potentials in apical SGNs (Tritsch et al., 2010a). Although these findings hint that synapse development initiates during late embryogenesis, it is generally agreed that afferent synaptogenesis in the mouse cochlea happens mainly postnatally (Sobkowicz et al., 1982).
Ultrastructural analyses of the developing organ of Corti in the intact mouse and in culture have shown that the initial formation of an individual IHC ribbon synapse can be broadly divided into two stages (Fig. 2A) (Sobkowicz et al., 1986): assembly of the presynaptic complex followed by development of the synaptic cleft and PSD. Presynaptic development appears to initiate autonomously in hair cells, with ribbons and the associated synaptic vesicles assembling in the cytoplasm independent of the presence of SGN terminals (Sobkowicz et al., 1986). As SGN neurites arrive and begin to form contacts during late embryonic to early postsynaptic stages (Fig. 2B), the pre-formed ribbons subsequently move toward the basolateral membrane of the IHC. Here, the ribbon is anchored by two filaments to a presynaptic thickening that forms in the IHC membrane, due in part to the activity of dense-core vesicles that transport essential building materials here (Pfenninger et al., 1969). The presynaptic thickening becomes straight and electron-dense, invaginating slightly to accommodate the ribbon. Once the entire presynaptic complex is built, the synaptic cleft begins to be filled with a dense filamentous matrix. At the same time, the postsynaptic membrane in the adjacent SGN becomes straight and electron-dense, gradually forming a PSD that enlarges over time. In the mature ribbon synapse, the presynaptic complex is attached to the active zone by a single curved density that fits over a concave PSD that extends beyond the presynaptic active zone (Sobkowicz et al., 1982). The developmental sequence of the OHC ribbon synapse is similar to that of the IHC, but lags behind the IHC by several days (Sobkowicz et al., 1986).
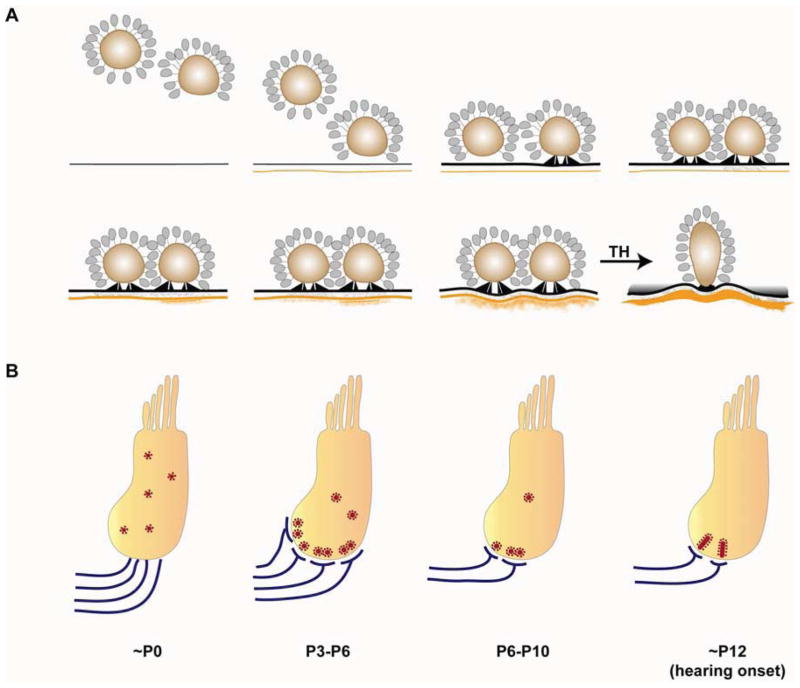
Fig. 2. Development of the mouse inner hair cell (IHC) ribbon synapse.
A. Presynaptic ribbon complexes are formed in IHCs and descend toward the basal cell membrane. Presynaptic densities are anchored by two rodlets to the membrane thickening. Synaptic clefts begin to be filled with a dense filamentous matrix. Subsequently, postsynaptic densities (PSDs) are assembled at sites apposing presynaptic active zones (Sobkowicz et al., 1986). In the mature ribbon synapse, the presynaptic complex is attached to the active zone by a single curved density and the PSD forms a concave shape that exceeds the territory of the presynaptic active zone (Sobkowicz et al., 1982). During early development, ribbons are round and tend to occur in clusters. Mature ribbon synapses are elliptical, and each individual afferent is juxtaposed to a single ribbon. This maturation process depends on thyroid hormone. B. Ribbons are gradually localized to the basolateral surface of the IHC in response to the innervation of SGN neurites during perinatal stages. After the first postnatal week, pruning, retraction and refinement of afferent fibers result in reduction of ribbon synapse number. Concurrently, clusters of ribbons consolidate. After hearing onset, each individual afferent terminal is apposed to a single ribbon.
The emergence of the proper number of functional ribbon synapses is dynamic, with many changes in the structure and organization of contacts as the animal matures. When contacts first form, the synapses are immature, with multiple ribbons clustered in large active zones (Sendin et al., 2007; Sobkowicz et al., 1982). On the post-synaptic side, GluR2/R3 AMPA receptors are present in the terminals at birth (Huang et al., 2012). As development progresses, both the number of ribbons and the level of AMPA receptors increase, peaking at the end of the first postnatal week and progressively declining to adult levels by the onset of hearing. Hence, there is a ~50% reduction in synapse number during the second postnatal week (Huang et al., 2012; Nemzou et al., 2006; Sendin et al., 2007; Sobkowicz et al., 1982). This reduction is likely a result of the pruning, retraction, and refinement of immature branched SGNs projections (Huang et al., 2007; Huang et al., 2012) together with the consolidation of multiple round ribbons into a single elongated ribbon at the presynaptic active zone (Fig. 2B) (Sendin et al., 2007). Similarly, whereas the immature ribbon synapse exhibits a diffuse pattern of AMPA receptor patches near the basolateral pole of the IHC (Sendin et al., 2007), in the mature SGN ending, AMPA receptors aggregate and cluster to form well-defined puncta (Khimich et al., 2005; Sendin et al., 2007). OHC synapses undergo even more profound pruning, initially forming a similar number of ribbons as each IHC over the first postnatal week, but losing most of them before the onset of hearing. This phenomenon probably results from the retraction of Type I fibers from the OHCs (Huang et al., 2012) and apoptosis of supernumerary Type II SGNs (Barclay et al., 2011).
Along with these morphological changes come a number of changes in the biophysical properties of the IHC ribbon synapse (see (Bulankina et al., 2012; Safieddine et al., 2012) for details). In mice, from the late embryonic stage up to the onset of hearing, immature IHCs are able to fire action potentials (Marcotti et al., 2003), which are generated from depolarization of the IHCs through voltage-gated Cav1.3 calcium channels and repolarization by voltage-gated delayed rectifier potassium channels (Kros et al., 1998; Marcotti et al., 2003). The amplitudes of both calcium and potassium currents increase during the first postnatal week. These calcium-based action potentials can generate sufficient calcium influx to trigger exocytosis at young IHC ribbon synapses (Beutner et al., 2001; Johnson et al., 2005) and evoke sodium-based action potentials in SGNs at birth (Tritsch et al., 2010a). In vivo recordings have confirmed that there are highly irregular patterns of activity in the pre-hearing pathway (Jones et al., 2007; Sonntag et al., 2009; Tritsch et al., 2010b) that likely originate in the cochlea, since firing ceases after cochlear ablation (Tritsch et al., 2010b). By analogy with other developing sensory systems, this early activity may be essential for the maturation of IHC innervation as well as the maintenance and refinement of central auditory projections (Friauf et al., 1999; Kandler et al., 2009; Kennedy, 2012).
Although it is well established that IHCs fire calcium-based action potentials before the onset of hearing, the mechanisms that promote IHC membrane depolarization and action potential firing are not fully understood. It is currently unclear whether pre-hearing IHCs in vivo are depolarized enough at rest to fire action potentials spontaneously (Johnson et al., 2013; Johnson et al., 2011), or whether their spiking requires an external depolarizing stimulus, such as ATP released from supporting cells in Kolliker’s organ (Tritsch et al., 2010a; Tritsch et al., 2007). The effects of ATP on IHCs are complex as nanomolar concentrations have been reported to hyperpolarize IHCs through activation of small-conductance Ca2+-activated K+ (SK2) channels, which may fine tune the IHC’s resting potential (Johnson et al., 2011). In addition, IHC discharge can also be modulated by acetylcholine (Johnson et al., 2011), which is released by the olivocochlear efferent fibers that form transient contacts with IHCs during early development (Simmons et al., 1996; Sobkowicz et al., 1994). Understanding how regulation of IHC firing by any of these cues influences the wiring of auditory circuits remains to be determined.
Around the onset of hearing, the expression of big potassium (BK) channels terminates this period of spontaneous spiking by preventing regenerative depolarization (Kros et al., 1998) and establishing a graded membrane potential in the mature IHC. The number of CaV1.3 Ca2+ channels also decreases, but at the same time, smaller Ca2+ currents are able to induce larger amounts of exocytosis in the mature IHC (Beutner et al., 2001; Brandt et al., 2005; Johnson et al., 2005). This increased release efficiency results from tighter association between Ca2+ channels and the synaptic machinery. Indeed, the majority of Ca2+ channel clusters are co-localized with presynaptic ribbons in the mature IHC (Brandt et al., 2005; Frank et al., 2010). Moreover, immature mouse IHCs switch from otoferlin-independent to otoferlin-dependent calcium-evoked exocytosis at P4 (Beurg et al., 2010). Concomitantly, synaptotagmins 1 and 2, the two most common calcium sensors of CNS fast synapses, are developmentally down-regulated before the second postnatal week and absent in the mature IHC after the onset of hearing (Beurg et al., 2010).
Although we still know little about the pathways that initiate and coordinate ribbon synapse development, a few key players have been identified. The overall maturation of the ribbon synapse appears to be under the control of thyroid hormone (Rusch et al., 1998; Sendin et al., 2007). In Pax8 null mice, which lack thyroid follicular cells, synapse elimination fails and synapse number therefore remains elevated after the onset of hearing. In addition, IHCs maintain their immature properties, with multiple ribbons anchored to a single active zone and large calcium currents with low calcium-evoked release efficiency (Sendin et al., 2007). A more specific function during the final steps of ribbon synapse development has been identified for Myosin VI, which is present in the active zone and interacts with otoferlin (Heidrych et al., 2009; Roux et al., 2009). Myosin VI mutant IHCs fail to transport BK channels to the membrane and show immature exocytotic calcium efficiency and reduced ribbon number. Understanding how thyroid hormone exerts its effects and whether Myosin VI is involved in these events are important challenges for the future.
Development of the post-synaptic SGN terminals is inherently linked with formation and maturation of the pre-synaptic ribbons in the IHCs. For instance, when SGN afferents are removed from cochlear explants, ribbons no longer localize properly to the basolateral surface of the hair cell (Sobkowicz et al., 1986). Nevertheless, pre-synaptic complexes still form, indicating that the ability to make a ribbon is under intrinsic control in the hair cells. Conversely, when ribbon formation is disrupted in zebrafish hair cells, the number of post-synaptic terminals is reduced (Sheets et al., 2011). Additionally, the size of the PSD correlates inversely with the size of the ribbon, with large ribbons apposed to small PSDs and vice versa. In cats, the larger ribbons form on the modiolar side, likely signaling to fibers with higher thresholds and lower spontaneous firing rates (SR). The smaller ribbons on the pillar side, on the other hand, correlate with innervation by fibers with lower thresholds and higher SR (Liberman et al., 2011). Thus, variation in synapse morphology may contribute to the functional heterogeneity of SGN signaling. How IHCs and SGNs interact to establish this systematic pattern is not known.
Although it has long been appreciated that pre-synaptic ribbons can form in IHCs without innervation of SGN fibers, how post-synaptic development is intrinsically controlled has only recently been investigated. One important player is the basic leucine-zipper transcription factor Mafb, which acts in SGNs to direct differentiation of the unusually large PSD at the IHC ribbon synapse (Yu et al., 2013). In Mafb mutant mice, SGN afferents fail to develop normal PSDs. Although Mafb is present only in SGNs, indirect effects on the presynaptic cell are also evident: IHC ribbons are smaller and reduced in number. These results suggest that pre- and post-synaptic development are initiated autonomously, but that subsequent signaling between IHCs and SGNs is necessary for formation of a morphologically normal synapse. Indeed, overall synapse number is reduced in mutant animals, which therefore exhibit impaired auditory responses.
Consistent with the idea that Mafb serves as a master regulator for PSD differentiation, overexpression of Mafb is sufficient to accelerate afferent synapse development (Yu et al., 2013). The onset of Mafb expression depends on the activity of Gata3, which serves as an upstream master regulator for SGN differentiation (Appler et al., 2013; Duncan et al., 2013; Luo et al., 2013; Sendin et al., 2007). Hence, as in Mafb mutants, ribbon synapse number is severely reduced in Gata3 mutant mice. Remarkably, restoration of Mafb significantly rescues the ribbon synapse defect in Gata3 mutants. Therefore, SGNs employ a Mafb-Gata3 transcriptional network to direct post-synaptic differentiation in afferent terminals. Identification of Mafb downstream genes in SGNs may shed some light on how the PSD is assembled at the IHC ribbon synapse.
Development of the endbulb of Held
Axon outgrowth and pathfinding of SGN central projections
At the same time that SGN peripheral processes are growing out towards the organ of Corti, the central axons of Type I SGNs project along the eighth nerve, first reaching the hindbrain around E11.5 and bifurcating one day later (Lu et al., 2011). Similar to what happens in peripheral branches, the outgrowth of central processes also occurs in a basal to apical progression (Angulo et al., 1990). By E15.5, central projections of SGNs from all regions of the cochlea are present in the cochlear nucleus and organized into coarse tonotopic maps, with apical and basal projections segregated along the dorsal-ventral axis of the cochlear nucleus (Fekete et al., 1984; Koundakjian et al., 2007). The descending branches of the central processes project to the PVCN and DCN and innervate several different kinds of target neurons via standard axodendritic boutons. In contrast, the ascending branches terminate in the anteroventral cochlear nucleus (AVCN) and give rise to a large axosomatic ending, the endbulb of Held (Ryugo et al., 1982), on the postsynaptic SBC. Smaller, medium-sized complex endings, which are categorized as modified endbulbs of Held, also make axosomatic contacts with GBCs near the posterior region of AVCN of the cat (Rouiller et al., 1986). In this review, we will focus mainly on the endbulb of Held received by SBCs.
Very little is known about the guidance cues that lead auditory nerve fibers to the cochlear nucleus. Several Eph receptors and Ephrin ligands are expressed in the eighth nerve of the chicken embryo, suggesting this signaling pathway may play a role in target selection and the emergence of topographic maps of the developing auditory projections (Huffman et al., 2007; Miko et al., 2007; Siddiqui et al., 2005). Normal auditory experience and neural activity are also essential, as the tonotopic organization of SGN axon terminals is significantly degraded in the cochlear nuclei of deafened animals (Leake et al., 2006). Interestingly, similar to the peripheral projection, the presence of target cells seems to be unnecessary for the initial guidance of central projections towards the AVCN. Genetic fate-mapping has demonstrated that neurons from AVCN are derived from the rhombic lip at the r2–r3 level (Farago et al., 2006), with GBCs originating exclusively from r3 (Renier et al., 2010). Most neurons of the ventral cochlear nucleus (VCN) are generated from an Atoh1-dependent cochlear extramural stream of the caudal rhombic lip between E11.5 to E13.5 (Wang et al., 2005). Mice with conditional disruption of Atoh1 lose most VCN neurons but SGN processes still project to the cochlear nuclei and bifurcate (Maricich et al., 2009). Identification of additional guidance cues and further analysis of available mutant mouse strains will help provide some clarity for how central wiring of SGNs is established.
Morphogenesis of the endbulb of Held synapse
Development of the endbulb of Held synapse involves dramatic changes in the size and shape of the endbulb and its contacts with the SBC cell body. The endbulb develops initially as a swelling at the end of a Type I SGN axon that has contacted a SBC (Fig. 3) (Limb et al., 2000). Much like the growth cone that navigated to the target, the ending begins with a relatively simple structure, with no obvious branches at these early stages (first postnatal week in mice). By P12, the ending has become 10–15 times larger and exhibits a more diverse array of morphologies, characterized by the emergence of occasional branches and thin filipodial-like extensions (Limb et al., 2000). The branches remain relatively confined, so the endbulb does not cover much of the SBC surface area. Over the next two weeks, the endbulb gradually acquires a more complex tree-like morphology, with many branches arborizing to form a reticulated network that envelops up to half of the SBC cell soma, similar to the appearance in adults (Fig. 3). Ultrastructural studies in the cat have shown that the earliest contacts between endbulbs and SBCs are irregular, with a ruffled “caterpillar-like” appearance (Ryugo et al., 2006). At these stages, the endbulbs appear to attach to the SBC cell surface via vesicle-free symmetrical membrane thickenings called puncta adherentia. Nascent PSDs at this stage are variable in length, with puncta adherentia often nearby. Over time, the puncta adherentia disappear, synaptic vesicle density increases, and the PSDs lengthen and bulge (Ryugo et al., 2006). In parallel, the apposition between the endbulb and the SBC becomes smoother and begins to be interrupted by occasional gaps called intermembranous cisternae (Ryugo et al., 2006), which have been proposed to play a role in transmitter clearance in mature animals.
Fig. 3. Morphological changes in the endbulb of Held in normal developing or deaf adult mice (adapted from (Limb et al., 2000) with permission).
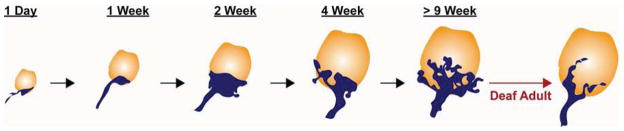
The endbulb of Held in mice is initially small and simple but gradually grows to form a highly branched and intricate structure. Endbulb complexity is severely reduced in adult mice that are congenitally deaf.
These morphological changes are paralleled by physiological changes that allow for effective signaling from SGNs to SBCs in the mature animal. After the onset of hearing, synchronous release from mature endbulbs triggers strong postsynaptic depolarization, which in turn activates KV1.x and SK channels in SBCs. Activation of these potassium channels prevents asynchronous pre-synaptic release from triggering spikes in postsynaptic SBCs, thereby increasing temporal precision (Yang et al., 2010a). In mice, each presynaptic endbulb terminal contains more than 1,000 readily releasable vesicles and expresses an estimated average of 6,400 P/Q type voltage-gated Ca2+ channels (Lin et al., 2011). The presynaptic endbulb terminal also expresses VGLUT1 and VGLUT2 (Gomez-Nieto et al., 2011) and employs complexin-I to regulate its release probability (Strenzke et al., 2009). Postsynaptically, SBCs produce the AMPA receptor subunits GluR2, 3, and 4 as well as the NMDA receptor subunit NR1 (Gomez-Nieto et al., 2011; Wang et al., 1998b). However, the major glutamate receptor here comprises mainly GluR3 and GluR4 subunits, which enables fast neurotransmission owing to the calcium permeability and rapid desensitization of this receptor composition (Wang et al., 1998b). Importantly, individual synapses exhibit a range of properties, indicating that subtle differences in synaptic transmission may contribute to how sound stimuli are encoded. Indeed, each SBC can receive inputs from several endbulbs (Nicol et al., 2002). Mature endbulbs are estimated to have 500–2000 release sites (Ryugo et al., 1996), and both the number of synaptic vesicles at individual release sites and the size of the apposing PSD can differ considerably (Nicol et al., 2002).
Several studies have highlighted the importance of neural activity for the emergence of the diverse morphologies typical of the mature endbulb of Held. The first hint was the observation that endbulb size and shape varies depending on the electrical properties of the central auditory nerve fibers in adult animals. Endbulbs from low spontaneous firing rate (SR) fibers have more complex terminals with smaller swellings and are apposed by larger PSDs than those of high SR fibers (Ryugo et al., 1991; Ryugo et al., 1996; Sento et al., 1989). Moreover, in congenitally deaf cats (Baker et al., 2010; Ryugo et al., 1997) and mice (Lee et al., 2003; Youssoufian et al., 2008), endbulbs are smaller and develop fewer terminal branches. In addition, the synapses that form have fewer synaptic vesicles and intermembraneous cisterns, but are larger, likely to compensate for the reduced signaling capabilities. These hypertrophic synapses lose their rounded shape, with longer and flatter PSDs. In deaf mice, the amplitudes of AMPA receptor-mediated excitatory post-synaptic currents (EPSCs) at the endbulb are also altered (McKay et al., 2007). Notably, electrical stimulation with a cochlear implant can restore endbulb synaptic morphology back to normal in young (3-month) but not old (6-month) deaf cats (O’Neil et al., 2010; O’Neil et al., 2011). This restoration can even be seen in contralateral endbulbs with unilateral cochlear implantation (O’Neil et al., 2010). Finally, bushy cells in immature mice have high input resistances and their excitatory postsynaptic potentials show longer and more variable latencies (Wu et al., 1987). Between P5 and P17, the input resistance lowers and timing of synaptic responses becomes more precise. These findings suggest that acoustic stimulation is required for the normal morphological and physiological maturation of the endbulb within a critical period.
Although the sequence of endbulb development has been documented in detail, we know nothing about the underlying mechanism. The remarkably close associations between the endbulb and the SBC membranes at all stages suggest an important role for cell-cell interactions, with the SBC promoting growth and arborization of the endbulb and the endbulb influencing maturation of the PSD in turn. To date, no molecule has been shown to be responsible for any of these events. However, a recent study identified bone morphogenetic proteins (BMPs) as candidate signaling molecules to specify the large nerve terminal size of another calyx-type synapse, the calyx of Held, in the MNTB (Xiao et al., 2013). This finding raises the interesting possibility that BMP signaling may also be involved in the growth of the endbulb. With the introduction of new molecular genetic tools, it will be exciting to witness how research on this specialized synapse blossoms in the coming years.
Development of the calyx of Held
The calyx of Held exhibits a number of unusual features that are particularly crucial for sound localization. First, these synapses are nearly exclusively localized to the contralateral side of the brain, with GBC axons forming a major auditory commissure from one ear that converges with input from the other ear. The calyx of Held is also enormous, covering up to half of the cell surface of the target principal neurons in the MNTB. In addition, most MNTB neurons receive only one calyceal ending (Smith et al., 1991). Hence, when a single GBC fires, synaptic vesicles are released simultaneously from hundreds of active zones, resulting in immediate and highly effective activation of a single principal neuron on the opposite side of the brain. Like the endbulb of Held, the calyx of Held stands apart from other synapses due to its size. The immature calyx of Held is one of the few central presynaptic terminals that is accessible to electrophysiologists, so much more is known about how growth of the synapse is coordinated with the emergence of its mature firing properties. Since calyx development has been reviewed in detail by several elegant articles (Borst et al., 2012; Nakamura et al., 2011; Schneggenburger et al., 2006), we will only briefly summarize our current understanding of this process with a focus on more recent discoveries.
Axon outgrowth and pathfinding of GBCs
A key feature of the calyx of Held is its strictly contralateral localization. To reach their final destinations, GBC axons must extend from the AVCN toward the midline and then cross to terminate on the contralateral MNTB. Importantly, the axons pass by the ipsilateral MNTB as they navigate, yet do not stop here to form a synapse (Tolbert et al., 1982). Efforts to understand how this important pattern of innervation is established have confirmed roles for canonical axon guidance molecules, and have raised the interesting possibility that these early wiring events may set the stage for subsequent synapse formation and maturation (Michalski et al., 2013).
Like other commissural neurons, GBCs rely on a combination of attractive and repulsive cues to send axons to and across the midline. Shortly after GBCs are born at E13, their axons project toward the midline, with many crossing over by E14.5 (Howell et al., 2007). Around the same time, netrin-1 is expressed in cells of the brainstem midline, and VCN axons are immunopositive for the netrin receptor DCC. In both netrin-1 and DCC mutant mice, VCN axons fail to reach the midline, suggesting that midline attraction is mediated by Netrin/DCC signaling (Fig. 4A) (Howell et al., 2007). As in the spinal cord, Netrin appears to act together with a Slit/Robo system here. Robo1, 2, and 3 are expressed in neurons of the cochlear nucleus, and all three Slit ligands are secreted by midline cells (Howell et al., 2007; Renier et al., 2010). Whereas Netrins provide an attractive cue, Slits are highly repellent and function to drive axons out of the midline so they can continue along their trajectory. This raises a conundrum: how can the midline be simultaneously attractive and repulsive to the GBC axons? The solution relies on the activity of Robo3, which prevents axons from sensing Slit cues until after they have reached the midline (Sabatier et al., 2004). Hence, in Robo3 mutant mice, GBC axons are prematurely repelled from the midline, resulting in ipsilateral mistargeting (Fig. 4B) (Renier et al., 2010). Remarkably, despite projecting to the wrong side of the brainstem, the misrouted axons in Robo3 mutant mice still form calyces of Held on the ipsilateral MNTB neurons. However, these synapses do not mature properly (Michalski et al., 2013), indicating that the act of crossing the midline is somehow necessary for the ability to form a mature synapse. Contact with the midline is known to affect production of proteins in spinal commissural neurons (Nawabi et al., 2010). Similarly, GBC axons may be exposed to signals at the midline that alter expression of proteins required for subsequent maturation of the calyx of Held.
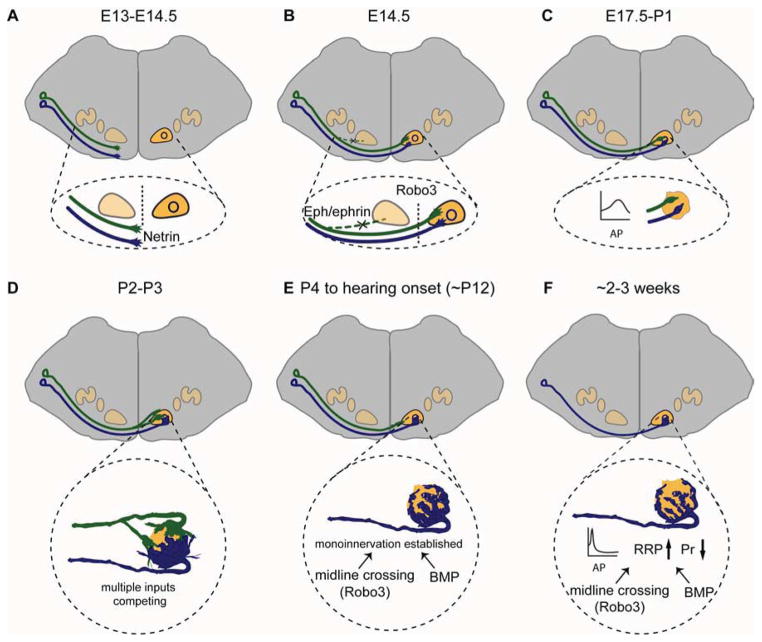
Fig. 4. Major stages in the development of the calyx of Held synapse in mice.
A. Around E13 to E14.5, GBC axons are attracted to the midline by Netrin-1/DCC signaling. B. By 14.5, the majority of GBC axons have crossed the midline of the brainstem due to the expression of Robo3, which prevents premature Slit responsiveness in pre-crossing axons. In parallel, EphB2/Ephrin-B2 signaling suppresses the formation of aberrant ipsilateral VCN-MNTB projections and is required for strictly contralateral VCN-MNTB projections. C. By E17, the earliest synaptic contacts between GBC axons and MNTB principal neurons are established. At P0 and P1, only a few small axosomatic contacts are formed on MNTB neurons. During this time, the presynaptic action potential (AP) has a long duration and small amplitude. D. Around P2-P3, presynaptic endings have formed large cup-shaped swellings called protocalyces, which contain many collaterals and are structurally dynamic. MNTB neurons receive inputs from several calyces at this stage. E. During the first and second postnatal weeks, collaterals are retracted and parts of the calyx become thinner and more intricate. Synaptic competition between multiple calyceal inputs is largely resolved, such that the strongest calyceal input wins, with monoinnervation of nearly all MNTB neurons by this stage. F. Adult calyx of Held synapses show a highly elaborate structure. Presynaptic AP kinetics are faster, with a shorter duration and larger amplitude. In addition, the readily releasable vesicle pool (RRP) size is increased and release probability (Pr) is decreased. Both the morphological and functional maturation of the calyx of Held require BMP signaling and Robo3-mediated axon midline crossing. The timeline is indicated as embryonic and postnatal ages in mice.
While crossing the midline, GBC axons must also ignore analogous targets in the ipsilateral MNTB in order to form uniquely contralateral contacts. This important feature of auditory wiring depends on Eph/Ephrin signaling, with EphB2 in VCN axons and the Ephrin-B2 ligand in MNTB (Hsieh et al., 2010). In mice with null mutations of EphB2/B3 or Ephrin-B2, VCN axons cross the midline as they should and form normal contacts in the contralateral MNTB. However, axons also elaborate additional calyceal contacts with neurons in the ipsilateral MNTB. Eph-Ephrin signaling can be bidirectional, with forward signaling characterized by a response of the Eph receptor-expressing cell to Ephrin ligand binding, and reverse signaling referring to a response of the Ephrin-expressing cell to Eph binding. Interestingly, the aberrant ipsilateral calyces occur only in mice with impaired Ephrin-B2 reverse signaling and not in mice lacking EphB2 forward signaling (Fig. 4B) (Hsieh et al., 2010). How EphrinB2 activation in the post-synaptic targets stabilizes abnormal projections from the GBCs remains unclear.
Formation and early structural maturation of the calyceal contact
Upon reaching the MNTB, GBC axons initiate a rapid program of synapse growth and pruning to form a single large and powerful calyx synapse on each target neuron. In mice, the earliest contacts between GBC axons and MNTB principal neurons are established by E17 (Hoffpauir et al., 2010) and are dendritic (Hoffpauir et al., 2006; Rodriguez-Contreras et al., 2008). The contacts gradually shift to the cell bodies (Fig. 4C) and quickly grow into large pre-synaptic endings called protocalyces, which extend multiple, dynamic collaterals (Rodriguez-Contreras et al., 2008). During these early neonatal stages in mice, many MNTB neurons are contacted by multiple large endings (Fig. 4D) (Hoffpauir et al., 2006), which can originate either from the same GBC axon (Rodriguez-Contreras et al., 2006) or from different parent axons (Holcomb et al., 2013). By P4, almost all MNTB neurons are contacted by young calyces, which form cup-shaped terminals that can cover over one-half of the cell body surface. As the calyces grow, they appear to compete with each other, with smaller contacts being eliminated in favor of one large input (Holcomb et al., 2013). Hence, by P9, nearly all MNTB neurons are innervated by a single calyx (Fig. 4E) (Bergsman et al., 2004; Holcomb et al., 2013; Rodriguez-Contreras et al., 2006).
Like the endbulb of Held, the calyx of Held gradually transforms from a simple cup-shape into an elaborate, highly branched structure. Around the end of the first postnatal week, the number of collaterals diminishes and openings begin to appear, a process known as fenestration (Fig. 4E) (Ford et al., 2009). Over time, more and more branches extend from the primary ending, forming a complex network that wraps around the soma of the MNTB neurons. This occurs in a gradient across the medial-lateral axis of MNTB, with calyces in the medial half acquiring their mature morphologies (Fig. 4F) a week before those in located more laterally, which do not fully mature until the end of the third postnatal week. This developmental gradient is disrupted after loss of afferent activity by cochlear removal (Ford et al., 2009), but calyces with normal morphologies can still form in the absence of sound stimulation. An important consequence of these structural changes is that astrocyte processes are able to fill in the spaces between the many branches of the mature calyx, where they are perfectly positioned to clear glutamate from the synaptic cleft at each active zone and thereby increase temporal precision of neurotransmission (Ford et al., 2009).
The final steps in calyx morphogenesis are accompanied by a refinement of the synaptic contacts with the MNTB neurons. At birth, the earliest axosomatic contacts consist of both puncta adherentia and immature synapses (Hoffpauir et al., 2006). The protocalyces gradually develop presynaptic terminals filled with densely packed vesicles and clusters of mitochondria, juxtaposed to large and long PSDs on the surface of the MNTB neuron (Hoffpauir et al., 2006; Rowland et al., 2000). As the protocalyces grow, the number of active zones, puncta adherentia, and PSDs increases accordingly (Hoffpauir et al., 2006), with each mature calyx ultimately signaling via ~400–1000 active zones opposite well-defined PSDs (Taschenberger et al., 2002). However, the active zones and PSDs tend to be smaller in mature animals, probably due to the splitting of larger active zones and PSDs into several smaller ones as the calyx becomes more and more branched. Similarly, within each active zone, the average number of docked vesicles decreases by half, so the total number of docked vesicles per calyx increases only slightly by the onset of hearing (Taschenberger et al., 2002). In the adult calyx of Held, synaptic vesicles form rings around mitochondrial networks (Wimmer et al., 2006), which are proposed to help calyces meet their high energy requirements (Rowland et al., 2000).
Functional maturation of the calyx of Held synapse
The physiological properties of the calyx and MNTB neurons evolve in parallel to these morphological changes, ensuring that synaptic transmission is sufficiently fast and reliable to relay faithfully the timing of firing of GBCs to their MNTB targets (Borst et al., 2012; Nakamura et al., 2011; Schneggenburger et al., 2006; von Gersdorff et al., 2002). During maturation, the duration of presynaptic action potentials becomes shorter and faster (Fig. 4F) (Taschenberger et al., 2000), brought about mainly by developmental changes affecting both Na+ and K+ channels (Leao RM, 3724, 2005; Takahashi, 1101, 2007; Ishikawa et al., 2003; Elezgarai et al., 2003; Nakamura et al., 2007). As the action potentials become faster, fewer voltage-gated calcium channels open, thereby reducing the amount of presynaptic calcium influx and decreasing the probability of release (Iwasaki et al., 2001; Taschenberger et al., 2000). To compensate for these changes, the size of the readily releasable pool increases (Fig. 4F) (Iwasaki et al., 2001; Taschenberger et al., 2000; Taschenberger et al., 2002) and the synaptic vesicles become larger, thereby increasing the quantal size within the mature calyx (Satzler et al., 2002; Taschenberger et al., 2002). Accordingly, the expression level of VGLUT1 is greatly upregulated during postnatal development (Billups, 2005).
Although less calcium enters the terminal, the Ca2+-secretion coupling becomes more efficient, due in part to increased use of P/Q type channels (Fedchyshyn et al., 2005; Iwasaki et al., 1998; Iwasaki et al., 2000). The channels also become more tightly associated with synaptic vesicles at the release sites, creating “nanodomains” capable of more restricted signaling (Fedchyshyn et al., 2005; Kochubey et al., 2009; Wang et al., 2008). The filamentous protein Septin 5 is involved in regulating this developmental transformation (Yang et al., 2010b). As a result of these coordinated changes, a briefer calcium influx can still cause the same number of vesicles to be released, thereby ensuring effective transmission at high frequencies (Yang et al., 2006). Calcium also decays more rapidly in the mature calyx of Held (Chuhma et al., 2001), probably due to increase levels of the Ca2+-binding protein parvalbumin (Felmy et al., 2004). The consequence of this accelerated decay is to decrease the amount of short-term facilitation and to reduce calcium channel inactivation, which in turn helps to minimize the effects of short-term depression. The decreased release probability, increased vesicle pool size and better Ca2+-secretion coupling prevent depletion of the readily releasable pool during high frequency transmission (Taschenberger et al., 2000; Taschenberger et al., 2002). These properties, together with the faster clearance of glutamate from the synaptic cleft to relieve AMPA receptor desensitization, are proposed to diminish the impact of short-term plasticity and thereby improve temporal precision after hearing onset (Crins et al., 2011; Wong et al., 2003). Interestingly, many of these events seem to be independent of cochlear nerve activity, as neurotransmission at the calyx of Held is largely unaffected in congenitally deaf mice (Erazo-Fischer et al., 2007; Youssoufian et al., 2005).
These changes in the presynaptic terminal are accompanied by similar changes in the properties of the post-synaptic response. First, after hearing onset, NMDA receptor levels are downregulated and NMDA receptor-mediated EPSCs decline (Futai et al., 2001; Joshi et al., 2002) such that synaptic transmission at the calyx is mediated mainly by fast AMPA-type currents in mature animals. At the same time, both AMPAR- and NMDAR-type currents become faster (Joshi et al., 2002; Taschenberger et al., 2000). For instance, AMPA receptors gate more rapidly due to increased use of the flop splice variant of GluR4 (Joshi et al., 2004; Koike-Tani et al., 2005). This shift in receptor subunit composition is critical for the rapid transmission typical of the calyx synapse, as deletion of GluR4 significantly slows the time course of AMPAR-type EPSCs, reduces the current amplitude, and exacerbates receptor desensitization (Yang et al., 2011b). Additionally, PSD-95 and Homer-1 may help to concentrate AMPA receptors within PSDs, further contributing to fast and precise neurotransmission (Hermida et al., 2010; Soria Van Hoeve et al., 2010).
These changes in synaptic transmission not only prepare the calyx of Held for its role as a relay neuron, but may also influence the synaptic competition events that drive monoinnervation. In mice, MNTB neurons can generate evoked action potentials as early as E17, with even more action potential-competent neurons present by P1 (Hoffpauir et al., 2010). During this time, MTNB neurons are quite excitable owing to their high input resistance (Hoffpauir et al., 2010; Rusu et al., 2011), such that each depolarizing current can evoke several action potentials. MNTB neurons at this stage therefore show a tonic firing pattern at the beginning of calyx growth. However, the input resistance of MNTB neurons progressively decreases during the first postnatal week (Hoffpauir et al., 2010). During this time, resting membrane potentials become more hyperpolarized and the thresholds for evoked action potentials increase. The MNTB neurons therefore become less excitable and convert to a phasic firing pattern, typically firing only one to three action potentials at the onset of depolarizing current steps (Hoffpauir et al., 2010). In addition, expression of high threshold KV3 channels shortens action potential duration (Brew et al., 1995; Wang et al., 1998a). The decreased excitability of MNTB neurons makes it progressively more difficult for non-calyceal or small calyceal inputs to trigger action potentials (Hoffpauir et al., 2010; Rusu et al., 2011). As a consequence, small calyceal inputs are gradually eliminated during development, leaving the strongest to take over as the only input on the MNTB neuron (Holcomb et al., 2013).
The molecules that drive the morphological and functional maturation of the calyx of Held are largely unknown. One early acting player is the neural recognition molecule NB-2, which seems to affect the initial formation of contacts between GBC axons and a subset of MNTB neurons. In NB-2 mutant mice, 8% of MNTB neurons lack calyces of Held at P6, followed by apoptosis of MNTB and VCN neurons between P10 to P15 (Toyoshima et al., 2009). More recently, efforts to understand how the calyx of Held becomes so large uncovered a role for BMP signaling (Xiao et al., 2013). In mutant mice lacking key BMP receptors, calyces remain small and synaptic competition is impaired, with many MNTB neurons still innervated by multiple small nerve terminals at P10. These synaptic contacts also exhibit less mature transmitter release properties with decreased precision. Disruption of BMP signaling does not affect development of small excitatory synapses in the LSO, suggesting that the BMP signal has a specific role promoting growth and hence competition to create the uniquely large calyx of Held (Fig. 4D–F). Intriguingly, similar defects occur in Robo3 conditional knock-out mice, where calyces of Held form exclusively on the wrong side of the brainstem (Renier et al., 2010). As in BMP signaling mutants, Robo3 mutant mice develop small calyces that fail to be pruned and retain immature transmitter release properties (Michalski et al., 2013; Xiao et al., 2013). Robo3’s primary role is to direct GBC axons to the contralateral side of the brainstem (Renier et al., 2010), suggesting that the act of crossing the midline prepares the nascent synaptic endings to become responsive to maturation signals in the environment (Michalski et al., 2013), such as BMP (Fig. 4E and F) or BMP-regulated trophic factors (Ji et al., 2012). These findings emphasize the impact that pathfinding can have on synaptogenesis, as well as the tight relationship between the morphological and physiological maturation of each synapse.
Conclusion
Over the past decade, we have made tremendous progress in understanding how auditory synapses develop, especially from a functional perspective. As for other synapses, it is clear that interactions between the pre- and post-synaptic neurons play a critical role at each of these synapses. In addition, in each case, changes in structure seem tightly paired with changes in physiology. With these discoveries have come many new questions regarding the molecular mechanisms that control these developmental events. For instance, we still have no idea what kinds of signals are produced by SGNs and IHCs to coordinate anchoring of the ribbon with assembly of the PSD, let alone how the size of the pre-synaptic ribbon and PSD are coordinated with each other and along the modiolus-to-pillar gradient of the cochlea. In addition, striking rearrangements occur in the developing cochlea, with synapse number increasing during the first week and then declining to adult levels at the onset of hearing. How are these pruning events controlled along the tonotopic axis of the cochlea, such that more synapses are retained in the most sensitive mid-frequency regions of the cochlea? Moreover, it is unclear whether the transiently present synapses hold any physiologically significance. This is of particular interest for the OHCs, which are initially well-innervated, but ultimately retain very few, weak synapses. Might the early synapses somehow influence the maturation of the SGN neurites or the hair cells themselves? Indeed, the development and functional maturation of OHC ribbon synapses remains largely unexplored. Another area for future studies will be to investigate why these highly specialized synapses form only between specific pre- and post-synaptic partners. For instance, SGN central axons interact with many post-synaptic partners, but only the contacts with SBCs go on to form endbulbs of Held. What is special about this interaction? Identification of the intrinsic regulators that specify each of these cell types during development may provide useful starting places for unraveling these mechanisms. With the identification of BMP as an important cue for calyx of Held development, it will be interesting to see whether the same type of pathway is at work in the endbulb of Held and whether additional molecules may be involved. Given the parallels between the BMP and Robo3 phenotypes, it will also be important to determine how midline crossing influences BMP responsiveness and how other cues contribute, including those that may promote parallel changes in physiology. With the introduction of high resolution and high-throughput optical imaging, newly developed electrophysiological recording approaches, large genome-wide screen and novel mouse genetic tools, we are now in a better position to tackle these questions.
Many auditory synapses are capable of unusually fast and reliable neurotransmission
Ribbon synapses, endbulbs of Held, and calyces of Held are morphologically unique
Mechanisms of synaptic transmission are also specialized in each of these synapses
The molecular pathways that create these specializations are gradually being defined
Acknowledgments
We gratefully thank Dr. David Ryugo who generously allowed us to adapt his figure for this article. We thank Dr. Cindy Lu, Dr. Donna Fekete, and Dr. Donata Oertel for critical reading of the manuscript; Dr. Nicolas Tritsch for insightful comments; and Ms. Chao-Yun Lu for help making figures. Funding for related work in our laboratory was supported by the NIH (R01DC009223, F32 DC012695), The March of Dimes, The Hellman Family Foundation, The Bertarelli Foundation, Action on Hearing Loss, and The Alice and Joseph Brooks Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angulo A, Merchan JA, Merchan MA. Morphology of the rat cochlear primary afferents during prenatal development: a Cajal’s reduced silver and rapid Golgi study. Journal of anatomy. 1990;168:241–55. [PMC free article] [PubMed] [Google Scholar]
- Appler JM, Goodrich LV. Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly. Progress in neurobiology. 2011;93:488–508. doi: 10.1016/j.pneurobio.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appler JM, Lu CC, Druckenbrod NR, Yu WM, Koundakjian EJ, Goodrich LV. Gata3 is a critical regulator of cochlear wiring. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:3679–91. doi: 10.1523/JNEUROSCI.4703-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF, Kolston PJ. Hair cell based amplification in the cochlea. Current opinion in neurobiology. 1994;4:503–8. doi: 10.1016/0959-4388(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Baker CA, Montey KL, Pongstaporn T, Ryugo DK. Postnatal development of the endbulb of held in congenitally deaf cats. Frontiers in neuroanatomy. 2010;4:19. doi: 10.3389/fnana.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay M, Ryan AF, Housley GD. Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development. Neural development. 2011;6:33. doi: 10.1186/1749-8104-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsman JB, De Camilli P, McCormick DA. Multiple large inputs to principal cells in the mouse medial nucleus of the trapezoid body. Journal of neurophysiology. 2004;92:545–52. doi: 10.1152/jn.00927.2003. [DOI] [PubMed] [Google Scholar]
- Beurg M, Michalski N, Safieddine S, Bouleau Y, Schneggenburger R, Chapman ER, Petit C, Dulon D. Control of exocytosis by synaptotagmins and otoferlin in auditory hair cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:13281–90. doi: 10.1523/JNEUROSCI.2528-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:4593–9. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B. Colocalization of vesicular glutamate transporters in the rat superior olivary complex. Neuroscience letters. 2005;382:66–70. doi: 10.1016/j.neulet.2005.02.071. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Sachs MB. The representations of the steady-state vowel sound /e/ in the discharge patterns of cat anteroventral cochlear nucleus neurons. Journal of neurophysiology. 1990;63:1191–212. doi: 10.1152/jn.1990.63.5.1191. [DOI] [PubMed] [Google Scholar]
- Borst JG, Soria van Hoeve J. The calyx of held synapse: from model synapse to auditory relay. Annual review of physiology. 2012;74:199–224. doi: 10.1146/annurev-physiol-020911-153236. [DOI] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11577–85. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9487–98. doi: 10.1523/JNEUROSCI.21-23-09487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:8011–22. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulankina AV, Moser T. Neural circuit development in the mammalian cochlea. Physiology. 2012;27:100–12. doi: 10.1152/physiol.00036.2011. [DOI] [PubMed] [Google Scholar]
- Buran BN, Strenzke N, Neef A, Gundelfinger ED, Moser T, Liberman MC. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7587–97. doi: 10.1523/JNEUROSCI.0389-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. The Journal of comparative neurology. 1983;215:359–69. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Ohmori H. Differential development of Ca2+ dynamics in presynaptic terminal and postsynaptic neuron of the rat auditory synapse. Brain research. 2001;904:341–4. doi: 10.1016/s0006-8993(01)02506-9. [DOI] [PubMed] [Google Scholar]
- Coate TM, Kelley MW. Making connections in the inner ear: recent insights into the development of spiral ganglion neurons and their connectivity with sensory hair cells. Seminars in cell & developmental biology. 2013;24:460–9. doi: 10.1016/j.semcdb.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crins TT, Rusu SI, Rodriguez-Contreras A, Borst JG. Developmental changes in short-term plasticity at the rat calyx of Held synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11706–17. doi: 10.1523/JNEUROSCI.1995-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Tingley D, Kachar B, Wenthold RJ, Petralia RS. Distribution of members of the PSD-95 family of MAGUK proteins at the synaptic region of inner and outer hair cells of the guinea pig cochlea. Synapse. 2001;40:258–68. doi: 10.1002/syn.1048. [DOI] [PubMed] [Google Scholar]
- Defourny J, Lallemend F, Malgrange B. Structure and development of cochlear afferent innervation in mammals. American journal of physiology. Cell physiology. 2011;301:C750–61. doi: 10.1152/ajpcell.00516.2010. [DOI] [PubMed] [Google Scholar]
- Defourny J, Poirrier AL, Lallemend F, Mateo Sanchez S, Neef J, Vanderhaeghen P, Soriano E, Peuckert C, Kullander K, Fritzsch B, Nguyen L, Moonen G, Moser T, Malgrange B. Ephrin-A5/EphA4 signalling controls specific afferent targeting to cochlear hair cells. Nature communications. 2013;4:1438. doi: 10.1038/ncomms2445. [DOI] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–86. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PloS one. 2013;8:e62046. doi: 10.1371/journal.pone.0062046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6324–7. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezgarai I, Diez J, Puente N, Azkue JJ, Benitez R, Bilbao A, Knopfel T, Donate-Oliver F, Grandes P. Subcellular localization of the voltage-dependent potassium channel Kv3.1b in postnatal and adult rat medial nucleus of the trapezoid body. Neuroscience. 2003;118:889–98. doi: 10.1016/s0306-4522(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Erazo-Fischer E, Striessnig J, Taschenberger H. The role of physiological afferent nerve activity during in vivo maturation of the calyx of Held synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:1725–37. doi: 10.1523/JNEUROSCI.4116-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–18. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6170–80. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of Held synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:4131–40. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Rouiller EM, Liberman MC, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats. The Journal of comparative neurology. 1984;229:432–50. doi: 10.1002/cne.902290311. [DOI] [PubMed] [Google Scholar]
- Felmy F, Schneggenburger R. Developmental expression of the Ca2+-binding proteins calretinin and parvalbumin at the calyx of held of rats and mice. The European journal of neuroscience. 2004;20:1473–82. doi: 10.1111/j.1460-9568.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Batra R, Stanford TR, Kuwada S. A neuronal population code for sound localization. Nature. 1997;388:871–4. doi: 10.1038/42246. [DOI] [PubMed] [Google Scholar]
- Ford MC, Grothe B, Klug A. Fenestration of the calyx of Held occurs sequentially along the tonotopic axis, is influenced by afferent activity, and facilitates glutamate clearance. The Journal of comparative neurology. 2009;514:92–106. doi: 10.1002/cne.21998. [DOI] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangrsic T, Khimich D, Fejtova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca(2)+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–38. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell and tissue research. 1999;297:187–95. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Dillard M, Lavado A, Harvey NL, Jahan I. Canal cristae growth and fiber extension to the outer hair cells of the mouse ear require Prox1 activity. PloS one. 2010;5:e9377. doi: 10.1371/journal.pone.0009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233:570–83. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Lehre KP. Immunocytochemical localization of a high-affinity glutamate-aspartate transporter, GLAST, in the rat and guinea-pig cochlea. The European journal of neuroscience. 1997;9:1961–9. doi: 10.1111/j.1460-9568.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11296–304. doi: 10.1523/JNEUROSCI.23-36-11296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Okada M, Matsuyama K, Takahashi T. High-fidelity transmission acquired via a developmental decrease in NMDA receptor expression at an auditory synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:3342–9. doi: 10.1523/JNEUROSCI.21-10-03342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN, Hutson KA, Masterton RB. Acoustic chiasm V: inhibition and excitation in the ipsilateral and contralateral projections of LSO. The Journal of comparative neurology. 1992;319:100–22. doi: 10.1002/cne.903190110. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nature neuroscience. 2002;5:147–54. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Gomez-Nieto R, Rubio ME. Ultrastructure, synaptic organization, and molecular components of bushy cell networks in the anteroventral cochlear nucleus of the rhesus monkey. Neuroscience. 2011;179:188–207. doi: 10.1016/j.neuroscience.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrych P, Zimmermann U, Kuhn S, Franz C, Engel J, Duncker SV, Hirt B, Pusch CM, Ruth P, Pfister M, Marcotti W, Blin N, Knipper M. Otoferlin interacts with myosin VI: implications for maintenance of the basolateral synaptic structure of the inner hair cell. Human molecular genetics. 2009;18:2779–90. doi: 10.1093/hmg/ddp213. [DOI] [PubMed] [Google Scholar]
- Hermida D, Mateos JM, Elezgarai I, Puente N, Bilbao A, Bueno-Lopez JL, Streit P, Grandes P. Spatial compartmentalization of AMPA glutamate receptor subunits at the calyx of Held synapse. The Journal of comparative neurology. 2010;518:163–74. doi: 10.1002/cne.22189. [DOI] [PubMed] [Google Scholar]
- Hoffpauir BK, Grimes JL, Mathers PH, Spirou GA. Synaptogenesis of the calyx of Held: rapid onset of function and one-to-one morphological innervation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5511–23. doi: 10.1523/JNEUROSCI.5525-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir BK, Kolson DR, Mathers PH, Spirou GA. Maturation of synaptic partners: functional phenotype and synaptic organization tuned in synchrony. The Journal of physiology. 2010;588:4365–85. doi: 10.1113/jphysiol.2010.198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb PS, Hoffpauir BK, Hoyson MC, Jackson DR, Deerinck TJ, Marrs GS, Dehoff M, Wu J, Ellisman MH, Spirou GA. Synaptic Inputs Compete during Rapid Formation of the Calyx of Held: A New Model System for Neural Development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:12954–12969. doi: 10.1523/JNEUROSCI.1087-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DM, Morgan WJ, Jarjour AA, Spirou GA, Berrebi AS, Kennedy TE, Mathers PH. Molecular guidance cues necessary for axon pathfinding from the ventral cochlear nucleus. The Journal of comparative neurology. 2007;504:533–49. doi: 10.1002/cne.21443. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Nakamura PA, Luk SO, Miko IJ, Henkemeyer M, Cramer KS. Ephrin-B reverse signaling is required for formation of strictly contralateral auditory brainstem pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9840–9. doi: 10.1523/JNEUROSCI.0386-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–33. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- Huang LC, Barclay M, Lee K, Peter S, Housley GD, Thorne PR, Montgomery JM. Synaptic profiles during neurite extension, refinement and retraction in the developing cochlea. Neural development. 2012;7:38. doi: 10.1186/1749-8104-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KJ, Cramer KS. EphA4 misexpression alters tonotopic projections in the auditory brainstem. Developmental neurobiology. 2007;67:1655–68. doi: 10.1002/dneu.20535. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nakamura Y, Saitoh N, Li WB, Iwasaki S, Takahashi T. Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10445–53. doi: 10.1523/JNEUROSCI.23-32-10445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. The Journal of physiology. 1998;509 (Pt 2):419–23. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. The Journal of physiology. 2001;534:861–71. doi: 10.1111/j.1469-7793.2001.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji SJ, Jaffrey SR. Intra-axonal translation of SMAD1/5/8 mediates retrograde regulation of trigeminal ganglia subtype specification. Neuron. 2012;74:95–107. doi: 10.1016/j.neuron.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. The Journal of physiology. 2005;563:177–91. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kuhn S, Franz C, Ingham N, Furness DN, Knipper M, Steel KP, Adelman JP, Holley MC, Marcotti W. Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8720–5. doi: 10.1073/pnas.1219578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, Roberts TP, Masetto S, Knipper M, Kros CJ, Marcotti W. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nature neuroscience. 2011;14:711–7. doi: 10.1038/nn.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Leake PA, Snyder RL, Stakhovskaya O, Bonham B. Spontaneous discharge patterns in cochlear spiral ganglion cells before the onset of hearing in cats. Journal of neurophysiology. 2007;98:1898–908. doi: 10.1152/jn.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi I, Wang LY. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. The Journal of physiology. 2002;540:861–73. doi: 10.1113/jphysiol.2001.013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi I, Shokralla S, Titis P, Wang LY. The role of AMPA receptor gating in the development of high-fidelity neurotransmission at the calyx of Held synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:183–96. doi: 10.1523/JNEUROSCI.1074-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nature neuroscience. 2009;12:711–7. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ. New developments in understanding the mechanisms and function of spontaneous electrical activity in the developing mammalian auditory system. Journal of the Association for Research in Otolaryngology : JARO. 2012;13:437–45. doi: 10.1007/s10162-012-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–94. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Han Y, Schneggenburger R. Developmental regulation of the intracellular Ca2+ sensitivity of vesicle fusion and Ca2+-secretion coupling at the rat calyx of Held. The Journal of physiology. 2009;587:3009–23. doi: 10.1113/jphysiol.2009.172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Tani M, Saitoh N, Takahashi T. Mechanisms underlying developmental speeding in AMPA-EPSC decay time at the calyx of Held. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:199–207. doi: 10.1523/JNEUROSCI.3861-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian EJ, Appler JL, Goodrich LV. Auditory neurons make stereotyped wiring decisions before maturation of their targets. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:14078–88. doi: 10.1523/JNEUROSCI.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–4. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Kubke MF, Carr CE. Development of the auditory brainstem of birds: comparison between barn owls and chickens. Hearing research. 2000;147:1–20. doi: 10.1016/s0378-5955(00)00116-7. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Chair L, Snyder RL. Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. The Journal of comparative neurology. 2006;497:13–31. doi: 10.1002/cne.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von Gersdorff H. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3724–38. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Cahill HB, Ryugo DK. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. Journal of neurocytology. 2003;32:229–43. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Wang H, Liberman MC. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:801–8. doi: 10.1523/JNEUROSCI.3389-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb CJ, Ryugo DK. Development of primary axosomatic endings in the anteroventral cochlear nucleus of mice. Journal of the Association for Research in Otolaryngology : JARO. 2000;1:103–19. doi: 10.1007/s101620010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KH, Oleskevich S, Taschenberger H. Presynaptic Ca2+ influx and vesicle exocytosis at the mouse endbulb of Held: a comparison of two auditory nerve terminals. The Journal of physiology. 2011;589:4301–20. doi: 10.1113/jphysiol.2011.209189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CC, Appler JM, Houseman EA, Goodrich LV. Developmental profiling of spiral ganglion neurons reveals insights into auditory circuit assembly. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10903–18. doi: 10.1523/JNEUROSCI.2358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XJ, Deng M, Xie X, Huang L, Wang H, Jiang L, Liang G, Hu F, Tieu R, Chen R, Gan L. GATA3 controls the specification of prosensory domain and neuronal survival in the mouse cochlea. Human molecular genetics. 2013;22:3609–23. doi: 10.1093/hmg/ddt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–82. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Rusch A, Kros CJ. Sodium and calcium currents shape action potentials in immature mouse inner hair cells. The Journal of physiology. 2003;552:743–61. doi: 10.1113/jphysiol.2003.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Xia A, Mathes EL, Wang VY, Oghalai JS, Fritzsch B, Zoghbi HY. Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11123–33. doi: 10.1523/JNEUROSCI.2232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs GS, Spirou GA. Embryonic assembly of auditory circuits: spiral ganglion and brainstem. The Journal of physiology. 2012;590:2391–408. doi: 10.1113/jphysiol.2011.226886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;234:633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4457–67. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay SM, Oleskevich S. The role of spontaneous activity in development of the endbulb of Held synapse. Hearing research. 2007;230:53–63. doi: 10.1016/j.heares.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Michalski N, Babai N, Renier N, Perkel DJ, Chedotal A, Schneggenburger R. Robo3-driven axon midline crossing conditions functional maturation of a large commissural synapse. Neuron. 2013;78:855–68. doi: 10.1016/j.neuron.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Miko IJ, Nakamura PA, Henkemeyer M, Cramer KS. Auditory brainstem neural activation patterns are altered in EphA4- and ephrin-B2-deficient mice. The Journal of comparative neurology. 2007;505:669–81. doi: 10.1002/cne.21530. [DOI] [PubMed] [Google Scholar]
- Nakamura PA, Cramer KS. Formation and maturation of the calyx of Held. Hearing research. 2011;276:70–8. doi: 10.1016/j.heares.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Takahashi T. Developmental changes in potassium currents at the rat calyx of Held presynaptic terminal. The Journal of physiology. 2007;581:1101–12. doi: 10.1113/jphysiol.2007.128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawabi H, Briancon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, Moret F, Abouzid K, Castellani V. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes & development. 2010;24:396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemzou NR, Bulankina AV, Khimich D, Giese A, Moser T. Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels. Neuroscience. 2006;141:1849–60. doi: 10.1016/j.neuroscience.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. Ultrastructural basis of synaptic transmission between endbulbs of Held and bushy cells in the rat cochlear nucleus. The Journal of physiology. 2002;539:713–23. doi: 10.1113/jphysiol.2001.012972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzielski AS, Wenthold RJ. Expression of AMPA, kainate, and NMDA receptor subunits in cochlear and vestibular ganglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:2338–53. doi: 10.1523/JNEUROSCI.15-03-02338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuys TG, Clark GM. Frequency discrimination following the selective destruction of cochlear inner and outer hair cells. Science. 1978;199:1356–7. doi: 10.1126/science.628846. [DOI] [PubMed] [Google Scholar]
- Nouvian R, Neef J, Bulankina AV, Reisinger E, Pangrsic T, Frank T, Sikorra S, Brose N, Binz T, Moser T. Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins. Nature neuroscience. 2011;14:411–3. doi: 10.1038/nn.2774. [DOI] [PubMed] [Google Scholar]
- O’Neil JN, Limb CJ, Baker CA, Ryugo DK. Bilateral effects of unilateral cochlear implantation in congenitally deaf cats. The Journal of comparative neurology. 2010;518:2382–404. doi: 10.1002/cne.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil JN, Connelly CJ, Limb CJ, Ryugo DK. Synaptic morphology and the influence of auditory experience. Hearing research. 2011;279:118–30. doi: 10.1016/j.heares.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger K, Sandri C, Akert K, Eugster CH. Contribution to the problem of structural organization of the presynaptic area. Brain research. 1969;12:10–8. doi: 10.1016/0006-8993(69)90051-1. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Renier N, Schonewille M, Giraudet F, Badura A, Tessier-Lavigne M, Avan P, De Zeeuw CI, Chedotal A. Genetic dissection of the function of hindbrain axonal commissures. PLoS biology. 2010;8:e1000325. doi: 10.1371/journal.pbio.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, de Lange RP, Lucassen PJ, Borst JG. Branching of calyceal afferents during postnatal development in the rat auditory brainstem. The Journal of comparative neurology. 2006;496:214–28. doi: 10.1002/cne.20918. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, van Hoeve JS, Habets RL, Locher H, Borst JG. Dynamic development of the calyx of Held synapse. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5603–8. doi: 10.1073/pnas.0801395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Cronin-Schreiber R, Fekete DM, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats: an analysis of terminal morphology. The Journal of comparative neurology. 1986;249:261–78. doi: 10.1002/cne.902490210. [DOI] [PubMed] [Google Scholar]
- Roux I, Hosie S, Johnson SL, Bahloul A, Cayet N, Nouaille S, Kros CJ, Petit C, Safieddine S. Myosin VI is required for the proper maturation and function of inner hair cell ribbon synapses. Human molecular genetics. 2009;18:4615–28. doi: 10.1093/hmg/ddp429. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–89. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:9135–44. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch A, Erway LC, Oliver D, Vennstrom B, Forrest D. Thyroid hormone receptor beta-dependent expression of a potassium conductance in inner hair cells at the onset of hearing. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15758–62. doi: 10.1073/pnas.95.26.15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu SI, Borst JG. Developmental changes in intrinsic excitability of principal neurons in the rat medial nucleus of the trapezoid body. Developmental neurobiology. 2011;71:284–95. doi: 10.1002/dneu.20856. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Fekete DM. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. The Journal of comparative neurology. 1982;210:239–57. doi: 10.1002/cne.902100304. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Rouiller EM. Central projections of intracellularly labeled auditory nerve fibers in cats: morphometric correlations with physiological properties. The Journal of comparative neurology. 1988;271:130–42. doi: 10.1002/cne.902710113. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Sento S. Synaptic connections of the auditory nerve in cats: relationship between endbulbs of held and spherical bushy cells. The Journal of comparative neurology. 1991;305:35–48. doi: 10.1002/cne.903050105. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Wu MM, Pongstaporn T. Activity-related features of synapse morphology: a study of endbulbs of held. The Journal of comparative neurology. 1996;365:141–58. doi: 10.1002/(SICI)1096-9861(19960129)365:1<141::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. The Journal of comparative neurology. 1997;385:230–44. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Montey KL, Wright AL, Bennett ML, Pongstaporn T. Postnatal development of a large auditory nerve terminal: the endbulb of Held in cats. Hearing research. 2006;216–217:100–15. doi: 10.1016/j.heares.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–69. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Safieddine S, Wenthold RJ. SNARE complex at the ribbon synapses of cochlear hair cells: analysis of synaptic vesicle- and synaptic membrane-associated proteins. The European journal of neuroscience. 1999;11:803–12. doi: 10.1046/j.1460-9568.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Safieddine S, El-Amraoui A, Petit C. The auditory hair cell ribbon synapse: from assembly to function. Annual review of neuroscience. 2012;35:509–28. doi: 10.1146/annurev-neuro-061010-113705. [DOI] [PubMed] [Google Scholar]
- Sanes DH. An in vitro analysis of sound localization mechanisms in the gerbil lateral superior olive. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:3494–506. doi: 10.1523/JNEUROSCI.10-11-03494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satzler K, Sohl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, Lubke JH. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:10567–79. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–72. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell and tissue research. 2006;326:311–37. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–75. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin G, Bulankina AV, Riedel D, Moser T. Maturation of ribbon synapses in hair cells is driven by thyroid hormone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3163–73. doi: 10.1523/JNEUROSCI.3974-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sento S, Ryugo DK. Endbulbs of held and spherical bushy cells in cats: morphological correlates with physiological properties. The Journal of comparative neurology. 1989;280:553–62. doi: 10.1002/cne.902800406. [DOI] [PubMed] [Google Scholar]
- Sheets L, Trapani JG, Mo W, Obholzer N, Nicolson T. Ribeye is required for presynaptic Ca(V)1.3a channel localization and afferent innervation of sensory hair cells. Development. 2011;138:1309–19. doi: 10.1242/dev.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnerson A, Devigne C, Pujol R. Age-related changes in the C57BL/6J mouse cochlea. II. Ultrastructural findings. Brain research. 1981;254:77–88. doi: 10.1016/0165-3806(81)90060-2. [DOI] [PubMed] [Google Scholar]
- Siddiqui SA, Cramer KS. Differential expression of Eph receptors and ephrins in the cochlear ganglion and eighth cranial nerve of the chick embryo. The Journal of comparative neurology. 2005;482:309–19. doi: 10.1002/cne.20396. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Liberman MC. Afferent innervation of outer hair cells in adult cats: I. Light microscopic analysis of fibers labeled with horseradish peroxidase. The Journal of comparative neurology. 1988;270:132–44. doi: 10.1002/cne.902700111. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Mansdorf NB, Kim JH. Olivocochlear innervation of inner and outer hair cells during postnatal maturation: evidence for a waiting period. The Journal of comparative neurology. 1996;370:551–62. doi: 10.1002/(SICI)1096-9861(19960708)370:4<551::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Carney LH, Yin TC. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. The Journal of comparative neurology. 1991;304:387–407. doi: 10.1002/cne.903040305. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM. The efferents interconnecting auditory inner hair cells. Hearing research. 1994;75:81–92. doi: 10.1016/0378-5955(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2:942–57. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GL, Levenick CV. Distribution of synaptic ribbons in the developing organ of Corti. Journal of neurocytology. 1986;15:693–714. doi: 10.1007/BF01625188. [DOI] [PubMed] [Google Scholar]
- Sonntag M, Englitz B, Kopp-Scheinpflug C, Rubsamen R. Early postnatal development of spontaneous and acoustically evoked discharge activity of principal cells of the medial nucleus of the trapezoid body: an in vivo study in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9510–20. doi: 10.1523/JNEUROSCI.1377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria Van Hoeve JS, Borst JG. Delayed appearance of the scaffolding proteins PSD-95 and Homer-1 at the developing rat calyx of held synapse. The Journal of comparative neurology. 2010;518:4581–90. doi: 10.1002/cne.22479. [DOI] [PubMed] [Google Scholar]
- Strenzke N, Chanda S, Kopp-Scheinpflug C, Khimich D, Reim K, Bulankina AV, Neef A, Wolf F, Brose N, Xu-Friedman MA, Moser T. Complexin-I is required for high-fidelity transmission at the endbulb of Held auditory synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7991–8004. doi: 10.1523/JNEUROSCI.0632-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:9162–73. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–43. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Morest DK, Yurgelun-Todd DA. The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: horseradish peroxidase labelling of identified cell types. Neuroscience. 1982;7:3031–52. doi: 10.1016/0306-4522(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Toyoshima M, Sakurai K, Shimazaki K, Takeda Y, Shimoda Y, Watanabe K. Deficiency of neural recognition molecule NB-2 affects the development of glutamatergic auditory pathways from the ventral cochlear nucleus to the superior olivary complex in mouse. Developmental biology. 2009;336:192–200. doi: 10.1016/j.ydbio.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010a;30:1539–50. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–5. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nature neuroscience. 2010b;13:1050–2. doi: 10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaiah RC, Hudspeth AJ. Molecular anatomy of the hair cell’s ribbon synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12387–99. doi: 10.1523/JNEUROSCI.1014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Borst JG. Short-term plasticity at the calyx of held. Nature reviews. Neuroscience. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- Wang LY, Neher E, Taschenberger H. Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential-evoked glutamate release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:14450–8. doi: 10.1523/JNEUROSCI.4245-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. The Journal of physiology. 1998a;509 (Pt 1):183–94. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wang YX, Wenthold RJ, Ottersen OP, Petralia RS. Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA-type glutamate receptor subunits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998b;18:1148–60. doi: 10.1523/JNEUROSCI.18-03-01148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber A, Raz Y. Axon guidance cues in auditory development. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2006;288:390–6. doi: 10.1002/ar.a.20299. [DOI] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–9. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Horstmann H, Groh A, Kuner T. Donut-like topology of synaptic vesicles with a central cluster of mitochondria wrapped into membrane protrusions: a novel structure-function module of the adult calyx of Held. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:109–16. doi: 10.1523/JNEUROSCI.3268-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AY, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4868–77. doi: 10.1523/JNEUROSCI.23-12-04868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Oertel D. Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1984;4:1577–88. doi: 10.1523/JNEUROSCI.04-06-01577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Oertel D. Maturation of synapses and electrical properties of cells in the cochlear nuclei. Hearing research. 1987;30:99–110. doi: 10.1016/0378-5955(87)90187-0. [DOI] [PubMed] [Google Scholar]
- Xiao L, Michalski N, Kronander E, Gjoni E, Genoud C, Knott G, Schneggenburger R. BMP signaling specifies the development of a large and fast CNS synapse. Nature neuroscience. 2013;16:856–64. doi: 10.1038/nn.3414. [DOI] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Developmental mechanisms for suppressing the effects of delayed release at the endbulb of Held. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010a;30:11466–75. doi: 10.1523/JNEUROSCI.2300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hearing research. 2011a;278:21–33. doi: 10.1016/j.heares.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Wang LY. Amplitude and kinetics of action potential-evoked Ca2+ current and its efficacy in triggering transmitter release at the developing calyx of held synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5698–708. doi: 10.1523/JNEUROSCI.4889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H, Ackerley CA, Trimble WS, Wang LY. Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse. Neuron. 2010b;67:100–15. doi: 10.1016/j.neuron.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Aitoubah J, Lauer AM, Nuriya M, Takamiya K, Jia Z, May BJ, Huganir RL, Wang LY. GluA4 is indispensable for driving fast neurotransmission across a high-fidelity central synapse. The Journal of physiology. 2011b;589:4209–27. doi: 10.1113/jphysiol.2011.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nature genetics. 1999;21:363–9. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. Journal of neurophysiology. 1990;64:465–88. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]
- Youssoufian M, Oleskevich S, Walmsley B. Development of a robust central auditory synapse in congenital deafness. Journal of neurophysiology. 2005;94:3168–80. doi: 10.1152/jn.00342.2005. [DOI] [PubMed] [Google Scholar]
- Youssoufian M, Couchman K, Shivdasani MN, Paolini AG, Walmsley B. Maturation of auditory brainstem projections and calyces in the congenitally deaf (dn/dn) mouse. The Journal of comparative neurology. 2008;506:442–51. doi: 10.1002/cne.21566. [DOI] [PubMed] [Google Scholar]
- Yu WM, Appler JM, Kim YH, Nishitani AM, Holt JR, Goodrich LV. A Gata3-Mafb transcriptional network directs post-synaptic differentiation in synapses specialized for hearing. eLife. 2013;2:e01341. doi: 10.7554/eLife.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]