Abstract
PURPOSE
The purpose of this study was to determine the effects of early outpatient exercise on muscle mass, function, and fractional synthetic rate in severely burned children.
METHODS
Forty-seven children with ≥40 % total body surface area burn performed 12-weeks standard of care rehabilitation (SOC: N=23) or rehabilitative exercise training (RET: N=24) immediately following hospital discharge. Dual-energy X-ray absorptiometry was used to assess lean body mass (LBM) at discharge, post-treatment, and 12 months post-burn. Muscle function was evaluated with a Biodex Isokinetic Dynamometer and peak aerobic fitness (VO2peak) measured using a modified Bruce treadmill protocol post-treatment. Stable isotope infusion studies were performed in a subset of patients (SOC: N=13; RET: N=11) at discharge and post-treatment to determine mixed-muscle fractional synthetic rate.
RESULTS
Relative peak torque (RET: 138 ± 9 N · m · kg−1 vs SOC: 106 ± 9 N · m · kg−1) and VO2peak (RET: 32 ± 1 ml · kg−1 · min−1 vs SOC: 28 ± 1 ml · kg−1 · min−1) was greater post-treatment with RET compared to SOC. In addition, RET increased whole-body (9 ± 2%) and leg (17 ± 3%) LBM compared to SOC. Furthermore, the percentage change in whole-body (18 ± 3%) and leg (31 ± 4%) LBM from discharge to 12 months post-burn was greater with RET compared to SOC. Muscle fractional synthetic rate decreased from discharge to post-treatment in both groups (6.9 ± 1.1% · d−1 vs 3.4 ± 0.4% · d−1); however no differences were observed between treatment groups at each time-point.
CONCLUSIONS
Early outpatient exercise training implemented at hospital discharge represents an effective intervention to improve muscle mass and function following severe burn injury.
Keywords: Severe Burn, Lean Body Mass, Muscle Strength, Cardiorespiratory Fitness, Fractional Synthetic Rate
INTRODUCTION
In the United States, it has been estimated approximately 450,000 people are admitted for burn treatment each year, with 30% of cases involving individuals less than 16 years of age (1). The risk of injury is influenced largely by socioeconomic status and geographic location (26). Major advances in critical care and treatment within the past two decades have improved survival rates (32). Despite these developments, survivors are often faced with long-term functional impairments that can impede a proper return to society (20). Consequently, the restoration of normal function and quality of life following severe burn is of clinical and social significance.
The pathophysiological response to major burn injury is characterized by elevated resting energy expenditure, systemic inflammation, and skeletal muscle catabolism (13, 17). In healthy adults, skeletal muscle comprises 70% of total lean body mass and represents the largest protein reservoir in humans. Thus, muscle protein is a major source of amino acids to support survival and recovery post-burn. Indeed, the release of amino acids from skeletal muscle is thought to facilitate wound healing, the production of inflammatory and acute phase proteins, and provide substrate for hepatic gluconeogenesis during the recovery from burn (13, 35, 36). This hypermetabolic and persistent catabolic response can persist for up to 2 years post burn injury (13, 18). Subsequently, the continuous muscle catabolism contributes to reduced muscle mass and strength, resulting in an impaired return to functional mobility. Therefore, strategies which promote the maintenance of skeletal muscle mass and function may provide long-term benefits in patients during the recovery from severe burn.
Skeletal muscle is a highly plastic tissue, in which the size is tightly regulated by the rates of protein synthesis and degradation. Net protein balance is responsive to several factors such as mechanical load, nutrient status/energy balance and hormone availability (34). Severe burn trauma increases both muscle protein and degradation, however favors an overall negative net protein balance (12, 25). Pharmacological treatments such as growth hormone (10), insulin (28), and oxandrolone (33) increase mixed-muscle fractional synthetic rate and results in the accretion of lean body mass (LBM) in severely burned patients. Therefore, the identification of additional treatments that regulate muscle mass and increase physical function is needed to continually improve standard of care rehabilitation. Exercise training is a non-pharmacological treatment that attenuates muscle catabolism associated with aging and chronic disease (7, 21). We have previously shown that rehabilitative exercise, initiated 6 months post-burn (typically 2-3 months after discharge), results in significant improvements to LBM, muscular strength and cardiorespiratory fitness in severely burned children (27, 30). However, the determination of fractional synthetic rate in these patients is needed in order to determine the impact of rehabilitative exercise on skeletal muscle protein metabolism. It is currently unknown if these benefits can be achieved if exercise is started immediately following hospital discharge, a time at which there is still pronounced post-burn hypermetabolism and hypercatabolism.
Despite significant improvements in muscle mass and function with exercise training, mechanistic insight as to how these adaptations occur is currently lacking. Therefore, the purpose of this clinical trial was to determine the effects of early outpatient exercise on muscle mass, function, and fractional synthetic rate in severely burned children. It was hypothesized that early rehabilitative exercise training in severely burned patients would increase muscle mass, muscular strength, and cardiorespiratory fitness when compared to standard of care. In addition, there would be no differences in basal muscle fractional synthetic rate between standard of care and rehabilitative exercise training.
METHODS
Patients
Forty-seven children with burns encompassing at least 40% of their total body surface area that were admitted for acute burn treatment at Shriners Hospital for Children-Galveston participated in the study. Patients received similar standard medical care and treatment from the time of admission until the time of discharge (see Patient Standard of Care). Patients received medications that are standard in the Burn Intensive Care Unit during acute burn treatment, which consists of pressors, diuretics, antibiotics or anesthetics. None of these drugs are known to be anabolic, and at no time did patients receive any investigational drugs. Total burn surface area was assessed by the “rule of nines” method during excisional surgery immediately following hospital admittance (15). Informed written consent was obtained from each patient’s guardian prior to enrollment. All procedures were approved by the University of Texas Medical Branch Institutional Review Board. After consent was obtained, patients were randomly assigned to standard of care (SOC) or rehabilitative exercise training (RET). The RET group participated in a 12-week in-hospital physiotherapy program (see Patient Standard of Care) supplemented with an individualized and supervised rehabilitative exercise training program (N=24). In contrast, the SOC group participated in the in-hospital physiotherapy program (N=23), but was not supplemented with the rehabilitative exercise training program. LBM assessment and 5-hour stable isotope studies were conducted at discharge and following the 12-week treatment intervention. In addition, muscle strength and cardiorespiratory fitness was assessed at post-treatment.
Patient Standard of Care
All patients received similar standard of care treatment during the acute phase of injury as previously described (14). Briefly, total fluid resuscitation needs were administered within the first 24 hours according the Galveston formula (5000 mL/m2 total body surface area burned plus 2000 mL/m2 total body surface area lactated Ringer solution). Early burn wound excision and placement of autograft or allograft was performed within 48 hours of admission, with excision and grafting procedures continued weekly until all burn sites were 95% healed (22). Following 4 days of bed rest, patients were allowed to ambulate daily between surgeries. Patients were fed a standardized Vivonex TEN enteral nutrition (3% fat, 82% carbohydrate, 15% protein) via a nasoduodenal or nasogastric tube. During the first week of hospitalization intake was determined using the formula 1500 kcal/m2 total body surface area plus 1500 kcal/m2 total body surface area burned (16, 23). During the remainder of hospitalization intake was then modified to 1.4 times the weekly measured resting energy expenditure. Prior to hospital discharge, each patient underwent nutrient counseling with a dietician in which the patient was instructed to consume 1.4 to 1.6 times their resting energy expenditure.
In addition to the aforementioned acute burn treatment, both treatment groups participated in a conventional physiotherapy program administered by an occupational therapist or physical therapy twice daily for one hour. The treatment program included positioning and splinting, range of motion and strengthening activities, and scar management techniques. In addition, caregiver education was also provided as part of the program. This therapy program is designed to improve overall range of motion and minimized deformities and contractures (6). At time of discharge, caregivers were given a home treatment program to follow during the treatment period. Patients were discharged when 95% of wounds were considered closed.
Lean Body Mass Assessment
Whole body LBM was assessed at discharge, post-treatment, and 12 months post-burn by dual-energy x-ray absorptiometry (DEXA) using QDR 4500A software (Hologic Inc, Waltham, MA). This methodology has been previously described by our group in detail (24, 27). Scans were taken with the patient lying supine on the scanning table, and pediatric analysis software was used to calculate LBM, bone mineral content, and fat mass. Whole-body and regional (trunk, leg, arm) LBM is reported in kilograms.
Functional Testing
Due to medical limitations such as impaired mobility and incomplete wound closure at the time of discharge, the assessment of muscle strength and cardiorespiratory fitness was conducted following the 12-week intervention. Muscle strength was assessed using the Biodex System-3 Dynamometer (Shirley, NY). The isokinetic test was performed at an angular velocity of 150° · s−1 on the dominant leg extensors. This speed has been shown to be well tolerated by burned children (3, 30, 31). Patients were seated upright with the anatomic axis of the knee joint aligned with the mechanical axis of the dynamometer. The patient’s position was stabilized with restraining straps over the mid-thigh, pelvis, and trunk. Patients were familiarized to the machine and testing procedures with visual and verbal explanations prior to testing. Following three submaximal repetitions without load, 10 maximal voluntary muscle contractions (full extension and flexion) were performed consecutively without rest between repetitions. Values of peak torque, total work, and average power were calculated by the Biodex software system. Peak torque was corrected for gravitational moments of the lower leg and the lever arm. The highest peak torque for the entire 10 repetitions was selected for analysis. Relative peak torque is presented to correct for differences in body weight.
Following muscular strength assessment, patients were given a period of rest between 30 minutes to an hour before cardiorespiratory fitness testing. Patients underwent a standardized treadmill exercise test using the modified Bruce protocol (9, 19). Heart rate and peak oxygen consumption (VO2peak) were measured throughout the exercise testing. Breath-by-breath analysis of expired gases, flow, and volume were made by using a Medgraphics CardiO2 system (St. Paul, MN) during rest and exercise. The exercise test began with the treadmill speed and elevation at 1.7 miles per hour and 0%, respectively. Thereafter, the speed and elevation increased every 3 minutes per the modified Bruce protocol. The patients were instructed and encouraged to complete 3-minutes stages, and the test was terminated once volitional fatigue was achieved. The heart rate and VO2peak achieved during testing were used to determine exercise intensities used throughout the 12-week training period for the RET group.
Three-Repetition Maximum Test
The first and second sessions of actual training were spent with patients randomized to the RET group being tested for a three repetition maximum to establish training loads that would be used during the training period as previously described (30). The exercises performed included bench press, leg press, shoulder press, leg extension, bicep curl, leg curl, and triceps curl. After an instruction period on correct lifting technique the patient performed a warm-up with the lever arm or bar to become familiar with the movement. Next, the weight was progressively increased to which the patient could successfully perform four repetitions with correct technique. If four repetitions were performed with correct technique a one minutes rest was given and the weight was increased. This was repeated until the patient could perform three repetitions, with the fourth repetition not volitionally possible with correct technique, and the test was terminated. The amount of weight lifted from the successful lift was recorded as the individuals three repetition maximum. From this information, the basic 3 sets of 8-12 repetitions RM’s were established as described below.
Rehabilitative Exercise Training Program
Rehabilitative exercise training was performed as previously described (30). Each exercise training session consisted of resistance and aerobic exercise under the supervision of a certified exercise physiologist. Eight basic resistance exercises were used, incorporating bench press, leg press, shoulder press, leg extension, biceps curl, leg curl, triceps curl, and toe raises. All exercises were performed using free weights and variable-resistance machines. Modifications to exercises were made when appropriate depending on the patient injury characteristics. During the first week of training, patients were familiarized to the equipment and instructed on proper weight lifting technique. Thereafter, the load was gradually increased from 50-60% of 3RM at the beginning of the program up to 80-85% of 3RM at the end of the program. Additionally, each exercise training session included aerobic exercise on a treadmill or cycle ergometer. Patients performed 20-40 minutes aerobic exercise 3-5 days · wk−1 at 70-85% of VO2peak. All exercise sessions were preceded by a 5-minute warm-up at <50% VO2peak. No strength training activities were permitted outside the supervised training session; however, both groups were encouraged to maintain normal daily activities.
Stable Isotope Infusion Study
Five hour stable isotope infusion studies were conducted in a subset of patients (SOC: N=13; RET: N=11) to determine mixed-muscle fractional synthetic rate. Stable isotope studies were performed following an overnight fast (10-12 hours) in the post-absorptive state. Catheters were placed in the antecubital vein of each arm under sedation for the purpose of stable isotope infusions and blood sampling. A baseline blood sample was taken before a primed (3.2 umol · kg−1) and constant (0.08 umol · kg−1 · min−1) infusion of L-[ring-2H5]-phenylalanine (Cambridge Isotopes Laboratories, Inc., Andover, MA) was initiated. Biopsies of the vastus lateralis were taken at hours 2 and 5 with a 5-mm Bergstrom needle (Stille, Stockholm, Sweden) for the determination of phenylalanine enrichment in bound and intracellular muscle proteins. Tissue was immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
Skeletal Muscle Analysis
Frozen muscle tissue (20-30 mg) was weighed and homogenized in 10% perchloric acid. The sample was centrifuged (3000 rpm for 10 min at 4°C) and the supernatant was recovered. The supernatant represents the free intracellular amino acid pool and was used to determine the intracellular phenylalanine concentration. The remaining pellet was washed in 2% perchloric acid, followed by ethanol, and ethyl ether before overnight incubation at 50°C. The next day, the dried muscle pellets were hydrolyzed in 6N hydrochloric acid at 100°C for 12 hours. The bound muscle hydrolysate and intracellular portion were then passed over a cation exchange column (Bio-Rad, Hercules, CA). The bound and intracellular phenylalanine enrichment (tracer/trace ratio) was determined in tert-butyldimethylsilyl derivatives by gas chromatography-mass spectrometry (Agilent, Santa Clara, CA) as previously described (25).
Mixed-Muscle Fractional Synthetic Rate Calculations
Mixed-muscle fractional synthetic rate was calculated using the precursor-product method as previously described (5, 25). The precursor is the mean enrichment of the intracellular free amino acid compartment and the product is the difference in the enrichment of the bound muscle protein between the two biopsies. Values were multiplied by 24 to express fractional synthetic rate as the percent per day (% · d−1) (25).
Statistical Analysis
Data are presented as means ± standard error. A repeated two-way ANOVA with factors of treatment and time was performed with Tukey’s post-hoc correction was used to assess differences in height, weight, LBM and muscle fractional synthetic rate. Unpaired students t-test was performed to assess differences in patient characteristics, functional assessments and the percentage change in LBM. Significance was set at P < 0.05 for all analysis. Statistical analysis was performed using SigmaStat version 3.5 (Systat Software Inc., Richmond, CA).
RESULTS
Patient Characteristics
Forty-seven children with ≥40% total burn surface area were enrolled in the current investigation. Baseline characteristics are presented in Table 1. There were no significant differences between groups in age, height, body weight, sex, length of stay, and percent total burn surface area burned at the time of discharge. The average patient age and total burn surface area were 13.0 ± 0.5 years and 60 ± 2%, respectively. There were no differences in height from discharge to post-treatment (P = 0.14). In contrast, both treatment groups increased body weight from discharge to post-treatment (7.5 ± 1.2%; Main effect of time: P < 0.001), however there were no differences between treatment groups.
Table 1.
Patient characteristics at hospital discharge.
| SOC (N=23) | RET (N=24) | P value | |
|---|---|---|---|
| Age, yr | 13 ± 1 | 13 ± 1 | 0.58 |
| Height, cm | 156 ± 4 | 152 ± 4 | 0.50 |
| Weight, kg | 47 ± 5 | 46 ± 3 | 0.87 |
| TBSA, % | 60 ± 3 | 59 ± 2 | 0.89 |
| 3rd, % | 49 ± 4 | 46 ± 4 | 0.62 |
| LOS, days | 38 ± 7 | 34 ± 4 | 0.96 |
| F:M | 5:18 | 4:20 | 0.72 |
Values are means ± standard error.
Abbreviations: SOC, standard of care. RET, rehabilitative exercise training. N, number of patients. yr, year. cm, centimeters. kg, kilogram. TBSA, total burn surface area determined at admittance. 3rd, third degree burn. F:M, number of female and male patients. LOS, length of hospital stay during acute burn care treatment.
Functional Testing
Muscle strength and cardiorespiratory fitness was assessed following the 12-week treatment (Figure 1). There was a trend towards an increase in absolute peak torque with RET when compared to SOC (Figure 1A; SOC: 52 ± 7.5 N · m vs RET: 72 ± 8.2 N · m; P = 0.08). When corrected for body weight, the RET group had a significantly greater relative peak torque compared to SOC (Figure 1B; SOC: 106 ± 8.7 N · m · kg−1 vs RET: 138 ± 8.6 N · m · kg−1; P = 0.01). Furthermore, VO2peak was greater with RET when compared to SOC (Figure 1C; SOC: 28 ± 1.3 ml · kg−1 · min−1 vs RET: 32.1 ± 1.3 ml · kg−1 · min−1; P = 0.04). Collectively, these data demonstrate that burn children in the RET group had higher levels of muscle strength and aerobic capacity after exercise training when compared to standard rehabilitation.
Figure 1. Effect of rehabilitative exercise training (RET) on muscle function in severely burned children.
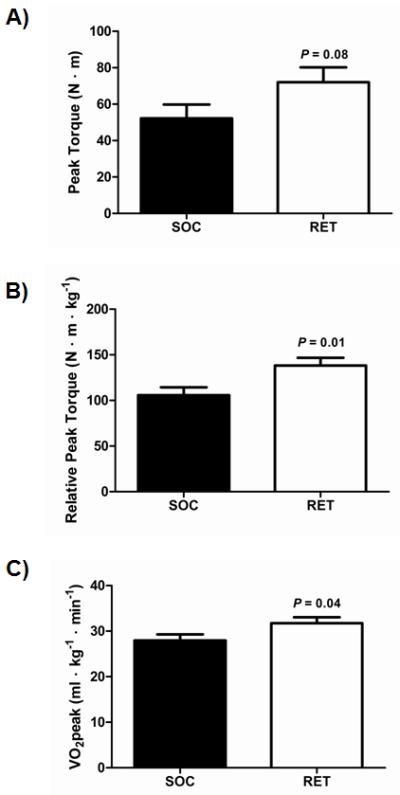
(A) Absolute peak torque (N·m) was assessed post-treatment using the Biodex isokinetic dynamometer at 150 ° · sec−1. (B) Relative peak torque (N · m · kg−1) was assessed post-treatment using the Biodex isokinetic dynamometer at 150 ° · sec−1. (C) Peak aerobic fitness (VO2peak) (ml · kg−1 · min−1) was assessed post-treatment using a modified Bruce treadmill protocol. SOC: N=23, RET: N=24. Values are means ± standard error. Abbreviations: N · m, newton-meter. kg, kilogram. SOC, standard of care. RET, rehabilitative exercise training.
Whole-body and Regional LBM
Whole-body and regional LBM are presented in Table 2. There were no differences between groups in whole-body and regional LBM at the time of discharge. There were no changes in LBM from discharge to post-treatment in the SOC group. In contrast, there was a significant increase in whole-body and regional LBM from discharge to post-treatment with RET (Table 2). In addition, the percent change in whole-body and leg LBM from discharge to post-treatment was greater with RET compared to SOC (Figure 2A). There was a trend towards an increase in arm LBM with RET compared to SOC (P = 0.06). To determine if early rehabilitative exercise training resulted in long-term muscle adaptations, LBM was examined 12 months post-burn injury. Similarly, the percentage change in whole-body and leg LBM from discharge to 12 months post-burn were greater with RET compared to SOC (Figure 2B; RET: 18 ± 3%, 31 ± 4%, respectively; P < 0.05). These results demonstrate that RET improved whole-body and regional LBM with training compared to SOC, and the positive changes in LBM were maintained for up to 12 months post-burn.
Table 2.
Effect of rehabilitative exercise training on lean body mass in severely burned children.
| SOC | RET | |||
|---|---|---|---|---|
| Discharge | Post-Treatment | Discharge | Post-Treatment | |
| Whole body, kg | 34.8 ± 3.3 | 35.9 ± 3.5 | 34.3 ± 2.2 | 37.6 ± 2.6* |
| Trunk, kg | 17.8 ± 1.6 | 18.3 ± 1.9 | 17.5 ± 1.1 | 18.5 ± 1.3* |
| Leg, kg | 10.6 ± 1.3 | 10.9 ± 1.1 | 10.3 ± 0.8 | 12.0 ± 0.9* |
| Arm, kg | 3.3 ± 0.4 | 3.4 ± 0.4 | 3.5 ± 0.2 | 4.0 ± 0.3* |
Values are means ± standard error.
Abbreviations: SOC, standard of care. RET, rehabilitative exercise training. kg, kilogram.
significant difference within group from discharge to post-treatment, P < 0.05.
Figure 2. Effect of rehabilitative exercise training (RET) on changes in whole-body and regional lean body mass (LBM) in severely burned children.

(A) Percentage change in LBM was assessed by dual-energy X-ray absorptiometry (DEXA) at discharge to post-treatment. SOC: N=23, RET: N=24. (B) Percentage change in LBM was assessed by DEXA at discharge to 12 months post-burn. SOC: N=18, RET: N=21. Values are means ± standard error. Abbreviations: SOC, standard of care. RET, rehabilitative exercise training.
Mixed-Muscle Fractional Synthetic Rate
In a subset of patients (SOC: N=13; RET: N=11), 5-hour stable isotope infusion studies were conducted to determine mixed-muscle fractional synthetic rate (Figure 3). No differences in muscle fractional synthetic rate were observed at the time of discharge (SOC: 6.1 ± 1.4% · d−1 vs RET: 7.9 ± 1.7% · d−1). Muscle fractional synthetic rate decreased from discharge to post-treatment (6.9 ± 1.1% · d−1 to 3.4 ± 0.4% · d−1; Main effect of time: P < 0.01), however no differences in muscle fractional synthetic rate were observed between SOC and RET post-treatment (SOC: 3.2 ± 0.4% · d−1 vs RET: 3.6 ± 0.9% · d−1). Lastly, no differences in the magnitude of change from discharge to post-treatment were observed between treatment groups (SOC: −2.9 ± 1.5% · d−1 vs RET: −4.3 ± 1.8% · d−1).
Figure 3. Effect of rehabilitative exercise training (RET) on muscle fractional synthetic rate in severely burned children.
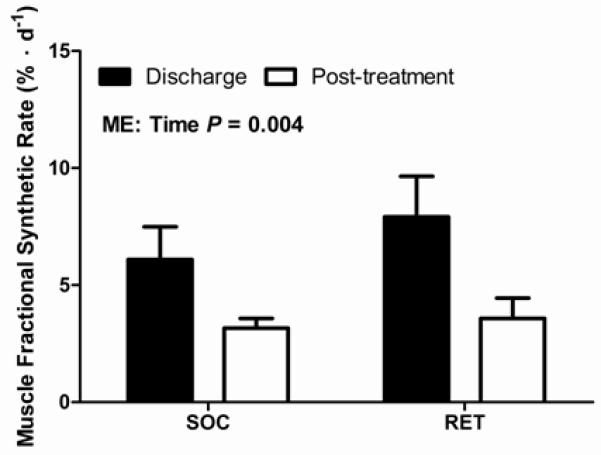
Muscle fractional synthetic rate is presented as the percent per day (% · d−1). SOC: N=13; RET: N=11. Values are means ± standard error. Significant main effect of time (P = 0.004). Abbreviations: SOC, standard of care. RET, rehabilitative exercise training. ME, Main effect.
DISCUSSION
Severe burn is a debilitating condition that results in prolonged metabolic and physical dysfunction. We have previously shown rehabilitative exercise training initiated 2-3 months following hospital discharge to have positive benefits on muscle mass and function in pediatric burn patients (24, 27, 29-31). However, many patients may return home following discharge to an environment with poor rehabilitative support which could hinder subsequent training-induced adaptations. In addition, in some circumstances patients do not return for exercise training. Thus, implementing early exercise is of interest to enhance rehabilitative efforts and increase exercise training compliance. To our knowledge, we are the first to report that early outpatient exercise training initiated immediately following hospital discharge represents an effective intervention to augment muscle mass and function following severe burn injury. Early rehabilitative exercise improved whole-body and regional LBM, and resulted in greater relative strength and VO2peak when compared to the standard of care rehabilitation. Interestingly, the changes in muscle mass and function were independent of changes in basal muscle fractional synthetic rate. These training-induced adaptations are similar, and for some parameters more robust, than improvements manifested by pharmacological agents such as oxandrolone (27) and growth hormone (31).
We have reported that a similar RET program, but implemented later in the continuum of burn injury improves LBM, muscle strength and cardiorespiratory fitness (24, 27, 29-31). The intent of the current study was to assess if implementing RET immediately after hospital discharge would offer benefits in these clinically and functionally relevant outcomes. Our results demonstrate that improvements to these clinically significant outcomes are attainable. More importantly, the early accretion of LBM was maintained 12 months post-burn despite the cessation of the rehabilitative exercise training program. Although direct comparisons between early and late implementation of RET during convalescence was not the intent of this study, there are similarities in the magnitude of changes (24, 29, 30). Collectively, these studies support the beneficial effects of rehabilitative exercise in the recovery from burn. However, an important consideration is that early rehabilitative exercise improves muscle mass and function during a period when severe hypermetabolism and hypercatabolism have been shown to be present (13, 17). Therefore, patients can continue their rehabilitative exercise and occupational therapy programs uninterrupted and in a hospital setting, as opposed to the current standard care of following guidelines and written instructions at home (6). It should also be noted that compliance to the RET program was nearly 100%, as outpatients were transported each weekday to and from the hospital for the training and therapy sessions. These findings support a positive role for early rehabilitation exercise on immediate and long-term improvements to muscle mass and function in pediatric burn survivors.
Skeletal muscle mass is regulated by the rate of protein turnover, which is the net balance between protein synthesis and degradation. Severe burn trauma increases protein catabolism resulting in the redistribution of skeletal muscle amino acids to support the hypermetabolic response (13, 35, 36). Thus, interventions targeting muscle protein turnover are of clinical interest. Increased intracellular free amino acids are thought to stimulate protein synthesis, and therefore muscle fractional synthetic rate may reflect overall protein turnover (4, 25). The current study examined basal fractional synthetic rate using stable isotope infusion studies performed at least 48 hours after the last exercise session in the resting state, and therefore any changes with exercise training reflect alterations in basal muscle protein synthesis. In agreement with others, we report that pediatric burn survivors basal muscle fractional synthetic rate is elevated at hospital discharge and then decreases during convalescence (4, 8, 11, 25), which is consistent with a decrease in hypermetabolism. With regards to exercise training, we were unable to detect differences in basal fractional synthetic rate between treatment groups at each time-point despite significant improvements in muscle mass and function. These findings further demonstrate that exercise training does not negatively affect hypermetabolism in burn patients (2). Future studies are warranted to determine if improved muscle mass and function with rehabilitative exercise in pediatric burn survivors is manifested through acute post-exercise increases in protein synthesis (0-24 hours), and if this post-exercise response is altered by exercise training.
The current clinical trial has several limitations that should be addressed. The primary limitation to this study is that nutrition and physical activity was not assessed during acute burn care treatment and the 12-week intervention. There is the potential that nutrition and physical activity during acute burn treatment may affect treatment interventions upon discharge. However, patients were given a standard nutrition based on resting energy expenditure during acute care, and we did not observe differences in clinically relevant variables such as total burn surface area and length of stay between treatment groups at discharge. Although these assessments during acute burn treatment were not a primary analysis of the current study, we are now focusing on future studies which will include patients wearing an accelerometer or pedometer during this time. Further, despite patients participating in nutritional counseling prior to hospital discharge, patients engaging in regular exercise training may have been more likely to adhere to dietary recommendations. Future studies are needed to determine the importance of a positive energy balance during the early stages of convalescence. Another important limitation is that the data was collected at a single burn hospital and the study population consisted primarily of Hispanic children that reside outside of the United States, which may limit the generalization of the current findings. Multicenter, randomized control trials are currently being discussed and would increase the consistency of standard of care and rehabilitative exercise training programs between burn care facilities. Lastly, we examined muscle fractional synthetic rate using stable isotope infusion studies in a subset of patients. In addition to the children being severely burned, these are highly complex studies and could not be completed in all patients. Nevertheless, our results yield important and robust information on potential mechanisms on how rehabilitative exercise might affect muscle protein metabolism in burned children.
In conclusion, a 12-week rehabilitative exercise training program following hospital discharge improved muscle mass and function in severely burned children. The changes were not accompanied with perturbations in hypermetabolism, further suggesting a positive role for exercise training on long-term improvements in the recovery from burn. Based on these results we continue to advocate on the importance of implementing RET immediately after discharge, with future efforts to determine optimal strategies to improve clinically and functionally relevant outcomes.
ACKNOWLEDGEMENTS
The authors thank the study participants and their families for their time and effort, and the clinical and research staff of Shriners Hospitals for Children, Galveston, TX for their help with conducting the stable isotope infusion studies, sample processing and GC-MS analyses. This study was supported by National Institute for Disabilities and Rehabilitation Research grant H133A120091; National Institutes of Health grants P50 GM060388, R01 HD049471, R01 GM056687; Shriner grants 71006, 71008, 71009, 84080, 84090. JPH was supported by Leon Hess Professorship Funds. EB was a faculty member and scientific staff at UTMB and SHC, and is presently at the University of Arkansas for Medical Sciences, Little Rock, AR. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Funding Sources: This research was supported by National Institute for Disabilities and Rehabilitation Research grant H133A120091; National Institutes of Health grants P50 GM060388, R01 HD049471, R01 GM056687; Shriner grants 71006, 71008, 71009, 84080, 84090. JPH was supported by Leon Hess Professorship Funds. EB was a faculty member and scientific staff at UTMB and SHC, and is presently at the University of Arkansas for Medical Sciences, Little Rock, AR. This is a registered clinical trial at Clincaltrials.gov NCT00675714.
Footnotes
CONFLICTS OF INTERESTS
The authors declare no conflicts of interest.
REFERENCES
- 1.Burn Incidence and Treatment in the United States: 2012 Fact Sheet. Sources: National Electric Injury Surveillance System-All Injury Project (NEISS-AIP); National Emegency Department Survey (HCUP-NEDS) (2010 Data); National Ambulatory Medical Care Survey; Dec 21, 2013. American Burn Association Web site [Internet] cited. Available from: http://www.ameriburn.org/resources_factsheet.php. [Google Scholar]
- 2.Al-Mousawi AM, Williams FN, Mlcak RP, Jeschke MG, Herndon DN, Suman OE. Effects of exercise training on resting energy expenditure and lean mass during pediatric burn rehabilitation. J Burn Care Res. 2010;31(3):400–8. doi: 10.1097/BCR.0b013e3181db5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alloju SM, Herndon DN, McEntire SJ, Suman OE. Assessment of muscle function in severely burned children. Burns. 2008;34(4):452–9. doi: 10.1016/j.burns.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87(7):3378–84. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995;268(1 Pt 1):E75–84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 6.Celis MM, Suman OE, Huang TT, Yen P, Herndon DN. Effect of a supervised exercise and physiotherapy program on surgical interventions in children with thermal injury. J Burn Care Rehabil. 2003;24(1):57–61. doi: 10.1097/00004630-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Charette SL, McEvoy L, Pyka G, et al. Muscle hypertrophy response to resistance training in older women. J Appl Physiol (1985) 1991;70(5):1912–6. doi: 10.1152/jappl.1991.70.5.1912. [DOI] [PubMed] [Google Scholar]
- 8.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229(1):11–8. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froelicher V, Quaglietti S. Handbook of Exercise Testing. Little, Brown and Company; Boston: 1996. p. 16. [Google Scholar]
- 10.Gore DC, Honeycutt D, Jahoor F, Wolfe RR, Herndon DN. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991;126(1):38–43. doi: 10.1001/archsurg.1991.01410250042006. [DOI] [PubMed] [Google Scholar]
- 11.Gore DC, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Extremity hyperinsulinemia stimulates muscle protein synthesis in severely injured patients. Am J Physiol Endocrinol Metab. 2004;286(4):E529–34. doi: 10.1152/ajpendo.00258.2003. [DOI] [PubMed] [Google Scholar]
- 12.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232(4):455–65. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 14.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg. 2012;256(3):402–11. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Rutan RL, Rutan TC. Management of the pediatric patient with burns. J Burn Care Rehabil. 1993;14(1):3–8. doi: 10.1097/00004630-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hildreth MA, Herndon DN, Desai MH, Duke MA. Caloric needs of adolescent patients with burns. J Burn Care Rehabil. 1989;10(6):523–6. doi: 10.1097/00004630-198911000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones NL. Clinical Exercise Testing. W.B. Saunders; Philadelphia: 1997. [Google Scholar]
- 20.Kimmo T, Jyrki V, Sirpa AS. Health status after recovery from burn injury. Burns. 1998;24(4):293–8. doi: 10.1016/s0305-4179(98)00007-2. [DOI] [PubMed] [Google Scholar]
- 21.LaStayo PC, Marcus RL, Dibble LE, Smith SB, Beck SL. Eccentric exercise versus usual-care with older cancer survivors: the impact on muscle and mobility--an exploratory pilot study. BMC Geriatr. 2011;11:5. doi: 10.1186/1471-2318-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller MJ, Herndon DN. The challenge of burns. Lancet. 1994;343(8891):216–20. doi: 10.1016/s0140-6736(94)90995-4. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TT, Gilpin DA, Meyer NA, Herndon DN. Current treatment of severely burned patients. Ann Surg. 1996;223(1):14–25. doi: 10.1097/00000658-199601000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porro LJ, Al-Mousawi AM, Williams F, Herndon DN, Mlcak RP, Suman OE. Effects of propranolol and exercise training in children with severe burns. J Pediatr. 2013;162(4):799–803 e1. doi: 10.1016/j.jpeds.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter C, Cotter M, Diaz EC, Jennings K, Herndon DN, Borsheim E. Amino acid infusion fails to stimulate skeletal muscle protein synthesis up to 1 year after injury in children with severe burns. J Trauma Acute Care Surg. 2013;74(6):1480–5. doi: 10.1097/TA.0b013e3182921651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruitt BA, Jr, Wolf SE, Mason AD., Jr . Epidemiological, demographic and outcome characteristics of burn injury. In: Herndon DN, editor. Total Burn Care. Elsevier; Edinburgh: 2012. pp. 19–23. [Google Scholar]
- 27.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007;119(1):e109–16. doi: 10.1542/peds.2006-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222(3):283–94. 94–7. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suman OE, Herndon DN. Effects of cessation of a structured and supervised exercise conditioning program on lean mass and muscle strength in severely burned children. Arch Phys Med Rehabil. 2007;88(12 Suppl 2):S24–9. doi: 10.1016/j.apmr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985) 2001;91(3):1168–75. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 31.Suman OE, Thomas SJ, Wilkins JP, Mlcak RP, Herndon DN. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol (1985) 2003;94(6):2273–81. doi: 10.1152/japplphysiol.00849.2002. [DOI] [PubMed] [Google Scholar]
- 32.Wolf SE, Rose JK, Desai MH, Mileski JP, Barrow RE, Herndon DN. Mortality determinants in massive pediatric burns. An analysis of 103 children with > or = 80% TBSA burns (> or = 70% full-thickness) Ann Surg. 1997;225(5):554–65. doi: 10.1097/00000658-199705000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf SE, Thomas SJ, Dasu MR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237(6):801–11. doi: 10.1097/01.SLA.0000071562.12637.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317(7):403–8. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe RR, Jahoor F, Herndon DN, Miyoshi H. Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery. 1991;110(1):54–67. [PubMed] [Google Scholar]


