Abstract
Background
High flow nasal cannula (HFNC) improves ventilation by washing out nasopharyngeal dead space while delivering oxygen. Heliox (Helium-oxygen gas mixture), a low density gas mixture, decreases resistance to airflow, reduces the work of breathing and facilitates distribution of inspired gas. Excessive lung work and potential injury increases the workload on the immature diaphragm predisposing the muscle to fatigue and can lead to inflammatory and oxidative stress, thereby contributing to impaired diaphragmatic function. We tested the hypothesis that HFNC with Heliox will decrease the work of breathing thereby unloading the neonatal diaphragm and potentially reducing diaphragmatic injury.
Methods
Spontaneously breathing neonatal pigs were randomized to Nitrox (Nitrogen-oxygen gas mixture) or Heliox and studied over 4 hrs following oleic acid injury. Gas exchange, pulmonary mechanics indices and systemic markers of inflammation were measured serially. Diaphragm inflammation biomarkers and histology for muscle injury were assessed at termination.
Results
Heliox breathing animals demonstrated decreased respiratory load and work of breathing with lower pressure- rate product, lower labored breathing index, and lower levels of diaphragmatic inflammatory markers and muscle injury score as compared to Nitrox.
Conclusion
These results suggest that HFNC with Heliox is a useful adjunct to attenuate diaphragmatic fatigue in the presence of lung injury by unloading the diaphragm, resulting in a more efficient breathing pattern and decreased diaphragm injury.
Keywords: High flow nasal cannula (HFNC), Helium-oxygen (Heliox), diaphragm injury, lung injury
Introduction
Premature infants are at increased risk for respiratory distress syndrome. Mechanical ventilation has improved respiratory failure and survival in these infants; however, it has also been associated with chronic lung injury or bronchopulmonary dysplasia (BPD). Upwards of 25-42% of extremely low birth weight (ELBW) infants develop BPD and there is no evidence that this incidence is on the decline.1,2 Lung injury can be caused by alveolar-capillary membrane damage, surfactant inactivation or deficiency, inflammation and oxidant injury leading to impaired gas exchange,3 all of which increase thoraco-abdominal asynchrony secondary to increased resistive and elastic load.4 These alterations in work of breathing lead to respiratory muscle contractile afterload causing fatigue and muscle injury, characterized by inflammatory, oxidative and structural damage of the diaphragm leading to respiratory failure. Due to developmental deficiencies in the chest wall and diaphragm, the infant with lung injury is predisposed to respiratory failure. 5-7 In this regard, the composition of diaphragm muscle fibers and immature arrangement of reduced apposition between the chest wall and diaphragm muscle contributes to respiratory fatigue in the face of higher respiratory load imposed by lung injury and alveolar instability. High chest wall compliance makes ventilation less efficient in the infant, adding to the increased load on the diaphragm and increasing probability of respiratory failure.8
Continuous positive airway pressure (CPAP) is the most common form of non-invasive respiratory support used in spontaneously breathing infants. Mechanisms by which CPAP provides support is through alveolar recruitment, pressure delivery to the lung, increasing functional residual capacity, improving ventilation, oxygenation and reducing work of breathing. However, excessive CPAP can lead to adverse effects, including air leak syndromes9, nasal injuries due to prolonged use and possible effect on renal dysfunction.10 Another mode of support is high flow nasal cannula (HFNC) which delivers warmed and humidified gas at higher flow. Mechanisms of HFNC for support include nasopharyngeal dead space washout with improved ventilation, warmed and humidified gas that improves conductance and pulmonary compliance and decreased metabolic work needed for gas conditioning, decreased inspiratory resistance and work of breathing and varying end-distending pressure.11-14
Helium-oxygen gas mixture (i.e. Heliox) has been used for decades to reduce work of breathing in the presence of high airway resistance. Helium is a biologically inert gas which is seven times less dense than nitrogen. Being less dense, Helium decreases turbulence, provides more laminar flow, thus decreases airflow resistance and requires less driving pressure for distribution. In addition, Heliox has high diffusivity and can act as a carrier mixture, favoring gas exchange.15-19 Clinical and pre-clinical studies have shown Heliox to decrease oxygen need, improve ventilation, improve thoraco-abdominal synchrony and work of breathing and also reduce lung inflammation by reducing oxidative and mechanical stress. 20-31
To test the hypothesis that an alternative gas delivery system, HFNC to deliver Heliox may decrease the work of breathing and lung injury, we studied the use of HFNC to deliver Heliox in a newborn porcine lung injury model in which we evaluated lung mechanics, diaphragm inflammation and structure. These data were compared to a control group of lung injured newborn pigs supported by Nitrox delivered by HFNC group.
Methods
Fourteen neonatal full-term, spontaneously breathing pigs (age 1-10 days, weight 2.4-4.5 kg) were anesthesized (intramuscular injections of ketamine (23 mg/kg), acepromazine (0.58 mg/kg) and xylazine (0.8 mg/kg) separated by 10 mins. 12 Umbilical catheters (5 Fr) were placed in a jugular vein and carotid artery. A saline- filled catheter (3.5 Fr) was placed midway in the trachea, transtracheally at a perpendicular orientation and attached to a pressure transducer for continuous dynamic tracheal pressure monitoring (Grass model 79D, Grass Instruments, Co, Quincy, MA). The animals were supported with double prong HFNC (Vapotherm 2000i (Vapotherm, Inc, Stevensville, MD) at 4L/min at initial FIO2 0.5. In addition to a calibrated blender for Heliox and Nitrox, gas concentrations were monitored with an in-line MiniOX oxygen monitor. Control animals were placed on HFNC receiving Nitrox (mixture of oxygen and nitrogen) and the experimental group received helium instead of nitrogen (Heliox). Following instrumentation, respiratory distress was induced by infusion of oleic acid (0.08 ml/kg; Sigma) that was emulsified in 0.5 ml/kg blood drawn acutely from the animal’s venous line. This blood emulsion was infused into the central venous line in four equal aliquots, each separated by 10 min to allow for physiologic equilibration and stabilization between doses. The injury model and oleic acid dose was refined in a pilot study by titrating oleic acid to achieve injury leading to a 50% reduction in PaO2 and respiratory compliance while preserving spontaneous respiration. In addition, this model has been previously reported by our group.30,32,33 Maintenance fluids were provided by a continuous infusion of 5% dextrose solution per standard practice. Respiratory parameters were assessed by respiratory inductance plethysmography (RIP) (SomnaStar PT; SensorMedics, Yorba Linda, CA), including relative tidal volume, respiratory rate, thoracoabdominal synchrony. Relative tidal volumes and minute ventilations were determined prior to lung injury during manual bagging (via 3.0 endotracheal tube) at a fixed rate (30 breaths/min) with low (2-4 ml/kg) and high (6-8 ml/kg) tidal volume strategies using a pneumotachograph within a pediatric pulmonary monitoring system (NICO, Cardiopulmonary Monitor, Philips HealthCare, Andover, MA.) in order to establish relative calibration standards for inductive plethysmography (e.g. Labored Breathing Index).34 Analyses involved pneumotachography to determine integrated tidal volumes and utilized a calibration algorithm for both Nitrox and Heliox. The bands containing inductance coils were carefully positioned at the level of the axillae (i.e. rib cage band) and midway between the umbilicus and xiphisternal junction (i.e. abdomen band). In the present study, we used an uncalibrated RIP method for phase and synchrony evaluations and used an abbreviated two point RIP-tidal breathing calibration for evaluation of tidal breaths and minute ventilation during the tidal breathing analysis. The Respitrace was used to construct Lissajous loops and subsequent calculation of phase angles between the rib cage and abdominal movement using RespiEvents software 5.2 (NIMS,Miami, FL)on at least 10 uniform breaths.5,35,36
At baseline, following injury and then every hour for 4 hrs , arterial blood samples were obtained to measure gas exchange , vital signs, SpO2, FIO2, tracheal pressure monitoring and pulmonary function were measured.. FIO2 was titrated for SpO2 95 ± 2% post injury from hour 1 through 4. Animals were deeply sedated after completion of the experiment. The diaphragm was perfused in situ via the vasculature with cold Millonig’s phosphate buffer. The animals were then euthanized and consistent regional samples of the anterior and posterior portion of right diaphragm were dissected, formalin-fixed and prepared for histomorphometrical analysis. Consistent regional samples of the anterior and posterior portion of left diaphragm were dissected, snap-frozen in liquid nitrogen and stored at −80°C for subsequent analysis of interleukin-8 (IL-8) and myeloperoxidase (MPO).
Diaphragm tissue IL-8 ELISA was analyzed from the supernatant generated from homogenized diaphragm tissue. Samples of fixed weight (100 mg) were washed twice with phosphate-buffered saline and homogenized in 500 μl RIPA solution (50 mMTrisHCl, 150 mMNaCl, 1% igepal, 0.5% NaDOC, and 0.1% SDS) with Complete™ protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). The homogenate was centrifuged at 12,00 × g for 10 min at 48°C to obtain a supernatant for measurement of IL-8. ELISAs on duplicate samples were performed using porcine-specific capture and detection antibodies and respective recombinant porcine cytokine proteins as positive controls (DuoSet Porcine IL 8, RD Systems, Minneapolis, MN). Optical density was read at 450 nm, and the limit of detection was 31 pg/ml.
Myeloperoxidase (MPO) in diaphragm tissue was analyzed following tissue homogenization (100 mg in 1 ml of 0.05 M potassium phosphate buffer, pH 6.0). The homogenate was centrifuged at 1,70 × g for 30 min at 4°C. The supernatant was then incubated at 60°C for 2 hr on a thermostated water bath, and again centrifuged at 10,00 × g for 5 min at 4 °C to obtain a supernatant for measurement of MPO activity, using a colorimetric bioassay ( Schierwagen et al.16) Duplicate 10-ml aliquots of standard (human leukocyte MPO, ICN Biomedicals, Aurora, OH) and samples were incubated in a 96-well plate with 100 ml of substrate buffer (0.1M sodium citrate, 0.1% o-dianisidine, 1 mM hydrogen peroxide, pH 5.5) for 1 min. The plate was read immediately at 560 nm in an automated plate reader (MRX RevelationTM, Thermo Labsystems, Franklin, MA), and the detection limit was 0.0625 U/ml. Total protein concentration in lung tissue homogenate was analyzed using the microtiter assay of Bradford. IL-8 and MPO levels were normalized for total protein concentration in the diaphragm.37
For histomorphometrical analyses, tissues were formalin- fixed and embedded in paraffin. Thin sections (5 μm) were cut and stained with hematoxylin and eosin. The slides were numbered with masking of treatment option such that all analyses were performed while blinded to treatment group. Specimens were analyzed qualitatively with gross light microscopy at x100 and x400 magnification. Diaphragm sections were first displayed under the microscope at low power, and then changed to higher power to randomize selection and avoid preselection of areas. Images at both low and high power were photographed and digitized. Histomorphometric quantitative assessments were performed using computerized software (Image Pro Plus, Silver Spring, MD) and included measurements of muscle fiber diameter and quantification of muscle damage. Myofiber diameter was defined as the maximum diameter across the lesser aspect of the muscle fiber and was measured in muscle fibers oriented in cross-section.38 The muscle damage score is a numerical scoring system where a 48 point grid is superimposed on fixed cross-sections and grids are scored as either normal or abnormal muscle morphology. A grid was categorized as abnormal based on the presence of inflammatory cells, pale or variable staining of the cytoplasm or central nucleated fibers. Grids were not scored if the space was empty or contained connective tissue, nerve or vessels. The muscle damage score was calculated by dividing the number of damaged grids by the total number of grids that were evaluated (muscle damage score (%) = [abnormal/ abnormal + normal] x 100).39,40
Data analysis was performed using Graph Pad PRISM and Microsoft Excel. Values are expressed as mean ± SEM. Group differences were analyzed statistically using Analysis of Variance (ANOVA) with Bonferonni post-hoc analysis to determine intergroup and time dependent differences for physiologic parameters, pressure rate product and labored breathing index. The Student t-test was used to analyze treatment group differences for inflammatory markers and muscle injury score. Significance was accepted at P ≤ 0.05.
Results
The neonatal pigs averaged 9.1 ± 1.8 postnatal days and 3.42 ± 0.66 kg body weight. There were no significant differences in age, gender, or weight between treatment groups.
Physiologic Parameters
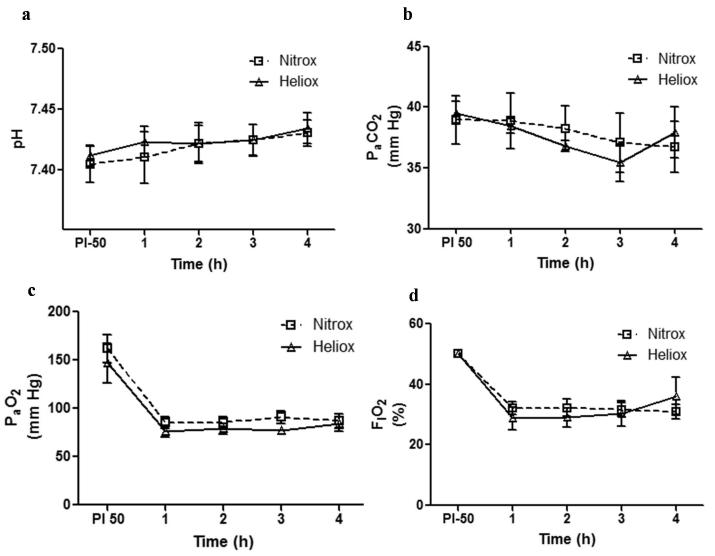
As shown in Figure 1, gas exchange and blood chemistry values are presented for both groups (Nitrox and Heliox) at post injury and for 4 hours of spontaneous breathing. Initially all animals were on FIO2 50%, 4 L/min HFNC under baseline conditions (no group differences noted). As indicated, the oleic acid (OA) injury (immediate response) resulted in a significant detriment in oxygenation (figure 1c) with little change in PaCO2 (figure 1b). With supplemental oxygen both groups were able to compensate effectively in response to the OA injury. Over hours 1 through 4, the inspired oxygen concentration for both groups was titrated to maintain oxygen saturation in the range of 95 ± 2% while the animals controlled their own depth and rate of breathing. The PaCO2 was lower in the Heliox group, though over time it did not reach statistical significance (P = 0.34). There was no acidosis or hypercapnia noted in either of the groups. FIO2 requirement was similar in both groups (P =0.43) and PaO2 was higher in the Heliox group compared to Nitrox at similar FIO2 exposures (P = 0.64).
Figure 1.
Lung physiological profile (mean ± SEM) in Heliox group (solid line with open triangles) vs Nitrox group (dashed line with open squares) from post lung injury and over 1 through 4 hours. (a) No significant difference in pH between the two groups (P = 0.21). (b) PaCO2 was lower for Heliox group, though over time did not reach statistical significance (P = 0.34). (c) PaO2 level was higher for Heliox group at all time points from hour 1 through 4, though not statistically significant (P = 0.64). (d) FIO2 requirement was similar between both groups (P = 0.43).
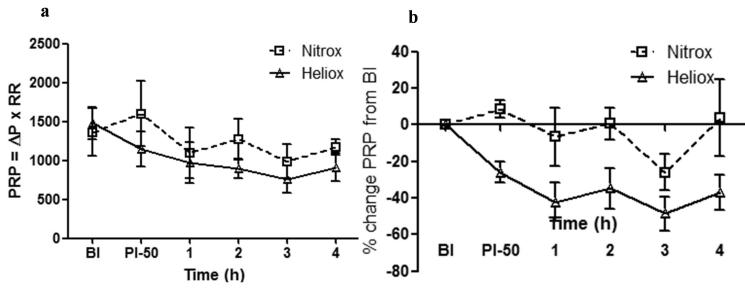
Figure 2 illustrates the pressure rate product and percent changes in this parameter for both groups (Nitrox and Heliox) at baseline injury, post injury on 50 % FIO2and for 4 hours of spontaneous breathing. As shown, no group difference was noted at baseline for pressure rate product (P > 0.05) and the product trended lower in the heliox group overtime post injury between 1 and 4 hours ( P= 0.06). When pressure rate product was normalized to baseline values (to reduce initial inter- animal variability), there was a significant decrease in the Heliox group (P < 0.01) over time, with the 4 hour measurement showing the greatest intergroup difference (P < 0.05).
Figure 2.
Pressure rate product (PRP) and percentage change of this parameter from baseline injury (BI) over time (post injury-50% FIO2 and over hour 1 through 4) in Heliox group (solid line with open triangles) vs Nitrox group (dashed line with open squares). (a) Pressure rate product values trended lower in the heliox group (P = 0.06). (b) Percentage change in PRP normalized to baseline injury on y-axis plotted against post injury and over 4 hours of spontaneous breathing. Heliox group has significantly greater percentage drop in pressure rate product over time (* P < 0.01).
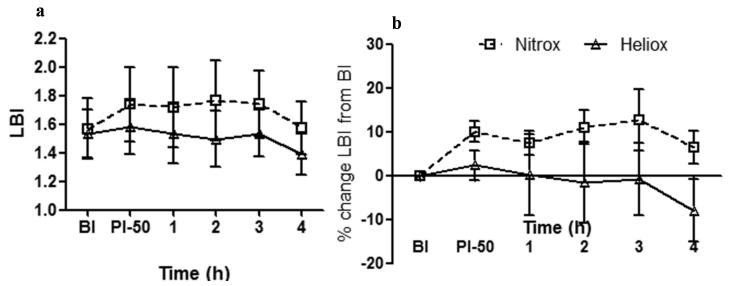
Labored Breathing Index (LBI) values and percent changes in this parameter are illustrated for both groups (Nitrox and Heliox) at baseline injury, post injury 50 % FIO2 and for 4 hours of spontaneous breathing in figure 3. As noted, LBI was lower in the Heliox group (figure 3a) (P < 0.01) and this improvement in work of breathing was maintained when LBI was normalized as percent change from baseline injury (figure 3b), there was greater decrease in LBI in the heliox group (P < 0.01). Heliox had a decrease in LBI over time compared to increasing index in the Nitrox group (figure 3b).
Figure 3.
Labored Breathing Index (LBI) and percentage change of this parameter from baseline injury (BI) over time (post injury-50% FIO2 and over hour 1 through 4) in Heliox group (solid line with open triangles) vs Nitrox group (dashed line with open squares). (a) Heliox group compared to Nitrox group had significantly lower LBI values over time (* P < 0.01). (b) Percentage change in LBI normalized to baseline injury on y-axis plotted against time from post injury and over 4 hours of spontaneous breathing. Heliox group has significantly greater percentage drop in LBI over time (* P < 0.01).
Inflammatory Markers
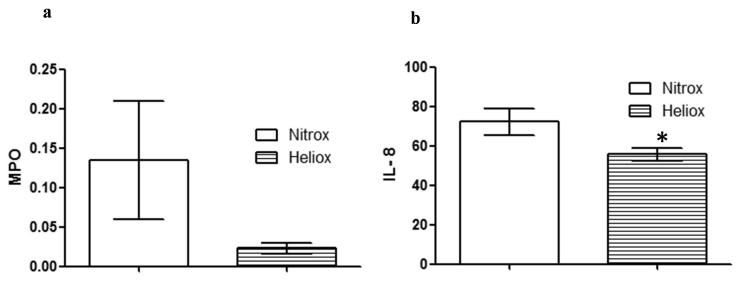
MPO (figure 4a) and IL-8 (figure 4b) for diaphragm tissue are shown for both groups (Nitrox and Heliox) after 4 hrs OA injury and spontaneous breathing. As noted, there was less inflammatory damage to the diaphragm as shown by significantly lower IL-8 levels in the Heliox group (P < 0.01). MPO levels trended lower in the Heliox group (P =0.07).
Figure 4.
Diaphragm Myeloperoxidase (MPO) and interleukin 8 (IL-8) levels after 4 hours of oleic acid injury in Heliox and Nitrox groups are presented as normalized for total protein concentration in the diaphragm. (a) MPO trended lower in the Heliox group compared to Nitrox group, not statistically significant (P 0.07). (b) IL-8 levels were significantly lower in the Heliox group after 4 hours of spontaneous breathing, post oleic acid injury (* P < 0.01).
Histological Parameters
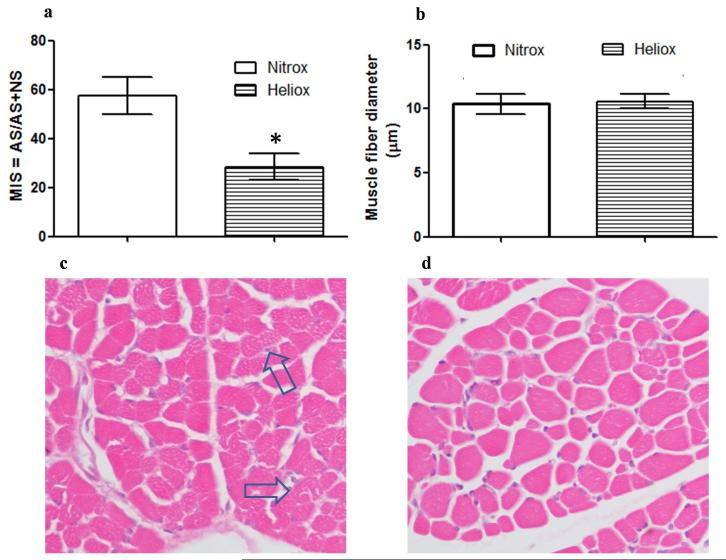
Histomorphology of cross sectional diaphragm sections showed evidence of injury in both Nitrox and Heliox support, with Nitrox breathing animals showing a greater abnormal score with uneven staining and fragmentation of fibers (figure 5a). In the Heliox sections there is significantly less fragmentation and more uniform staining (as shown in figure 5b). There was no centralization of nuclei noted in either group. The muscle injury score (MIS) (figure 5c) of the Heliox group (25%) was significantly lower (P < 0.01) as compared to the Nitrox group (58%) There was no difference noted in the fiber diameter between Nitrox and Heliox group (10.4 ± 0.9 and 10.6 ± 0.7 respectively; P = 0.38) (figure 5d).
Figure 5.
Diaphragm muscle injury score (MIS), cross-sectional muscle fiber diameter and H & E stained cross-sections of diaphragm. (Clear bars are Nitrox breathing animals and dashed bars are Heliox breathing). (a) Muscle injury Score comparing nitrox to heliox sections. Muscle Injury was attenuated in heliox breathing pigs compared to nitrox group (* P < 0.01). (b) Muscle fiber diameter was similar between both groups (P > 0.05). (c & d) H&E stained cross-sections of diaphragm (magnification 400x) obtained from one newborn pig in each group. (c) Nitrox section, arrows points to uneven staining and fragmented fibers. (d) Heliox section showing more uniform staining and less fragmentation.
Discussion
Over the last 2 decades, there have been a handful of studies in the neonatal population emphasizing the improvement in work of breathing when Nitrox is replaced with Heliox as a carrier air mixture. These studies have mainly been limited to obstructive lung disease processes in children. There have been very few studies in premature neonates since the original observation in 1984 that showed reduced airway resistance, work of breathing, and improved thoracoabdominal synchrony.28 More recent clinical use of Heliox with non-invasive ventilation in neonates has been limited to conventionally delivered CPAP. Colnaghi et al.31 showed Heliox to be safe and effective in reducing the need for intubation and decreased surfactant requirements. Migliori et al.26 noted reduced resistive work of breathing and mean peak inspiratory pressure and improved gas exchange with lower TcCO2 levels in preterm infants, but there was no control group in this non-blinded study. Recent review of non-invasive ventilation approaches has described the advantages of Heliox over Nitrox as a carrier gas for non-invasive ventilation.9,25
The airways in the premature neonate are especially vulnerable to high airway resistance due to small caliber, increased secretions, airway edema, rapid breathing rates and collapsibility of airway. Various modes of non-invasive ventilation have been used to maintain airway patency and nasopharyngeal dead space washout. HFNC is becoming a commonly used mode of noninvasive ventilation which has the above advantages and also provides warmed and humidified gas which improves conductance and pulmonary compliance13 decreases metabolic work needed for gas conditioning, decreases inspiratory resistance and work of breathing and can also provide varying end-distending pressure.11,14 These benefits of HFNC are amplified with the use of Heliox, since it’s unique biophysical properties aid in improving work of breathing, improves ventilation, decreases oxygen needs, may enhance nasopharyngeal washout (more effective diffusion of Heliox) and further reduces lung inflammation.30 In addition, Heliox provides an effective steady state thermal environment during early development.41
In this study, spontaneously breathing lung injured newborn pigs supported with high flow nasal Heliox demonstrated lesser effort and work of breathing compared to Nitrox breathing animals. The benefit of lower density of helium gas mixture is proportional to the FIO2, with the greatest density difference occurring at lower FIO2 (20-40%).16,17,21,26,28,42 FIO2 requirement was <40% in the Heliox group. FIO2 requirement and PaO2were similar in both groups, but there was lower work of breathing in the Heliox group. This indicates greater efficiency when exposed to helium as compared to nitrogen even when exposed to similar oxygen concentration. The improved efficiency is likely related to decreasing turbulence, decreased resistance to flow and greater diffusivity. Whereas PaCO2 levels were similar in both groups, as compared to the Nitrox group, the Heliox group had lower effort of breathing and load to achieve similar level of ventilation. This is reflected by improvement in thoraco-abdominal asynchrony and labored breathing index, Heliox showed a greater decrease in resistive load with greater decrease in PRP. Similar results have been shown in spontaneously breathing BPD infants where Heliox breathing decreased pulmonary resistance, resistive work of breathing and mechanical power of breathing.26,28
The RIP Lissajous approach was developed for sinusoidal breathing patterns, and not complex patterns associated with some distressed breathing patterns. However, it has been shown by our group that even in distorted loops, the calculated phase angle is still a good estimate of synchrony and the error has been estimated at <10%.4,5 In our experience, we have found that the phase angle and pressure rate product, as well as several other parameters (even with a two point calibration) provides very reliable and reproducible information on breathing synchrony which in turn indirectly characterizes respiratory load.
The Heliox breathing group had more homogenously stained diaphragm fibers compared to samples obtained from the Nitrox group in which there was a significantly greater muscle injury mainly characterized by uneven staining and fragmentation of fibers. While there was no difference in diaphragm fiber diameter, we posit that the absence of differences is likely due to the short exposure time; longer injury time is most likely needed to note hypertrophy or atrophy of the fibers with time. There are limitations to the comparison of the neonatal pig diaphragm with premature human neonatal diaphragm muscle with a paucity of studies assessing structure of human neonatal diaphragm. Nonetheless, during early development in both species the chest wall is relatively more compliant that the lung. Within this context, given that a compliant chest wall, coupled with a stiff lung presents increased work of breathing as a challenge to the easy fatigability of respiratory pump muscle in preterm neonates makes the findings of this study in neonatal pig model clinically relatable to preterm infants with RDS/BPD.
The greater muscle damage in the Nitrox group is likely due to greater initial mechanical injury, followed by inflammatory cell infiltrate, cytotoxic injury and oxidant damage compared to heliox group.39 IL-8, which is a neutrophil chemotactic factor43 and an established inflammatory marker was significantly lower in the Heliox group, thus strengthening our contention that Heliox unloads the diaphragm, lessens muscle fatigue, improves work of breathing resulting in decreased diaphragm inflammatory injury. While the MPO levels were lower in heliox group, statistical significance at our stated level was not reached, a finding that may be explained by similar oxygen exposure in the two groups and similar oxidant injury.
Due to the non-invasive support (gas leaks around the nares and mouth), this study could not directly determine the work of breathing. However, using inductance plethysmography and the direct invasive determination of airway pressure (only possible in an animal model), we were able to estimate indices of effort of breathing. More specifically, we determined pressure rate product and labored breathing index. In this oleic acid lung injury model, we showed that for the same oxygen requirement (FIO2), the Heliox group was able to maintain oxygen saturation and arterial PaCO2 in a stable target range more efficiently than the Nitrox group. This conclusion is based on the finding that work of breathing indices (pressure-rate product and labored breathing index) were reduced in the Heliox group during the treatment period (4 hours) as compared to the Nitrox group. Concomitant with this finding, there was a reduction in diaphragmatic inflammatory biomarkers associated with the reduction in breathing effort, thus supporting more efficient breathing effort.
In lung disease, in order to expand the lung, respiratory muscles must generate sufficient force to overcome elastic forces associated with the low lung compliance and resistive forces associated with high airway resistance. The diaphragm plays a greater role in the work of breathing compared to the compliant chest wall. In neonates, the diaphragm is flattened, has smaller apposition zone, low oxidative capacity and lesser percentage of fatigue resistant fibers, thus less endurance to sustain increased work load.8 In the setting of lung injury, Heliox with HFNC decreased resistive load, decreased thoraco-abdominal asynchrony, caused less diaphragm inflammatory and structural damage. These findings strongly suggest that Heliox via HFNC may be a useful adjunct to improve the energetics of breathing and attenuate diaphragm fatigue in presence of lung injury. We speculate that the use of Heliox will reduce the total cost of neonatal intensive care by substantially reducing length of respiratory care and length of stay in the hospital.
In summary, this study suggests that Heliox with HFNC, by unloading the lung, decreases the workload of the diaphragm, results in a more efficient breathing pattern and thereby decreases diaphragm injury. This approach is supportive of attenuating respiratory failure in the setting of premature lung predisposed to RDS or in injured lungs characterized by BPD. Future studies need to focus on reciprocating similar results of Heliox with noninvasive ventilation in human neonates over a longer duration to assess the impact on reducing RDS severity and/or BPD, and decreasing calories expended on work of breathing, thereby improving growth and also possibly shortening length of stay.
Acknowledgments
This study was funded through Nemours Research Programs, NIH COBRE Grant no. 8 P20 GM103464 (T. H. S.), NIH Grant no. 5T32 GM008562-17 (ER) Pediatric pharmacology fellowship funded by NICHD under the Best Pharmaceuticals for Children Act, and restricted research funds (MRW). This work was performed at the Nemours/Alfred I. duPont Hospital for Children and Temple University School of Medicine. The study was IACUC approved.
References
- 1.Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123(6):1562–1573. doi: 10.1542/peds.2008-1962. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, 3rd, Watterberg KL, Saha S, Das A, Higgins RD. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Northway WH, Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. The New England journal of medicine. 1967;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 4.Deoras KS, Greenspan JS, Wolfson MR, Keklikian EN, Shaffer TH, Allen JL. Effects of inspiratory resistive loading on chest wall motion and ventilation: differences between preterm and full-term infants. Pediatr Res. 1992;32(5):589–594. doi: 10.1203/00006450-199211000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Allen JL, Wolfson MR, McDowell K, Shaffer TH. Thoracoabdominal asynchrony in infants with airflow obstruction. The American review of respiratory disease. 1990;141(2):337–342. doi: 10.1164/ajrccm/141.2.337. [DOI] [PubMed] [Google Scholar]
- 6.Jiang TX, Reid WD, Belcastro A, Road JD. Load dependence of secondary diaphragm inflammation and injury after acute inspiratory loading. American journal of respiratory and critical care medicine. 1998;157(1):230–236. doi: 10.1164/ajrccm.157.1.9702051. [DOI] [PubMed] [Google Scholar]
- 7.Reid WD, Belcastro AN. Chronic resistive loading induces diaphragm injury and ventilatory failure in the hamster. Respiration physiology. 1999;118(2-3):203–218. doi: 10.1016/s0034-5687(99)00089-4. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan JS, Miller TL, Shaffer TH. The neonatal respiratory pump: a developmental challenge with physiologic limitations. Neonatal network : NN. 2005;24(5):15–22. doi: 10.1891/0730-0832.24.5.15. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer TH, Alapati D, Greenspan JS, Wolfson MR. Neonatal non-invasive respiratory support: physiological implications. Pediatric pulmonology. 2012;47(9):837–847. doi: 10.1002/ppul.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squires AJ, Hyndman M. Prevention of nasal injuries secondary to NCPAP application in the ELBW infant. Neonatal network : NN. 2009;28(1):13–27. doi: 10.1891/0730-0832.28.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400–1405. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, Stump A, Shaffer TH, Dysart K. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatric pulmonology. 2011;46(1):67–74. doi: 10.1002/ppul.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenspan JS, Wolfson MR, Shaffer TH. Airway responsiveness to low inspired gas temperature in preterm neonates. The Journal of pediatrics. 1991;118(3):443–445. doi: 10.1016/s0022-3476(05)82165-1. [DOI] [PubMed] [Google Scholar]
- 14.Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, Pyon KH. Work of breathing using high-flow nasal cannula in preterm infants. J Perinatol. 2006;26(8):476–480. doi: 10.1038/sj.jp.7211530. [DOI] [PubMed] [Google Scholar]
- 15.Barach AL. The Therapeutic Use of Helium. JAMA. 1936;107(16):1273–1280. [Google Scholar]
- 16.Barach AL, Eckman M. The Effects of Inhalation of Helium Mixed with Oxygen on the Mechanics of Respiration. J Clin Invest. 1936;15(1):47–61. doi: 10.1172/JCI100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess DR, Fink JB, Venkataraman ST, Kim IK, Myers TR, Tano BD. The history and physics of heliox. Respir Care. 2006;51(6):608–612. [PubMed] [Google Scholar]
- 18.Mink SN, Wood LD. How does HeO2 increase maximum expiratory flow in human lungs? J Clin Invest. 1980;66(4):720–729. doi: 10.1172/JCI109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otis AB, Bembower WC. Effect of gas density on resistance to respiratory gas flow in man. Journal of applied physiology. 1949;2(6):300–306. doi: 10.1152/jappl.1949.2.6.300. [DOI] [PubMed] [Google Scholar]
- 20.Allan PF, Thomas KV, Ward MR, Harris AD, Naworol GA, Ward JA. Feasibility study of noninvasive ventilation with helium-oxygen gas flow for chronic obstructive pulmonary disease during exercise. Respir Care. 2009;54(9):1175–1182. [PubMed] [Google Scholar]
- 21.Elleau C, Galperine RI, Guenard H, Demarquez JL. Helium-oxygen mixture in respiratory distress syndrome: a double-blind study. The Journal of pediatrics. 1993;122(1):132–136. doi: 10.1016/s0022-3476(05)83506-1. [DOI] [PubMed] [Google Scholar]
- 22.Hess D, Chatmongkolchart S. Techniques to avoid intubation: noninvasive positive pressure ventilation and heliox therapy. IntAnesthesiolClin. 2000;38(3):161–187. doi: 10.1097/00004311-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Hess DR. Heliox and noninvasive positive-pressure ventilation: a role for heliox in exacerbations of chronic obstructive pulmonary disease? Respir Care. 2006;51(6):640–650. [PubMed] [Google Scholar]
- 24.Liet JM, Ducruet T, Gupta V, Cambonie G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database Syst Rev. 2010;(4):CD006915. doi: 10.1002/14651858.CD006915.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Martinon-Torres F. Noninvasive ventilation with helium-oxygen in children. J Crit Care. 2012;27(2):220, e221–229. doi: 10.1016/j.jcrc.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Migliori C, Gancia P, Garzoli E, Spinoni V, Chirico G. The Effects of helium/oxygen mixture (heliox) before and after extubation in long-term mechanically ventilated very low birth weight infants. Pediatrics. 2009;123(6):1524–1528. doi: 10.1542/peds.2008-0937. [DOI] [PubMed] [Google Scholar]
- 27.Phatak RS, Pairaudeau CF, Smith CJ, Pairaudeau PW, Klonin H. Heliox with inhaled nitric oxide: a novel strategy for severe localized interstitial pulmonary emphysema in preterm neonatal ventilation. Respir Care. 2008;53(12):1731–1738. [PubMed] [Google Scholar]
- 28.Wolfson MR, Bhutani VK, Shaffer TH, Bowen FW., Jr Mechanics and energetics of breathing helium in infants with bronchopulmonary dysplasia. The Journal of pediatrics. 1984;104(5):752–757. doi: 10.1016/s0022-3476(84)80961-0. [DOI] [PubMed] [Google Scholar]
- 29.Orsini AJ, Stefano JL, Leef KH, Jasani M, Ginn A, Tice L, Nadkarni VM. Heliox improves pulmonary mechanics in a pediatric porcine model of induced severe bronchospasm and independent lung mechanical ventilation. Critical care. 1999;3(2):65–70. doi: 10.1186/cc311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nawab US, Touch SM, Irwin-Sherman T, Blackson TJ, Greenspan JS, Zhu G, Shaffer TH, Wolfson MR. Heliox attenuates lung inflammation and structural alterations in acute lung injury. Pediatric pulmonology. 2005;40(6):524–532. doi: 10.1002/ppul.20304. [DOI] [PubMed] [Google Scholar]
- 31.Colnaghi M, Pierro M, Migliori C, Ciralli F, Matassa PG, Vendettuoli V, Mercadante D, Consonni D, Mosca F. Nasal continuous positive airway pressure with heliox in preterm infants with respiratory distress syndrome. Pediatrics. 2012;129(2):e333–338. doi: 10.1542/peds.2011-0532. [DOI] [PubMed] [Google Scholar]
- 32.Wolfson MR, Hirschl RB, Jackson JC, Gauvin F, Foley DS, Lamm WJ, Gaughan J, Shaffer TH. Multicenter comparative study of conventional mechanical gas ventilation to tidal liquid ventilation in oleic acid injured sheep. Asaio J. 2008;54(3):256–269. doi: 10.1097/MAT.0b013e318168fef0. [DOI] [PubMed] [Google Scholar]
- 33.Miller TL, Blackson TJ, Shaffer TH, Touch SM. Tracheal gas insufflation-augmented continuous positive airway pressure in a spontaneously breathing model of neonatal respiratory distress. Pediatric pulmonology. 2004;38(5):386–395. doi: 10.1002/ppul.20094. [DOI] [PubMed] [Google Scholar]
- 34.Giordano K, Rodriguez E, Green N, Armani M, Richards J, Shaffer TH, Attia MW. Pulmonary function tests in emergency department pediatric patients with acute wheezing/asthma exacerbation. Pulm Med. 2012:724139. doi: 10.1155/2012/724139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen JLGJ, Deoras KS, Keklikian MR, Wolfson MR, Shaffer TH. Interaction between chest wall motion and lung mechanics in normal infants and infants with bronchopulmonary dysplasia. Pediatric Pulmonology. 1991;11(1):37–43. doi: 10.1002/ppul.1950110107. [DOI] [PubMed] [Google Scholar]
- 36.Locke RGJ, Shaffer TH, Rubenstein SD, Wolfson MR. Effect of nasal CPAP on thoracoabdominal motion in neonates with respiratory insufficiency. Pediatric Pulmonology. 1991;11(3):259–264. doi: 10.1002/ppul.1950110313. [DOI] [PubMed] [Google Scholar]
- 37.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 38.Muscle biopsy: a practical approach. Saunders Elsevier Limited; Philadelphia, PA: 2007. A DVaSC. [Google Scholar]
- 39.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol. 1990;258(3 Pt 1):C429–435. doi: 10.1152/ajpcell.1990.258.3.C429. [DOI] [PubMed] [Google Scholar]
- 40.Reid WD, Huang J, Bryson S, Walker DC, Belcastro AN. Diaphragm injury and myofibrillar structure induced by resistive loading. Journal of applied physiology. 1994;76(1):176–184. doi: 10.1152/jappl.1994.76.1.176. [DOI] [PubMed] [Google Scholar]
- 41.Singhaus CJ, Utidjian LH, Akins RE, Miller TL, Shaffer TH, Touch SM. Growth and development in a heliox incubator environment: a long-term safety study. Neonatology. 2007;91(1):28–35. doi: 10.1159/000096968. [DOI] [PubMed] [Google Scholar]
- 42.Barnett TB. Effects of helium and oxygen mixtures on pulmonary mechanics during airway constriction. Journal of applied physiology. 1967;22(4):707–713. doi: 10.1152/jappl.1967.22.4.707. [DOI] [PubMed] [Google Scholar]
- 43.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Archives of disease in childhood Fetal and neonatal edition. 2008;93(6):F455–461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]