Abstract
Objective
The primary aim of this exploratory investigation was to determine if there are differences in cortical activation of children with spastic diplegic cerebral palsy (CP) and typically developing children during gait.
Methods
Functional near-infrared spectroscopy was used to measure the concentration of oxygenated hemoglobin that was present in the supplementary motor area, pre-central gyrus, post-central gyrus and superior parietal lobule as the children walked on a treadmill. A sagittal plane video was concurrently collected and later digitized to quantify the temporal gait variations.
Results
1) The children with CP had an increased amount of activation in the sensorimotor cortices and superior parietal lobule during gait, 2) The children with CP had a greater amount of variability or error in their stride time intervals, and 3) an increased amount of error in the temporal gait kinematics was associated with an increased amount of activity across the cortical network.
Conclusion
Our results suggest that the perinatal damage and subsequent neural reorganization that occurs with spastic diplegic CP may impact the functional cortical activity for controlling gait. Furthermore, our results imply the increased cortical activity of the somatosensory cortices and superior parietal cortices may underlie the greater amount of error in the temporal gait kinematics.
Keywords: Walking, Variability, Motor Control, Mobile Brain Imaging
Introduction
One of the leading causes of childhood disability is cerebral palsy (CP), which often results from a perinatal brain injury. Over 90% of these children present motor impairments that result in a gait pattern that is slower, less coordinated, and has a greater amount of variability or errors in the temporal kinematics [1,2]. A considerable amount of effort has been devoted to using structural magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) to understand if damage in the respective lobules and fiber tracts is related to the atypical motor performance seen in children with CP. The general consensus is that children who have a higher Gross Motor Function Classification Score (GMFCS) tend to have more extensive damage to the corticospinal and thalamocortial pathways [3–5]. In addition, it has been further reported that the extent of the damage to the these tracts is related to lower extremity weakness and deficient gait biomechanics [5,6].
Despite the noted structural damage, numerous transcranial magnetic stimulation (TMS) investigations have shown that the sensorimotor cortices of children with CP often dynamically rewire themselves throughout development. For example, in children with more severe hemiplegic presentations, the ipsilateral homologue cortices often assume the role of the damaged contralateral cortices that would normally be involved in the control of movement [7,8]. Likewise, in children with less severe brain injury, it has been shown that the locale of neuronal populations that control the leg muscles are more lateral in the homunculus topology [9], which suggests cortical reorganization of neural representations of the leg.
Very few investigations have evaluated the activity of the sensorimotor cortices during movement in children with CP [10–14]. The outcomes of these functional MRI (fMRI) studies have indicated that the unaffected hemispheres in children with hemiplegic CP often assume the role of the damaged hemispheres while performing a finger-to-thumb opposition motor task [10,11,13]. In addition, it has been shown that bilateral activation of the sensorimotor cortices and the contralateral premotor cortex often occurs when children with spastic diplegic and quadraplegic presentations perform a finger-to-thumb opposition task [12]. It is unknown how these differences in neural activity affect the motor performance of children with CP since biomechanical data was not concurrently collected during these experiments. In addition, the ecological validity of these experimental outcomes is limited because they are based on simplified motor tasks. Although these experimental outcomes are enlightening, they still do not address if the neuroplastic changes normalize the activity of the sensorimotor cortices, or result in additional challenges to nervous system function that may increase the probability for errors in the motor performance.
Functional near-infrared spectroscopy (fNIRS) is an emerging neuroimaging technique that measures the hemodynamic changes that occur in cortical tissues during movement [15]. During fNIRS experiments, children wear a cap that contains a series of photon emitters and detectors, and this cap is situated on the scalp near a brain region of interest. The emitters produce infrared light that penetrates the skull and is absorbed or refracted by hemoglobin in the underlying neural tissues. The total refraction measured by the detectors is used to quantify the amount of oxygenated (oxyHb) and de-oxygenated (deoxyHb) hemoglobin in local neural tissues. It has been well established that a greater concentration of oxyHb is associated with a greater amount of activity in the underlying neural tissues [15].
fNIRS has an advantage over other imaging modalities because it is less susceptible to head movements, quiet, does not require a confined environment, and it can be used to evaluate cortical activity during ecologically valid motor tasks such as walking [16,17]. Prior fNIRS experiments have shown that the amount of cortical activity is greater during challenging walking conditions [16,17]. For example, during walking, the amount of activity across the cortical network is reduced when stroke patients are supported by an overhead support system [17]. Additionally, it has also been shown that a greater amount of cortical activity is associated with a more variability or errors in the gait temporal kinematics [16]. Based on these novel insights, we suspect that children with CP would have a greater amount of cortical activity while walking because their spatiotemporal kinematics are more variable and potentially more challenging to control [16].
In this exploratory investigation, we used fNIRS to measure the concentration of oxyHb that was present in the sensorimotor cortices as children with and without CP walked on a treadmill. We quantified the differences in the amount of activation in the cortical networks that are involved in the control of gait by monitoring the change in the concentration of oxyHb. Our primary aim was to explore if children with CP have an increased amount of neural activity across the cortical network during gait compared with typically developing (TD) children. Our secondary aims were to further probe the relationship between the amount of neural activity, and the amount of variability seen in the gait temporal kinematics of the children with CP.
Materials and Methods
Participants
The University of Nebraska Medical Center’s Institutional Review Board approved this investigation. The participating children were recruited from the physical therapy clinic at the University of Nebraska Medical Center, where they had previously received treatment or had undergone a gait analysis. Four children with spastic diplegic CP (Age = 11.0 ± 4 yrs.) and eight TD children (Age = 13.2 ± 3 yrs.) volunteered to participate in this investigation. Written informed consent was acquired from the parents and the children assented to participate in the experiment. The children with CP had a previously defined diagnosis of CP by a pediatric neurologist. For all of the children, the resulting CP was a result of periventricular leukomalacia, and they had a spastic diplegia presentation. Children with known large occupying lesions and/or volume loss that would have affected the cortical tissue were not included in our investigation. Three of the children with CP had GMFCS of II and wore orthotics during ambulation. The other child with CP was classified as GMFCS III and required forearm crutches for ambulation. The participating TD children were free of any neurologic and/or orthopedic impairment that would have affected their gait. Further descriptions of the participating children are detailed in Table 1.
Table 1.
Participant Demographics.
| Group | Height (meters) | Mass (kg) | Gender | Age (year) | GMFCS |
|---|---|---|---|---|---|
| CP 1 | 1.38 | 32.6 | Female | 9 | II |
| CP 2 | 1.10 | 16.3 | Female | 7 | II |
| CP 3 | 1.53 | 30.4 | Male | 11 | III |
| CP 4 | 1.63 | 52.6 | Male | 14 | II |
| TD 1 | 1.72 | 70.3 | Female | 18 | N/A |
| TD 2 | 1.56 | 51.7 | Female | 11 | N/A |
| TD 3 | 1.77 | 47.6 | Male | 14 | N/A |
| TD 4 | 1.57 | 54.4 | Female | 12 | N/A |
| TD 5 | 1.66 | 57.6 | Female | 11 | N/A |
| TD 6 | 1.30 | 23.6 | Female | 7 | N/A |
| TD 7 | 1.56 | 44.9 | Female | 14 | N/A |
| TD 8 | 1.44 | 41.7 | Female | 9 | N/A |
GMFCS = Gross Motor Function Classification Score, N/A = Not applicable.
Experimental Paradigm
Each child performed two sequential sessions and each session consisted of five alternating blocks of standing-still for 30 seconds and 30 seconds of walking at a speed of 0.45 m/s on a programmable treadmill (RTM 4000, Biodex, Shirley, NY). Participants held onto the handrails at all times, maintained a stable head position, and kept their eyes fixated on a visual target that was positioned 30.5 cm away at head level.
fNIRS Data Acquisition
A continuous wave fNIRS system (ETG-4000 Optical System; Hitachi Medical Corporation) utilizing two different wavelengths (~695 and ~830 nm) was used for this study. Relative changes in the absorption of near-infrared light were sampled at 10 Hz, and these measures were converted into relative concentration changes of oxyHb based on the modified Beer-Lambert approach [18]. The overall optical system consisted of eight infrared optode emitters and eight optode detectors, arranged in a 4 X 4 square array with 3 cm inter-optode spacing, that were affixed to a custom Lycra hat that was worn on the head. This configuration provided 24-channels that measured the hemodynamic changes in the underlying neural tissues. The optodes were positioned on the participant’s head using the International 10/20 system for EEG recording, with Cz located beneath the center of the front two rows of optodes (i.e., between channels 5–6). All optodes were connected to lightweight fiber optic cables that allowed for transmission of the infrared light to the Hitachi ETG-4000 workstation. The fiber optic cables were tethered to an overhead support system, which reduced movement in the cabling, and thereby stabilized the cap’s position during the respective walking conditions.
fNIRS Data Analysis
The measured (oxyHb) hemodynamic waveforms were filtered using a 0.01 Hz high pass filter and a 5.0 s moving average. To increase the signal-to-noise ratio and to exclude extraneous biological noise (e.g., respiratory and cardio-artifacts), principal component analyses were conducted on the respective waveforms taken from all channels [15]. Components with 0.25 or lower correlation with the reference waveform, which corresponded to the expected hemodynamic function, were filtered from the data while those with correlations above 0.25 were passed and included in the final reconstruction of the individual channel time series. The reference waveform was a trapezoidal function with an upward slope that started at the onset of walking and had a 5 second time to peak, a 25 second peak duration, and a 5 second downward slope. These data were used to create average oxyHb trial waveforms for each channel. Changes in the amount of activation in the sensorimotor cortices were evaluated relative to their specific baselines, which were determined from the 2.5 seconds that immediately preceded the onset of walking.
Changes in the amount of activation in the sensorimotor area were assessed by separating the sampled channels into groups that were situated over the supplementary motor area (SMA), precentral gyrus, postcentral gyrus, and superior parietal lobule (for a complete description of the methodology, see Okamoto et al. [19] and Wilson et al. [20]). The average maximum concentration of oxyHb in each of the channel groups was evaluated. Additionally, the average maximum concentration of oxyHb across all the channels was evaluated to quantify the overall cortical activity.
Gait Analysis
Two-dimensional sagittal plane video was collected at 60 Hz and the respective heel-strikes were manually digitized with the SIMI motion capture software (SIMI Reality Motion Systems, Unterschleissheim, Germany). The stride, stance and swing times were subsequently calculated with custom software. The coefficient of variation of the respective temporal kinematic measures was calculated to quantify the amount of variability present in the gait pattern.
Statistical Analysis
A mixed model (group × cortical area) ANOVA was used to determine if there were significant differences in the concentration of oxyHb in the SMA, precentral gyrus, postcentral gyrus, and superior parietal lobule between the children with CP and the TD children. Significant interaction effects were followed-up with t-tests. Independent t-tests were also used to examine the differences between children with CP and TD children for the respective temporal kinematic measures, and the average maximum oxyHb concentration across all channels. Pearson product moment correlations were used to determine if there was a relationship between the amount of variability seen in the stride-time intervals, and the oxyHb concentration in each of the four regions of interest. These correlations were performed with the data collected from all children. We did not perform the correlations for the children with CP in isolation since our exploratory study was based on a few children with CP that had similar presentation. All statistical tests were performed at a 0.05 alpha level. Results in the text and graphs are presented as mean ± standard error of the mean.
Results
fNIRS Results
A representative oxyHb time series for a child with CP and a TD child are displayed in Figure 1. As this figure shows, the concentration of oxyHb increased during the active walking period and decreased during the non-active period (i.e., when the child was standing still). Moreover, there was a substantially greater amount of oxyHb across many of the channels for this representative child with CP during the walking period.
Figure 1.
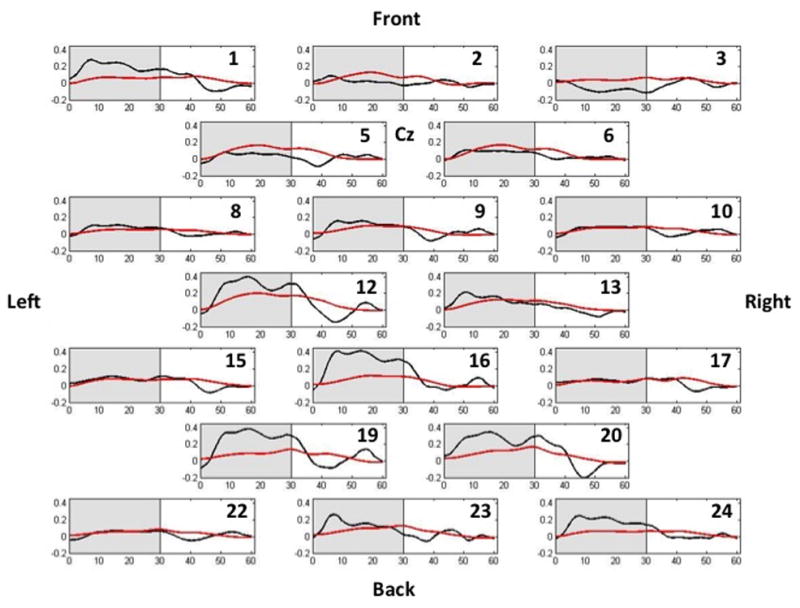
Representative time series of the oxyHb concentrations for all channels in children with CP (black lines) and TD children (red lines). The grayed area represents the active walking period, while the white areas represent the baseline period where the child is standing still. The approximate correspondence between channels and cortical areas was as follows: channels 1–3 and 5–6 were over the supplementary motor area, channels 8–10 and 12–13 were above the precentral gyrus, channels 12–13 and 15–17 were near the postcentral gyrus, and channels 19–20 and 22–24 were over the superior parietal lobule; see the Methods section for further information. As can be clearly discerned, the concentration of oxyHb was greater in children with CP across most channels. Units on the ordinate are Mmol × mm and seconds on the abscissa.
For the mixed model ANOVA, there was no significant group effect for oxyHb concentration across the cortical areas examined (F(1,10)= 3.92, P = 0.08). However, there was a significant cortical area × group interaction (F(3,30)= 4.20; P = 0.01), and post hoc tests revealed that the oxyHb concentration in the precentral gyrus (t(10)= 1.92; P= 0.03), postcentral gyrus (t(10)= 1.8; P= 0.04) and superior parietal lobule (t(10)= 2.20; P= 0.018) was greater in the children with CP compared with the TD children (Figure 2). The oxyHb concentration of the SMA was not different between the children with CP and TD children (t(10)= −1.10; P= 0.10).
Figure 2.
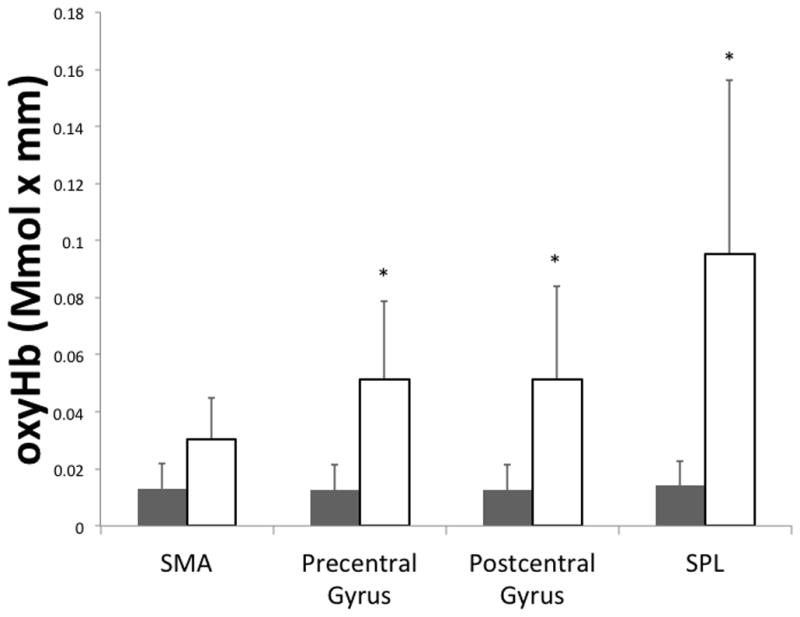
Average maximum oxyHb concentration in the respective cortical areas for children with CP (white) and TD children (gray). SMA is the supplementary motor area, and SPL is the superior parietal lobule. Error bars reflect the standard error of the mean. * p < 0.05.
Furthermore, our results showed that the children with CP had significantly greater oxyHb concentration across all channels (CP = 0.06 ± 0.06 Mmol × mm, TD = 0.01 ± 0.02 Mmol × mm; t(10)= 3.25; P=0.009), indicating a greater amount of neural activation overall.
Gait Analysis Results
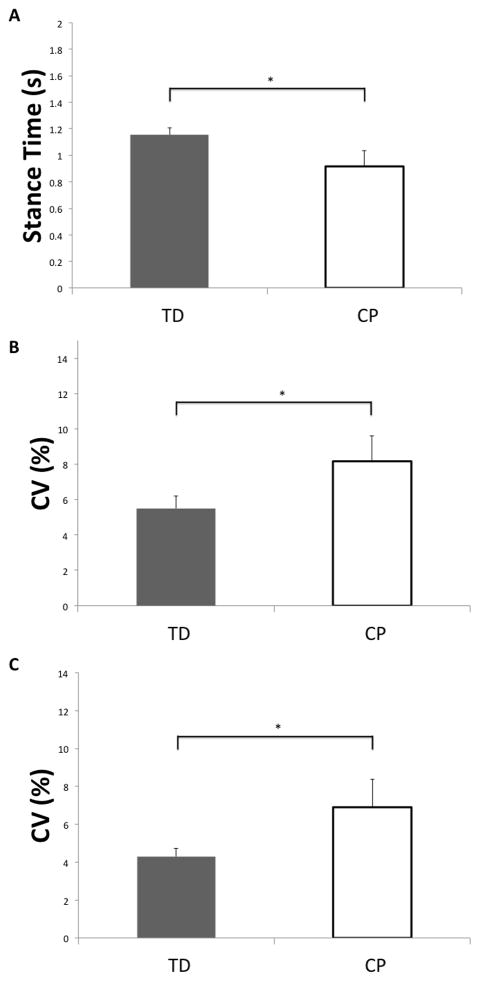
For the temporal kinematics, children with CP had a shorter stance time interval (t(10)= −2.40; P = 0.03; Figure 3A), and a more variable stance time CV (t(10)=2.1; P= 0.05; Figure 3B). Children with CP also had a more variable stride time (t(10)=2.5; P= 0.03; Figure 3C), but no differences were detected between the two groups for the stride time interval (CP = 1.55 ± 0.22 s, TD = 1.74 ± 0.08 s; t(10) = −1.1; P=0.30). Finally, no differences were detected between the two groups for swing time interval (CP = 0.64 ± 0.31 s, TD = 0.58 ± 0.03 s; t(10)= 0.32; P= 0.75) or swing time variability (CP = 10.07 ± 1.69 %, TD = 8.34 + 0.66 %; t(10)= 1.30; P= 0.22).
Figure 3.
A) Mean stance time intervals for children with CP and TD children, B) coefficient of variation for the stance time intervals, C) coefficient of variation for the stride time intervals. Error bars reflect the standard error of the mean. * p < 0.05.
Neurobehavioral Correlations
The correlations between the amount of oxyHb concentration in the respective cortical areas and the measured gait variables are shown in Table 2. Our results show that there were strong positive correlations between the oxyHb concentration in the superior parietal lobule and variability in the stride and stance time intervals for all the children. We also found a moderate correlation between variability in the stride time intervals and the oxyHb concentration in the postcentral gyrus. Lastly, moderate positive correlations were found between the stance time intervals and the respective oxyHb concentration in the pre and post central gyri.
Table 2.
Pearson correlations between the oxyHb concentration in respective cortical areas and the biomechanical variables.
| Cortical Area | Stance Time | Stance Time CV | Stride Time | Stride Time CV |
|---|---|---|---|---|
| Supplementary Motor Area | −0.30 | 0.40 | −0.42 | 0.28 |
| Pre Central Gyrus | −0.32 | 0.56* | −0.56* | 0.47 |
| Post Central Gyrus | −0.33 | 0.63* | −0.49* | 0.55* |
| Superior Parietal Lobule | −0.50* | 0.76* | −0.33 | 0.77* |
CV= Coefficient of Variation
= P < 0.05
Discussion
This exploratory investigation was innovative in that we used a combination of fNIRS and biomechanical analysis to understand how CP may impact the connection between the brain and the body for the control of gait. The biomechanical results were consistent with the previous studies that have also showed that the children with CP spent less time in single support and have a more variable gait [21–24]. The current consensus is that less time is spent in single support because the center of mass is inherently unstable in this position and has a higher probability of exceeding the foot support boundaries, which would result in a fall [24]. The variability that comprises the gait temporal kinematics may result from an increased amount of errors and/or intentional corrections in the timings to accommodate for internal or external perturbations [21–24]. Based on this notion, we suspect that the increased variability seen in the gait of the children with CP indicates that they had greater errors in selecting the proper adjustments for controlling the walking pattern. In other words, the heightened variability indicates that the control of the gait pattern was more burdensome for the children with CP.
The concentration of oxyHb found across the SMA, precentral gyrus, postcentral gyrus and superior parietal lobule was used as a proxy for the degree of cortical involvement during gait [15]. Overall our results show that children with CP require greater cortical activity to control their gait, and that the increased neural demand is associated with greater amount of variability or errors in the gait temporal kinematics. These results are the first to suggest that perinatal damage and the subsequent neural reorganization that occurs with CP may not normalize the functional cortical activity that is involved in the control of gait. However, based on the fNIRS data alone it is difficult to determine if the hyper-activation across the cortical network represents an increased neural demand and/or a recruitment of compensatory networks that aid the redistributed neuronal groups that that are involved in the control of gait.
It is fairly well accepted that the primary motor and somatosensory cortices areas are involved in the control of gait [16, 17, 25, 26]. Furthermore, it has recently been shown that the amount of activity present in these cortical areas is dependent upon the complexity of gait task [16]. The heightened cortical activity seen in a more complex task is thought to represent greater demand on the sensorimotor cortices for regulating the precision of the gait kinematics [16]. Based on this notion, the greater activity in sensorimotor cortices of the children with CP may reflect an increased demand for controlling the accuracy of the gait kinematics. This demand may partly arise from faults in the performance of the musculoskeletal machinery (i.e., spasticity, joint contractures, skeletal torsions, weakness), and/or sensory deficits that impact the ability to properly monitor the ongoing gait kinematics [30]. Essentially, less predictable performance in the musculoskeletal and sensory systems may require greater neural computations for the maintenance of the ongoing gait kinematics.
The children with CP also had an increased amount of activity in the superior parietal cortex. The superior parietal cortex has been implicated in the sensorimotor integration process, maintenance of the internal representation of the body’s state, and the sustainment of attention to peripheral sensory feedback [27–30]. We suspect that the greater neural activity in this cortical area indicates that children with CP require greater neural resources to actively maintain an internal model of the ongoing gait kinematics, and to detect errors in their planned movement trajectories. This may explain why we found that the children with CP had greater variability or errors in their gait temporal kinematics.
When we combined the data from all the children, we found a significant positive correlation between gait variations and the amount of neural activity in the postcentral gyrus and superior parietal lobule. This correlation implies that a greater amount of errors in the gait temporal kinematics are related to higher demands being placed on these cortical areas. This also suggests that the greater activations observed in the primary somatosensory and superior parietal cortices of children with CP are related to errors in the temporal gait kinematics. We suspect that this link may be mediated by the sensory deficits seen in children with CP, since both of these cortical areas are involved in the processing and communication of sensory information. These results further highlight the notion that sensory deficits may play a substantial role in the motor control problems seen in children with CP.
This investigation is novel in that we have provided insight that link between the activation of the sensorimotor cortices and the gait temporal kinematics. Very few investigations have attempted to make this connection largely due to the inability of the imaging technology to monitor the brain’s performance during walking. The results shown here and elsewhere indicate that it is feasible to use fNIRS to monitor changes in cortical activity during walking [16, 17]. These results are promising because fNIRS has been shown to have good spatial correlation between the cortical hemodynamic responses measured with fMRI [31, 32]. Our preliminary work has established that fNIRS is a viable technology that can be used to elucidate the role the sensorimotor cortices have on the walking patterns of children with CP. These results are promising because they represent a springboard for our future investigations that will use a combination of biomechanical measures (i.e., joint kinematic, electromyography) and fNIRS to assess the efficacy of the current therapeutic trends that are being used to improve the walking patterns of children with CP. Based on the results presented in this investigation, we suspect that those children who are classified as unresponsive to the current gait training trends will have a higher amount of oxyHb than children with CP who are classified as responders.
Conclusions
Our exploratory results show that the children with spastic diplegic CP have an increased amount of activation in sensorimotor and superior parietal cortices during gait. This suggests that the cortical networks in children with spastic diplegic CP are more challenged to control the temporal gait kinematics. In addition, our results show that there is a link between the amount of cortical activity and the gait kinematic variations. Further exploration of this connection will enhance our understanding of how the aberrant cortical activity seen in children with CP is related to the noted motor impairments during gait.
Acknowledgments
We would like to thank Joan Deffeyes for her assistance with the data collections. Funding for this project was provided by the Hattie B. Munroe Foundation and the NICHD division of the National Institutes of Health (1R21HD077532-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Able MF, Damiano DL. Strategies for increasing walking speed in diplegic cerebral palsy. J Ped Orthop. 1996;16:753–8. doi: 10.1097/00004694-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Kurz MJ, Arpin DJ, Corr B. Differences in the dynamic gait stability of children with cerebral palsy and typically developing children. Gait Posture. 2012;36:600–4. doi: 10.1016/j.gaitpost.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Murakami A, Morimoto M, Yamada K, Kizu O, Nishimura A, Nichimura T, et al. Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics. 2008;122:500–6. doi: 10.1542/peds.2007-2816. [DOI] [PubMed] [Google Scholar]
- 4.Rha DW, Chang WH, Kim J, Sim EGS, Park ES. Comparing quantitative tractography metrics of motor and sensory pathways in children with periventricular leukomalacia and different levels of gross motor function. Neuroradiol. 2012;54:615–21. doi: 10.1007/s00234-011-0996-2. [DOI] [PubMed] [Google Scholar]
- 5.Hoon AH, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49:745–50. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 7.Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–47. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- 8.Holstrom L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52:145–52. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 9.Maegaki Y, Maeoka Y, Ishii S, Eda I, Ohtagaki A, Kitahara T, et al. Central motor reorganization in creegral palsy patients with bilateral cerebral lesions. Pediatric Res. 1999;45:559–67. doi: 10.1203/00006450-199904010-00016. [DOI] [PubMed] [Google Scholar]
- 10.Vandermeeren Y, Sebire G, Garndin CB, Thonnard JL, Schlogel X, de Volder AG. Functional reorganization of brain in children affected with congenital hemiplegia: fMRI study. Neuroimage. 2003;20:289–301. doi: 10.1016/s1053-8119(03)00262-3. [DOI] [PubMed] [Google Scholar]
- 11.Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL. Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol. 2001;49:320–7. [PubMed] [Google Scholar]
- 12.Lee JJ, Lee DR, Shin YK, Lee NG, Han BS, You SU. Comparative neuroimaging in children with cerebral palsy using fMRI and a novel EEG-based brain mapping during a motor task – a preliminary investigation. Neuro Rehabilitation. 2013;32:279–85. doi: 10.3233/NRE-130845. [DOI] [PubMed] [Google Scholar]
- 13.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis a TMS and fMRI study. Brain. 2002;125:2222–37. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- 14.Phillips JP, Sulivan KJ, Burtner PA, Caprihan A, Provost B, Bernitsky-Beddingfield A. Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive bodyweight-supported treadmill training: a pilot study. Dev Med Child Neurol. 2007;49:39–44. doi: 10.1017/s0012162207000102.x. [DOI] [PubMed] [Google Scholar]
- 15.Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23:S275–88. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage. 2012;59:1602–7. doi: 10.1016/j.neuroimage.2011.08.084. [DOI] [PubMed] [Google Scholar]
- 17.Miyai I, Suzuki M, Hatakenaka M, Kubota K. Effect of body weight support on cortical activation during gait in patients with stroke. Exp Brain Res. 2006;169:85–91. doi: 10.1007/s00221-005-0123-x. [DOI] [PubMed] [Google Scholar]
- 18.Obrig H, Villringer A. Beyond the visible – imaging the human brain with light. J Cerebral Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Wilson TW, Kurz MJ, Arpin DJ. Functional specialization within the supplementary motor area: A fNIRS study of bimanual coordination. Neuroimage. doi: 10.1016/j.neuroimage.2013.04.112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies BL, Kurz MJ. Children with cerebral palsy have greater stochastic features present in their variability of their gait kinematics. Res Developl Disabil. 2013;34:3648–3653. doi: 10.1016/j.ridd.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kurz MJ, Arpin DJ, Corr B. Differences in the dynamic gait stability of children with cerebral palsy and typically developing children. Gait Posture. 2012;36:600–604. doi: 10.1016/j.gaitpost.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Katz-Leurer, Rotem H, Keren O, Meyer S. Balance abilities and gait characteristics in post-traumatic brain injury, cerebral palsy and typically developed children. Develop Neurorehab. 2009;12:100–105. doi: 10.1080/17518420902800928. [DOI] [PubMed] [Google Scholar]
- 24.Hsue BJ, Miller F, Su FC. The dynamic balance of the children with cerebral palsy and typical developing during gait. Part I: Spatial relationship between COM and COP trajectories. Gait Posture. 2009;29:465–70. doi: 10.1016/j.gaitpost.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228:183–6. doi: 10.1016/s0304-3940(97)00381-9. [DOI] [PubMed] [Google Scholar]
- 26.La Fourgere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, et al. Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–98. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 27.Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nature Neurosci. 1999;2:563–7. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- 28.Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nature Neurosci. 1998;1:529–33. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- 29.Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–4. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 30.Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano DL. Joint-position sense and kinesthesia in cerebral palsy. Arch Phys Med Rehabili. 2009;90:447–53. doi: 10.1016/j.apmr.2008.08.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 2006;29:368–82. doi: 10.1016/j.neuroimage.2005.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17:719–31. [PubMed] [Google Scholar]