Abstract
Background
Reduced exercise tolerance from impaired cardiac output is an important criterion for left ventricular assist device (LVAD) implantation. However, little is known about how exercise capacity changes following LVAD, and how changes compare with patients undergoing heart transplant.
Methods and Results
We compared changes in cardiopulmonary exercise testing performed pre- and post-operatively in patients who underwent HeartMate II LVAD implantation (n=25) and heart transplantation (n=74) at the Mayo Clinic in Rochester, Minnesota from 2007–2012. Pre-operatively, patients undergoing LVAD and transplant had markedly reduced exercise time (mean 5.1 minutes [45% predicted] and 5.0 minutes [44% predicted], respectively), low peak VO2 (mean 11.5 ml/kg/min [43% predicted] and 11.9 mL/kg/min [38% predicted]), and abnormal ventilatory gas exchange (VE/VCO2 nadir 39.4 and 37.4). Following LVAD and transplant, there were similar improvements in exercise time (mean Δ +1.2 vs. 1.7 minutes, respectively, p=0.27) and VE/VCO2 nadir (mean Δ −3.7 vs. −4.2, p=0.74). However, peak VO2 increased post-transplant but did not change post-LVAD (mean Δ +5.4 vs. +0.9 mL/kg/min, respectively, p<0.001). Most patients (72%) had a peak VO2<14 mL/kg/min post-LVAD.
Conclusions
While improvements in exercise capacity and gas exchange are seen following LVAD and heart transplant, peak VO2 doesn’t improve post-LVAD and remains markedly abnormal in most patients.
Keywords: exercise capacity, left ventricular assist device, heart transplantation
INTRODUCTION
A limitation in exercise capacity is one of the hallmark features of patients with advanced heart failure (HF). Cardiopulmonary exercise testing (CPET) is a mainstay in the objective assessment of exercise capacity in patients with advanced HF. A marked reduction in peak oxygen consumption (VO2) is a widely established marker of adverse prognosis1, 2, and an important criterion in determining candidacy for heart transplantation3. However, the limited supply of donor organs and prolonged wait list times have prompted the development of alternative cardiac replacement strategies such as left ventricular assist devices (LVAD). With advances in technology and mechanical circulatory support, LVADs are increasingly being used in patients with advanced HF as a bridge to transplant (BTT) or as destination therapy (DT). Early on, patients were implanted with LVADs that provided pulsatile flow, but in the current era, patients primarily receive LVADs that deliver continuous flow. While pulsatile LVADs more closely mimicked normal physiologic conditions, continuous flow devices are smaller, more durable, and associated with better outcomes4.
However, information on exercise capacity changes after implantation of a continuous flow LVAD is limited, and how this may compare to patients treated with heart transplantation has not been comprehensively explored. Earlier reports have suggested that exercise performance remains suboptimal in some post-LVAD patients5–7, however, these studies are limited as CPET was not performed pre-LVAD for comparison and hence the effect of this intervention on exercise parameters is unknown. There have also been disparate reports regarding whether exercise capacity is comparable in patients who have had LVAD as compared to heart transplantation5–7, which is important given that LVADs are being considered as an alternative to heart transplantation in some patients. In addition, it is unknown how exercise capacity may change in women, older patients, and especially those treated with LVAD as DT rather than BTT, as these populations have not been represented in prior studies.
Therefore, we undertook the present study to investigate how CPET parameters change following continuous flow LVAD when implanted as DT and BTT, and how these changes compare to patients treated with heart transplantation.
METHODS
Patient Population
This was an observational retrospective cohort study including patients that underwent heart transplantation or LVAD implantation at the Mayo Clinic from 2007–2012. We included all patients who underwent LVAD implantation who had cardiopulmonary exercise testing (CPET) performed both pre- and post-LVAD. As we were interested in comparing changes in CPET parameters post-LVAD to post-transplant, we also included patients undergoing cardiac transplantation from 2007–2012 who had CPET performed pre- and post-transplant. We excluded patients who underwent transplantation of multiple organs. Given the age distribution in the LVAD population (youngest patient 37 years old), we excluded transplant patients who were <30 years old at the time of transplant. This study was approved by the Mayo Clinic Institutional Review Board.
Cardiopulmonary Exercise Testing
Symptom-limited treadmill exercise testing with respiratory gas exchange analysis was performed using a modified Naughton protocol (2-min workloads, 2 METs/min increments in work) as previously described8. Electrocardiograms were continuously monitored and blood pressure was assessed the last 30 s of each 2-min workload. Patients were encouraged to exercise to exhaustion. Exercise duration was expressed in minutes and in percent of age- and gender-predicted value9. Predicted exercise time was calculated according to the following formula: 17.1−0.13*age (in men) and 14.3−0.10*age (in women). The ratio of minute ventilation to carbon dioxide production (VE/VCO2), a measure of the efficiency of respiratory gas exchange, and peak oxygen consumption (peak VO2) were measured using a Medical Graphics metabolic cart (St. Paul, Minnesota). Calibration used gravimetric quality gases before each test and physiologic calibration for weekly quality control. VE/VCo2 nadir was taken as the lowest 30 second average during exercise. Peak VO2 was the highest averaged 30-s VO2 during exercise and was expressed as absolute peak-VO2 or normalized peak VO2 (percent of age, gender, and weight predicted). Quality of exercise effort was assessed by respiratory exchange ratio (RER) and the Borg Rating of Perceived Exertion Scale. This scale measures patient-perceived exertion with numeric values ranging from 0 to 20; values of 17 and above are indicative of very hard, extremely hard, and maximal exertion. Abnormal heart rate (HR) recovery was defined as a decline in HR of ≤12 bpm at one-minute after maximal exercise. CPET is routinely done as a part of the pre-transplant evaluation (unless the patient is too unstable to complete) and annually post-transplant. CPET is not a requirement at our institution pre-LVAD, as patients are often too unstable to complete the testing. Since 2010, the performance of CPET at one-year post LVAD has been encouraged. When two or more CPET were performed pre-LVAD or transplant, we used the one closest to the date of surgery. When two or more CPET were performed post-LVAD or transplant, we used the test performed closest to one year post-LVAD or transplant. All LVAD patients were on full LVAD support at the time of CPET.
Clinical Characteristics
Diabetes was defined by physician’s diagnosis or use of medications to treat diabetes at the time of LVAD implantation or heart transplant. Peripheral vascular disease was defined by physician’s diagnosis or the presence of >50% stenosis in a carotid artery on duplex ultrasound routinely performed pre-LVAD and heart transplant. Chronic obstructive pulmonary disease (COPD) was defined by physician’s diagnosis or the presence of moderate or severe obstruction on pulmonary function testing performed pre-LVAD and heart transplant. Body mass index (BMI) was calculated based on the height and weight at the time of LVAD implantation or heart transplantation. Left ventricular ejection fraction (EF) was recorded from transthoracic echocardiograms performed during routine clinical practice as previously described10.
Sensitivity Analysis
On average, the patients undergoing LVAD implantation were older than patients undergoing heart transplantation. To address the issue of whether differences in age may have impacted results observed, we performed a sensitivity analysis were we matched LVAD patients and heart transplant patients by age (± 3 years) and sex. To maximize the number of patients included, we matched up to 4 heart transplant patients per LVAD patient. As the overall findings were similar compared with the primary analysis, the results of the sensitivity analysis are presented in the Supplementary Materials.
Statistical Analysis
Baseline patient characteristics are presented as mean ± standard deviation, median (interquartile range) or number (%) as appropriate. Differences in baseline characteristics between LVAD and heart transplant patients were compared using t-tests for normally-distributed continuous variables, Wilcoxon rank sum test for non-normally distributed continuous variables, and χ2 or Fisher’s exact test for binary variables. To determine whether changes in CPET parameters occurred following LVAD and heart transplant, pre- and postoperative values were compared using paired t-tests. To detect whether changes occurring post-LVAD differed from changes post-transplant, we compared paired differences between groups using t-tests. Least-squares regression analysis modeling was used to estimate the relative contributions of the clinical variables to the change in normalized peak VO2 following LVAD implantation or heart transplantation. Variables with a significance level of p<0.1 were included in the multivariable model. Data were analyzed using Stata/SE 13.0. A p value of <0.05 was used as the level of significance in all analyses.
RESULTS
Study Population
From 2007–2012, there were 198 patients who had LVAD implantation, 181 of whom received the HeartMate II device, a continuous axial flow pump. In total, 25 patients who underwent LVAD (all HeartMate II) had CPET performed both pre- and post-operatively, and are included in this analysis. In the same time frame, there were 116 patients ≥30 years old that had a heart transplant, of whom 74 completed CPET both pre- and post-transplant and are included in this analysis. Patients undergoing LVAD implantation were older than transplant patients (mean 63 vs. 54 years, Table 1), though other baseline characteristics were similar. Baseline CPET was performed on average 10.9 ± 8.1 months prior to heart transplant and 3.0 ± 4.5 months prior to LVAD. The type of beta blocker prescribed at the time of CPET (69% carvedilol, 21% metoprolol succinate, 9% metoprolol tartrate) and total daily dose (median carvedilol dose 25 mg total daily, median metoprolol succinate/tartrate dose 50 mg daily) did not differ in patients pre-LVAD compared with pre-transplant. At CPET, the resting HR, blood pressure, exercise time, and peak VO2 were similar pre-LVAD and pre-transplant.
Table 1.
Baseline Patient Characteristics
| LVAD Patients (n=25) | Heart Transplant Patients (n=74) | P value | |
|---|---|---|---|
| Demographics, Comorbidities, and Medications | |||
| Age at transplant/LVAD, years | 63.4 (9.9) | 53.7 (10.5) | <0.001 |
| Female, N(%) | 6 (24.0) | 17 (23.0) | 0.92 |
| Ischemic etiology, N(%) | 10 (40.0) | 27 (36.5) | 0.75 |
| Diabetes, N(%) | 7 (28.0) | 13 (17.6) | 0.26 |
| Peripheral vascular disease, N(%) | 2 (8.0) | 4 (5.4) | 0.99 |
| COPD, N(%) | 3 (12.0) | 8 (10.8) | 0.99 |
| Body mass index, kg/m2 | 28.6 (4.5) | 28.2 (5.4) | 0.69 |
| * Atrial fibrillation, N(%) | 4 (16.0) | 16 (21.6) | 0.59 |
| Left ventricular ejection fraction, % | 22.0 (10.3) | 25.8(13.4) | 0.21 |
| Taking beta blocker, N(%) | 22 (88.0) | 67 (90.5) | 0.72 |
| LVAD parameters | |||
| Destination therapy, N(%) | 21 (84.0) | -- | |
| Pump speed at CPET (median, IQR) | 9400 (9200, 9600) | -- | |
| Preoperative CPET parameters | |||
| Resting HR, bpm | 75.6 (11.9) | 73.2 (12.5) | 0.42 |
| Resting blood pressure, mmHg | |||
| Systolic | 102.3 (16.6) | 97.1 (15.0) | 0.14 |
| Diastolic | 63.0 (9.0) | 63.4 (11.8) | 0.88 |
| Exercise time, minutes | 5.1 (1.4) | 5.0 (1.5) | 0.73 |
| Peak VO2, mL/kg/min | 11.5 (2.5) | 11.9 (3.4) | 0.54 |
| VE/VCO2 nadir | 39.4 (5.2) | 37.4 (7.3) | 0.21 |
All values shown are mean (standard deviation) unless otherwise noted.
Proportion of patients in atrial fibrillation at time of pre-operative CPET.
BPM= beats per minute, COPD= chronic obstructive pulmonary disease, CPET= cardiopulmonary exercise testing, HR= heart rate, IQR= interquartile range, LVAD= left ventricular assist device, VO2 = peak oxygen consumption, Ve/VCO2 = ratio of minute ventilation to carbon dioxide production
Changes in Cardiopulmonary Exercise Testing After LVAD
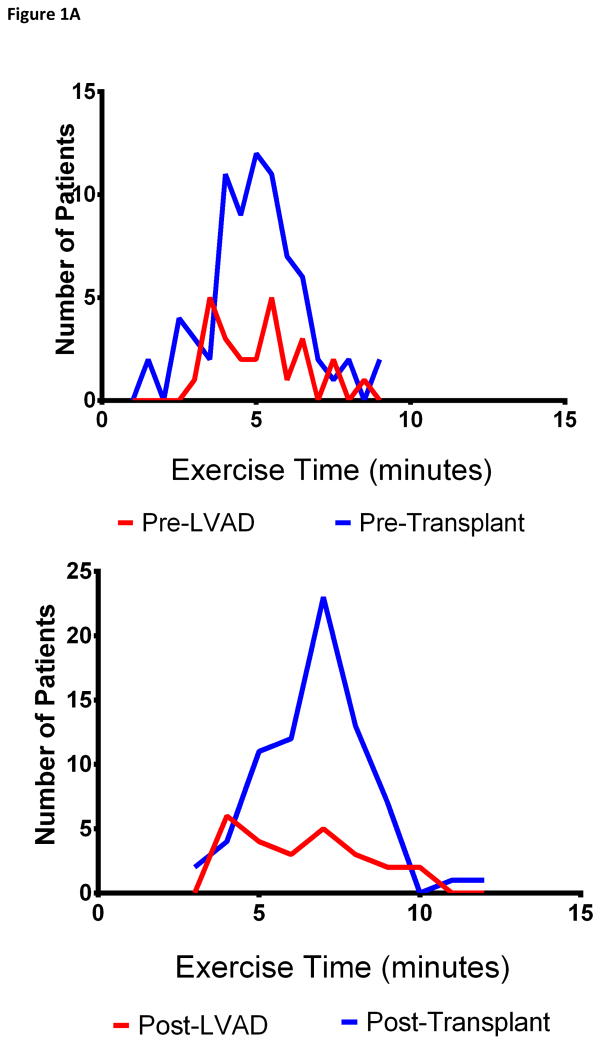
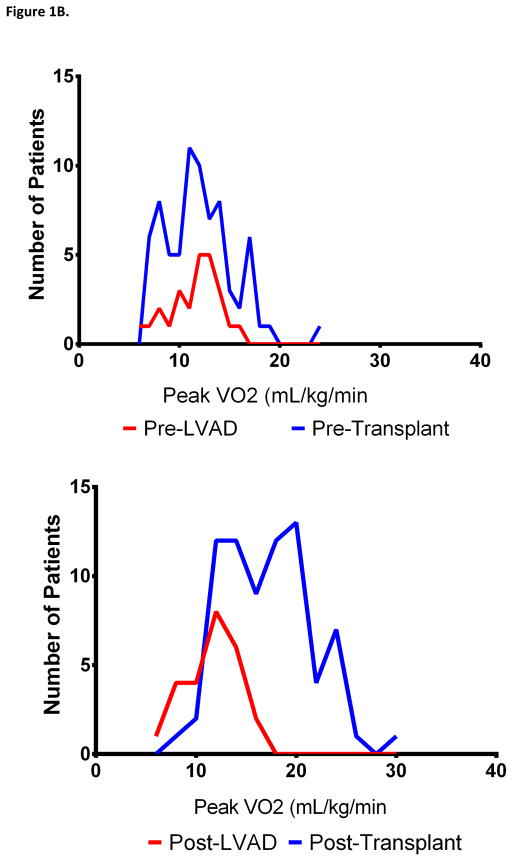
Pre-LVAD, patients had markedly reduced exercise time (5.1 ± 1.4 minutes, 45% ± 15% predicted) and peak VO2 (11.5 ± 2.5 mL/kg/min, normalized peak VO2 43% ± 10%, Table 2, Figure 1). They often had impaired gas exchange with exercise as noted by an elevated VE/VCO2 nadir (39.4 ± 5.2). Nearly all patients (96%) had abnormal HR recovery. Post-LVAD, patients underwent CPET testing a median (25th, 75th percentile) of 12 months (4, 25 months) after LVAD implantation. Nine patients (36%) were in atrial fibrillation at the time of CPET; baseline HR was similar to patients who were not in atrial fibrillation. It was not feasible to obtain blood pressure during exercise in most patients post-LVAD. Post-LVAD patients were able to exercise an average of 1.2 minutes longer than pre-LVAD, with substantial improvements in gas exchange during exercise (mean ΔVE/VCO2 nadir −3.7). While abnormal HR recovery persisted in most patients (72%), the magnitude of HR recovery improved post-LVAD (mean 1-minute decline 4.7 bpm pre-LVAD compared with 11.6 bpm post-LVAD, p=0.008). However, the modest increases seen in peak VO2 (mean Δ +0.9 mL/kg/min) were not statistically significant, and peak VO2 remained markedly abnormal in most patients (normalized peak VO2 48% ± 12%, Figure 1). Notably, 18 patients (72%) had a peak VO2 of <14 mL/kg/min post-LVAD. There were no differences in the change in exercise capacity or peak VO2 in patients who had LVAD implanted as DT vs. BTT, in patients with ischemic cardiomyopathy vs. non-ischemic cardiomyopathy, or by age at implant. However, women had greater improvements in exercise time (mean Δ +3.1 vs. + 0.6 minutes, p=0.033) and had a trend toward more frequent improvement of their normalized peak VO2 (100% vs. 63%, p=0.10) compared with men following LVAD. There was no relationship between the timing of CPET post-LVAD and peak VO2 (r=0.014, p=0.95) and change in VO2 (r=−0.18, p=0.39) compared with pre-LVAD. There was no relationship between EF at the time of CPET post-LVAD and peak VO2. Quality of exercise effort (as measured by the RER and Borg Rating of Perceived Exertion scale) was similar on CPET pre- and post-LVAD.
Table 2.
Cardiopulmonary Exercise Test Parameters Before and After LVAD and Heart Transplant
| Parameter | Pre-LVAD | Post-LVAD | * P value Change LVAD | Pre-Transplant | Post-Transplant | † P value Change Transplant | ‡ P Value LVAD vs. Transplant |
|---|---|---|---|---|---|---|---|
| Vital Signs | |||||||
| Baseline heart rate, bpm | 75.6 (11.9) | 80.7 (8.8) | 0.16 | 73.2 (12.5) | 97.3 (12.2) | <0.001 | --- |
| Peak heart rate, bpm | 103.0 (17.5) | 109.5 (18.6) | 0.25 | 106.3 (21.3) | 132.0 (19.2) | <0.001 | --- |
| Heart rate reserve, bpm | 27.5 (14.2) | 28.8 (17.9) | 0.73 | 33.1 (20.5) | 34.8 (16.7) | 0.23 | --- |
| HR recovery, beats | 4.7 (5.1) | 11.6 (13.2) | 0.008 | 6.9 (11.2) | −3.0 (5.7) | <0.001 | --- |
| Abnormal HR recovery, N(%) | 24 (96.0) | 18 (72.0) | 0.11 | 61 (82.4) | 72 (97.3) | 0.51 | --- |
| Exercise Parameters | |||||||
| Exercise time (minutes) | 5.1 (1.4) | 6.3 (1.9) | 0.023 | 5.0 (1.5) | 6.7 (1.6) | <0.001 | 0.27 |
| % Predicted exercise time | 45% (15%) | 57% (18%) | <0.001 | 44% (15%) | 65% (18%) | <0.001 | 0.010 |
| METS | 3.3 (0.7) | 3.5 (0.8) | 0.17 | 3.4 (1.0) | 4.9 (1.3) | <0.001 | <0.001 |
| Peak VO2 (mL/kg/min) | 11.5 (2.5) | 12.4 (2.8) | 0.17 | 11.9 (3.4) | 17.3 (4.4) | <0.001 | <0.001 |
| Normalized peak VO2 | 43% (10%) | 48% (12%) | 0.051 | 38% (11%) | 56% (19%) | <0.001 | <0.001 |
| AT as % peak VO2 | 78% (9%) | 77% (8%) | 0.69 | 78% (10%) | 74% (9%) | 0.019 | 0.45 |
| VE/VCO2 nadir | 39.4 (5.2) | 35.7 (7.1) | 0.017 | 37.4 (7.3) | 33.2 (4.5) | <0.001 | 0.74 |
| RER | 1.14 (0.2) | 1.16 (0.12) | 0.47 | 1.16 (0.13) | 1.22 (0.12) | <0.001 | 0.31 |
| ¥Rating of Perceived Exertion Very Hard/Maximal, N(%) | 24 (96.0) | 23 (92.0) | 0.63 | 64 (88.9) | 70 (95.9) | 0.09 | 0.20 |
AT= anaerobic threshold, HR= heart rate, All values shown are mean (standard deviation) unlesss otherwise noted.
P value compares pre- and post-measurements for patients undergoing LVAD
P value compares pre- and post-exercise parameters for patients undergoing cardiac transplantation
P value compares the change in CPET measurements for patients undergoing LVAD vs. cardiac transplantation
Borg Rating of Perceived Exertion at Peak Exercise ≥17. Data missing on 2 patients pre-transplant and 1 patient post-transplant
Figure 1. Distribution of Exercise Time and Peak VO2 Before and After LVAD and Heart Transplant.
The distribution of exercise time (Panel A) and peak VO2 (panel B) before and after LVAD (blue) and heart transplant (red) are shown
Changes in Cardiopulmonary Exercise Testing After Heart Transplant
Pre-transplant, both exercise time (5.0 ± 1.5 minutes, 44% ± 15% predicted) and peak VO2 (11.9 ± 3.4 mL/kg/min, normalized peak VO2 38% ± 11%) were markedly reduced, with impairment in VE/VCO2 nadir (Table 2, Figure 1). Post-transplant, patients underwent CPET a median (25th, 75th percentile) of 12 months (12, 13 months) after heart transplantation. Post-transplant, both exercise duration (mean Δ +1.7 minutes) and peak VO2 (mean Δ +5.4 mL/kg/min) significantly increased. Consistent with cardiac denervation post-transplant, the baseline and peak HR was higher post-transplant, and HR recovery was markedly abnormal. There was no relationship between the timing of CPET post-transplant and peak VO2 (r= −0.038, p=0.75) and change in VO2 (r=−0.13, p=0.26) compared with pre-transplant.
Comparing Changes in Cardiopulmonary Exercise Testing Post-LVAD and Post-Transplant
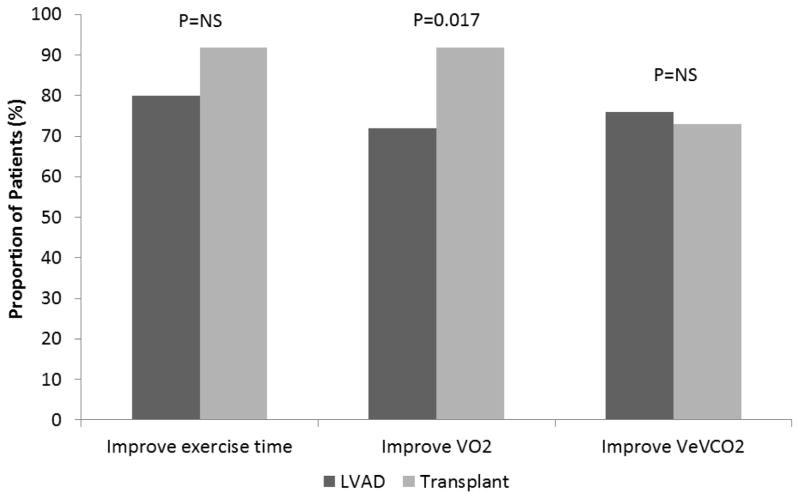
Patients who underwent heart transplantation had greater improvements in peak VO2 compared with patients who underwent LVAD implantation (Table 2, Figure 1B and 2). On multivariable analysis, normalized peak VO2 pre-LVAD or pre-transplant, BMI, and LVAD implant (compared with heart transplant) were independent predictors of the change in normalized peak VO2 following LVAD or heart transplant (Table 3). Patients receiving a heart transplant had 11.6% greater improvement in their normalized peak VO2 compared with patients who underwent LVAD (p=0.001). In contrast, improvements in VE/VCO2 nadir were similar post-LVAD and transplant. Comparing changes in exercise time following heart transplant and LVAD, the absolute difference in exercise time (mean increase 1.7 vs. 1.2 minutes, respectively, p=0.27) and the proportion of patients who improved their exercise time (92% vs. 80%, p=0.13) were similar. However, the change in age and sex-predicted exercise time was greater post-transplant compared with post-LVAD (mean increase 22% vs. 12%, respectively, p=0.010). Less patients were taking beta blockers post-transplant compared with post-LVAD (7% vs. 76%, p<0.001), though heart rate reserve during CPET was similar (p=0.13). There were two LVAD patients (both men) who had CPET performed at baseline, with an LVAD, and after heart transplant. Both had improvements in their exercise time and peak VO2 after heart transplant compared to LVAD.
Figure 2. Improvement in Cardiopulmonary Exercise Testing Following LVAD and Heart Transplant.
The proportion of patients with improvement in exercise time, peak VO and VE/VCO2 post-LVAD (blue) and post-heart transplant (red) are shown
Table 3.
Associations of Clinical Variables with Change in Normalized Peak VO2 after LVAD and Heart Transplant
| Univariate analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | Estimate | P Value | Estimate | P Value |
| Normalized Peak VO2 Pre-LVAD/Heart Transplant | −0.34 | 0.002 | −0.32 | 0.027 |
| Age at LVAD or Transplant | 0.034 | 0.82 | --- | --- |
| Male sex | −2.7 | 0.49 | --- | --- |
| Body mass index (kg/m2) | −0.66 | 0.058 | −0.84 | 0.010 |
| LVAD implant (compared with heart transplant) | −12.9 | <0.001 | −11.6 | 0.001 |
| Effort quality (RER post LVAD or transplant) | 19.1 | 0.17 | --- | --- |
LVAD= left ventricular assist device; RER= respiratory exchange ratio
DISCUSSION
This study provides important information comparing changes in CPET parameters pre- and post-LVAD and orthotopic heart transplant. First, while improvements in exercise duration and gas exchange were seen following LVAD, peak VO2 did not change, and most patients continued to have markedly impaired exercise capacity. Results were similar in patients who had LVAD as DT vs. BTT, though women had greater improvements in exercise time compared with men. Patients who underwent heart transplantation had improvements in peak VO2 in addition to exercise duration and gas exchange.
There are limited data informing us on how exercise capacity and CPET parameters change following LVAD. Previous studies have reported the average peak VO2 after LVAD implant to range from 14.5 to 24.2 mL/kg/min5–7, 11–13. By comparison, the peak VO2 post-LVAD was only 12.4 ± 2.8 mL/kg/min in this study, though this may reflect that our population was much older (mean age 63 years) than patients included in prior studies, who were primarily in their 30s and 40s. When compared with normal age- and sex-predicted values, similar to prior reports, we found that exercise capacity and peak VO2 were still low. Both Kugler and deJonge reported that workload and peak VO2 were 50–60% of age and sex-predicted after LVAD5, 7, which is similar to our population.
Comparing pre-LVAD exercise parameters to post-LVAD values in each patient allowed us to assess the improvement in exercise capacity conferred by the LVAD, a comparison that was lacking in most prior studies. A single previous study assessed peak VO2 in 9 patients both pre and 3–6 months post-LVAD, and reported improvement in 8 of 9 patients14. However, quantification was limited by sample size, and this was primarily a younger population with LVAD implanted as BTT. Other prior reports have solely reported on CPET post-LVAD. Interestingly, we found that patients had a greater improvement in exercise duration than peak VO2 following implantation of a continuous flow LVAD. Exercise time is affected by many factors and is generally considered at best only a fair surrogate for cardiac output. Physiologically, the suboptimal oxygen consumption post-LVAD may be in part due to the fact that continuous flow devices have limited ability to modulate cardiac output during exercise. Unlike pulsatile flow LVADs, continuous flow devices are set at a fixed speed. The speed is chosen with a goal of effectively offloading the left ventricle while avoiding excessive emptying (i.e. “suck down”). While cardiac output will increase with increasing venous return, even at fixed pump speed15, this response may be limited. As such, one might hypothesize that an individual’s ability to augment their cardiac output with exercise may be partially reliant on their native cardiac function. Accordingly, a previous report suggested that LVAD patients with lower EF (18% vs. 24%) had lower peak VO2 and their exercise capacity was more sensitive to changes in pump speed13. However, we saw no relationship between EF at the time of exercise testing after LVAD and peak VO2. In addition, a separate report found only a moderate correlation (r=0.53) between measured cardiac output and peak VO2 during treadmill testing of patients with LVADs11.
In contrast with an LVAD, patients who have received a heart transplant may have a greater ability to increase their cardiac output in response to exercise, as they don’t have to rely on a device with a fixed pump speed. Certainly these physiologic differences may help to explain the differential improvement in peak oxygen consumption we observed in patients treated with heart transplant vs. LVAD. However, while improvements were greater in heart transplant recipients, they still remained well below age- and sex-predicted normal values. This suboptimal improvement in exercise capacity and VO2 has been previously reported after orthotopic heart transplant16, 17. Potential contributing factors are numerous and may include chronotropic incompetence due to cardiac denervation16, effects of chronic corticosteroid use18, elevated body mass index of the recipient17, allograft hypertrophy19, and advanced age of the donor18. Chronic and likely minimally reversible changes at the level of the skeletal muscle resulting in inefficiency in extracting oxygen20 may contribute to persistently low oxygen consumption in both LVAD and transplant recipients.
While peak VO2 did not improve following LVAD, we did see improvements in both exercise time and ventilatory gas exchange. Continuous flow LVADs maintained at adequate pump speed are extremely efficacious at offloading the left ventricle. Morgan et al recently reported their experience of 130 patients who had right heart catheterization performed one month after continuous flow LVAD insertion21. They found marked improvements in the pulmonary capillary wedge pressure, which decreased from an average of 23.0 mmHg (markedly elevated) to 12.9 mmHg (normal). This marked reduction in left ventricular filling pressure as a consequence of LVAD therapy could account for the improvement in VE/VCO2 seen herein. As patients are experiencing less dyspnea with exercise due to their improved gas exchange, they are likely to feel better and become more active, leading to improved muscular strength and stamina resulting in the ability to exercise longer. Similarly, it has been previously reported that most patients demonstrate an improvement in 6 minute walk distance following LVAD22, 23.
Limitation, Strengths, and Clinical Implications
There are limitations that should be acknowledged to aid in interpretation of these data. First, CPET was performed as a part of routine clinical practice rather than a study protocol, so timing of CPET post-LVAD and transplant varied. However, no relationship between the duration of time after LVAD and exercise duration or peak VO2 was observed in this or other recent studies14. Furthermore, the 25 patients with LVAD included in this analysis represent only a subset of those who underwent LVAD implantation at our institution, as most LVAD patients did not have CPET performed both pre- and post-LVAD, and patients who were not well enough to undergo CPET would not have been included. Second, we did not control for the number of CPETs a patient might have performed prior to their index pre-LVAD or transplant CPET, meaning some patients may have experienced a test-retest improvement in their exercise time on their post-procedure CPET based on increased test familiarity. Third, our small sample size limits our ability to draw definitive conclusions based on these data, which would benefit from replication in other populations. However, there are several important strengths. First, we were able to rigorously perform CPET in a group of patients both pre- and post-LVAD and compare these findings with changes following heart transplantation. As our program implants a large number of LVADs as DT, we were uniquely positioned to examine changes in CPET in this growing population. Knowing what the destination exercise performance might be after LVAD implantation will help to provide realistic expectations regarding improvement in exercise capacity post-LVAD. A patient with a peak VO2 that is too high and/or VE/VCO2 nadir that reflects normal ventilatory gas exchange prior to implantation may not derive improvement in exercise capacity with left ventricular offloading.
Conclusions
While LVAD therapy results in improvement in exercise duration and gas exchange, most patients still exhibit substantial limitations in exercise capacity with use of continuous flow devices. As device technology continues to rapidly advance and evolve, improvements may be seen.
Supplementary Material
Supplementary Materials Table. Cardiopulmonary Exercise Test Parameters Before and After LVAD and Heart Transplant: Results From Matched Analysis
Acknowledgments
Funding Sources. Dr. Dunlay is funded by a National Institutes of Health Career Development Award (K23HL 116643). Dr. Pereira is funded by a grant from the National Center for Advancing Translational Sciences (UL1 TR000135).
Footnotes
DISCLOSURES. None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21:154–61. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 2.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25:1024–42. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 5.de Jonge N, Kirkels H, Lahpor JR, Klopping C, Hulzebos EJ, de la Riviere AB, et al. Exercise performance in patients with end-stage heart failure after implantation of a left ventricular assist device and after heart transplantation: an outlook for permanent assisting? J Am Coll Cardiol. 2001;37:1794–9. doi: 10.1016/s0735-1097(01)01268-2. [DOI] [PubMed] [Google Scholar]
- 6.Haft J, Armstrong W, Dyke DB, Aaronson KD, Koelling TM, Farrar DJ, et al. Hemodynamic and exercise performance with pulsatile and continuous-flow left ventricular assist devices. Circulation. 2007;116:I8–15. doi: 10.1161/CIRCULATIONAHA.106.677898. [DOI] [PubMed] [Google Scholar]
- 7.Kugler C, Malehsa D, Tegtbur U, Guetzlaff E, Meyer AL, Bara C, et al. Health-related quality of life and exercise tolerance in recipients of heart transplants and left ventricular assist devices: a prospective, comparative study. J Heart Lung Transplant. 2011;30:204–10. doi: 10.1016/j.healun.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Daida H, Allison TG, Johnson BD, Squires RW, Gau GT. Comparison of peak exercise oxygen uptake in men versus women in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;80:85–8. [PubMed] [Google Scholar]
- 9.Wasserman K, Hansen J, Sue D. Principles of Exercise Testing and Interpretation. 3. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 10.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–6. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakovljevic DG, Birks EJ, George RS, Trenell MI, Seferovic PM, Yacoub MH, et al. Relationship between peak cardiac pumping capability and selected exercise-derived prognostic indicators in patients treated with left ventricular assist devices. Eur J Heart Fail. 2011;13:992–9. doi: 10.1093/eurjhf/hfr069. [DOI] [PubMed] [Google Scholar]
- 12.Jaski BE, Kim J, Maly RS, Branch KR, Adamson R, Favrot LK, et al. Effects of exercise during long-term support with a left ventricular assist device. Results of the experience with left ventricular assist device with exercise (EVADE) pilot trial. Circulation. 1997;95:2401–6. doi: 10.1161/01.cir.95.10.2401. [DOI] [PubMed] [Google Scholar]
- 13.Noor MR, Bowles C, Banner NR. Relationship between pump speed and exercise capacity during HeartMate II left ventricular assist device support: influence of residual left ventricular function. Eur J Heart Fail. 2012;14:613–20. doi: 10.1093/eurjhf/hfs042. [DOI] [PubMed] [Google Scholar]
- 14.Leibner ES, Cysyk J, Eleuteri K, El-Banayosy A, Boehmer JP, Pae WE. Changes in the functional status measures of heart failure patients with mechanical assist devices. ASAIO J. 2013;59:117–22. doi: 10.1097/MAT.0b013e3182816cb7. [DOI] [PubMed] [Google Scholar]
- 15.Brassard P, Jensen AS, Nordsborg N, Gustafsson F, Moller JE, Hassager C, et al. Central and peripheral blood flow during exercise with a continuous-flow left ventricular assist device: constant versus increasing pump speed: a pilot study. Circ Heart Fail. 2011;4:554–60. doi: 10.1161/CIRCHEARTFAILURE.110.958041. [DOI] [PubMed] [Google Scholar]
- 16.Borrelli E, Pogliaghi S, Molinello A, Diciolla F, Maccherini M, Grassi B. Serial assessment of peak VO2 and VO2 kinetics early after heart transplantation. Med Sci Sports Exerc. 2003;35:1798–804. doi: 10.1249/01.MSS.0000093610.71730.02. [DOI] [PubMed] [Google Scholar]
- 17.Leung TC, Ballman KV, Allison TG, Wagner JA, Olson LJ, Frantz RP, et al. Clinical predictors of exercise capacity 1 year after cardiac transplantation. J Heart Lung Transplant. 2003;22:16–27. doi: 10.1016/s1053-2498(02)00475-8. [DOI] [PubMed] [Google Scholar]
- 18.Renlund DG, Taylor DO, Ensley RD, O’Connell JB, Gilbert EM, Bristow MR, et al. Exercise capacity after heart transplantation: influence of donor and recipient characteristics. J Heart Lung Transplant. 1996;15:16–24. [PubMed] [Google Scholar]
- 19.Raichlin E, Al-Omari MA, Hayes CL, Edwards BS, Frantz RP, Boilson BA, et al. Cardiac allograft hypertrophy is associated with impaired exercise tolerance after heart transplantation. J Heart Lung Transplant. 2011;30:1153–60. doi: 10.1016/j.healun.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 21.Morgan JA, Paone G, Nemeh HW, Murthy R, Williams CT, Lanfear DE, et al. Impact of continuous-flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant. 2013;32:398–403. doi: 10.1016/j.healun.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–34. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 23.Hasin T, Topilsky Y, Kremers WK, Boilson BA, Schirger JA, Edwards BS, et al. Usefulness of the six minute walk test after continuous axial flow left ventricular device implantation to predict survival. Am J Cardiol. 2012;110:1322–8. doi: 10.1016/j.amjcard.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials Table. Cardiopulmonary Exercise Test Parameters Before and After LVAD and Heart Transplant: Results From Matched Analysis