Abstract
Mitochondrial Ca2+ controls numerous cell functions, such as energy metabolism, reactive oxygen species generation, spatiotemporal dynamics of Ca2+ signaling, cell growth and death in various cell types including neurons. Mitochondrial Ca2+ accumulation is mainly mediated by the mitochondrial Ca2+ uniporter (MCU), but recent reports also indicate that mitochondrial Ca2+-influx mechanisms are regulated not only by MCU, but also by multiple channels/transporters. We previously reported that ryanodine receptor (RyR), which is a one of the main Ca2+-release channels at endoplasmic/sarcoplasmic reticulum (SR/ER) in excitable cells, is expressed at the mitochondrial inner membrane (IMM) and serves as a part of the Ca2+ uptake mechanism in cardiomyocytes. Although RyR is also expressed in neuronal cells and works as a Ca2+-release channel at ER, it has not been well investigated whether neuronal mitochondria possess RyR and, if so, whether this mitochondrial RyR has physiological functions in neuronal cells. Here we show that neuronal mitochondria express RyR at IMM and accumulate Ca2+ through this channel in response to cytosolic Ca2+ elevation, which is similar to what we observed in another excitable cell-type, cardiomyocytes. In addition, the RyR blockers dantrolene or ryanodine significantly inhibits mitochondrial Ca2+ uptake in permeabilized striatal neurons. Taken together, we identify RyR as an additional mitochondrial Ca2+ uptake mechanism in response to the elevation of [Ca2+]c in neurons, suggesting that this channel may play a critical role in mitochondrial Ca2+-mediated functions such as energy metabolism.
Keywords: Striatal neurons, calcium, ryanodine receptor, [3H]ryanodine binding, mitochondria, dantrolene
Introduction
Mitochondria play an important role in shaping the intracellular Ca2+ concentration as they can take up Ca2+ in response to physiological changes in the cytosolic Ca2+ concentration ([Ca2+]c) in various cell-types/tissues including neurons [7,10,24]. Mitochondrial Ca2+ accumulation was first recognized as an important mechanism for the acceleration of oxidative phosphorylation and electron transport chain activity, which results in the stimulation of ATP synthesis [12]. In addition, mitochondrial dysfunction and the loss of cellular Ca2+ homeostasis are frequently observed together in pathophysiological conditions such as neuronal excitotoxicity, apoptosis and neurodegenerative disorders [8]. However, the detailed mechanisms of how altered mitochondrial Ca2+ handling and/or mitochondrial dysfunction affect these neurological pathogenesis are not yet fully understood.
Mitochondrial Ca2+ influx was originally considered as a single transport mechanism through mitochondrial Ca2+ uniporter (MCU) which can be inhibited by ruthenium red and lanthanides (see reviews [7,24]). However, the molecular identities responsible for mitochondrial Ca2+ accumulation have remained an unsolved question until very recently. Recently, several groups have discovered the molecular identity of MCU and its regulatory proteins and confirmed it as the main mitochondrial Ca2+ uptake mechanism (see reviews[19,24]). Although in these studies MCU was confirmed as the most dominant Ca2+ influx mechanism, previous studies have identified additional Ca2+ uptake pathways, which display different physiological and pharmacological characteristics from MCU theory (see reviews [7,24]).
Among these studies, we reported that ryanodine receptor (RyR) is one of the mitochondrial Ca2+ influx mechanisms in another excitable cell-type, cardiomyocytes, termed mRyR (mitochondrial RyR) [2,3] (see also reviews [24,25]). Our group first identified that a low level of RyR is expressed in the mitochondrial inner membrane (IMM) in cardiomyocytes through a combination of biochemical, cell biological and electrophysiological experiments. Since cardiac mRyR exhibits a bell-shaped Ca2+-dependent activation (bimodal activation) in the physiological range of [Ca2+]c, this unique property places mRyR as an ideal candidate for sequestering Ca2+ quickly and transiently during physiological [Ca2+]c oscillation in excitable cells. In addition, using not only native cardiomyocytes, but also RyR overexpression/knock-out in cultured cardiac myoblasts and knock-out mouse hearts, we showed that the molecular identity of mRyR is possibly a skeletal-muscle type-isoform RyR type 1 (RyR1) and is required for Ca2+-dependent acceleration of ATP production in cardiomyocytes even though the expression level is much lower than RyR2 which is the main RyR isoform expressed in cardiac sarcoplasmic reticulum (SR) /endoplasmic reticulum (ER) [3,23].
Although RyR is expressed [9,11,31] in the brain and serves as a Ca2+-release channel of the intracellular Ca2+ store (ER) in addition to inositol 1,4,5-trisphosphate (IP3) receptors [15,17,18], the interaction of between the RyR expression and mitochondrial functions under physiological and pathophysiological conditions in brain has been completely unknown. Therefore, the objective of this study was to investigate the possibility whether neuronal mitochondria possess mRyR similar to cardiomyocytes and to assess if mRyR takes part in the mitochondrial Ca2+ influx mechanism. Our finding suggests that RyR is expressed at IMM and takes up Ca2+ into mitochondria in response to [Ca2+]c elevations.
Materials and Methods
An expanded Material and Methods section is available in the online supplementary file.
Reagents and antibodies
All reagents and antibodies were purchased from Sigma-Aldrich Corporation (St. Louis, MO) unless otherwise indicated.
Cells
All procedures involving animal use were in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the University Committee on Animal Resources. Primary striatal neurons were prepared from Sprague-Dawley rat embryos (embryonic day 18) [1].
Preparation of rat brain mitochondria and mitochondrial subfractions
Mitochondria-enriched protein fractions and mitochondrial subfractions were prepared using differential centrifugation [3,23].
Fluorescence microscopy
Localization of RyR was observed in live or fixed cultured striatal neurons using confocal microscopy (Leica, Heidelberg Germany). For live cells, RyR, SR/ER and mitochondria were labeled with boron-dipyrromethene (Bodipy)-conjugated ryanodine (Bodipy-Ry), Bodipy-conjugated sarco/endoplasmic Ca2+-ATPase (SERCA) inhibitor thapsigargin (Bodipy-Thap) and Mitotracker Deep Red (MTR) (Molecular Probes, Eugene, OR), respectively. The pre-treatment of unlabeled ryanodine completely abolished the Bodipy-Ry staining, confirmed that Bodipy-Ry specifically binds to RyRs in live striatal neurons (Supplementary Fig.1). For time-lapse studies, live cell images were collected by TILL system (TILL Photonics, München, Germany). For detection of RyR in fixed striatal neurons, cells were probed with primary antibodies against RyR (Santa Cruz biotechnology, Santa Cruz, CA) and cytochrome c oxidase (Cox) (for labeling mitochondria) (Molecular Probes) followed by fluorescence-conjugated secondary antibodies. Scatter 2D plots of pixel intensities and Pearson's correlation coefficient were obtained by Image J software (NIH) [23].
Measurements of cytosolic and mitochondrial Ca2+ concentration
[Ca2+]c in permeabilized striatal neurons was measured by Ca2+ indicator Fura-2 with a fluorescent microscope (TILL Photonics) [26]. For mitochondrial Ca2+ concentration ([Ca2+]m) measurements, cells were skinned by saponin and then stained by an acetoxymethyl (AM) ester form of Fura-2 or Rhod-2 [26]. Fura-2 calibration was performed as previously reported [1].
[3H]ryanodine binding
[3H]ryanodine binding assays were performed as we previously described [3].
Data and statistical analysis
All results are shown as mean standard error or otherwise indicated. Unpaired Student's t-test was performed. Statistical significance was set as a p value of <0.05.
Results
Dantrolene and Ryanodine block mitochondrial Ca2+ uptake in striated neurons
To test whether RyR is involved in the mitochondrial Ca2+ uptake mechanism in neurons, the changes in [Ca2+]m in response to [Ca2+]c elevation were measured in permeabilized neurons in the presence and absence of a RyR blocker, dantrolene using Fura-2 [3]. First, we stimulated the cells with IP3 and mobilized IP3 receptor-based SR Ca2+ release. Because RyRs were expressed at ER [3,11,21], this protocol is enable to match the magnitude of cytosolic Ca2+ transient in the presence and absence of dantrolene [23]. We confirmed that the application of 10 µM IP3 induced Ca2+ release from intracellular stores, resulted in an increase of the [Ca2+]c (from 108 ± 11.4 to 550 ± 47.3 nM) (Fig.1A). We also confirmed that magnitude of Ca2+ release from ER by IP3 treatment did not changed significantly changed in the presence or in the absence of dantrolene (490 ± 51.2 versus 550 ± 47.3 nM, P=1.00). Under this experimental condition, we next observed the changes in [Ca2+]m in response to IP3 treatment (Fig.1B). We confirmed that the application of IP3 quickly increased [Ca2+]m (from 110 ± 0.6 to 700 ± 59.6 nM), but 10-min pretreatment of dantrolene significantly inhibited IP3-induced increase in [Ca2+]m. In addition, the IP3-induced increase in [Ca2+]m (from 90 ± 7.8 to 250 ± 19.6 nM) partially recovered after washing out dantrolene, suggesting that this inhibitory effect by dantrolene is reversible.
Fig.1. Dantrolene inhibits mitochondrial Ca2+ uptake induced by IP3-mediated Ca2+ release from the ER.
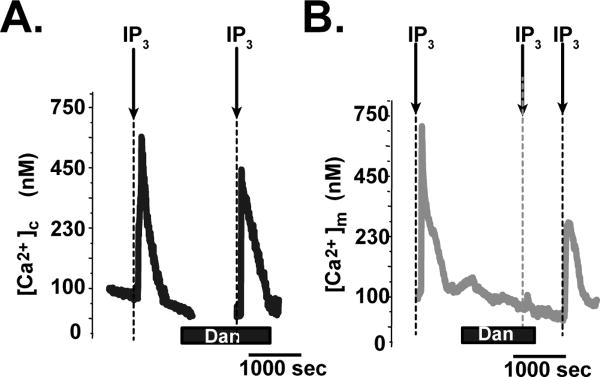
A. Representative time course of the changes in [Ca2+]c in response to 10 µM IP3 treatment in saponin-permeabilized striatal neurons. First, permeabilized cells were exposed to IP3 to evoke first [Ca2+]c elevation. Then IP3 was washed out and the cells were treated with 10 µM dantrolene (Dan) for 10 minutes, followed by a second exposure to IP3. B. Representative time course of the changes in [Ca2+]m in response to 10 µM IP3 treatment in saponin-permeabilized striatal neurons under the same protocols in A.
We also observed that the treatment of another RyR blocker, ryanodine, also significantly inhibited mitochondrial Ca2+ uptake in response to the application of Ca2+ into the extracellular solution (Supplementary Fig.2). These results indicate that in neurons, RyR is involved in the mitochondrial Ca2+ uptake mechanism.
RyR is expressed in the inner mitochondrial membrane in neurons
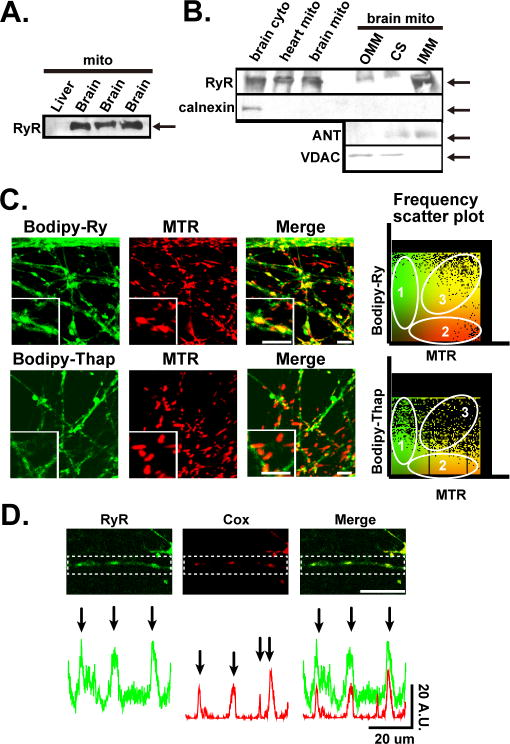
We next tested whether RyR is expressed in mitochondria (i.e. mRyR) using biochemical approaches. Using specific antibody against RyR, we confirmed that RyR was detectable in mitochondria-enriched protein fractionation obtained from whole brain (Fig.2A and B). Because RyR is mainly expressed in SR/ER, the purity of our mitochondria-enriched protein fractionation was evaluated by detection of voltage-dependent anion channel (VDAC) and calnexin by Western blotting as mitochondrial and ER/SR markers, respectively [3]. The mitochondria-enriched protein fractionation obtained from whole brain showed that RyR is found in both in cytosolic (including SR)- and mitochondria-enriched protein fraction (Fig.1B). To determine the submitochondrial localization of RyR in brain mitochondria, the IMM-enriched proteins were separated by osmotic shock from the outer mitochondrial membrane (OMM) and the contact site (CS) fractions. Separation of OMM-, CS-, and IMM-enriched fractions was confirmed by the detection of the levels of marker proteins for IMM and OMM, adenine-nucleotide translocator (ANT) and VDAC, respectively (Fig.2B). RyR was detectable mainly from IMM, which is similar to the results we reported in cardiomyocytes [3].
Fig.2. RyR is expressed in neuronal mitochondria.
A. Representative Western blots of mitochondria-enriched fractions from rat liver and brain (from three different preparations). B. Representative Western blots of mitochondria-enriched fraction and mitochondrial subfractions from rat brain. Cytosolic/ER-protein enriched fraction from rat brain and mitochondria-enriched fraction from rat heart were shown as a comparison. C. Labeling of RyR or SERCA in live striatal neurons using Bodipy-Ry or Bodipy-Thap, respectively. Images were taken from the areas of neurites. Top: Neurons were also labeled with MTR (red emission) to visualize the localizations of mitochondria. Color scatter plottings obtained from the representative pictures at left were shown in right. Region 1 and 2 pixels represent signal in channel 1 (green) or 2 (Red) only, respectively and region 3 represents co-localized pixels. Scale bars, 5 μm. D. Immunocytochemical detection of RyR. Images were taken from the areas of neurites. The fluorescence profile from a dot square region at each image is shown at the bottom. Arrows show the location of peak fluorescence. Scale bars, 20 μm. A.U., Arbitrary unit.
Expression of RyR in mitochondria was further confirmed in cultured live striatal neurons. First, we labeled RyR with fluorescence-dye (Bodipy) conjugated ryanodine (Bodipy-Ry) in live cultured striatal neurons (Fig.2C). Bodipy-Ry showed a mesh-like staining pattern with punctuate staining region. We also found that punctuate staining regions of Bodipy-Ry were colocalized with MTR staining, suggesting that RyR is partially expressed in mitochondria. Time-lapse imaging showed that these spots moved, which further verified that this punctuate structures were indeed mitochondria (Supplementary Fig.3). For comparison, we also stained the neuron cells with Bodipy-Thap for labeling ER. The Bodipy-Thap signals showed almost no colocalization with MTR (Fig.2C). Scatter 2D plots of pixel intensities in red (MTR) and green (Bodipys) channels indicated that the cells stained by Bodipy-Ry possessed higher amount of co-localized pixels (yellow in segment 3 in Fig.2C) compared to those observed in the cells stained by Bodipy-Thap. We next quantitatively assessed an efficiency of the mitochondrial colocalization by calculating the values of Pearson's correlation [23] (Fig.2C). The Pearson's value for Bodipy-Ry-MTR staining was higher than that in Bodipy-Thap-MTR staining (0.716 vs 0.322 in Fig.2C), suggesting that RyR was not only localized at ER, but also partially localized at mitochondria. In contrast to neurons, no colocalization of the Bodipy-Ry and MTR was observed in glia cells (Supplementary Fig.4) (Pearson's value=0.276). In addition, red-range Bodipy-Ry (Bodipy-TR-Ry) was almost colocalized with Bodipy-Thap, suggesting that RyR is expressed in ER, but not in mitochondria in glia cells (Pearson's value=0.828).
Expression of RyR in mitochondria was further confirmed by immunocytochemistry using the same RyR-specific antibody used in Western blotting (Fig.2D, see also Fig.2A and B). Confocal analysis revealed a mesh-like staining pattern with punctuate staining region for RyR. Double labeling against Cox (an IMM protein) confirmed that RyR partially localized at mitochondrial localization (see fluorescence profiles in Fig.2D).
Characterization of mRyR in brain mitochondria
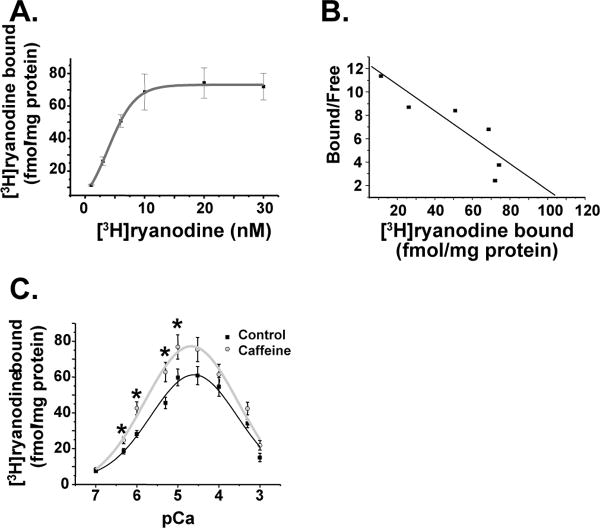
Finally, we further characterized the neuronal mRyR using [3H]ryanodine binding assays (Fig.3). [3H]ryanodine bound with high affinity to isolated brain mitochondria in [3H]ryanodine-concentration dependent manner (Fig.3A). The apparent affinity (Kd) of [3H]ryanodine binding was 8.5 nM in the presence of 20 µM Ca2+ and the maximal number of binding sites (Bmax) was 103 fmol/ mg protein obtained by Scatchard plot (Fig.3B). We next investigated modulation of [3H]ryanodine binding by Ca2+ and caffeine, which are well known modulators of ryanodine binding to RyR of ER/SR [3]. Indeed, brain mitochondria showed both Ca2+- and caffeine-sensitive manner in [3H]ryanodine binding (Fig.3C). We found a bell-shaped curve for Ca2+ dependence with maximal [3H]ryanodine binding occurring at pCa 5.2. In addition, application of caffeine increased [3H]ryanodine binding (Fig.3C). This increase in [3H]ryanodine binding by caffeine was also Ca2+-dependent. Maximal increase (≅50%) in [3H]ryanodine binding was obtained at pCa 6.
Fig.3. [3H]ryanodine binding assay in isolated brain mitochondria.
A. [3H]ryanodine binds to isolated mitochondria from rat brain with high affinity. B. Scatchard plot of [3H]ryanodine binding. C. [3H]ryanodine binding in various free Ca2+ concentration in the presence (gray circles) or in the absence (black squares) of 20 mM caffeine. p<0.05, compared to the control in each pCa.
Discussion
In the present study, we report the molecular and functional identification of mRyR in neuron. First, we showed in cultured primary cells that neuronal mitochondrial Ca2+ accumulation is sensitive to both ryanodine and dantrolene (Fig.1 and Supplementary Fig.2). Second, using brain mitochondria and cultured primary cells, we found that RyR is not only expressed in ER, but also in mitochondria possibly at IMM (Fig.2). Third, we characterized neuronal mRyR and found that the properties of ryanodine binding are similar to those we observed in cardiac mRyR [3] (Fig.3). These results indicate that, in addition to MCU, RyR is involved in the mitochondrial Ca2+ influx mechanism in neurons, similar to our previous observation in another excitable cell-type, cardiomyocytes [3].
Our finding that neurons express mRyR adds a new component to the complexity of mitochondrial Ca2+ uptake in neuron. The prevailing view is that the ruthenium red-inhibitive MCU is exclusively responsible for mitochondrial Ca2+ influx (see review [19]). However, the growing evidence also advances the idea that multiple Ca2+ influx mechanisms coexist, including our findings of mRyR [24,25]. Indeed, we showed that ryanodine or dantrolene treatment significantly inhibited the mitochondrial Ca2+ accumulation in neuron, but did not completely abolish, suggesting that other ion channel/transporters are engaged in mitochondrial Ca2+ mechanism and mRyR serves as a part of this mechanism (Fig.1 and Supplementary Fig.2). Elucidation of the unique biophysical characteristics and physiological functions for each Ca2+ influx mechanism is significant for our understanding of mitochondrial Ca2+ signaling in regulating physiology and pathophysiology in excitable cells including neurons. Recent studies revealed that dantrolene treatment is significantly protective from cytotoxicity in neurons and/or the progression of neurodegenerative disorders [5,14,22], suggesting the potential importance of RyR in neuronal cell death signaling under the pathophysiological conditions. Future studies will address our next hypothesis: mRyR in neurons is involved in the regulation of ATP production through Ca2+ accumulation, as we previously showed in the heart, [3,23] and how mRyR expression levels and functions might be altered under pathophysiological conditions.
In brain, all three RyR subtypes (RyR1, 2 and 3) are expressed [9,11,27,31]. However, in our biochemical and cell biological studies (Figs.2&3), we used RyR-isoform non-specific antibody and Bodipy-Ry to identify mRyR in neuronal cells, which could not distinguish which isoform(s) is (are) expressed in mitochondria. We previously reported that RyR2 is expressed at SR and RyR1 can be expressed only in mitochondria, but not in SR in cardiac cells, suggesting that the molecular identity of mRyR in cardiomyocytes is RyR1 [3,23]. RyR2 is not only expressed in SR/ER, but also at the plasma membrane [20] or in the Golgi complex [6], which supports our idea of RyR isoform-dependent subcellular distribution/retention. Thus, it is a reasonable idea that multiple RyR subtypes are expressed in a single neuronal cell and each isoform has a different subcellular localization as shown in cardiomyocytes. Further studies will be required to precisely identify the isoform(s) of mRyR in neuron (whether neuronal mRyR is RyR1 or not) using genetic knockdown or overexpression as we performed in cardiac cells [3,23].
In addition, RyR subtypes show different spatial and temporal expression patterns among various brain regions [9,11,27,31]. The main RyR isoform is RyR2 [15,17,18] , which is evenly expressed throughout the brain at relatively high levels. RyR3 is also ubiquitously expressed in the brain at lower levels compared to RyR2 [9,11,31] and serves as a part of the Ca2+-release mechanism in ER [21,29]. Interestingly, skeletal-muscle type isoform RyR1 is expressed in the brain [11,31], particularly at the cerebellum, cortex, and hippocampus [16,30], but the physiological relevance of the uneven distribution pattern of RyR1 is completely unknown. The brain is an organ with a high metabolic demand and energy availability determines the power and speed of the neuronal transmission system. Since the cerebellum, cortex, and hippocampus have a high number of synapses [28] and use ATP to accelerate synaptic transmission, act as a neurotransmitter [4], generate action potential, and maintain resting potentials [13]: it is a feasible idea that the neuronal cells in these regions possess an additional molecular mechanism to enhance the efficiency of Ca2+-dependent ATP production in mitochondria (mRyR), similar to cardiomyocytes. Further studies will address the different expression levels of mRyR between various brain regions and also will clarify whether the mRyR expression pattern in the brain has a correlation to the mitochondrial bioenergetics and cellular energy demands in the central nervous system.
Conclusion
Our studies show the molecular and functional identification of mRyR in neuronal mitochondria. Our results suggest that mRyR may function to sequester Ca2+ to mitochondria in response to the elevation of [Ca2+]c in neurons.
Supplementary Material
Highlights.
Dantrolene and Ryanodine block mitochondrial Ca2+ uptake in striated neurons.
Ryanodine receptor (RyR) is expressed in the inner mitochondrial membrane in neurons
Brain mitochondria bind [3H]ryanodine both in Ca2+- and caffeine-sensitive manner.
Mitochondrial RyR takes part in the mitochondrial Ca2+ influx mechanism in neurons.
Acknowledgments
The authors thank Mr. Mark Gallagher for culturing the striatal neurons. This work was supported by NIH grants (RO1HL-033333, RO1HL-093671, and R21HL-110371 to S.-S.S. and 5T32AA007463-26 to S.H.) and AHA grants (0335425T to Y.D. and 14BGIA18830032 to J.O.-U.).
List of abbreviations
- OMM
outer mitochondrial membrane
- CS
contact sites
- IMM
inner mitochondrial membrane
- ER/SR
endo/sarcoplasmic reticulum
- SERCA
sarco/endoplasmic Ca2+-ATPase
- VDAC
voltage-dependent anion channel
- ANT
adenine-nucleotide translocase
- [Ca2+]c
cytosolic Ca2+ concentration
- [Ca2+]m
mitochondrial Ca2+ concentration
- RyR
ryanodine receptor
- MTR
Mitotracker deep Red
- Bodyipy
boron-dipyrromethene
- Bodipy-Ry
Bodipy-conjugated ryanodine
- Bodipy-Thap
Bodipy-conjugated thapsigargin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alano CC, Beutner G, Dirksen RT, Gross RA, Sheu SS. Mitochondrial permeability transition and calcium dynamics in striatal neurons upon intense NMDA receptor activation. J Neurochem. 2002;80(3):531–538. doi: 10.1046/j.0022-3042.2001.00738.x. [DOI] [PubMed] [Google Scholar]
- 2.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276(24):21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 3.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005;1717(1):1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 5.Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG, Stutzmann GE. Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer's disease. PLoS One. 2012;7(12):e52056. doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cifuentes F, Gonzalez CE, Fiordelisio T, Guerrero G, Lai FA, Hernandez-Cruz A. A ryanodine fluorescent derivative reveals the presence of high-affinity ryanodine binding sites in the Golgi complex of rat sympathetic neurons, with possible functional roles in intracellular Ca(2+) signaling. Cell Signal. 2001;13(5):353–362. doi: 10.1016/s0898-6568(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 7.Dedkova EN, Blatter LA. Calcium signaling in cardiac mitochondria. J Mol Cell Cardiol. 2013;58:125–133. doi: 10.1016/j.yjmcc.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch. 2012;464(1):111–121. doi: 10.1007/s00424-012-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci. 1994;14(8):4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellerich FN, Gizatullina Z, Gainutdinov T, Muth K, Seppet E, Orynbayeva Z, Vielhaber S. The control of brain mitochondrial energization by cytosolic calcium: the mitochondrial gas pedal. IUBMB Life. 2013;65(3):180–190. doi: 10.1002/iub.1131. [DOI] [PubMed] [Google Scholar]
- 11.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128(5):893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51(14):2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32(7):1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth Analg. 2010;111(6):1400–1410. doi: 10.1213/ANE.0b013e3181f7181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johenning FW, Zochowski M, Conway SJ, Holmes AB, Koulen P, Ehrlich BE. Distinct intracellular calcium transients in neurites and somata integrate neuronal signals. J Neurosci. 2002;22(13):5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakizawa S, Yamazawa T, Chen Y, Ito A, Murayama T, Oyamada H, Kurebayashi N, Sato O, Watanabe M, Mori N, Oguchi K, Sakurai T, Takeshima H, Saito N, Iino M. Nitric oxide-induced calcium release via ryanodine receptors regulates neuronal function. EMBO J. 2012;31(2):417–428. doi: 10.1038/emboj.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Yun HM, Baik JH, Chung KC, Nah SY, Rhim H. Functional interaction of neuronal Cav1.3 L-type calcium channel with ryanodine receptor type 2 in the rat hippocampus. J Biol Chem. 2007;282(45):32877–32889. doi: 10.1074/jbc.M701418200. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen BX, Arancio O, Marks AR. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell. 2012;150(5):1055–1067. doi: 10.1016/j.cell.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchi S, Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol. 2014 doi: 10.1113/jphysiol.2013.268235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moonga BS, Li S, Iqbal J, Davidson R, Shankar VS, Bevis PJ, Inzerillo A, Abe E, Huang CL, Zaidi M. Ca(2+) influx through the osteoclastic plasma membrane ryanodine receptor. Am J Physiol Renal Physiol. 2002;282(5):F921–32. doi: 10.1152/ajprenal.00045.2000. [DOI] [PubMed] [Google Scholar]
- 21.Murayama T, Ogawa Y. Properties of Ryr3 ryanodine receptor isoform in mammalian brain. J Biol Chem. 1996;271(9):5079–5084. doi: 10.1074/jbc.271.9.5079. [DOI] [PubMed] [Google Scholar]
- 22.Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One. 2008;3(11):e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O-Uchi J, Jhun BS, Hurst S, Bisetto S, Gross P, Chen M, Kettlewell S, Park J, Oyamada H, Smith GL, Murayama T, Sheu SS. Overexpression of ryanodine receptor type 1 enhances mitochondrial fragmentation and Ca2+-induced ATP production in cardiac H9c2 myoblasts. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00094.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O-Uchi J, Pan S, Sheu SS. Perspectives on: SGP Symposium on Mitochondrial Physiology and Medicine: Molecular identities of mitochondrial Ca2+ influx mechanism: Updated passwords for accessing mitochondrial Ca2+-linked health and disease. J Gen Physiol. 2012;139(6):435–443. doi: 10.1085/jgp.201210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu SY, Beutner G, Dirksen RT, Kinnally KW, Sheu SS. Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 2010;584(10):1948–1955. doi: 10.1016/j.febslet.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32(1):97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 27.Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13(7):3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soreq H, Seidman S. Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci. 2001;2(4):294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 29.Supnet C, Grant J, Kong H, Westaway D, Mayne M. Amyloid-beta-(1-42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J Biol Chem. 2006;281(50):38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- 30.Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, Pessah IN, Lein PJ. PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ Health Perspect. 2012;120(7):997–1002. doi: 10.1289/ehp.1104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu B, Yamaguchi H, Lai FA, Shen J. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc Natl Acad Sci U S A. 2013;110(37):15091–15096. doi: 10.1073/pnas.1304171110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.